扩展功能
文章信息
- 黄晓斌, 仲蕾蕾
- HUANG Xiao-Bin, ZHONG Lei-Lei
- 遗传改造酿酒酵母生产植物萜类药物的策略
- The strategy of genetic engineering Saccharomyces cerevisiae to produce the plant-derived terpenoid drugs
- 微生物学通报, 2015, 42(10): 2019-20215
- Microbiology China, 2015, 42(10): 2019-20215
- 10.13344/j.microbiol.china.140974
-
文章历史
- 收稿日期: 2014-12-02
- 接受日期: 2015-06-11
- 优先数字出版日期(www.cnki.net): 2015-07-14
萜类化合物(Terpenoids),又称类异戊二烯,在自然界中分布十分广泛,是植物次生代谢产物,由异戊二烯(Isoprene)单元组成的化合物及其衍生物。根据所含异戊二烯数目的不同可以分为单萜(C10)、倍半萜(C15)、二萜(C20)、三萜(C30)、四萜(C40)和多萜等。由于萜类化合物分子中含有不同的碳环数目,又可因此分为链萜、单环萜、双环萜和三环萜等[1]。
萜类具有重要的商业价值,被广泛应用于工业、农业、医药卫生等领域。近几年来,植物萜类在医药上的成功应用引起了人们的广泛关注。比如青蒿素作为倍半萜类衍生物用于治疗疟疾[2, 3];紫杉醇作为二萜类衍生物用于治疗各种癌症[4, 5, 6];人参皂苷作为三萜类化合物常用于中药配方[7, 8];番茄红素作为抗氧化剂用于各种保健品[9, 10, 11];银杏内酯B作为迄今发现的最强的血小板活化因子拮抗剂,用于治疗缺血性脑中风的痰瘀阻络症[12];长春花萜类吲哚生物碱广泛应用于临床抗肿瘤、降血压、降血糖等[13];柠檬烯具有良好的镇咳、祛痰、抑菌作用[14, 15];榄香烯也被开发为抗肿瘤新药[16, 17, 18],等等。因为资源的有限性,直接从植物里提取这些萜类物质远远不能满足人们的需要,而且植物生长周期长、投入大、不易管理。
对酵母的萜类生物合成途径进行人为设计来生产萜类物质是近年来迅速发展的生物合成技术在应用上的具体表现。通过改造酵母的方式来生产这些萜类,不但成本低,周期短,而且不受地域环境影响,表达稳定,便于工业化生产及下游分离,使这些萜类物质更能为人类所应用[19, 20]。
1 萜类物质的生物合成萜类在植物中包括初生代谢物和次生代谢物两大部分。初生代谢物在植物基本生命活动中发挥着重要作用,例如植物甾醇参与细胞生物膜的构建[21];叶绿素、类胡萝卜素和质体醌参与其光合作用[22, 23];泛醌参与呼吸作用里的氧化还原反应[24];脱落酸、赤霉素、油菜素内酯和细胞分裂素则调节植物生长发育[25, 26, 27];多萜醇和异戊二烯基团参与蛋白修饰等[28]。植物的次生萜类代谢物的生物学功能主要表现在植物与环境的相互作用方面,如吸引传粉动物、防御食草动物吸食和病原微生物感染等[29, 30]。
植物萜类生物合成主要有两条途径。第一条是甲羟戊酸(Mevalonic acid,MVA)途径。它定位于细胞质中,以乙酰辅酶A作为原初供体生成异戊烯焦磷酸(Isopentenyl diphosphate,IPP),倍半萜类化合物、类固醇(甾体类)等经由这一途径合成[31];第二条途径是2-甲基赤藓糖醇-4-磷酸(2-Methyl-D-erythritol-4-phosphate,MEP)途径,它主要在植物质体中进行,以IPP为中间产物,分别合成叶绿素、叶醌、赤霉素、脱落酸、生育酚、单萜和质体醌等[32, 33]。在以酵母为宿主菌的基因改造研究中,主要是集中于MVA途径的改造。
为便于研究,可把植物萜类生物合成分为3个阶段:(1) 前体形成。包括起始单元IPP及其异构体二甲基烯丙基焦磷酸(Dimethylallyl diphosphate,DMAPP)、直接前体牻牛儿基焦磷酸(Geranyl diphosphate,GPP),法尼基焦磷酸(Farnesyl diphosphate,FPP),牻牛儿基牻牛儿焦磷酸(Geranyl geranyl diphosphate,GGPP),牻牛儿基法尼基焦磷酸(Geranylfarnesyldiphosphate,GFPP);(2) 骨架构建。分别包括单萜、倍半萜、二萜、二倍半萜、三萜等萜类骨架的形成;(3) 后修饰过程。根据修饰反应类型分为羟化反应、酰化反应、糖基化反应等。IPP与DMAPP是植物中萜类合成的共同前体,在相关酶GPPS (Geranyl diphosphate synthase)、FPPS (Farnesyl diphosphate synthase)、GGPPS (Geranylgeranyl diphosphate synthase)的作用下,IPP与DMAPP头尾缩合生成线性前体GPP、FPP、GGPP。其中,FPP是倍半萜、三萜、多萜的共同前体。在质体中,GPP是单萜的前体,GGPP是二萜、四萜、叶绿醌等的前体[34, 35]。植物萜类化合物的生物合成示意图过程见图 1。
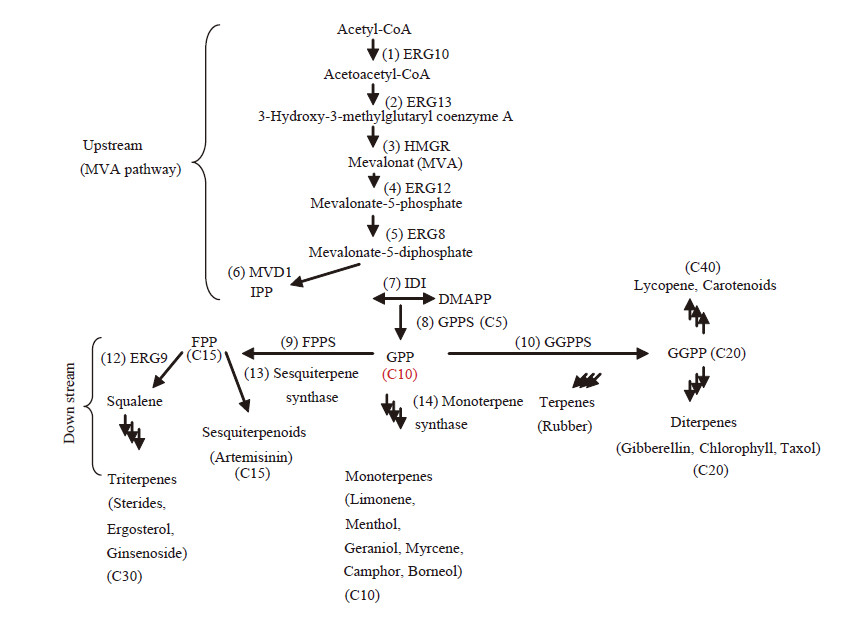
|
|
图 1
植物萜类 MVA 生物合成途径示意图
Figure 1
Schematic diagram for the biologic MVA synthesis pathway of plant terpenoid
注:ERG10:乙酰乙酰CoA硫解酶;ERG13:3-羟基-3-甲基戊二酰CoA氧化酶;HMGR:3-羟基-3-甲基戊二酰CoA还原酶;ERG12:甲羟戊酸激酶;ERG8:甲羟戊酸二磷酸激酶;MVD1:甲羟戊酸二磷酸脱羧酶;IDI:异戊烯基焦磷酸异构酶;IPP:异戊烯基焦磷酸;DMAPP:二甲基烯丙基焦磷酸;GPS:牻牛儿基焦磷酸分酶;GGPPS:牻牛儿基牻牛儿基焦磷酸合酶;FPPS:法尼基焦磷酸合酶;ERG9:角鲨烯合酶. Note: ERG10: Acetoacety1-CoA thiolase; ERG13: Hydroxymethylglutaryl-CoA oxidase; HMGR: Hydroxymethylglutaryl-CoA reductase; ERG12: Mevalonate kinas; ERG8: Phosphomevalonate kinas; MVD1: Mevalonate pyrophosphate decarboxylase; IDI: Isopentenyl diphosphate isomerase; IPP: Isopentenyl pyrophosphate; DMAPP: Dimethylallyl pyrophosphate; GPS: Geranyl diphosphate synthas; GGPPS: Geranyl geranyl diphosphate synthase; FPPS: Farnesyl diphosphate synthase; ERG9: Squalene synthase. |
酿酒酵母作为一种安全有效的真核微生物,遗传背景广为熟知,其基因序列早在1996年已被测出。目前,很多实验室研究把植物里特有的一些合成途径转到酿酒酵母,通过发酵,使其生产出植物才能生产的药物、保健品、化工原料等。这既扩展了原料来源渠道,对环境保护也起到了积极作用,而且在经济上也可以使制造加工成本降低[36]。
纵观目前世界各实验室改造酿酒酵母并通过发酵来生产萜类物质,所做研究工作主要集中于如下几方面:(1) 改变上游的HMGR基因;(2) 导入单个或多个下游靶基因;(3) 反义RNA或RNAi降低某基因表达强度;(4) 改变分叉代谢途径的流向;(5) 通过更换基因启动子来控制基因表达强弱;(6) 物种间同源基因的利用;(7) 转录因子基因的表达调节[37]。
表 1具体显示了近年来用酵母作为宿主表达菌研究的萜类物质种类、采用的方法策略及研究所取得的成果。
| 目标萜类 Terpenoids | 研究策略方法 Research strategies | 产量 Yield | 参考文献 References |
| 番茄红素 Lycopene | 采用酵母启动子、终止子控制,异源表达细菌类胡萝卜素类基因,使麦角固醇代谢流部分转向到类胡萝卜素类代谢流(图 1中的第9步转向第10、11步) | 0.113 mg/g番茄红素 | [38] |
| β-胡萝卜素 β-Carotene | 通过整合外源基因进入酵母染色体的方式超表达HMGR (第3步)和GGPPS (第11步) | 5.9 mg/g β-胡萝卜素 | |
| 青蒿素前体(紫穗槐-4,11-二烯、青蒿酸) Artemisinin precursor (Amorpha-4,11-diene, Arteannuic acid) | 分别以质粒和整合到染色体的方式向酵母中转入紫穗槐-4,11-二烯合酶(ADS)基因(第13步),使酵母合成紫穗槐-4,11-二烯,首次在真核生物中制备出青蒿素前体物质 | 0.6 mg/L紫穗槐二烯 | [39] |
| 改造酵母上游MVA途径(第1−7步);转入下游ADS基因(第13步),细胞色素P450单氧化酶(CYP71AV1)基因,第一次使酵母菌成功生产出青蒿酸 | 153 mg/L紫穗槐二烯32 mg/L青蒿酸 | [40] | |
| 改造上游途径MVA的HMGR (第3步);线粒体靶向转入FDP合成酶基因(第9步)和ADS基因(第13步) | 1.5 mg/L瓦伦烯20 mg/L紫穗槐烯 | [41] | |
| 在酵母中超表达乙醛脱氢酶基因(图 1第1步以前),并引入沙门氏菌的乙酰辅酶A合成酶基因(第1步),来增加MVA代谢途径的前体乙酰辅酶A的供应量 | 120 mg/L紫穗槐烯 | [42] | |
| 整体优化MVA上游途径和下游途径;转入青蒿醛脱氢酶(ALDH1)基因;控制下游氧化过程中的CYP71AV1、CPR1和CYB5基因表达比例(第13步);优化发酵底物和培养条件;减弱竞争途径ERG9的表达(第12步) | 25 g/L青蒿酸 | [43] | |
| 紫杉醇前体 Taxol precursor | 向酵母中转入合成途径前面部分的5个基因并显示活性(第1−5步);但继续到第一步细胞色素P450羟化酶就遇到困难(第15步以后) | 1.0 mg/L 紫杉二烯, 0.025 mg/L紫杉二 烯-5a-醇 | [44] |
| 改造HMGR (第3步);向酵母中转入优化的硫化叶菌GGPPS (第11步)和经优化的TXS基因(第15步),使紫杉二烯的含量提高40倍。 | 8.7 mg/L 紫杉二烯 | [45] | |
| α-檀香萜 α-Santalene | 通过更换启动子,对鲨烯合成酶ERG9表达进行控制(第12步),使甾醇方向的代谢流向檀香萜合成方向增加3.4倍;同时超表达HMGR (第3步);敲除脂质磷酸磷酸酶基因(LPP1),以控制第二条FPP代谢流出。 | 92 mg/L α-檀香萜 | [46] |
| 人参皂苷 Ginsenoside | 向酵母中转入达玛烯二醇-II合酶基因、原人参萜二醇合酶基因和拟南芥里的一个NADPH依赖的细胞色素P450还原酶基因;通过超表达HMGR,FPPS,ERG9和2,3-氧鲨烯合酶基因来增加鲨烯和2,3-氧鲨烯的浓度,同时通过密码子优化增加原人参二醇合酶活性,使原人参二醇含量增高262倍到1.189 g/L | 1.189 g/L原人参二醇 | [47] |
| 香叶醇 Geraniol | 在酵母中异源表达香叶醇合酶(第14步),并过表达MAF1以负调控tRNA。 | 36.04 mg/L香叶醇 | [48] |
目前用酵母代替高等植物来生产合成其次生代谢物萜类化合物还存在很多的问题,归纳起来,主要有以下几方面原因:(1) 植物中,后翻译修饰酶种类繁多[49]。不但已经分离得到的萜类结构复杂,其中间代谢产物也多种多样,多种酶多步参与催化;改变代谢途径中的一种酶活性就会影响到多种中间产物浓度,也因此影响其它酶活性。(2) 某些表达调控机制还不清楚。因为后修饰酶调控网络复杂,有些酶只有痕量水平表达,分离难度大。(3) 萜类代谢过程复杂。对上、下游某些所谓关键基因进行改造后,中间各种代谢产物的浓度就会相应变化,使各方向的代谢流此消彼长,其规律难以把握。(4) 酵母经过改造后,其生长稳定性可能会受到很大影响,发酵过程不易控制,副产物量比重大,导致关键产物产量不高。
针对这些可能存在的问题,结合前人大量研究结果(表 1),可以采取一些适当的策略,总结如下:(1) 适当提高上游关键酶(比如HMGR、IDI)的活性,直接增强前体物质IPP和DMAPP的供应[50, 51]。(2) 除了提高关键酶的活性,同时也要关注那些非限速酶的活性,找到他们最佳配合的平衡点。(3) 在酵母中异源表达植物基因时,要注意密码子优化,使其适合酵母菌的调控生长环境。(4) 根据目标产物,提高关键前体底物浓度。比如要提高青蒿素的产量,可以通过提高FPPS活性来提高FPP的量;要提高番茄红素的产量,则可通过加强GGPPS的活性来提高GGPP的量。(5) 抑制竞争代谢途径。方法有反义RNA技术及同源阻断等。比如鲨烯合酶和紫穗槐-4,l1-二烯合酶竞争性地利用FPP,则可通过反义RNA技术抑制鲨烯合酶的表达,增加青蒿素代谢流中FPP的量。(6) 合理利用选择压力,挑选耐受突变菌株。比如在培养基中施以适当浓度的制霉菌素、角鲨抑素、外源胆固醇等来选择固醇耐受菌株,可能其甲羟戊酸途径某些部分会得到加强,然后根据目标生产萜类,突变其关键基因,使其前体物质合成量增加,继而达到目标产物的增加。
总之,在对微生物进行代谢途径改造和再造时,可以根据目标产物选取关键的中间产物和主要路径,使关键中间产物的前一步反应的底物浓度增高,即“Push”策略;同时要加强催化此关键中间产物的酶活性水平,即直接“Pull”策略;然后考虑所有有竞争性的旁路代谢,改变旁路代谢流方向,即“Restrain”的策略[52]。综合多种方法,找到菌株适应生产的最佳平衡点,同时优化下游发酵及提纯工艺,从而达到比较理想的改造目的。
4 讨论自然界中,萜类化合物种类众多,但因资源有限、提取工艺复杂,难以大量生产。如青蒿素虽然是很好的抗疟疾药物,但受资源限制,导致供不应求。而尝试用微生物改造的方法大量获得青蒿素,则是可持续发展战略,但目前也只能得到中间产物青蒿酸,还需要借助化学半合成的方法才能获得功能完全的青蒿素。在酵母中青蒿酸的成功生物合成对人们利用微生物发酵生产其他药物是一个很大的鼓舞。目前很多实验室也在试图用类似的策略来生产紫杉醇等高效、高价药物,但仍然没有成功。归纳起来,问题主要有两个方面,一是紫杉醇本身的生物合成通路复杂,在酵母中难以再建一条这样的通路而不影响酵母生长;二是还有些通路处于模糊状态,比如MVA通路中一些关键前体FPP、GGPP等流向其它通路,一些关键酶还有待进一步进行鉴定及克隆,如紫杉烷1β-羟化酶、紫杉烷9α-羟化酶、紫杉烷9-酮基-氧化酶、紫杉烷C4,C20-β-环氧酶等。
酵母作为一种相对简单、安全的微生物,其正常的生长环境通过不断进化已经达到了一个平衡状态,人为地从基因组水平彻底地对其进行改造,它的一些生理功能有可能会受到影响而不能正常生长,或许经过改变后产生一些对人类有害的物质,所以在产品应用时也要加强评估。对它的一些旁路途径进行改造后要保证它的正常生长,以便最终正常发酵。选择性地在酵母中调控类萜生物合成途径;优化植物基因在其体内表达;从总体上来提高萜类合成前体和下游萜类产物的表达量。这样,随着生物技术的成熟,可以直接将某个物种的代谢途径转入工程菌实现工业化生产,但前提是要将代谢途径和调控研究透彻,而后修饰酶因其多样性和复杂性将是难点。但我们有理由相信,在不久的将来,利用微生物发酵技术来生产紫杉醇、人参皂苷等药物必将有很大的突破。
| [1] | Baunach M, Franke J, Hertweck C. Terpenoid biosynthesis off the beaten track: unconventional cyclases and their impact on biomimetic synthesis[J]. Angewandte Chemie, 2015, 54(9): 2604-2626 |
| [2] | Visser BJ, Wieten RW, Kroon D, et al. Efficacy and safety of artemisinin combination therapy (ACT) for non-falciparum malaria: a systematic review[J]. Malaria Journal, 2014, 13(1): 463 |
| [3] | Auparakkitanon, S. Discovery and development of antiplasmodial compounds in Thailand during the 21st century[J]. The Southeast Asian Journal of Tropical Medicine Public Health, 2014, 45(4): 761-782 |
| [4] | Tran TA, Gillet L, Roger S, et al. Non-anti-mitotic concentrations of taxol reduce breast cancer cell invasiveness[J]. Biochemical and Biophysical Research Communications, 2009, 379(2): 304-308 |
| [5] | Miller K, Neilan B, Sze DM. Development of Taxol and other endophyte produced anti-cancer agents[J]. Recent Patents on Anticancer Drug Discovery, 2008, 3(1): 14-19 |
| [6] | Walsh V, Goodman J. From taxol to Taxol: the changing identities and ownership of an anti-cancer drug[J]. Medical Anthropology, 2002, 21(3/4): 307-336 |
| [7] | Wang XY, Wang YG, Wang YF. Ginsenoside Rb1, Rg1 and three extracts of traditional Chinese medicine attenuate ultraviolet B-induced G1 growth arrest in HaCaT cells and dermal fibroblasts involve down-regulating the expression of p16, p21 and p53[J]. Photodermatology Photoimmunology and Photomedicine, 2011, 27(4): 203-212 |
| [8] | Lee CH, Kim JH. A review on the medicinal potentials of ginseng and ginsenosides on cardiovascular diseases[J]. Journal of Ginseng Research, 2014, 38(3): 161-166 |
| [9] | Feofilova EP, Tereshina VM, Memorskaia AS, et al. Fungal lycopene: the biotechnology of its production and prospects for its application in medicine[J]. Mikrobiologiia, 2006, 75(6): 725-730 |
| [10] | Lee CO. Complementary and alternative medicine patients are talking about: lycopene[J]. Clinical Journal of Oncology Nursing, 2005, 9(2): 245-246 |
| [11] | Kitade Y, Watanabe S, Masaki T, et al. Inhibition of liver fibrosis in LEC rats by a carotenoid, lycopene, or a herbal medicine, Sho-saiko-to[J]. Hepatology Research, 2002, 22(3): 196-205 |
| [12] | Johnston GA. Bilobalide, a sesquiterpene trilactone from Ginkgo biloba, is an antagonist at recombinant alpha1beta2gamma2L GABA(A) receptors[J]. European Journal of Pharmacology, 2003, 464(1): 1-8 |
| [13] | Liu DS, CE Krebs, SJ Liu. Proliferation of human breast cancer cells and anti-cancer action of doxorubicin and vinblastine are independent of PKC-alpha[J]. Journal of Cellular Biochemistry, 2007, 101(2): 517-528 |
| [14] | Liu T, Guo Y, Gao Z, et al. Bioequivalence evaluation of two D-limonene capsule formulations in healthy Chinese volunteers[J]. Die Pharmazie, 2008, 63(10): 718-720 |
| [15] | Krishnaiah YS, Chandrasekhar DV, Rama B, et al. In vivo evaluation of limonene-based transdermal therapeutic system of nicorandil in healthy human volunteers[J]. Skin Pharmacology and Physiology, 2005, 18(6): 263-272 |
| [16] | Chen M, Zhang J, Yu S, et al. Anti-lung-cancer activity and liposome-based delivery systems of beta-elemene[J]. Evidence-Based Complementary and Alternative Medicine, 2012, 2012: 259523 |
| [17] | Edris AE. Anti-cancer properties of Nigella spp. essential oils and their major constituents, thymoquinone and beta-elemene[J]. Current Clinical Pharmacology, 2009, 4(1): 43-46 |
| [18] | Tao L, Zhou L, Zheng L, et al. Elemene displays anti-cancer ability on laryngeal cancer cells in vitro and in vivo[J]. Cancer Chemotherapy and Pharmacology, 2006, 58(1): 24-34 |
| [19] | Ajikumar PK, Tyo K, Carlsen S, et al. Terpenoids: Opportunities for biosynthesis of natural product drugs using engineered microorganisms[J]. Molecular Pharmaceutics, 2008, 5(2): 167-190 |
| [20] | Bernhardt P, O’Connor SE. Opportunities for enzyme engineering in natural product biosynthesis[J]. Current Opinion in Chemical Biology, 2009, 13(1): 35-42 |
| [21] | Neelakandan AK, Nguyen HT, Kumar R, et al. Molecular characterization and functional analysis of Glycine max sterol methyl transferase 2 genes involved in plant membrane sterol biosynthesis[J]. Plant Molecular Biology, 2010, 74(4/5): 503-518 |
| [22] | Green BR, Durnford DG. The chlorophyll-carotenoid proteins of oxygenic photosynthesis[J]. Annual Review of Plant Physiology and Plant Molecular Biology, 1996, 47: 685-714 |
| [23] | Trebst A, Depka B, Jäger J, et al. Reversal of the inhibition of photosynthesis by herbicides affecting hydroxyphenylpyruvate dioxygenase by plastoquinone and tocopheryl derivatives in Chlamydomonas reinhardtii[J]. Pest Management Science, 2004, 60(7): 669-674 |
| [24] | Ribas-Carbo M, Wiskich JT, Berry JA, et al. Ubiquinone redox behavior in plant mitochondria during electron transport[J]. Archives of Biochemistry and Biophysics, 1995, 317(1): 156-160 |
| [25] | Kim SK, Sohn EY, Joo GJ, et al. Influence of jasmonic acid on endogenous gibberellin and abscisic acid in salt-stressed chard plant[J]. Journal of Environmental Biology, 2009, 30(3): 333-338 |
| [26] | Vriet C, Russinova E, Reuzeau C. From squalene to brassinolide: the steroid metabolic and signaling pathways across the plant kingdom[J]. Molecular Plant, 2013, 6(6): 1738-1757 |
| [27] | Werner T, Schmülling T. Cytokinin action in plant development[J]. Current Opinion in Plant Biology, 2009, 12(5): 527-538 |
| [28] | Siwko ME, Marrink SJ, de Vries AH, et al. Does isoprene protect plant membranes from thermal shock? A molecular dynamics study[J]. Biochimica et Biophysica Acta-Biomembranes, 2007, 1768(2): 198-206 |
| [29] | Büchel K, Malskies S, Mayer M, et al. How plants give early herbivore alert: Volatile terpenoids attract parasitoids to egg-infested elms[J]. Basic and Applied Ecology, 2011, 12(5): 403-412 |
| [30] | Bohlmann J, Martin D, Oldham NJ, et al. Terpenoid secondary metabolism in Arabidopsis thaliana: cDNA cloning, characterization, and functional expression of a myrcene/(E)-beta-ocimene synthase[J]. Archives of Biochemistry and Biophysics, 2000, 375(2): 261-269 |
| [31] | Lange BM, Croteau R. Isoprenoid biosynthesis via a mevalonate-independent pathway in plants: cloning and heterologous expression of 1-deoxy-D-xylulose-5-phosphate reductoisomerase from peppermint[J]. Archives of Biochemistry and Biophysics, 1999, 365(1): 170-174 |
| [32] | Lichtenthaler HK. The 1-deoxy-D-xylulose-5-phosphate pathway of isoprenoid biosynthesis in plants[J]. Annual Review of Plant Physiology and Plant Molecular Biology, 1999, 50: 47-65 |
| [33] | Lichtenthaler HK, Rohmer M, Schwender J. Two independent biochemical pathways for isopentenyl diphosphate and isoprenoid biosynthesis in higher plants[J]. Physiologia Plantarum, 1997, 101(3): 643-652 |
| [34] | Guo BH, Kai GY, Jin HB, et al. Taxol synthesis[J]. African Journal of Biotechnology, 2006, 5(1): 15-20 |
| [35] | Nagegowda DA. Plant volatile terpenoid metabolism: Biosynthetic genes, transcriptional regulation and subcellular compartmentation[J]. FEBS Letters, 2010, 584(14): 2965-2973 |
| [36] | Khor GK, Uzir MH. Saccharomyces cerevisiae: a potential stereospecific reduction tool for biotransformation of mono- and sesquiterpenoids[J]. Yeast, 2011, 28(2): 93-107 |
| [37] | Kuranda K, Francois J, Palamarczyk G. The isoprenoid pathway and transcriptional response to its inhibitors in the yeast Saccharomyces cerevisiae[J]. FEMS Yeast Research, 2010, 10(1): 14-27 |
| [38] | Yamano S, Ishii T, Nakagawa M, et al. Metabolic engineering for production of beta-carotene and lycopene in Saccharomyces cerevisiae[J]. Bioscience Biotechnology and Biochemistry, 1994, 58(6): 1112-1114 |
| [39] | Lindahl AL, Olsson ME, Mercke P, et al. Production of the artemisinin precursor amorpha-4, 11-diene by engineered Saccharomyces cerevisiae[J]. Biotechnology Letters, 2006, 28(8): 571-580 |
| [40] | Ro DK, Paradise EM, Ouellet M, et al. Production of the antimalarial drug precursor artemisinic acid in engineered yeast[J]. Nature, 2006, 440(7086): 940-943 |
| [41] | Farhi M, Marhevka E, Masci T, et al. Harnessing yeast subcellular compartments for the production of plant terpenoids[J]. Metabolic Engineering, 2011, 13(5): 474-481 |
| [42] | Shiba Y, Paradise EM, Kirby J, et al. Engineering of the pyruvate dehydrogenase bypass in Saccharomyces cerevisiae for high-level production of isoprenoids[J]. Metabolic Engineering, 2007, 9(2): 160-168 |
| [43] | Paddon CJ, Westfall PJ, Pitera DJ, et al. High-level semi-synthetic production of the potent antimalarial artemisinin[J]. Nature, 2013, 496(7446): 528-532 |
| [44] | Dejong JM, Liu Y, Bollon AP, et al. Genetic engineering of Taxol biosynthetic genes in Saccharomyces cerevisiae[J]. Biotechnology and Bioengineering, 2006, 93(2): 212-224 |
| [45] | Engels B, Dahm P, Jennewein S. Metabolic engineering of taxadiene biosynthesis in yeast as a first step towards Taxol (Paclitaxel) production[J]. Metabolic Engineering, 2008, 10(3/4): 201-206 |
| [46] | Scalcinati G, Knuf C, Partow S, et al. Dynamic control of gene expression in Saccharomyces cerevisiae engineered for the production of plant sesquitepene alpha-santalene in a fed-batch mode[J]. Metabolic Engineering, 2012, 14(2): 91-103 |
| [47] | Dai Z, Liu Y, Zhang X, et al. Metabolic engineering of Saccharomyces cerevisiae for production of ginsenosides[J]. Metabolic Engineering, 2013, 20: 146-156 |
| [48] | Liu J, Zhang W, Du G, et al. Overproduction of geraniol by enhanced precursor supply in Saccharomyces cerevisiae[J]. Journal of Biotechnology, 2013, 168(4): 446-451 |
| [49] | Li H, Horiguchi T, Croteau R et al. Studies on Taxol biosynthesis: preparation of taxadiene-diol and triol derivatives by deoxygenation of taxusin[J]. Tetrahedron, 2008, 64(27): 6561-6567 |
| [50] | Rico J, Pardo E, Orejas M. Enhanced production of a plant monoterpene by overexpression of the 3-Hydroxy-3-Methylglutaryl coenzyme a reductase catalytic domain in Saccharomyces cerevisiae[J]. Applied and Environmental Microbiology, 2010, 76(19): 6449-6454 |
| [51] | Ohto C, Muramatsu M, Obata S, et al. Overexpression of the gene encoding HMG-CoA reductase in Saccharomyces cerevisiae for production of prenyl alcohols[J]. Applied Microbiology and Biotechnology, 2009, 82(5): 837-845 |
| [52] | Lü X, Xie W, Lu W, et al. Enhanced isoprene biosynthesis in Saccharomyces cerevisiae by engineering of the native acetyl-CoA and mevalonic acid pathways with a push-pull-restrain strategy[J]. Journal of Biotechnology, 2014, 186: 128-136 |
 2015, Vol. 42
2015, Vol. 42




