中国科学院微生物研究所、中国微生物学会主办
文章信息
- 喻伟艳, 许跃强, 李建军, 李志敏, 王琪, 杜昱光
- YU Weiyan, XU Yueqiang, LI Jianjun, LI Zhimin, WANG Qi, DU Yuguang
- 新型冠状病毒的糖基化、糖受体识别及糖链抑制剂的发现
- Glycosylation, glycan receptors recognition of SARS-CoV-2 and discoveries of glycan inhibitors against SARS-CoV-2
- 生物工程学报, 2022, 38(9): 3157-3172
- Chinese Journal of Biotechnology, 2022, 38(9): 3157-3172
- 10.13345/j.cjb.220175
-
文章历史
- Received: March 7, 2022
- Accepted: June 1, 2022
2. 中国科学院过程工程研究所 生化工程国家重点实验室, 北京 100190;
3. 大连医科大学附属第二医院 肺癌转化医学中心, 辽宁 大连 116023
2. State Key Laboratory of Biochemical Engineering, Institute of Processing and Engineering, Chinese Academy of Sciences, Beijing 100190, China;
3. Lung Cancer Translational Medicine Center, The Second Affiliated Hospital of Dalian Medical University, Dalian 116023, Liaoning, China
2020年1月30日世界卫生组织宣布由SARS-CoV-2感染人类引发的新型冠状病毒肺炎为国际关注的突发公共卫生事件,并于2020年3月11日宣布该病为大流行病。新型冠状病毒SARS-CoV-2属于冠状病毒科的β-冠状病毒属[1],其与非典冠状病毒SARS-CoV和中东呼吸综合征相关病毒MERS-CoV是3种对人类生命最具威胁的冠状病毒[2]。SARS-CoV-2传播力强,有较高的临床发病率,截至2022年5月底,全球已有超过5亿人感染,其中死亡人数超过600万(WHO coronavirus (COVID-19) dashboard)。SARS-CoV-2主要通过喷嚏和咳嗽从上呼吸道排出的飞沫传播,随后会黏附在鼻腔、口腔、眼睛以及呼吸道的黏膜表面[3-4]。SARS-CoV-2会感染呼吸道上皮细胞,如肺中分泌纤毛黏液支气管上皮细胞和Ⅰ型肺细胞,以及胃肠道上皮细胞[5-6]。SARS-CoV-2的刺突(spike, S) 蛋白可以与宿主细胞膜表面的受体血管紧张素转化酶2 (human angiotensin-converting enzyme 2, hACE2) 结合,再经TMPRSS2蛋白酶及Furin蛋白酶切割后与细胞膜融合进而实现入侵,病毒进入宿主体内后会释放遗传物质进一步复制和扩增。新型冠状病毒对人体的入侵会引起炎症因子风暴,并导致弥漫性肺泡损伤,最终引起肺水肿和呼吸衰竭。针对SARS-CoV-2的结合、蛋白酶加工、融合、内吞及复制等过程,全球的科学家及各大药厂研发了一系列的抗病毒药物,包括老药新用的瑞德西韦、法匹拉韦等,而新开发的靶向病毒RNA合成的莫纳匹拉韦和3CL蛋白酶抑制剂帕罗维德目前已逐步在全球上市。国内也有普克鲁胺、阿兹夫定、VV116、DC402234、SHEN26等多个抗新冠小分子药物进入临床试验[7]。中医药在预防及治疗新型冠状病毒肺炎中也占据着重要的地位,连花清瘟颗粒、香霍喷雾剂及基于四性五味的中药新处方等在新型冠状病毒肺炎的治疗中得到了应用[8-11],此外,中和抗体和疫苗也是对抗新冠病毒的有力武器。目前,全球已有超过300种新冠候选疫苗,198种新冠疫苗处于临床前研究阶段,156种进入临床试验,11种获得世界卫生组织紧急使用授权,其中包括灭活疫苗(BBIBP-CorV、PiCoVacc等)、重组蛋白质疫苗(ZF2001等)、mRNA疫苗(mRNA-1273、BNT162 mRNA等) 和腺病毒疫苗(Ad5-nCoV等)。这些疫苗在全球的大规模接种有效预防了新型冠状病毒的感染及减轻感染引起的症状。在抗体药物方面,包括礼来、阿斯利康、葛兰素史克、再生元等在内的全球各大药企及国内的创新药企已开发一系列产品进入临床试验或得到紧急授权使用,但部分抗体对Omicron突变株无效[7, 12]。中国腾盛博药研发的新冠病毒中和抗体联合药物安巴韦单抗/罗米司韦单抗注射液(BRII-196/BRII-198) 于2021年12月8日获得国家药品监督管理局的紧急审批,并进入国家卫健委于2022年3月15日发布的《新型冠状病毒肺炎诊疗方案》[7]。虽然疫苗及药物的出现有效降低了SARS-CoV-2的传播和感染,但是随着新型冠状病毒变异株的不断出现,如Alpha突变株(B.1.1.7)、Beta突变株(B.1.351)、Gamma突变株(P.1)、Kappa突变株(B.1.671.1)、Delta突变株(B.1.617.2) 及Omicron (B.1.1.529) 等,这些疫苗及药物对新型冠状病毒“群防”的效果却不尽如人意。特别是对于感染力更强的Omicron突变株,人们以SARS-CoV-2武汉株为模本开发的抗体药物及疫苗已出现了效价的大幅下滑[12-14]。面对这种情况,寻找新的预防和治疗手段迫在眉睫。
我们注意到,新型冠病毒的S蛋白是一个高度糖基化的蛋白,而在其对宿主细胞受体的识别、结合及入侵过程中,糖识别机制发挥了重要的作用,这也为抗SARS-CoV-2糖类药物的开发提供了契机。因此,本文将从新冠病毒的糖基化、糖链介导的入侵机制及抗新型冠状病毒糖类药物开发及机制研究几个方面阐述糖链与新型冠状病毒的关系,旨在为开发抗冠状病毒糖类药物提供参考依据。
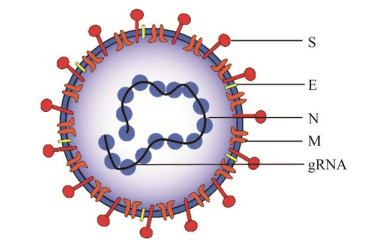
1 SARS-CoV-2的糖基化修饰SARS-CoV-2属于包膜病毒,具有约30 kb的正义单链RNA基因组,可转录多达29种蛋白质,其中主要的4种结构蛋白包括刺突糖蛋白、包膜(envelope, E) 蛋白、膜(membrane, M) 蛋白和核衣壳(nucleocapsid, N) 蛋白[15] (图 1)。
|
| 图 1 新型冠状病毒的结构 Fig. 1 Structure model of SARS-CoV-2. |
| |
S蛋白是一种高度糖基化的跨膜蛋白,以三聚体形式存在,由两个亚基组成:S1亚基包含N端域(N-terminal domain, NTD)、受体结合域(receptor binding domain, RBD) 和其他几个结构域,S2亚基介导病毒-细胞膜融合[16-17]。其中受体结合域RBD负责与hACE2受体结合,而在S1和S2之间有一个PRRA序列基序,该序列包含一个Furin蛋白酶裂解位点,该位点对于病毒进入人体细胞至关重要[18]。研究显示,宿主细胞表面丝氨酸蛋白酶TMPRSS2参与许多冠状病毒包括SARS-CoV-2的质膜融合[19-21],而溶酶体组织蛋白酶通常参与内体膜的融合过程[22]。冠状病毒的S蛋白对于病毒颗粒与宿主受体的相互作用至关重要,如SARS-CoV与血管紧张素转换酶2 (ACE2)[19, 23]、MERS-CoV与二肽基肽酶4 (DPP4)[24]以及HCoV-229E与氨肽酶N (APN) 结合[25]。
S蛋白具有广泛的糖基化,其单体含有22个N-糖基化位点和17个O-糖基化位点[26-27],其糖基化位点的分布如图 2所示。S蛋白表面覆盖着大量的糖链,这些糖链的存在可以避免宿主免疫系统对病毒的识别,从而逃避免疫系统的杀伤。值得注意的是,不论从哺乳动物细胞还是从昆虫系统表达所得的S蛋白,以及从SARS-CoV-2中提取的S蛋白,22个N-糖基化位点始终存在,而S蛋白在不同宿主表达系统中获得的O-糖基化修饰存在差异[28],这意味着O-糖基化修饰可能使新型冠状病毒S蛋白在结构上具有更高的灵活性。

|
| 图 2 新型冠状病毒S蛋白的糖基化位点分布 Fig. 2 Distribution of the glycosylation sites in S protein of SARS-CoV-2 (A) Distribution of glycosylation sites in S protein. The lower branch with blue indicates N-glycosites and the upper branch with red indicates O-glycosites; NTD: N-terminal structural domain; RBD: receptor binding domain; FP: fusion peptide; HR1: heptad repeat 1; HR2: heptad repeat 2; CH: central helix; CD: connector domain; TM: transmembrane structural domain; CT: cell tail. (B) Structural simulation of glycosylated S proteins (PDB: 6XR8[29]). Pink indicates S protein A chain, purple indicates S protein B chain, yellow indicates S protein C chain, red dots indicates O-glycosylation sites, green dots indicates N-glycosylation sites. |
| |
S蛋白融合前以亚稳态构象存在,这种状态称为“向下” (down) 构象;当S1亚基与宿主细胞受体结合时,蛋白结构进行重排,暴露出与受体结合的关键因素,这种状态称为“向上” (up) 构象。受体结合后,三聚体不稳定,导致S1亚基脱落,S2亚基转变为稳定的融合后构象,介导病毒膜与宿主细胞膜融合[29]。有研究发现,当S蛋白处于“向下”构象时,受体结合位点可被近端糖基化位点(N165、N243或N343) 隐蔽,从而保护关键氨基酸残基和其他表位不受细胞和抗体的识别。这几个糖基化位点对于S蛋白处于“打开”还是“关闭”状态非常重要,也直接影响了对hACE2的结合[30]。
1.2 新型冠状病毒其他蛋白的糖基化有证据表明,除S蛋白,E、M蛋白的表达也具有N-糖基化修饰[31]。SARS-CoV-2的N蛋白与甘露糖结合相关丝氨酸蛋白酶2 (MASP-2) 结合,该酶是MBL补体激活途径中的关键酶,异常结合会导致补体激活和加重肺损伤[32]。N蛋白还与11种人类趋化因子高度结合,可导致白细胞的趋化作用被抑制[33]。E蛋白则通过糖链识别结合TLR2受体,引起炎症细胞因子的释放,如肿瘤坏死因子(tumor necrosis factor α, TNF-α) 和干扰素γ (interferon gamma, INF-γ)[34]。
1.3 病毒糖基化修饰的生理功能及意义与其他病原体相比,大部分病毒自身不能进行糖基化修饰,病毒在细胞内复制过程中,可利用宿主的糖基化系统对自身蛋白进行修饰。病毒的糖基化通常具有以下功能[35]:第一,有助于糖蛋白的折叠和运输,进而完成病毒组装。第二,糖基化有利于病毒的释放。第三,病毒可以通过脱落或分泌糖蛋白误导免疫反应进行免疫逃避。这些分泌的糖蛋白类似于“替身”,可以结合中和抗体或引起非中和抗体的产生。如埃博拉病毒分泌的sGP糖蛋白[36]及呼吸道合胞病毒分泌的G糖蛋白[37]。第四,病毒表面糖链可以遮蔽抗原表位,逃避免疫系统的识别。第五,病毒表面糖链可被宿主体内的糖受体识别,便于病毒在体内的运输及感染宿主。第六,病毒表面糖链本身还可作为抗原表位。
对于新型冠状病毒来说,糖基化不仅影响自身的折叠及结构稳定,而且对于其入侵、识别及免疫逃避都非常重要,相关的研究还在不断深入中。
2 糖链介导的新型冠状病毒入侵 2.1 SARS-CoV-2对宿主细胞表面糖胺聚糖的结合研究表明,SARS-CoV-2通过S蛋白的RBD与hACE2结合从而进入宿主细胞[19, 38] (图 3A)。通过对SARS-CoV-2 S蛋白序列分析,与SARS- CoV相比,该病毒已进化出潜在的糖胺聚糖(glycosaminoglycan, GAG) 结合域[39-40]。GAG是一个线性硫酸多糖家族,几乎存在于所有哺乳动物细胞表面,通常包括硫酸乙酰肝素(heparan sulfate, HS)、硫酸软骨素(chondroitin sulfate, CS)、透明质酸(hyaluronic acid, HA)、硫酸皮肤素(dermatan sulfate, DS) 和硫酸角质素(keratan sulfate, KS),这些多糖已被证明可促进多种病毒附着[41-42]。SARS-CoV-2与宿主细胞表面的硫酸乙酰肝素结合可促进S蛋白的RBD与hACE2的结合。在这种相互作用下,S蛋白结构由“向下”构象变为“向上”构象,使其更易与宿主受体结合[43-44] (图 3B)。此外,硫酸乙酰肝素的分子多样性在支持病毒进入、运输和复制过程中起着重要作用,而这些过程跨越了病毒生命周期的大多数细胞过程[45]。

|
| 图 3 SARS-CoV-2感染宿主的过程及S蛋白的构象变化[46] Fig. 3 The process of SARS-CoV-2 infection and conformation change of S protein (A) SARS-CoV-2 infection processes through cell surface HSPG. HSPG: acetyl heparan sulfate proteoglycan; hACE2: human angiotensin-converting enzyme 2; TMPRSS2: transmembrane serine protease. (B) Structure conformation simulation of SARS-CoV-2 (one RBD-up conformation, PBD: 7EB3[46]; two RBD-up conformation, PBD: 7EB5[46]). |
| |
hACE2有7个N-糖基化和1个O-糖基化位点[47],其中N90、N322和N546会促进S蛋白与hACE2的结合[48]。此外,在人类基因组中发现hACE2会存在单核苷酸多态性(single nucleotide polymorphisms, SNPs),其改变可能会影响hACE2的糖基化以及对病毒S蛋白的亲和力[49]。之前报道,hACE2在各种类型细胞中表达,如心脏、肾脏和睾丸的内皮细胞和肺泡上皮细胞[50-51]。最近研究发现,hACE2在肺、肝脏、皮肤和肠道内皮细胞表达水平相对较低以及在肺组织中检测不到,仅在肺泡上皮Ⅱ型、基底细胞和杯状细胞亚群上表达[52–55]。
虽然SARS-CoV-2通过hACE2受体进入细胞的机制已明确,但该病毒在不表达或低表达hACE2的细胞中也被检测出,以及在病毒引起的“过度免疫激活”的免疫系统中依旧生存,这似乎表明还存在其他作用机制。
2.3 宿主免疫系统凝集素受体对SARS- CoV-2的识别病毒侵入宿主细胞,一方面可由进入受体介导,通过内吞和融合等多种机制进入细胞;另一方面,细胞表面的聚糖和凝集素可作为黏附受体与病毒结合,介导病毒的入侵。宿主免疫系统有一类进化保守的糖蛋白——凝集素,能够结合不同的糖链表位,并以糖链依赖的形式启动先天性免疫反应[56]。细胞表面的凝集素可作为进入受体直接介导病毒的入侵,也可作为黏附分子,以转染的方式加强进入受体(ACE2) 介导的传播[57-58]。SARS-CoV的8个糖基化位点与受体DC-SIGN、L-SIGN发生相互作用,其中有6个糖基化位点在SARS-CoV-2中是保守的[59-60]。同样,S蛋白糖链表型的鉴定结果中,包含一些凝集素的配体,这似乎暗示SARS-CoV-2可能具有类似的相互作用。
新型冠状病毒RBD的N331和N343位点上的N-糖链可以特异性结合巨噬细胞半乳糖凝集素(macrophage galactose-type lectin, MGL)、人半乳糖凝集素galectin-3/7/8、Siglec-10和DC-SIGN[61]。而S蛋白的NTD及CTD结构域与C型凝集素DC-SIGN、L-SIGN、LSECtin、ASGR1及CLEC10A等受体结合[62]。此外,S蛋白上Thr323/Ser325的O-糖链在MGL与SARS-CoV-2的结合中发挥重要作用[60, 63]。
在缺乏hACE2的内皮细胞中,L-SIGN和DC-SIGN可作为进入受体介导病毒的感染。L-SIGN可以与hACE2相互作用,形成异二聚体,说明L-SIGN可以通过依赖hACE2及不依赖hACE2两种方式介导SARS-CoV-2的感染。而S蛋白与DC-SIGN的结合依赖于糖链[60],去除L-SIGN的N92位的N-糖链,L-SIGN和S蛋白的结合更紧密[64]。这说明在hACE2低表达的细胞中,凝集素可能是SARS-CoV-2感染细胞的协同受体。
表 1展示了最近研究报道的细胞表面与SARS-CoV-2结合的凝集素受体,从表中看出,这些受体大部分位于免疫细胞表面,这似乎表明病毒通过与受体结合参与了免疫调节,从而说明在免疫系统过度活化的患者体内,病毒依旧生存的可能原因之一。
| Receptors | Organ | Cell types | Glycan ligand | References |
| DC-SIGN | Lung | Dendritic cells | High mannose and fucosylated glycans | [57-58, 60, 63-64] |
| L-SIGN | Lung | Lung endothelial cells, type Ⅱ alveoli cells | High mannose and complex N-glycans | [57-58, 60, 64] |
| MGL | Lung Upper respiratory tract |
Dendritic cells and macrophage |
Gal and GalNAc terminated glycans, complex N-glycans | [58, 60, 63-64] |
| MR | Lung Upper respiratory tract |
Dendritic cells and macrophage | High mannose N-glycans | [60] |
| Langerin | Marrow | Langerhans cells | High mannose and sulfated glycans | [58] |
| Siglec-1 | Lung | Myeloid cells, macrophage | GM1 | [57, 65] |
| Siglec-3/9/10 | Marrow | Monocytes, macrophage, neutrophil, DCs, NK cells, T-lymphocytes, B-lymphocytes, eosinophilic granulocyte | Fucosylated or sulfated α2, 3-sialic acid, α2, 6-sialic acid | [63] |
如上所述,新型冠状病毒可利用糖链对宿主细胞侵染和逃避免疫反应,但是,糖类抗病毒的效果也是显而易见的[66-69],糖链可以通过多种作用机制作用于病毒,其潜在的抗病毒靶点及作用机制见图 4。

|
| 图 4 抗病毒糖链化合物的靶点及作用机制 Fig. 4 Multi-targets of antiviral glycans and the underpinning molecular mechanisms. |
| |
一方面,大多数多糖携带电荷,可与病毒表面直接作用并改变病毒结构导致病毒失去感染力或直接杀伤病毒。例如角叉菜胶可与疱疹病毒(herpes simplex virus, HSV) 发生不可逆结合导致病毒粒子失活使其无法感染,从而有效减少病毒的增殖[70]。另一方面糖类疫苗佐剂也相继问世,并且显示出抗病毒效果,如壳聚糖、岩藻酸盐、透明质酸和β-葡聚糖已被用作抗病毒疫苗佐剂[71]。
3.2 糖链可阻断病毒对宿主细胞的黏附及膜融合病毒可以通过表面糖链来遮蔽关键氨基酸位点,根据病毒表面糖链的结构和特点“投其所好”,在宿主细胞表面预先加入特定糖链保护剂,使糖链与宿主受体结合,隐藏宿主细胞可被病毒识别与结合的位点,此方法即通过添加外源糖链竞争性抑制病毒与受体的结合。
3.3 糖链可抑制病毒在细胞中的复制病毒遗传物质进入细胞后,利用宿主细胞的遗传及表达系统,进行复制和完成生物大分子的合成,最后装配和释放子代病毒。多糖可以干扰病毒复制过程中相关酶的活性从而影响病毒复制[72]。黄芪多糖APS在体外以剂量依赖性方式抑制禽类传染性支气管炎病毒IBV的复制[73],灰树花多糖GFP1可以抑制肠道病毒71 (EV71) RNA基因组的合成[74]。多种亚氨基糖衍生物也具有抑制感染性病毒粒子释放和减少感染细胞数量的功能[75],来自乳酸菌的EPS26a可以完全抑制人腺病毒5 (HAdV-5) 的形成和释放[76]。
3.4 糖链可以调节宿主免疫功能进而抵抗病毒病毒可通过糖链和宿主免疫细胞结合,同理,可通过外源糖类调节免疫系统,从而提高机体抗病毒效力或缓解病毒带来的伤害。多糖可与免疫细胞表面的特异性受体结合,从而激活受体介导的免疫反应。如Toll样受体(TLR2、TLR4) 是多糖激活免疫反应的主要受体,激活后的受体通过接头蛋白My D88将信号传递出去,最后激活NK-κB或AP-1,引起炎症细胞因子的释放[77]。不同来源的多糖已被证明可以调节巨噬细胞的活性[78-82]。甘露聚糖可以和巨噬细胞表面的MR受体结合,从而增强吞噬作用和促炎症因子释放[83]。1, 3-β-d-葡聚糖(GOS) 与巨噬细胞表面CR3和TLR2结合,激活MAPK和NF-κB通路,增强内吞作用和杀菌能力[84]。Dectin-1受体可与β-d-葡聚糖结合,通过免疫受体酪氨酸激活基序(immunoreceptor tyrosine-based activation motif, ITAM),激活下游MAPK信号通路[85];此外海带多糖也可以结合Dectin-1,参与免疫调节作用[86]。
对于新型冠状病毒给机体带来的“细胞因子风暴”,似乎更需要激活免疫抑制通路,减少炎症反应对机体的伤害[87]。目前已有多种免疫调节剂在临床上减轻了过度炎症,但带来效果的同时也带来了巨大的风险,如抗IL-6受体抗体Sarilumab不仅没有改善临床结果和死亡率,反而引起了严重的并发症[88]。因此,需要开发有效的免疫调节剂,在不损害宿主免疫保护的情况下抑制炎症。
多糖还可以通过控制促炎症因子的产生和调节氧化还原的平衡,从而调节免疫抑制反应。板蓝根多糖在抗流感病毒中,可诱导TLR-3的蛋白表达,通过抑制TLR-3信号通路的激活来削弱流感病毒诱导的促炎因子的上调[89]。紫菜多糖不仅抑制由内毒素(lipopolysaccharide, LPS) 诱导的小鼠骨髓来源树突状细胞(bone marrow-derived dendritic cell, BMDC) 和脾脏树突状细胞中共刺激分子的表达,还可抑制促细胞炎症因子的产生以及小鼠中Th1和Tc1细胞的分化[90]。其他糖类也显示出抑制促炎因子(TNF-α、IL-1β、IL-6和IL-8) 的产生[91–93]。硫酸化多糖可提高细胞过氧化氢酶(catalase, CAT) 和超氧化物歧化酶(super oxide dismutase, SOD) 的水平[94],防止脂质过氧化引起的组织损伤[95],减少细胞内活性氧(reactive oxygen species, ROS) 的产生并保护细胞免受ROS损伤[96]。从葡萄酒中分离的甘露聚糖样多糖可抑制内毒素刺激的细胞炎症因子和一氧化氮的生成[97]。此外,岩藻多糖和硫酸化鼠李糖可以干扰或抑制EGFR通路,从而防止病毒感染引起的肺过度纤维化反应[98–100]。
综上所述,糖类作为抗病毒药物的潜力是不可否认的,且同一种糖具有多种抗病毒机制,这可能暗示其具有广谱抗病毒的潜能,表 2为不同多糖的抗病毒活性研究总结。
| Types | Virus | Possible mechanisms | References |
| Polysaccharide extract from Laminaria japonica | RSV | Inhibit RSV replication and induce IFN-α secretion | [101] |
| Fucoidan | IAV | Target viral neuraminidase and cellular EGFR pathway | [98] |
| Sulphated polysaccharides from Enteromorpha compressa | HSV | Inhibit virus replication | [102] |
| Polysaccharide from Laminaria japonic | EV71 | Inhibit viral proliferation, suppress viral-induced apoptosis, induce IFN-β expression | [103] |
| Iota-carrageenan | VZV | Inhibit viral proliferation | [104] |
| EPS 26a from Lactic Acid Bacteria | HAdV-5 | Inhibit viral particles formation and release | [76] |
| Fucoidan from Sargassum henslowianum | HSV-2 | Inhibit viral attachment | [105] |
截至目前,研究者已发现部分糖链具有抗SARS-CoV-2的功能,其中以硫酸化多糖及肝素类寡糖为主。
岩藻多糖、未降解肝素及三聚体肝素分子(Tris-HP) 可以有效抑制SARS-CoV-2的感染[106],而硫酸化的海参多糖SCSP、褐藻多糖和卡拉胶也具有抗病毒活性[107]。对海带多糖中抗SARS-CoV-2的活性结构单元进行鉴定,发现葡萄糖醛酸甘露寡糖(glucuronomannan) 和硫酸化半乳糖岩藻多糖(sulfated galactofucan) 是主要的抗病毒成分[108]。硫酸化海参多糖中抗SARS-CoV-2的活性结构单元也得到了鉴定[109]。进一步的研究发现昆布多糖可以靶向3CLpro从而抑制SARS-CoV-2的感染[110]。
宿主细胞表面的硫酸乙酰肝素作为辅助受体(co-receptors) 可以与新型冠状病毒的S蛋白结合,从而协助病毒入侵[43]。而外源性硫酸乙酰肝素可通过竞争性抑制阻止或延缓黄病毒、疱疹病毒、流感病毒、HIV及冠状病毒的入侵。相关报道显示肝素及硫酸乙酰肝素可抑制SARS-CoV-2的入侵,且其抑制活性与肝素分子的长度及硫酸化程度相关[39, 43, 111]。
此外,细菌的LPS[63, 112]、白桦茸多糖[113]也展现出与新型冠状病毒S蛋白良好的结合力,这同样意味着自然界中存在更多的糖链化合物具有开发成为糖链药物的潜力。
5 总结与展望由于迫切需要针对SARS-CoV-2的抗病毒药物,许多研究人员将重点放在重新利用现有药物上,利用体外高通量筛选已批准的药物,发现一些潜在的抗病毒分子,这些分子在新型冠状病毒患者的临床治疗上仍有待检测[114-115]。
新型冠状病毒目前还在持续变异,这可能导致疫苗或抗体药物无效。此外,非中和抗体带来的抗体依赖的病毒感染增强效应(antibody-dependent enhancement, ADE),对疫苗及中和抗体的研发也是一大挑战,所以应对未来的新变种病毒,亟需研发安全、有效的广谱抗病毒疫苗和药物。
糖类物质具有生物相容性、安全性、低成本、低毒性等特点,且众多研究表示糖类具有抗病毒效果。但作为自然界结构最为复杂的生物大分子,糖链抗病毒的机制还在不断挖掘中,一种多糖可以抵抗多种病毒,开发针对SARS-CoV-2的糖类药物是具有前景的,未来需要从以下几个方面入手。
第一,加强对抗病毒功能糖链的筛选。目前已有许多关于抗病毒的糖类制品。硫酸化多糖SPMG已在中国进入Ⅱ期临床试验,它是第一种具有成为抗艾滋病药物潜力的多糖。阳离子改性壳聚糖(N-(2-hydroxypropyl)-3-trimethylammonium chitosan chloride hydrophobically-modified derivative, HM-HTCC) 是冠状病毒HCoV-NL的有效抑制剂[116]。Iota-角叉菜胶鼻喷雾剂可以减少由鼻病毒、冠状病毒和流感病毒等引起的普通感冒的持续时间[117-118]。根据已有的抗病毒糖链类型及结构特征,结合我国丰富的糖资源,可预见会有更多抗病毒功能糖链被发现。
第二,深入开展抗病毒功能糖链结构的解析工作。众所周知,糖类独特的结构和生物活性相关,分子量、组成、官能团和结构构象都可能影响其生物活性,提取和纯化方法也会影响糖类的生物活性[119-120]。糖链结构的复杂性和异质性使得多糖的提取和纯化异常繁琐,而通过化学合成的方法来制备均一化糖链也充满挑战,因此也阻碍了糖类药物开发。功能糖链结构的解析将有助于明确活性结构单元,以便进一步大规模生产及运行作用机理的相关研究。
第三,明确糖类药物的抗病毒机制。由于从自然界获得的糖链特别是多糖往往结构不均一,由此导致糖类抗病毒机制不唯一,也导致药物的选择性不强,难以获得高效专一的糖类药物。如亚氨基糖尚未在临床上被批准用于抗病毒适应症,在治疗病毒感染所需的浓度下,该药物存在一个脱靶效应,该药会阻碍宿主肠道葡萄糖苷酶活性,导致腹泻和腹痛[121]。阐明作用机制是当前抗病毒药物研发面临的巨大挑战。
新型冠状病毒利用宿主细胞糖基化系统完成自身蛋白的糖基化,一方面有助于其蛋白的稳定与运输,另一方面携带宿主细胞的表位糖链,可以使其躲避免疫防御,并且可能参与调节免疫系统从而对宿主产生巨大威胁。糖基化赋予了SARS-CoV-2更强的适应性,我们也可以采取“以糖攻糖”的策略,筛选和开发更多的糖类药物对抗病毒。
| [1] |
Coronaviridae Study Group of the International Committee on Taxonomy of Viruses. The species Severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat Microbiol, 2020, 5(4): 536-544. DOI:10.1038/s41564-020-0695-z
|
| [2] |
Dawood FS, Ricks P, Njie GJ, et al. Observations of the global epidemiology of COVID-19 from the prepandemic period using web-based surveillance: a cross-sectional analysis. Lancet Infect Dis, 2020, 20(11): 1255-1262. DOI:10.1016/S1473-3099(20)30581-8
|
| [3] |
Ferioli M, Cisternino C, Leo V, et al. Protecting healthcare workers from SARS-CoV-2 infection: practical indications. Eur Respir Rev, 2020, 29(155): 200068. DOI:10.1183/16000617.0068-2020
|
| [4] |
Peiris JSM, Yuen KY, Osterhaus ADME, et al. The severe acute respiratory syndrome. N Engl J Med, 2003, 349(25): 2431-2441. DOI:10.1056/NEJMra032498
|
| [5] |
Hui KPY, Cheung MC, Perera RAPM, et al. Tropism, replication competence, and innate immune responses of the coronavirus SARS-CoV-2 in human respiratory tract and conjunctiva: an analysis in ex-vivo and in-vitro cultures. Lancet Respir Med, 2020, 8(7): 687-695. DOI:10.1016/S2213-2600(20)30193-4
|
| [6] |
Lamers MM, Beumer J, Van Der Vaart J, et al. SARS-CoV-2 productively infects human gut enterocytes. Science, 2020, 369(6499): 50-54. DOI:10.1126/science.abc1669
|
| [7] |
郑楠, 赵明, 田晓鑫, 等. 全球新型冠状病毒疫苗及治疗药物研发现状与趋势. 中国新药杂志, 2022, 31(1): 69-76. Zheng N, Zhao M, Tian XX, et al. Global research and development of COVID-19 vaccine and therapeutics: status and trend. Chin J New Drugs, 2022, 31(1): 69-76 (in Chinese). |
| [8] |
Wang ZL, Yang LY. Chinese herbal medicine: fighting SARS-CoV-2 infection on all fronts. J Ethnopharmacol, 2021, 270: 113869. DOI:10.1016/j.jep.2021.113869
|
| [9] |
杨柳, 杨海琴, 李俊灿, 等. 新型冠状病毒肺炎用药. 世界科学技术-中医药现代化, 2021, 23(4): 1013-1020. Yang L, Yang HQ, Li JC, et al. Medication for COVID-19. Mod Tradit Chin Med Mater Med World Sci Technol, 2021, 23(4): 1013-1020 (in Chinese). |
| [10] |
杨宏志, 林瑞超, 董汛, 等. 香藿喷雾剂联合基础康复疗法治疗新型冠状病毒肺炎恢复期余毒未清证60例临床研究. 中医杂志, 2021, 62(17): 1509-1513. Yang HZ, Lin RC, Dong X, et al. Xiang huo spray combined with basic rehabilitation therapy in treating 60 Cases of COVID-19 with syndrome of residual toxin during convalescence period. J Tradit Chin Med, 2021, 62(17): 1509-1513 (in Chinese). |
| [11] |
周政, 朱春胜, 张冰. 基于数据挖掘的中医药治疗新型冠状病毒肺炎用药规律研究. 中国中药杂志, 2020, 45(6): 1248-1252. Zhou Z, Zhu CS, Zhang B. Study on medication regularity of traditional Chinese medicine in treatment of COVID-19 based on data mining. China J Chin Mater Med, 2020, 45(6): 1248-1252 (in Chinese). |
| [12] |
Cao YL, Wang J, Jian FC, et al. Omicron escapes the majority of existing SARS-CoV-2 neutralizing antibodies. Nature, 2022, 602(7898): 657-663. DOI:10.1038/s41586-021-04385-3
|
| [13] |
Lu L, Mok BW, Chen LL, et al. Neutralization of SARS-CoV-2 Omicron variant by sera from BNT162b2 or coronavac vaccine recipients. Clin Infect Dis, 2021, ciab1041.
|
| [14] |
Edara VV, Manning KE, Ellis M, et al. mRNA-1273 and BNT162b2 mRNA vaccines have reduced neutralizing activity against the SARS-CoV-2 Omicron variant. bioRxiv, 2021: 2021Dec22;2021.12. 20.473557.
|
| [15] |
Kim D, Lee JY, Yang JS, et al. The architecture of SARS-CoV-2 transcriptome. Cell, 2020, 181(4): 914-921.e10. DOI:10.1016/j.cell.2020.04.011
|
| [16] |
Walls AC, Park YJ, Tortorici MA, et al. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell, 2020, 181(2): 281-292.e6. DOI:10.1016/j.cell.2020.02.058
|
| [17] |
Wrapp D, Wang NS, Corbett KS, et al. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science, 2020, 367(6483): 1260-1263. DOI:10.1126/science.abb2507
|
| [18] |
Hoffmann M, Kleine-Weber H, Pöhlmann S. A multibasic cleavage site in the spike protein of SARS-CoV-2 is essential for infection of human lung cells. Mol Cell, 2020, 78(4): 779-784.e5. DOI:10.1016/j.molcel.2020.04.022
|
| [19] |
Hoffmann M, Kleine-Weber H, Schroeder S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell, 2020, 181(2): 271-280.e8. DOI:10.1016/j.cell.2020.02.052
|
| [20] |
Matsuyama S, Nagata N, Shirato K, et al. Efficient activation of the severe acute respiratory syndrome coronavirus spike protein by the transmembrane protease TMPRSS2. J Virol, 2010, 84(24): 12658-12664. DOI:10.1128/JVI.01542-10
|
| [21] |
Shirato K, Kawase M, Matsuyama S. Middle East respiratory syndrome coronavirus infection mediated by the transmembrane serine protease TMPRSS2. J Virol, 2013, 87(23): 12552-12561. DOI:10.1128/JVI.01890-13
|
| [22] |
Belouzard S, Millet JK, Licitra BN, et al. Mechanisms of coronavirus cell entry mediated by the viral spike protein. Viruses, 2012, 4(6): 1011-1033. DOI:10.3390/v4061011
|
| [23] |
Li WH, Moore MJ, Vasilieva N, et al. Angiotensin- converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature, 2003, 426(6965): 450-454. DOI:10.1038/nature02145
|
| [24] |
Raj VS, Mou HH, Smits SL, et al. Dipeptidyl peptidase 4 is a functional receptor for the emerging human coronavirus-EMC. Nature, 2013, 495(7440): 251-254. DOI:10.1038/nature12005
|
| [25] |
Yeager CL, Ashmun RA, Williams RK, et al. Human aminopeptidase N is a receptor for human coronavirus 229E. Nature, 1992, 357(6377): 420-422. DOI:10.1038/357420a0
|
| [26] |
Shajahan A, Supekar NT, Gleinich AS, et al. Deducing the N- and O-glycosylation profile of the spike protein of novel coronavirus SARS-CoV-2. Glycobiology, 2020, 30(12): 981-988. DOI:10.1093/glycob/cwaa042
|
| [27] |
Tian WM, Li DL, Zhang N, et al. O-glycosylation pattern of the SARS-CoV-2 spike protein reveals an "O-follow-N" rule. Cell Res, 2021, 31(10): 1123-1125. DOI:10.1038/s41422-021-00545-2
|
| [28] |
Zhang Y, Zhao WJ, Mao YH, et al. O-glycosylation landscapes of SARS-CoV-2 spike proteins. Front Chem, 2021, 9: 689521. DOI:10.3389/fchem.2021.689521
|
| [29] |
Cai YF, Zhang J, Xiao TS, et al. Distinct conformational states of SARS-CoV-2 spike protein. Science, 2020, 369(6511): 1586-1592. DOI:10.1126/science.abd4251
|
| [30] |
Casalino L, Gaieb Z, Goldsmith JA, et al. Beyond shielding: the roles of glycans in the SARS-CoV-2 spike protein. ACS Cent Sci, 2020, 6(10): 1722-1734. DOI:10.1021/acscentsci.0c01056
|
| [31] |
Huang YJ, Zhao H, Huang X, et al. Identification of oligosaccharyltransferase as a host target for inhibition of SARS-CoV-2 and its variants. Cell Discov, 2021, 7(1): 116. DOI:10.1038/s41421-021-00354-2
|
| [32] |
Gao T, Hu MD, Zhang XP, et al. Highly pathogenic coronavirus N protein aggravates lung injury by MASP-2-mediated complement over-activation. Infect Dis, 2020.
|
| [33] |
López-Muñoz AD, Kosik I, Holly J, et al. Cell surface SARS-CoV-2 nucleocapsid protein modulates innate and adaptive immunity. bioRxiv, 2021: 2021Dec13;2021.12. 10.472169.
|
| [34] |
Zheng M, Karki R, Williams EP, et al. TLR2 senses the SARS-CoV-2 envelope protein to produce inflammatory cytokines. Nat Immunol, 2021, 22(7): 829-838. DOI:10.1038/s41590-021-00937-x
|
| [35] |
Watanabe Y, Bowden TA, Wilson IA, et al. Exploitation of glycosylation in enveloped virus pathobiology. Biochim Biophys Acta Gen Subj, 2019, 1863(10): 1480-1497.
|
| [36] |
Mohan GS, Li WF, Ye L, et al. Antigenic subversion: a novel mechanism of host immune evasion by Ebola virus. PLoS Pathog, 2012, 8(12): e1003065. DOI:10.1371/journal.ppat.1003065
|
| [37] |
Bukreyev A, Yang LJ, Fricke J, et al. The secreted form of respiratory syncytial virus G glycoprotein helps the virus evade antibody-mediated restriction of replication by acting as an antigen decoy and through effects on Fc receptor-bearing leukocytes. J Virol, 2008, 82(24): 12191-12204. DOI:10.1128/JVI.01604-08
|
| [38] |
Nguyen HL, Lan PD, Thai NQ, et al. Does SARS-CoV-2 bind to human ACE2 more strongly than does SARS-CoV. J Phys Chem B, 2020, 124(34): 7336-7347. DOI:10.1021/acs.jpcb.0c04511
|
| [39] |
Kim SY, Jin WH, Sood A, et al. Characterization of heparin and severe acute respiratory syndrome-related coronavirus 2 (SARS-CoV-2) spike glycoprotein binding interactions. Antiviral Res, 2020, 181: 104873. DOI:10.1016/j.antiviral.2020.104873
|
| [40] |
Mycroft-West CJ, Su DH, Pagani I, et al. Heparin inhibits cellular invasion by SARS-CoV-2: structural dependence of the interaction of the spike S1 receptor-binding domain with heparin. Thromb Haemost, 2020, 120(12): 1700-1715. DOI:10.1055/s-0040-1721319
|
| [41] |
Patel M, Yanagishita M, Roderiquez G, et al. Cell-surface heparan sulfate proteoglycan mediates HIV-1 infection of T-cell lines. AIDS Res Hum Retroviruses, 1993, 9(2): 167-174. DOI:10.1089/aid.1993.9.167
|
| [42] |
O'Donnell CD, Shukla D. The importance of heparan sulfate in herpesvirus infection. Virol Sin, 2008, 23(6): 383-393. DOI:10.1007/s12250-008-2992-1
|
| [43] |
Clausen TM, Sandoval DR, Spliid CB, et al. SARS-CoV-2 infection depends on cellular heparan sulfate and ACE2. Cell, 2020, 183(4): 1043-1057.e15. DOI:10.1016/j.cell.2020.09.033
|
| [44] |
Yang Y, Du Y, Kaltashov IA. The utility of native MS for understanding the mechanism of action of repurposed therapeutics in COVID-19: heparin as a disruptor of the SARS-CoV-2 interaction with its host cell receptor. Anal Chem, 2020, 92(16): 10930-10934. DOI:10.1021/acs.analchem.0c02449
|
| [45] |
Shukla D, Liu J, Blaiklock P, et al. A novel role for 3-O-sulfated heparan sulfate in Herpes simplex virus 1 entry. Cell, 1999, 99(1): 13-22. DOI:10.1016/S0092-8674(00)80058-6
|
| [46] |
Yang TJ, Yu PY, Chang YC, et al. D614G mutation in the SARS-CoV-2 spike protein enhances viral fitness by desensitizing it to temperature-dependent denaturation. J Biol Chem, 2021, 297(4): 101238. DOI:10.1016/j.jbc.2021.101238
|
| [47] |
Shajahan A, Archer-Hartmann S, Supekar NT, et al. Comprehensive characterization of N- and O- glycosylation of SARS-CoV-2 human receptor angiotensin converting enzyme 2. Glycobiology, 2021, 31(4): 410-424. DOI:10.1093/glycob/cwaa101
|
| [48] |
Zhao P, Praissman JL, Grant OC, et al. Virus-receptor interactions of glycosylated SARS-CoV-2 spike and human ACE2 receptor. Cell Host Microbe, 2020, 28(4): 586-601.e6. DOI:10.1016/j.chom.2020.08.004
|
| [49] |
Li WH, Zhang CS, Sui JH, et al. Receptor and viral determinants of SARS-coronavirus adaptation to human ACE2. EMBO J, 2005, 24(8): 1634-1643. DOI:10.1038/sj.emboj.7600640
|
| [50] |
Hamming I, Timens W, Bulthuis MLC, et al. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol, 2004, 203(2): 631-637.
|
| [51] |
Danilczyk U, Eriksson U, Crackower MA, et al. A story of two ACEs. J Mol Med (Berl), 2003, 81(4): 227-234. DOI:10.1007/s00109-003-0419-x
|
| [52] |
Zou X, Chen K, Zou JW, et al. Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection. Front Med, 2020, 14(2): 185-192. DOI:10.1007/s11684-020-0754-0
|
| [53] |
Hikmet F, Méar L, Edvinsson Å, et al. The protein expression profile of ACE2 in human tissues. Mol Syst Biol, 2020, 16(7): e9610.
|
| [54] |
Hou YJ, Okuda K, Edwards CE, et al. SARS-CoV-2 reverse genetics reveals a variable infection gradient in the respiratory tract. Cell, 2020, 182(2): 429-446.e14. DOI:10.1016/j.cell.2020.05.042
|
| [55] |
Ganier C, Du-Harpur X, Harun N, et al. CD147 (BSG) but not ACE2 expression is detectable in vascular endothelial cells within single cell RNA sequencing datasets derived from multiple tissues in healthy individuals. bioRxiv, 2020, DOI: 10.1101/2020.05.29.123513.
|
| [56] |
Van Kooyk Y, Rabinovich GA. Protein-glycan interactions in the control of innate and adaptive immune responses. Nat Immunol, 2008, 9(6): 593-601. DOI:10.1038/ni.f.203
|
| [57] |
Lempp FA, Soriaga LB, Montiel-Ruiz M, et al. Lectins enhance SARS-CoV-2 infection and influence neutralizing antibodies. Nature, 2021, 598(7880): 342-347. DOI:10.1038/s41586-021-03925-1
|
| [58] |
Thépaut M, Luczkowiak J, Vivès C, et al. DC/L-SIGN recognition of spike glycoprotein promotes SARS-CoV-2 trans-infection and can be inhibited by a glycomimetic antagonist. PLoS Pathog, 2021, 17(5): e1009576. DOI:10.1371/journal.ppat.1009576
|
| [59] |
Han DP, Lohani M, Cho MW. Specific asparagine- linked glycosylation sites are critical for DC-SIGN- and L-SIGN-mediated severe acute respiratory syndrome coronavirus entry. J Virol, 2007, 81(21): 12029-12039. DOI:10.1128/JVI.00315-07
|
| [60] |
Gao C, Zeng J, Jia N, et al. SARS-CoV-2 spike protein interacts with multiple innate immune receptors. bioRxiv, 2020: 2020Jul30;2020.07. 29.227462.
|
| [61] |
Lenza MP, Oyenarte I, Diercks T, et al. Structural characterization of N-linked glycans in the receptor binding domain of the SARS-CoV-2 spike protein and their interactions with human lectins. Angew Chem Int Ed Engl, 2020, 59(52): 23763-23771. DOI:10.1002/anie.202011015
|
| [62] |
Lu Q, Liu J, Zhao S, et al. SARS-CoV-2 exacerbates proinflammatory responses in myeloid cells through C-type lectin receptors and Tweety family member 2. Immunity, 2021, 54(6): 1304-1319.e9. DOI:10.1016/j.immuni.2021.05.006
|
| [63] |
Chiodo F, Bruijns SCM, Rodriguez E, et al. Novel ACE2-independent carbohydrate-binding of SARS-CoV-2 spike protein to host lectins and lung microbiota. bioRxiv, 2020, 2020, 05.13.092478.
|
| [64] |
Amraei R, Yin W, Napoleon MA, et al. CD209L/L-SIGN and CD209/DC-SIGN act as receptors for SARS-CoV-2. bioRxiv, 2021: 2021Jun14;2020.06. 22.165803.
|
| [65] |
Perez-Zsolt D, Basagoiti J, Rodon Aldrufeu J, et al. Siglec-1 on dendritic cells mediates SARS-CoV-2 trans -infection of target cells while on macrophages triggers proinflammatory responses. bioRxiv, 2021, 2021, 05.11.443572.
|
| [66] |
Girond S, Crance JM, Van Cuyck-Gandre H, et al. Antiviral activity of carrageenan on hepatitis A virus replication in cell culture. Res Virol, 1991, 142(4): 261-270. DOI:10.1016/0923-2516(91)90011-Q
|
| [67] |
Cardozo FT, Camelini CM, Mascarello A, et al. Antiherpetic activity of a sulfated polysaccharide from Agaricus brasiliensis mycelia. Antiviral Res, 2011, 92(1): 108-114. DOI:10.1016/j.antiviral.2011.07.009
|
| [68] |
Zhu W, Chiu LCM, Ooi VEC, et al. Antiviral property and mechanisms of a sulphated polysaccharide from the brown alga Sargassum patens against herpes simplex virus type 1. Phytomedicine, 2006, 13(9/10): 695-701.
|
| [69] |
Reichert M, Bergmann SM, Hwang J, et al. Antiviral activity of exopolysaccharides from Arthrospira platensis against koi herpesvirus. J Fish Dis, 2017, 40(10): 1441-1450. DOI:10.1111/jfd.12618
|
| [70] |
Carlucci MJ, Scolaro LA, Damonte EB. Herpes simplex virus type 1 variants arising after selection with an antiviral carrageenan: lack of correlation between drug susceptibility and syn phenotype. J Med Virol, 2002, 68(1): 92-98. DOI:10.1002/jmv.10174
|
| [71] |
Mallakpour S, Azadi E, Hussain CM. Chitosan, alginate, hyaluronic acid, gums, and β-glucan as potent adjuvants and vaccine delivery systems for viral threats including SARS-CoV-2: a review. Int J Biol Macromol, 2021, 182: 1931-1940. DOI:10.1016/j.ijbiomac.2021.05.155
|
| [72] |
Shi QM, Wang AJ, Lu ZH, et al. Overview on the antiviral activities and mechanisms of marine polysaccharides from seaweeds. Carbohydr Res, 2017, 453/454: 1-9. DOI:10.1016/j.carres.2017.10.020
|
| [73] |
Zhang PJ, Liu XF, Liu HY, et al. Astragalus polysaccharides inhibit avian infectious bronchitis virus infection by regulating viral replication. Microb Pathog, 2018, 114: 124-128. DOI:10.1016/j.micpath.2017.11.026
|
| [74] |
Zhao C, Gao LY, Wang CY, et al. Structural characterization and antiviral activity of a novel heteropolysaccharide isolated from Grifola frondosa against Enterovirus 71. Carbohydr Polym, 2016, 144: 382-389. DOI:10.1016/j.carbpol.2015.12.005
|
| [75] |
Alonzi DS, Scott KA, Dwek RA, et al. Iminosugar antivirals: the therapeutic sweet spot. Biochem Soc Trans, 2017, 45(2): 571-582. DOI:10.1042/BST20160182
|
| [76] |
Biliavska L, Pankivska Y, Povnitsa O, et al. Antiviral activity of exopolysaccharides produced by lactic acid bacteria of the genera Pediococcus, Leuconostoc and Lactobacillus against human adenovirus type 5. Medicina (Kaunas), 2019, 55(9): 519. DOI:10.3390/medicina55090519
|
| [77] |
Barbosa JR, Dos Santos Freitas MM, Da Silva Martins LH, et al. Polysaccharides of mushroom Pleurotus spp. : new extraction techniques, biological activities and development of new technologies. Carbohydr Polym, 2020, 229: 115550.
|
| [78] |
Ren JY, Hou CL, Shi CC, et al. A polysaccharide isolated and purified from Platycladus orientalis (L. ) Franco leaves, characterization, bioactivity and its regulation on macrophage polarization. Carbohydr Polym, 2019, 213: 276-285.
|
| [79] |
Fu AK, Wang Y, Wu YP, et al. Echinacea purpurea extract polarizes M1 macrophages in murine bone marrow-derived macrophages through the activation of JNK. J Cell Biochem, 2017, 118(9): 2664-2671. DOI:10.1002/jcb.25875
|
| [80] |
Minato KI, Laan LC, Van Die I, et al. Pleurotus citrinopileatus polysaccharide stimulates anti- inflammatory properties during monocyte-to- macrophage differentiation. Int J Biol Macromol, 2019, 122: 705-712. DOI:10.1016/j.ijbiomac.2018.10.157
|
| [81] |
Zhang MM, Wu WJ, Ren Y, et al. Structural characterization of a novel polysaccharide from Lepidium meyenii (maca) and analysis of its regulatory function in macrophage polarization in vitro. J Agric Food Chem, 2017, 65(6): 1146-1157. DOI:10.1021/acs.jafc.6b05218
|
| [82] |
Wei W, Li ZP, Bian ZX, et al. Astragalus polysaccharide RAP induces macrophage phenotype polarization to M1 via the notch signaling pathway. Molecules, 2019, 24(10): 2016. DOI:10.3390/molecules24102016
|
| [83] |
Wileman TE, Lennartz MR, Stahl PD. Identification of the macrophage mannose receptor as a 175-kDa membrane protein. PNAS, 1986, 83(8): 2501-2505. DOI:10.1073/pnas.83.8.2501
|
| [84] |
Liu J, Tang JQ, Li XT, et al. Curdlan (Alcaligenes faecalis) (1→3)-β-d-glucan oligosaccharides drive M1 phenotype polarization in murine bone marrow-derived macrophages via activation of MAPKs and NF-κB pathways. Molecules, 2019, 24(23): 4251. DOI:10.3390/molecules24234251
|
| [85] |
Deng C, Fu HT, Shang JY, et al. Dectin-1 mediates the immunoenhancement effect of the polysaccharide from Dictyophora indusiata. Int J Biol Macromol, 2018, 109: 369-374. DOI:10.1016/j.ijbiomac.2017.12.113
|
| [86] |
Smith AJ, Graves B, Child R, et al. Immunoregulatory activity of the natural product laminarin varies widely as a result of its physical properties. J Immunol, 2018, 200(2): 788-799. DOI:10.4049/jimmunol.1701258
|
| [87] |
Zhou Z, Ren LL, Zhang L, et al. Heightened innate immune responses in the respiratory tract of COVID-19 patients. Cell Host Microbe, 2020, 27(6): 883-890.e2. DOI:10.1016/j.chom.2020.04.017
|
| [88] |
Cheng C, Zhang F. Correspondence on: 'interleukin-6 blockade with sarilumab in severe COVID-19 pneumonia with systemic hyperinflammation-an open-label cohort study' by Della-Torre et al. Ann Rheum Dis, 2020: 2020Aug19;annrheumdis-2020Aug19; annrheu2020-218616.
|
| [89] |
Li ZT, Li L, Zhou HX, et al. Radix isatidis polysaccharides inhibit influenza a virus and influenza A virus-induced inflammation via suppression of host TLR3 signaling in vitro. Molecules, 2017, 22(1): 116. DOI:10.3390/molecules22010116
|
| [90] |
Wang YH, Hwang JY, Park HB, et al. Porphyran isolated from Pyropia yezoensis inhibits lipopolysaccharide-induced activation of dendritic cells in mice. Carbohydr Polym, 2020, 229: 115457. DOI:10.1016/j.carbpol.2019.115457
|
| [91] |
Cheng JJ, Chao CH, Chang PC, et al. Studies on anti-inflammatory activity of sulfated polysaccharides from cultivated fungi Antrodia cinnamomea. Food Hydrocoll, 2016, 53: 37-45. DOI:10.1016/j.foodhyd.2014.09.035
|
| [92] |
Wu GJ, Shiu SM, Hsieh MC, et al. Anti-inflammatory activity of a sulfated polysaccharide from the brown alga Sargassum cristaefolium. Food Hydrocoll, 2016, 53: 16-23. DOI:10.1016/j.foodhyd.2015.01.019
|
| [93] |
Dinić M, Pecikoza U, Djokić J, et al. Exopolysaccharide produced by probiotic strain Lactobacillus paraplantarum BGCG11 reduces inflammatory hyperalgesia in rats. Front Pharmacol, 2018, 9: 1. DOI:10.3389/fphar.2018.00001
|
| [94] |
Alencar POC, Lima GC, Barros FCN, et al. A novel antioxidant sulfated polysaccharide from the algae Gracilaria caudata: in vitro and in vivo activities. Food Hydrocoll, 2019, 90: 28-34. DOI:10.1016/j.foodhyd.2018.12.007
|
| [95] |
Dore CM, Das C Faustino Alves MG, Will LS, et al. A sulfated polysaccharide, fucans, isolated from brown algae Sargassum vulgare with anticoagulant, antithrombotic, antioxidant and anti-inflammatory effects. Carbohydr Polym, 2013, 91(1): 467-475. DOI:10.1016/j.carbpol.2012.07.075
|
| [96] |
Fernando IPS, Sanjeewa KKA, Samarakoon KW, et al. FTIR characterization and antioxidant activity of water soluble crude polysaccharides of Sri Lankan marine algae. ALGAE, 2017, 32(1): 75-86. DOI:10.4490/algae.2017.32.12.1
|
| [97] |
Bezerra IL, Caillot ARC, Palhares LCGF, et al. Structural characterization of polysaccharides from Cabernet Franc, Cabernet Sauvignon and Sauvignon Blanc wines: Anti-inflammatory activity in LPS stimulated RAW 264. 7 cells. Carbohydr Polym, 2018, 186: 91-99. DOI:10.1016/j.carbpol.2017.12.082
|
| [98] |
Wang W, Wu JD, Zhang XS, et al. Inhibition of influenza A virus infection by fucoidan targeting viral neuraminidase and cellular EGFR pathway. Sci Rep, 2017, 7: 40760. DOI:10.1038/srep40760
|
| [99] |
Venkataraman T, Frieman MB. The role of epidermal growth factor receptor (EGFR) signaling in SARS coronavirus-induced pulmonary fibrosis. Antiviral Res, 2017, 143: 142-150. DOI:10.1016/j.antiviral.2017.03.022
|
| [100] |
Wang SY, Wang W, Hao C, et al. Antiviral activity against Enterovirus 71 of sulfated rhamnan isolated from the green alga Monostroma latissimum. Carbohydr Polym, 2018, 200: 43-53. DOI:10.1016/j.carbpol.2018.07.067
|
| [101] |
Cao YG, Hao Y, Li ZH, et al. Antiviral activity of polysaccharide extract from Laminaria japonica against respiratory syncytial virus. Biomed Pharmacother, 2016, 84: 1705-1710. DOI:10.1016/j.biopha.2016.10.082
|
| [102] |
Lopes N, Ray S, Espada SF, et al. Green seaweed Enteromorpha compressa (Chlorophyta, Ulvaceae) derived sulphated polysaccharides inhibit Herpes simplex virus. Int J Biol Macromol, 2017, 102: 605-612. DOI:10.1016/j.ijbiomac.2017.04.043
|
| [103] |
Yue YY, Li ZH, Li P, et al. Antiviral activity of a polysaccharide from Laminaria japonica against Enterovirus 71. Biomed Pharmacother, 2017, 96: 256-262. DOI:10.1016/j.biopha.2017.09.117
|
| [104] |
Abu-Galiyun E, Huleihel M, Levy-Ontman O. Antiviral bioactivity of renewable polysaccharides against varicella zoster. Cell Cycle, 2019, 18(24): 3540-3549. DOI:10.1080/15384101.2019.1691363
|
| [105] |
Sun QL, Li Y, Ni LQ, et al. Structural characterization and antiviral activity of two fucoidans from the brown algae Sargassum henslowianum. Carbohydr Polym, 2020, 229: 115487. DOI:10.1016/j.carbpol.2019.115487
|
| [106] |
Kwon PS, Oh H, Kwon SJ, et al. Sulfated polysaccharides effectively inhibit SARS-CoV-2 in vitro. Cell Discov, 2020, 6(1): 50. DOI:10.1038/s41421-020-00192-8
|
| [107] |
Song S, Peng HR, Wang QL, et al. Inhibitory activities of marine sulfated polysaccharides against SARS-CoV-2. Food Funct, 2020, 11(9): 7415-7420. DOI:10.1039/D0FO02017F
|
| [108] |
Jin WH, Zhang WJ, Mitra D, et al. The structure-activity relationship of the interactions of SARS-CoV-2 spike glycoproteins with glucuronomannan and sulfated galactofucan from Saccharina japonica. Int J Biol Macromol, 2020, 163: 1649-1658. DOI:10.1016/j.ijbiomac.2020.09.184
|
| [109] |
Dwivedi R, Samanta P, Sharma P, et al. Structural and kinetic analyses of holothurian sulfated glycans suggest potential treatment for SARS-CoV-2 infection. J Biol Chem, 2021, 297(4): 101207. DOI:10.1016/j.jbc.2021.101207
|
| [110] |
Zhang SH, Pei RJ, Li MX, et al. Cocktail polysaccharides isolated from Ecklonia kurome against the SARS-CoV-2 infection. Carbohydr Polym, 2022, 275: 118779. DOI:10.1016/j.carbpol.2021.118779
|
| [111] |
Hao W, Ma B, Li ZH, et al. Binding of the SARS-CoV-2 spike protein to glycans. Sci Bull (Beijing), 2021, 66(12): 1205-1214. DOI:10.1016/j.scib.2021.01.010
|
| [112] |
Petruk G, Puthia M, Petrlova J, et al. SARS-CoV-2 spike protein binds to bacterial lipopolysaccharide and boosts proinflammatory activity. J Mol Cell Biol, 2020, 12(12): 916-932.
|
| [113] |
Eid JI, Das B, Al-Tuwaijri MM, et al. Targeting SARS-CoV-2 with Chaga mushroom: an in silico study toward developing a natural antiviral compound. Food Sci Nutr, 2021, 9(12): 6513-6523. DOI:10.1002/fsn3.2576
|
| [114] |
Riva L, Yuan SF, Yin X, et al. Discovery of SARS-CoV-2 antiviral drugs through large-scale compound repurposing. Nature, 2020, 586(7827): 113-119. DOI:10.1038/s41586-020-2577-1
|
| [115] |
Touret F, Gilles M, Barral K, et al. In vitro screening of a FDA approved chemical library reveals potential inhibitors of SARS-CoV-2 replication. Sci Rep, 2020, 10(1): 13093. DOI:10.1038/s41598-020-70143-6
|
| [116] |
Milewska A, Kaminski K, Ciejka J, et al. HTCC: broad range inhibitor of coronavirus entry. PLoS One, 2016, 11(6): e0156552. DOI:10.1371/journal.pone.0156552
|
| [117] |
Koenighofer M, Lion T, Bodenteich A, et al. Carrageenan nasal spray in virus confirmed common cold: individual patient data analysis of two randomized controlled trials. Multidiscip Respir Med, 2014, 9(1): 57. DOI:10.1186/2049-6958-9-57
|
| [118] |
Ludwig M, Enzenhofer E, Schneider S, et al. Efficacy of a carrageenan nasal spray in patients with common cold: a randomized controlled trial. Respir Res, 2013, 14(1): 124. DOI:10.1186/1465-9921-14-124
|
| [119] |
Marks RM, Lu H, Sundaresan R, et al. Probing the interaction of dengue virus envelope protein with heparin: assessment of glycosaminoglycan-derived inhibitors. J Med Chem, 2001, 44(13): 2178-2187. DOI:10.1021/jm000412i
|
| [120] |
Liu ZH, Niu FJ, Xie YX, et al. A review: natural polysaccharides from medicinal plants and microorganisms and their anti-herpetic mechanism. Biomed Pharmacother, 2020, 129: 110469. DOI:10.1016/j.biopha.2020.110469
|
| [121] |
Fischl MA, Resnick L, Coombs R, et al. The safety and efficacy of combination N-butyl-deoxynojirimycin (SC-48334) and zidovudine in patients with HIV-1 infection and 200‒500 CD4 cells/mm3. J Acquir Immune Defic Syndr (1988), 1994, 7(2): 139-147.
|
 2022, Vol. 38
2022, Vol. 38