扩展功能
文章信息
- 张伟, 侯书宁, 何婷, 徐淼, 黄新祥, 杨瑞馥, 周冬生, 张义全
- ZHANG Wei, HOU Shu-Ning, HE Ting, XU Miao, HUANG Xin-Xiang, YANG Rui-Fu, ZHOU Dong-Sheng, ZHANG Yi-Quan
- AphA 蛋白对副溶血弧菌 vopT 的转录调控研究
- Transcriptional regulation of vopT by AphA in Vibrio parahemolyticus
- 微生物学通报, 2015, 42(10): 1971-1976
- Microbiology China, 2015, 42(10): 1971-1976
- 10.13344/j.microbiol.china.140957
-
文章历史
- 收稿日期: 2014-11-27
- 接受日期: 2015-01-15
- 优先数字出版日期(www.cnki.net): 2015-03-09
2. 江苏大学医学院 江苏 镇江 212013;
3. 北京微生物流行病研究所病原微生物生物安全国家重点实验室 北京 100071
2. School of Medicine, Jiangsu University, Zhenjiang, Jiangsu 212013, China;
3. State Key Laboratory of Pathogen and Biosecurity, Beijing Institute of microbiology and Epidemiology, Beijing 100071, China
副溶血弧菌(Vibrio parahemolyticus)是一种嗜盐的革兰氏阴性弧菌,主要栖息于海水和海产品中。当人们食用生的或未被彻底煮熟的海产品时,可能会被其感染而引起以腹痛、腹泻、恶心、发热等为主要症状的急性胃肠炎[1]。流行病学调查显示副溶血弧菌已成为沿海地区引发食物中毒的首要病原菌[1, 2]。
III型分泌系统(T3SS)是一种由多组分蛋白复合体在细菌表面形成的“针状”注射装置,通过该装置可将分泌性效应蛋白直接注入宿主细胞内而发挥致病作用[3]。副溶血弧菌O3:K6型菌株RIMD2210633拥有两套T3SS基因簇,分别称为T3SS1和T3SS2[4]。T3SS1位于大染色体,主要与副溶血弧菌对巨噬细胞、HeLa细胞等的细胞毒性有关,可引起细胞凋亡[5];T3SS2位于小染色体致病岛(Vp-PAI)内,主要与肠毒性有关,但是也具有一定的细胞毒性[6, 7]。T3SS2与其他细菌的T3SS同源性较低,对其结构与功能的认识还比较肤浅,但是该系统一些转位蛋白(如VopB2和VopD2)和效应蛋白(如VopA/P、VopT、VopL和VopC等)的功能已经被研究得比较详细[8, 9, 10, 11]。其中,VopT具有ADP-糖基转移酶活性,能通过对靶蛋白特定的氨基酸残基进行ADP基团化修饰,而改变靶蛋白的生物学活性[9]。
T3SS是副溶血弧菌毒力因子之一,其转录表达应是一个被紧密调控的过程。已有研究表明T3SS1的表达受ExsA的激活,而受ExsD、ToxR、OpaR、H-NS和CalR等调控子的抑制[12, 13, 14, 15];另外,T3SS1的表达还受Ca2+和Fe2+的调节,高Ca2+或低Fe2+条件下表达量增高[16]。然而,T3SS2的转录调控研究还处于起步阶段,目前仅发现其表达受胆汁酸盐的诱导和VtrA及VtrB的激活[17, 18],而是否有其他调控子蛋白参与其表达调控,还有待于进一步研究。AphA是弧菌密度感应(Quorum sensing,QS)系统在低密度下的核心调控子[19, 20, 21, 22],研究表明霍乱弧菌的AphA可促进霍乱毒素基因ctx和菌毛基因tcp的转录[21],也可促进生物膜形成[22];表型结果显示副溶血弧菌的AphA也能增强生物膜形成、运动能力和对小鼠的致死毒性[20]。可见,弧菌的AphA主要起激活毒力因子表达和生物膜形成的作用。本文中,利用引物延伸、LacZ报告基因融合实验,实时定量RT-PCR、EMSA等经典的分子生化实验研究了AphA蛋白对T3SS2的效应蛋白基因——vopT的调控,结果发现AphA能间接抑制其转录,这丰富了我们对AphA功能的认识。
1 材料与方法 1.1 材料 1.1.1 菌株和质粒:实验所用的副溶血弧菌RIMD2210633株(野生型,WT)及其aphA非极性突变株(ΔaphA)[19, 20]、重组质粒vopT::pHRP309 (pHRP309质粒无启动子区的β-半乳糖苷酶基因上游分别克隆入vopT的启动子区序列,庆大霉素抗性)均由北京微生物流行病研究所病原微生物国家重点实验室保存。 1.1.2 主要试剂:HI肉汤(2.5% Bacto heart infusion)购自BD Bioscience;TRIzol Reagent购自Invitrogen公司;TaqDNA聚合酶和dNTPs购自Fermentas公司;PCR产物纯化试剂盒购自QIAGEN公司;Primer Extension System和fmol® DNA Cycle Sequencing System购自Promega公司;DNA-free™ Kit购自Amibion公司;LightCycler® 480 SYBR Green Master购自Roche公司。 1.2 细菌培养取10−20 μL甘油菌种接种至15 mL的HI肉汤中(50 mL的三角烧瓶,下同),37℃、200 r/min培养至平台期(12−14 h),而后按1:50稀释接种至15 mL新鲜的HI肉汤中,37℃、200 r/min培养至OD600大约为1.0,再按1:1 000稀释接种至15 mL新鲜的HI肉汤中,37℃、200 r/min培养至OD600约为0.15,收集菌体待用。
1.3 引物延伸实验[19]将能与vopT的mRNA互补的特异性引物(表 1)的5′-末端进行放射性标记,进而分别以WT和ΔaphA的总RNA为模板(总RNA的量一致),在逆转录酶的作用下,将其逆转录成cDNA。cDNA产物配伍测序反应的条带进行6%聚丙烯酰胺变性凝胶电泳,经放射自显影后,分析结果。
1.4 实时定量RT-PCR利用TRIzol Reagent提取细菌总RNA,进而利用DNA-free™ Kit去除总RNA中可能污染的基因组DNA,最后利用N6随机引物将总RNA逆转录为cDNA。用Roche的LightCycler system作RT-PCR分析。以16S rRNA基因的表达量为内参(引物序列见表 1),利用经典的2-ΔΔCt的方法对基因表达水平进行相对定量。
| Gene name | Sequences (5′→3′) |
| EMSA | |
| vopT | AAAAGATGTTTGTCGATTATTC/CTGCTTTTCAGATATGGAGG |
| vtrA | TACGCTTCCAATAATCACC/CCGATCTTGTGAGCCTAGAC |
| 16S rRNA | GACACGGTCCAGACTCCTAC/GGTGCTTCTTCTGTCGCTAAC |
| RT-PCR | |
| vopT | CGGCGGAGCAATTACTGG/TCTGGGTCTCGTGAGGTTG |
| vtrA | AGTCTAGGCTCACAAGATCG/AAATGGGCTCTGATGTTACG |
| Primer extension | |
| vopT | TCTACAAACCTTCACATCTGC |
| lacZ fusion | |
| vopT | GCGCGTCGACAAAAGATGTTTGTCGATTATTC/GCGCGAATTCCTGCTTTTCAGATATGGAGG |
PCR扩增vopT的整个启动子区序列(引物序列见表 1),并将其直接克隆入pHRP309质粒的β-半乳糖苷酶基因(无启动子区)的上游,以构建LacZ重组质粒。将重组质粒分别转入WT和ΔaphA中。细菌按1.2的方法培养收集后,通过检测并比较WT和ΔaphA菌株中β-半乳糖苷酶活性的差异,即可判断AphA对vopT的调控关系,一般以t检验的P<0.01为标准。
1.6 凝胶阻滞实验(EMSA)[23]PCR扩增vopT的启动子区(引物序列见表 1),并纯化回收,用T4多聚核苷激酶(T4 PNK)对DNA片段5′-末端进行放射性标记。利用Ni-NTA柱法纯化His-AphA蛋白。将不同浓度的His-AphA蛋白与标记的DNA探针在10 μL结合反应体系中,室温共同孵育20 min后,进行4%非变性聚丙烯酰胺凝胶中进行电泳,放射自显影后分析结果。
2 结果与分析 2.1 AphA抑制vopT的转录首先利用引物延伸实验搜寻vopT的转录起始位点,其结果如图 1a所示:vopT的转录起始位点为−86位的A (翻译起始位点为+1),且只能在ΔaphA中检测出引物延伸产物,而在WT中检测不到,这表明AphA能抑制vopT的转录;进一步采用LacZ报告基因融合实验研究AphA对vopT的调控关系,如图 1b所示:在WT中所检测到的β-半乳糖苷酶活性(Miller units)明显低于ΔaphA中的,且二者具有显著性差异(P<0.01),这说明AphA能抑制vopT的转录;图 1c为AphA对vopT调控的实时定量RT-PCR结果,可以看出:ΔaphA中vopT的mRNA表达水平明显高于WT中,二者相差大约7倍,这

|
|
图1
AphA负调控vopT的转录
Figure 1
AphA represses the transcription of vopT
注:G、A、T、C:Sanger测序条带;正、负数值表示距离起始密码子上下游的碱基数(翻译起始位点为“+1”). Note: Lanes G, A, T and C represented the Sanger sequencing reactions. The positive and minus numbers in the brackets indicated the nucleotide sites upstream and downstream of the translation start (+1), respectively. |
进一步说明vopT的转录受AphA的抑制。总之,在低密度下,AphA负调控vopT的转录。
2.2 AphA不能结合到vopT的启动子区图 1的结果显示AphA能抑制vopT的转录,为了验证这种转录抑制作用是否是直接通过蛋白-DNA相互作用而实现的,表达纯化了His-AphA重组蛋白,并采用体外的EMSA实验进行验证,结果如图 2所示:当His-AphA蛋白使用量达到60.0 pmol时,vopT仍未出现阻滞条带,而在前期研究中His-AphA在低浓度下(15.0 pmol)就能结合到aphA的启动子区[19],说明AphA不能结合到vopT的启动子区。

|
|
图2
His-AphA对vopT启动子区结合的EMSA实验结果
Figure 2
EMSA assay of binding of His-AphA to vopT promoter region
注:正、负数值表示距离起始密码子上下游的碱基数(翻译起始位点为“+1”). Note: The positive and minus numbers in the brackets indicated the nucleotide sites upstream and downstream of the translation start (+1), respectively. |
上文结果显示AphA只能间接抑制vopT的转录,而Kodama等[18]的研究结果表明VtrA蛋白能激活Vp-PAI内基因(包括vopT)的表达。据此推测:AphA可能是通过抑制VtrA的转录而间接抑制vopT的表达。RT-PCR结果(图 3a)显示vtrA的转录确实受AphA的抑制,但是体外的EMSA结果(图 3b)却表明His-AphA对vtrA启动子区没有直接的结合作用,这说明AphA不是通过VtrA而间接抑制vopT的转录。
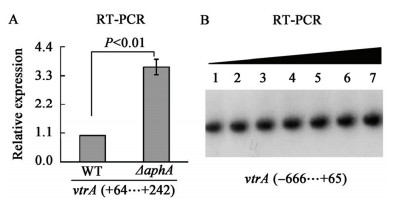
|
|
图3
AphA间接抑制vtrA的转录
Figure 3
AphA represses the transcription of vtrA in an indirect manner
注:正、负数值表示距离起始密码子上下游的碱基数(翻译起始点为“+1”). Note: The positive and minus numbers in the brackets indicated the nucleotide sites upstream and downstream of the translation start (+1), respectively. |
AphA是弧菌的毒力调控子,参与调控众多细胞途径。霍乱弧菌的AphA能直接结合到vpsT的启动子区并激活其表达,VpsT再通过激活胞外多糖的合成而促进生物膜形成[23]。AphA与AphB相互作用可激活tcpPH的转录,TcpP/TcpH再通过激活毒力调控子ToxT的表达而促进ctx (编码霍乱毒素)
和tcp (编码菌毛)的转录[22]。在副溶血弧菌中,对AphA功能的研究还处于起始阶段,仅有表型结果显示它能增强生物膜形成、运动能力和对小鼠的致死毒性等,但对其分子机制还一无所知[20]。本文利用引物延伸、LacZ报告基因融合实验和实时定量RT-PCR实验证明了AphA能抑制vopT(VPA1327)的转录,而随后的EMSA结果显示His-AphA不能作用于vopT的启动子区。vopT位于Vp-PAI内,编码产物为T3SS2的效应蛋白[9]。Kodama等的研究结果表明Vp-PAI内的基因转录均受VtrA和VtrB蛋白的激活,而VtrA又能激活vtrB的转录[18]。我们推测AphA可能是通过抑制VtrA的转录而间接抑制vopT的表达。然而,RT-PCR和EMSA结果(图 3)表明AphA只能间接抑制vtrA的转录,这说明我们的推测是不成立的。因此,AphA对T3SS2基因转录的间接抑制机制还有待于进一步研究。VopT是一种ADP-糖基转移酶,能将NAD+上的ADP基团转移至靶蛋白特定的氨基酸残基上,从而改变靶蛋白的生物学活性[9]。VopT对Caco-2和HCT-8细胞也具有一定的细胞毒性[9]。可见,在低密度时,AphA对副溶血弧菌毒力因子的表达具有正、负调控的双重模式。本研究结果不仅丰富了对AphA蛋白功能的认识,还有助于理解该菌的致病机制。
| [1] | Daniels NA, MacKinnon L, Bishop R, et al. Vibrio parahaemolyticus infections in the United States, 1973-1998[J]. The Journal of Infectious Diseases, 2000, 181(5): 1661-1666 |
| [2] | Wang S, Duan H, Zhang W, et al. Analysis of bacterial foodborne disease outbreaks in China between 1994 and 2005[J]. FEMS Immunology & Medical Microbiology, 2007, 51(1): 8-13 |
| [3] | Yang F, Yang QL, Du ZM. Function and regulation of type Ⅲ secretion system in Yersinia species[J]. Letters in Biotechnology, 2013, 24(3): 418-422 (in Chinese)阳芬, 杨秋林, 杜宗敏. 耶尔森菌 Ⅲ 型分泌系统研究进展[J]. 生物技术通讯, 2013, 24(3): 418-422 |
| [4] | Makino K, Oshima K, Kurokawa K, et al. Genome sequence of Vibrio parahaemolyticus: a pathogenic mechanism distinct from that of V. cholerae[J]. The Lancet, 2003, 361(9359): 743-749 |
| [5] | Park KS, Ono T, Rokuda M, et al. Functional characterization of two type Ⅲ secretion systems of Vibrio parahaemolyticus[J]. Infection and Immunity, 2004, 72(11): 6659-6665 |
| [6] | Caburlotto G, Lleo MM, Hilton T, et al. Effect on human cells of environmental Vibrio parahaemolyticus strains carrying type Ⅲ secretion system 2[J]. Infection and Immunity, 2010, 78(7): 3280-3287 |
| [7] | Hiyoshi H, Kodama T, Iida T, et al. Contribution of Vibrio parahaemolyticus virulence factors to cytotoxicity, enterotoxicity, and lethality in mice[J]. Infection and Immunity, 2010, 78(4): 1772-1780 |
| [8] | Kodama T, Hiyoshi H, Gotoh K, et al. Identification of two translocon proteins of Vibrio parahaemolyticus type Ⅲ secretion system 2[J]. Infection and Immunity, 2008, 76(9): 4282-4289 |
| [9] | Kodama T, Rokuda M, Park KS, et al. Identification and characterization of VopT, a novel ADP-ribosyltransferase effector protein secreted via the Vibrio parahaemolyticus type Ⅲ secretion system 2[J]. Cellular Microbiolory, 2007, 9(11): 2598-2609 |
| [10] | Liverman AD, Cheng HC, Trosky JE, et al. Arp2/3-independent assembly of actin by Vibrio type Ⅲ effector VopL[J]. Proceedings of The National Academy of Sciences, 2007, 104(43): 17117-17122 |
| [11] | Trosky JE, Mukherjee S, Burdette DL, et al. Inhibition of MAPK signaling pathways by VopA from Vibrio parahaemolyticus[J]. Journal of Biological Chemistry, 2004, 279(50): 51953-51957 |
| [12] | Henke JM, Bassler BL. Quorum sensing regulates type Ⅲ secretion in Vibrio harveyi and Vibrio parahaemolyticus[J]. Journal of Bacteriology, 2004, 186(12): 3794-3805 |
| [13] | Kodama T, Yamazaki C, Park KS, et al. Transcription of Vibrio parahaemolyticus T3SS1 genes is regulated by a dual regulation system consisting of the ExsACDE regulatory cascade and H-NS[J]. FEMS Microbiology Letters, 2010, 311(1): 10-17 |
| [14] | Whitaker WB, Parent MA, Boyd A, et al. The Vibrio parahaemolyticus ToxRS regulator is required for stress tolerance and colonization in a novel orogastric streptomycin-induced adult murine model[J]. Infection and Immunity, 2012, 80(5): 1834-1845 |
| [15] | Zhou X, Konkel ME, Call DR. Regulation of type Ⅲ secretion system 1 gene expression in Vibrio parahaemolyticus is dependent on interactions between ExsA, ExsC, and ExsD[J]. Virulence, 2010, 1(4): 260-272 |
| [16] | Gode-Potratz CJ, Chodur DM, McCarter LL. Calcium and iron regulate swarming and type Ⅲ secretion in Vibrio parahaemolyticus[J]. Journal of Bacteriology, 2010, 192(22): 6025-6038 |
| [17] | Gotoh K, Kodama T, Hiyoshi H, et al. Bile acid-induced virulence gene expression of Vibrio parahaemolyticus reveals a novel therapeutic potential for bile acid sequestrants[J]. PLoS One, 2010, 5(10): e13365 |
| [18] | Kodama T, Gotoh K, Hiyoshi H, et al. Two regulators of Vibrio parahaemolyticus play important roles in enterotoxicity by controlling the expression of genes in the Vp-PAI region[J]. PLoS One, 2010, 5(1): e8678 |
| [19] | Sun F, Zhang Y, Wang L, et al. Molecular characterization of direct target genes and cis-acting consensus recognized by quorum-sensing regulator AphA in Vibrio parahaemolyticus[J]. PLoS One, 2012, 7(9): e44210 |
| [20] | Wang L, Ling Y, Jiang H, et al. AphA is required for biofilm formation, motility, and virulence in pandemic Vibrio parahaemolyticus[J]. International Journal of Food Microbiology, 2013, 160(3): 245-251 |
| [21] | Childers BM, Klose KE. Regulation of virulence in Vibrio cholerae: the ToxR regulon[J]. Future Microbiology, 2007, 2(3): 335-344 |
| [22] | Yang M, Frey EM, Liu Z, et al. The virulence transcriptional activator AphA enhances biofilm formation by Vibrio cholerae by activating expression of the biofilm regulator VpsT[J]. Infection and Immunity, 2010, 78(2): 697-703 |
| [23] | Zhang YQ, Gao H, Wang L, et al. Purification of recombinant H-NS protein of Yersinia pestis and characterization of its DNA-binding activity[J]. Acta Microbiologica Sinica, 2011, 51(5): 615-621 (in Chinese)张义全, 高鹤, 王丽, 等. 鼠疫菌H-NS蛋白的表达与纯化及其DNA结合活性分析[J]. 微生物学报, 2011, 51(5): 615-621 |
 2015, Vol. 42
2015, Vol. 42




