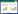中国科学院微生物研究所、中国微生物学会主办
文章信息
- 江瑞妮, 叶康, 甘恬, 陆跃乐, 朱林江, 陈小龙, 陈翰驰
- Jiang Ruini, Ye Kang, Gan Tian, Lu Yuele, Zhu Linjiang, Chen Xiaolong, Chen Hanchi
- 蔗糖磷酸化酶及其在糖基化反应中的应用
- Application of sucrose phosphorylase in glycosylation
- 生物工程学报, 2021, 37(1): 112-129
- Chinese Journal of Biotechnology, 2021, 37(1): 112-129
- 10.13345/j.cjb.200213
-
文章历史
- Received: April 16, 2020
- Accepted: June 8, 2020
- Published: June 16, 2020
2. 浙江工业大学 发酵工程研究所,浙江 杭州 310014
2. Institute of Fermentation Engineering, Zhejiang University of Technology, Hangzhou 310014, Zhejiang, China
糖基化作用是自然界中最重要的生化反应之一[1],能赋予天然产物结构多样性,改善化合物水溶性、稳定性、生物利用度等性质,所得产物能广泛应用于食品、医药及个人护理等领域[2-3]。有机小分子化合物的糖基化方法包括化学法和生物酶法。化学法常被用于合成不同的糖苷化合物,其根据供体底物的不同主要分为4类:银盐活化的糖基卤化物(Koenigs-Knorr糖苷化反应)[4-6]、路易斯酸活化的糖基三氯乙酰亚氨酯[7]、亲电子试剂活化的含硫甙以及n-戊烯基糖苷[8]。化学糖基化反应的区域选择性难以有效预测、控制[9],反应过程还伴随着许多副产物(如糖基供体水解副产物)的生成,影响产率[10]。与化学法相比,生物酶法专一性高,避免了对非作用基团的保护措施,且反应条件温和,是一类高效、绿色的糖基化修饰方法。De Roode等计算得出酶法糖基化反应产生的废料比化学法低5倍,时空产量高15倍[11]。生物体有1%–3%的基因编码与糖基化反应相关的酶[12],这些酶在碳水化合物活性酶(Carbohydrate- active enzyme,CAZy)数据库中[13],根据序列相似性被分为两大类:糖基转移酶类(Glycosyltransferases,GTs)和糖苷水解酶类(Glycoside hydrolases,GHs)[14]。GTs具有广泛的底物特异性,参与自然界中大部分的糖基化反应(图 1A)。GTs的催化效率很高,但其大规模应用被糖基供体高昂的价格所限制[15]。GHs包含糖苷酶和转糖苷酶,能够参与糖苷键的水解和合成(图 1B)。GHs分布广泛,糖基供体廉价,在生物技术和生物医学中有广泛的应用[16]。

|
| 图 1 GTs和GHs催化的生物反应 Fig. 1 Reactions catalyzed by (A) GTs and (B) GHs. X: the activating group of a donor substrate including pyrophosphate, phospholipid and nucleoside diphosphate. |
| |
GHs中存在许多出色的能够以蔗糖(Sucrose,Suc)为供体底物的葡萄糖基转移酶,如葡聚糖蔗糖酶(Glucansucrase)、淀粉蔗糖酶(Amylosucrase,ASase,EC 2.4.1.4)和蔗糖磷酸化酶(Sucrose phosphorylase,SPase,EC 2.4.1.7)等。葡聚糖蔗糖酶是GH70家族的一系列能够催化Suc生成α-葡聚糖的生物催化剂。同时,这类酶具有广泛的受体底物特异性,能够实现非碳水化合物的糖基化修饰[17]。但是,葡聚糖蔗糖酶类的一个显著特征是能够转移多个葡萄糖基至一个受体底物中,形成的混合产物不仅会影响糖基化反应的效率,还会增加下游分离难度[18]。淀粉蔗糖酶是葡聚糖蔗糖酶类中的一种,不同于该类酶中的其他酶,ASase属于GH13家族,可以催化以Suc为底物的聚合、异构化和水解反应[19],并且ASase具有将Suc的葡萄糖基转移至其他糖基受体(如糖原、淀粉、黄酮类化合物等)的能力[20-22]。ASase的糖基化产率往往高于其他酶[23],但是大多数的ASase表现出较差的热稳定性,而工业中碳水化合物的转化反应通常在高温条件下进行以避免微生物污染[24]。同属于GH13家族的SPase能够可逆地催化Suc与无机磷酸盐反应生成D-果糖(D-Fru)和α-D-葡萄糖-1-磷酸(α-D-Glc-1-P)[25-26]。SPase具有广泛的受体底物特异性,能将Suc的葡萄糖基转移至多种受体化合物,根据参与反应的类型可将其作用受体分为3类(图 2):水;无机磷酸;含醇羟基、酚羟基或羧基的糖基受体化合物[27]。SPase在糖基化应用中具有突出表现,尤其是对非碳水化合物的糖基化活性使其逐渐成为酶工程研究的热点[27]。

|
| 图 2 SPase催化反应类型 Fig. 2 Three types of reactions catalyzed by sucrose phosphorylases including hydrolysis, phosphorylysis and transglycosylation. A: acceptors. |
| |
以下从SPase的结构与催化特性、SPase的定向改造、SPase在糖基化反应中的应用3个方面系统性地总结了SPase的研究进展。最后,在此基础上对未来该课题研究的发展方向进行了展望。
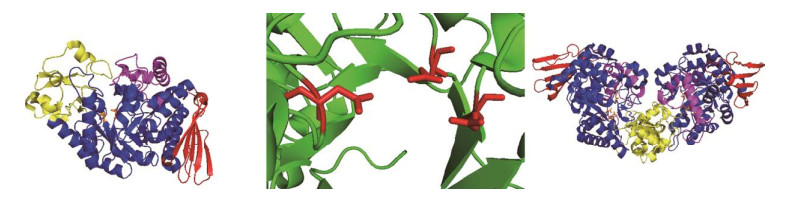
1 SPase的结构和催化特性 1.1 SPase的结构SPase主要分布于肠膜明串珠菌Leuconostoc mesenteroides[28]、嗜糖假单胞菌Pseudomonas saccharophila[29]、变形链球菌Streptococcus mutans、长双歧杆菌Bifidobacterium longum[30]、青春双歧杆菌Bifidobacterium adolescentis等微生物中[31-35],以单体或同型二聚体的形式发挥作用(表 1)。B. adolescentis来源的SPase (BaSPase)在结构上是一个同型二聚体,其中每个单体由4个结构域组成:结构域A (残基1–85,167–291,356–435)是由8个交替平行的β-折叠和α-螺旋组成的(β/α)8-桶结构;结构域B (残基86–166)主要由2个反向平行的β-折叠和2个短的α-螺旋构成;结构域B' (残基292–355)主要是一长一短的2个α-螺旋;结构域C含有5个反相平行的β-折叠,拓扑学结构为1, 1, 1, 1 (图 3A)[36]。催化相关位点定点突变体的动力学研究证实了SPase的活性中心是结构域A中的催化三联体结构(Asp192:催化亲核试剂;Asp290:过渡态稳定剂、Glu232:酸/碱催化剂) (图 3B),其在序列和结构上都具有保守性[37-39]。结构域B和B'是结构元件,与SPase的功能密切相关,位于其上的两个回环(Loop A,336AAASNLDLY344和Loop B,132YRPRP136)接近催化活性中心,在催化过程中参与酶构型的转变[40]。结构域C的长回环顶端接近结构域B',封锁了相当于ASase的寡糖结合位点的区域,因此导致了两种酶催化特性的差异[41]。单体之间通过结构域B相互作用在二聚体内部形成空腔,底物因此能进入活性位点(图 3C)[36]。
| Organism | Amino acid | Natural molecular mass (kDa) | Oligomeric state | Domains | 3D structure status | Residues involved in catalysis | Reference | |
| B. adolescentis | 504 | 129.0 | dimeric | A, B, B′, C | (β/α)8 -barrel | Asp192, Glu232, Asp290 | [32] | |
| L. mesenteroides | 492 | 56.1 | monomeric | Asp196, Glu237, Asp295 | [33] | |||
| P. saccharophila | 498 | 78.0 | dimeric | Asp200, Glu240, Asp298 | [34] | |||
| S. mutans | 480 | 55.7 | monomeric | Asp193, Glu234, Asp292 | [35] | |||
SPase遵循保留型双取代作用机制,催化过程中存在共价连接的β-葡萄糖基-酶中间体[34, 42-43]。如图 4所示,在催化的起始阶段,催化谷氨酸Glu232对Suc糖苷键的质子化以及天冬氨酸Asp192对葡萄糖基异头碳原子的亲核攻击同时进行,形成β-葡萄糖基-酶中间体,释放D-Fru。随后,受体化合物(如磷酸盐)与中间体反应,生成α-D-葡萄糖基化合物。
SPase的催化亚位点残基在催化过程中也起着至关重要的作用。Wildberger等研究发现在L. mesenteroides来源的SPase (LmSPase)中底物Glc-1-P在活性位点的定位是通过与催化三联体残基及催化亚位点残基Arg137形成氢键实现的,并且氨基酸残基Phe53与过渡态的稳定作用密切相关[44]。催化亚位点Asp49、Arg395能与被转移的葡萄糖基形成带电荷的氢键网络,从而有利于催化反应的进行[45]。Verhaeghe等[46]通过丙氨酸扫描技术绘制了BaSPase的受体位点图谱,发现与SPase磷酸盐底物特异性相关的位点是Pro134、Arg135、Tyr196、His234、Leu343、Tyr344、Gln345,与Fru底物特异性相关的位点是Tyr132、Pro134、Tyr196、His234、Asp342、Gln345。
SPase的糖基供体底物特异性较专一,只有Suc、Glc-1-P及α-D-葡萄糖-1-氟化物能够作为其糖基供体底物[47]。但其能够作用的糖基受体范围较广,尤其是LmSPase和BaSPase[28-29, 48-51]。SPase的最小作用受体底物是乙二醇,其参与糖基化反应的区域及立体选择性取决于受体底物的结构[52],如SPase作用于1, 2-丙二醇类受体化合物时,能够催化2位羟基的区域选择性糖基化反应[53]。Wildberger等证实了SPase参与的甘油酰胺的糖基化反应存在完全的立体选择性和非对映体选择性,因此能够实现外消旋甘油酰胺的手性拆分[54]。
Silverstein等研究了P. saccharophila来源的SPase (PsSPase)的催化反应动力学,发现在不同受体浓度下,供体底物浓度倒数(1/[S])与初始反应速率倒数(1/[v])组成的线形图谱是相互平行的,该现象与双取代作用机制一致[34]。双取代作用机制存在着一个显著的缺点,即反应体系中的水会与葡萄糖基-酶共价中间体反应造成糖基供体底物不可逆的水解。因此,SPase的催化过程存在糖基化反应与水解反应的竞争关系。糖基供体底物的水解产物葡萄糖会抑制SPase的催化活性[34, 55],影响最终产率。虽然底物的转化水平受到热力学限制,最终的糖基化产率也受到糖基化/水解反应选择性的影响,但是对反应过程进行动力学控制(如提高受体底物的浓度等)能够推动反应向理想方向进行[52, 56-57]。
2 SPase的定向改造虽然SPase的糖基受体底物特异性广泛,具有较好的工业应用前景,但其对一些受体底物(如芳香类化合物)的亲和力不佳,导致产率低[58],且SPase的有机溶剂与热稳定性也有待提高。因此,需要借助酶工程手段(如酶的固定化技术)改变SPase的作用形式,或通过理性突变、定向进化等策略进行天然SPase的基因修饰,实现其稳定性和催化特性的定向改造[59-62]。
2.1 SPase的热稳定性改造SPase的热稳定性可以通过酶固定化技术、定点突变和定向进化技术进行改善。SPase与酶载体Sapebeads EC-HFA的共价连接使其在60 ℃条件下的半衰期超过10 h[63]。并且,其在60 ℃的填充床反应器中进行Glc-1-P的生物合成时,2周时间内未见酶活损失[64]。将SPase固定化为交联酶聚集体形式能使其最适温度提高17 ℃,且其在60 ℃下孵育10 d活性不丧失[65]。LmSPase与硅结合模块Zbasic2的融合表达能够实现前者以固定化酶形式参与微生物反应器中的连续流式生物催化反应,从而提高酶活性利用率[66]。Fujii等通过向S. mutans来源的SPase (SmSPase)中引入随机突变的策略构建突变体文库,经高温筛选得到热稳定性改善的突变体[67]。Cerdobbel等将BaSPase结构中最易弯曲的氨基酸残基替换为相应序列位点最常见的氨基酸,同时结合定点突变技术促进蛋白质晶体结构中的静电相互作用,最终获得60 ℃条件下半衰期延长1倍以上的突变体,值得一提的是,该突变体的有机溶剂稳定性也有所改善[68]。
2.2 SPase的催化性质改造SPase的转糖苷活性、底物特异性、糖基化反应的区域及立体选择性等性质可以通过定点突变和定向进化技术得以改造。LmSPase经定点突变获得的突变体E237Q在酚类化合物的糖基化反应中无Suc水解活性,转化率因此提高了约7倍[39]。何贺贺等通过向LmSPase的活性中心近距离位点引入突变,得到了转糖苷活性增强的突变体[69]。Dirks-Hofmeister等将热解糖热厌氧杆菌Thermoanaerobacterium thermosaccharolyticum来源的SPase (TtSPase)活性回环中位阻效应较大的Arg134突变为Ala,获得的突变体TtSPase (R134 A)能实现白藜芦醇饱和水溶液中受体化合物的完全转化[70]。Kraus等将BaSPase的Gln345突变为Phe,结构域继而发生可逆性的移动,使其底物特异性发生改变[71-72]。该突变体能够高效催化一系列芳香族化合物的糖基化反应,尤其是以白藜芦醇作为受体底物时糖基化反应产率可达到97%[73]。并且,BaSPase (Q345F)参与糖基化反应的区域选择性也发生了转变,从而实现稀有二糖黑曲霉糖(3-O-a-D-吡喃葡萄糖基-D-葡萄糖,3-O-a-D-glucopyranosyl-D-glucose)的高效合成[74]。Verhaeghe等通过半理性突变和低通量筛选的方法获得了糖基化区域选择性改变的双突变体BaSPase (L341I_Q345S),实现了益生元曲二糖(2-O-α-D-吡喃葡萄糖基-D-葡萄糖,2-O-α-D- glucopyranosyl-D-glucose)的选择性合成[75]。并且利用该突变体,高效、规模化的曲二糖生产流程得以建立[76]。
2.3 SPase的高通量筛选与生产不论是在自然界优质酶的筛选过程中,还是在工业酶的定向进化过程中,高通量筛选策略常常被用于高活性目标的高效筛选。Choi等发现SPase作用于Glc-1-P释放的无机磷酸根离子能与钼酸铵络合成蓝色复合物,通过比色法能够实现SPase高活性菌株的快速筛选[77-78]。Vilozny等利用选择性碳水化合物传感系统检测SPase的作用产物Fru,实现了SPase的高通量筛选[79]。此外,Aerts等开发了基于组成型启动子的高通量筛选系统,有效减少了筛选过程中的移液步骤[80]。
在宿主细胞中克隆不可培养生物体来源的SPase基因以实现SPase在工程菌株中的异源表达能够提高SPase在生物催化领域的应用潜力[81]。不同生物体的SPase的基因可通过基因工程技术获得,包括聚合酶链式反应[82]以及利用密码子优化技术实现目的基因的合成[83]等。工业上SPase的生产主要通过工程菌的发酵来实现,结合发酵条件(如初始细胞密度、诱导剂浓度等)优化,能够实现SPase在宿主细胞中的过量表达[84]。Su等通过SPase与磷脂酶C的共表达,实现了胞质蛋白在胞外的高效表达,从而简化SPase生产中的蛋白提纯步骤[85]。
3 SPase的应用 3.1 SPase在糖基化反应中的应用SPase的糖基化受体根据涉及的反应基团的不同可分为3类(表 2):多羟基化合物、含酚基化合物、含羧基化合物。
| Enzyme | UniProt number | Donor | Acceptor | Conditions (pH, T) | Product | Yield (%) | Reference |
| LmSP | Q54495 | Glc-1-P (20%) | Xylitol (20%) | 6.9 37 ℃ | 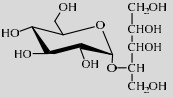
|
5.1 | [49] |
| LmSP | Q03Z66 | Suc (0.8 mol/L) | Glycerol (2.0 mol/L) | 7.0 30 ℃ | 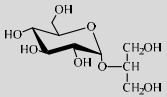
|
90 | [56] |
| BlSP | V6XTV6 | Suc (0.8 mol/L) | L-ascorbic acid (1.2 mol/L) | 5.2 40 ℃ | 
|
~50 | [94] |
| LmSP | Q54495 | Suc (40%) | Kojic acid (2%) | 7.5 42 ℃ | 
|
7.5/12.2 | [51] |
| LmSP | Q54495 | Suc (50 mg) | EHMF/HDMF (5.0 mg) | 7.2 32 ℃ | 
|
48/45 | [99] |
| TtSP(R134A) | D9TT09 | Suc (34%) | 3-hydroxy-β-lactams (15 mmol/L) | 55 ℃ | 
|
3–17 | [100] |
| LmSP | Q54495 | Suc (50%) | Hydroquinone (1%) | 6.5 37–42 ℃ | 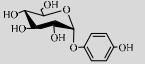
|
60 | [50] |
| BaSP | Q84HQ2 | Suc (68%) | Pyrogallol (10%) | 7.5 50 ℃ | 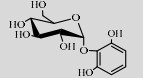
|
72.3 | [106] |
| TtSP(R134A) | D9TT09 | Suc (34%) | Resveratrol (1%) | 6.5 55 ℃ | 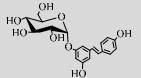
|
100 | [70] |
| LmSP | Q54495 | Suc (30%) | EGCG (1%) | 6.0 42 ℃ | 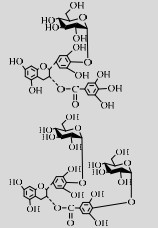
|
30.4/39.8 | [48] |
| LmSP | Q54495 | Suc (30%) | Catechin (1%) | 7.5 42 ℃ | 
|
81 | [114] |
| SmSP | P10249 | Suc (30 %) | Benzoic acid (0.8%) | 3.9 40 ℃ | 
|
55 | [117] |
| SmSP | P10249 | Suc (40%) | Acetic acid (1%) | 4.2 37 ℃ | 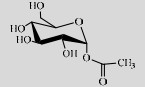
|
> 90 | [118] |
| BlSP | V6XTV6 | Suc (30%) | Caffeic acid (1%) | 6.8 42 ℃ | 
|
-b | [120] |
| a: R1=iPr, Pr, Pr; R2=Bn, iPr. b: –: Not reported. | |||||||
SPase能够作用于一系列单糖、糖醇类受体,如:D-Fru[34]、D-Glc[86]、L-山梨糖[29]、L-阿拉伯糖[32]、木糖醇[49]、L-阿拉伯醇[87],生成的产物能够作为食品、化妆品添加剂[49]。SPase还能催化二糖、寡糖的糖基化反应,如乳糖、纤维二糖、麦芽三糖、异麦芽三糖[88],糖基化寡糖能抑制淀粉酶和糖苷酶的活性,因此具有潜在的血糖调节功能[89]。
SPase还能作用于非碳水化合物的醇羟基。LmSPase能催化Suc和甘油,实现2-O-α-D-吡喃葡萄糖基-sn-甘油(2-O-a-D-glucopyranosyl-sn- glycerol,2-αGG)的高效合成[56]。2-αGG具有强效保湿、抗衰老、舒缓修复、激活细胞等生理活性,被广泛应用为化妆品添加剂[90]。并且其甜度是Suc的0.55倍,可被应用为无致龋性的甜味剂[91]。
B. longum来源的SPase (BlSPase)能以L-抗坏血酸作为糖基受体合成2-O-α-D-吡喃葡糖基-L-抗坏血酸(2-O-α-D-glucopyranosyl-L-ascorbic acid,AA- 2G)[92]。AA-2G有更好的体外稳定性,在体内AA-2G能被α-糖苷酶水解为L-抗坏血酸,从而发挥其抗氧化活性[93]。利用SPase生物合成AA-2G的过程具有特殊的pH依赖性:pH为7.0–7.5时,主要发生Suc的水解,副产物AA-6G积累;当pH低于6.0时,BlSPase高效催化AA-2G的合成,不产生AA-6G,而且随着pH的降低,催化活性逐渐增强,在pH为5.2时,AA-2G的合成活性达到最大[94]。
LmSPase能催化Suc与曲酸反应生成5-O-α-D-吡喃葡萄糖基-曲酸(Kojic acid 5-O-α-D-glucopyranosyl-kojic acid,KA-5G)和7-O-α-D-吡喃葡萄糖基-曲酸(Kojic acid 7-O-α-D-glucopyranosyl-kojic acid,KA-7G)。其中,KA-7G不仅保留了酪氨酸酶的抑制活性,并且其口味、水溶性、稳定性均优于曲酸,能应用于化妆品、食品等行业[51, 95]。
5-乙基-4-羟基-2-甲基-3(2H)-呋喃酮(5-ethyl-4- hydroxy-2-methyl-3(2H)-furanone,EHM F)与4-羟基-2, 5-二甲基-3(2H)-呋喃酮(4-hydroxy-2, 5- dimethyl-3(2H)-furanone,HDMF)不仅能被应用为食品加工行业的芳香物质,还能抑制致癌物诱发的小鼠前胃癌的发生[96-97]。但是EHMF和HDMF极易被氧化,且易挥发[98]。LmSPase能够分别催化EHMF和HDMF与Suc反应,生成稳定性显著改善的2-乙基-5-甲基-3(2H)-呋喃酮- 4-O-α-D-葡萄糖苷(2-ethyl-5-methyl-3(2H)-furanone- 4-O-α-D-glucopyranoside,EMG-G)和2, 5-二甲基- 3(2H)-呋喃酮-4-O-α-D-葡萄糖苷(2, 5-dimethyl-3 (2H)-furanone-4-O-α-D-glucopyranoside,DMF-G),其在生物体内经α-糖苷酶水解后发挥抗氧化活性[99]。
TtSPase经酶改造后获得的突变体R134A能够催化3-羟基-β-内酰胺类化合物的糖基化反应[100]。该催化反应具有高度的对映体选择性,为其他手性化合物的合成奠定了基础。
3.1.2 SPase参与含酚基化合物的糖基化反应许多酚类化合物都具有如抗肿瘤、抗氧化、抗菌、抗炎症等生物活性,甚至能预防和治疗心脑血管疾病[101]。但酚类化合物普遍存在水溶性差、稳定性不佳、毒性高等缺点,糖基化作用能够有效改善这些缺陷[102-103]。SPase参与的酚类化合物的糖基化反应总结如下。
对苯二酚能抑制生物体内酪氨酸酶活性从而抑制黑色素的合成,因此具有较强的美白作用[104]。但是对苯二酚对人体有很大毒副作用,且易被氧化成醌。LmSPase能催化Suc和对苯二酚专一性合成α-熊果苷(α-arbutin),该产物对酪氨酸酶的抑制能力是β-arbutin的10倍以上且对人体无毒[50, 105]。
BaSPase能催化Suc和邻苯三酚合成稳定性改善的邻苯三酚-2-O-α-D-葡萄糖苷(Pyrogallol-2-O-α-D-glucopyranoside)[106]。并且,De Winter等还设计了缓冲液/乙酸乙酯(5/3)的双相体系作为上述反应的溶剂系统,使受体底物的转化率提高约7倍[107]。
白藜芦醇是一种植物抗毒素,能够抵抗多种疾病,包括Ⅱ型糖尿病、肥胖、动脉粥样硬化、阿兹海默症等[108-109]。研究发现白藜芦醇的糖基化修饰能够改善其水溶性、稳定性、生物利用度,有利于该化合物的临床应用[110]。酶改造突变体TtSPase (R134A)能够高效作用于白藜芦醇生成水溶性、稳定性均得到改善的糖基化产物[70]。儿茶素类化合物具有抗炎、抗菌、抗病毒及抗氧化等特性[111-112]。其中,含量最丰富的表没食子儿茶素没食子酸酯(Epigallocatechin gallate, EGCG)抗氧化性最显著[113]。但EGCG在水中易降解,LmSPase能以EGCG为受体生成稳定性改善的4ʹ-O-吡喃葡萄糖基-表没食子儿茶素没食子酸酯(4ʹ-O-D-glucopyranosyl-epigallocatechin gallate,EGCG-G)和4ʹ, 4ʺ-O-二吡喃葡萄糖基-表没食子儿茶素没食子酸酯(4ʹ, 4ʺ-O-D-diglucopyranosyl- epigallocatechin gallate,EGCG-2G)[48]。此外,LmSPase还能催化Suc和儿茶素合成儿茶素-3ʹ-O-D-葡萄糖苷(Catechin-3ʹ-O-D-glucopyranoside,C-G)[114]。在保留其抗氧化能力的同时,C-G的抗褐变能力、水溶性都显著优于儿茶素[106]。
3.1.3 SPase参与含羧基化合物的糖基化反应许多结构上含有羧基的化合物能被运用于食品、化妆品行业,如乙酸、苯甲酸、丁酸等[115-116],但当中许多化合物具有较强的气味和酸度,或是较差的水溶性。Sugimoto等利用SmSPase催化Suc和苯甲酸合成1-O-苯甲酰-α-D-吡喃葡萄糖苷(1-O-benzoyl-α-D-glucopyranoside,BA-G),并且SmSPase对乙酸、甲酸、丙酸、马来酸、丙二酸、富马酸、乳酸、苹果酸、阿魏酸都有糖基化活性[117]。SmSPase作用于乙酸生成的1-O-乙酰-α-D-吡喃葡萄糖(1-O-acetyl-α-D-glucopyranose)的酸味阈值是乙酸的100倍,在口味上有较好的改善[118]。
咖啡酸具有强大的抗氧化活性,能够作为食品及化妆品添加剂[119]。咖啡酸的应用受到其水溶性、稳定性的限制。BlSPase能催化Suc和咖啡酸合成咖啡酸的单糖苷和二糖苷[120]。
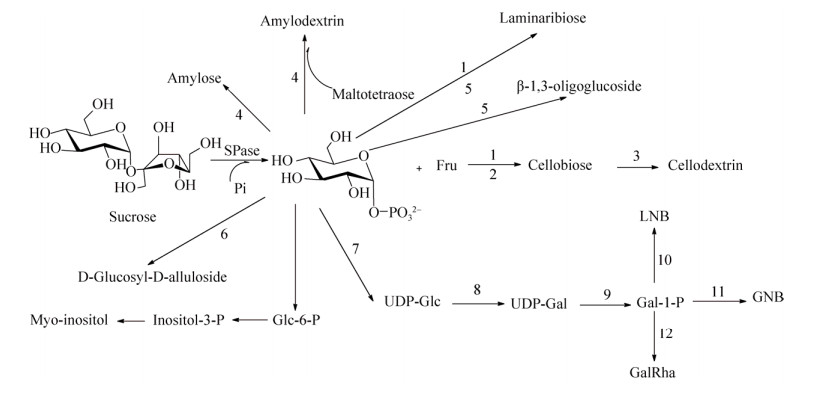
3.2 SPase与其他酶的联合应用SPase还能通过与其他酶的联合应用发挥其生物活性,形成的酶耦合系统主要分为以下3类:(1) SPase能够催化Suc与无机磷酸生成Glc-1-P,为其他酶提供底物,实现高附加值产物的合成(图 5)。如LmSPase催化合成Glc-1-P的过程联用木糖异构酶(Xylose isomerase, EC 5.3.1.5)、纤维二糖磷酸化酶(Cellobiose phosphorylase,EC 2.4.1.20)能实现食品原料纤维二糖的高产量合成[121-122]。上述催化过程联合纤维糊精磷酸化酶(Cellodextrin phosphorylase, EC 2.4.1.49)能高效合成可溶性短链麦芽糊精[123]。葡聚糖磷酸化酶(Glucan phosphorylase,EC 2.4.1.1)能作用于SmSPase的产物Glc-1-P高效合成直链淀粉[124-125]。SPase产生的Glc-1-P与麦芽四糖共同作为葡聚糖磷酸化酶的底物,构成了直链糊精的低成本合成途径[126]。SPase、木糖异构酶、昆布二糖磷酸化酶(Laminaribiose phosphorylase,EC 2.4.1.31)的联合应用实现了昆布二糖的生物合成[127]。功能糖β-1, 3-葡寡糖的大规模制备是通过SPase和昆布二糖磷酸化酶的联合应用实现的[128]。在D-葡萄糖基-D-阿洛酮糖苷的合成过程中,SPase、D-塔格糖-3-差向异构酶(D-Tagatose-3- epimerase)的共同作用能减少投入成本[129]。Nishimoto等设计了SPase在内的耦合酶催化体系,合成了乳糖-N-二糖Ⅰ (Lacto-N- bioseⅠ,LNB Ⅰ)[130]、半乳糖-N-二糖(Galacto-N-biose,GNB)[131]、半乳糖基-β1→4-L-鼠李糖(Galatosyl-β1→4-L- rhamnose,GalRha)[132]。由SPase、葡萄糖磷酸变位酶(Phosphoglucomutase, EC 5.4.2.2)、肌醇-1-磷酸合酶(Inositol 1-phosphate synthase,EC 5.5.1.4)、肌醇单磷酸化酶(Inositol monophosphatase,EC 3.1.3.25)组成的多酶体系能以热循环级联催化的方式高效合成肌醇[133]。
|
| 图 5 SPase与一系列酶偶联催化合成多种化合物 Fig. 5 Applications of SPase combined with other biocatalysts by supplying Glc-1-P. 1: xylose isomerase; 2: cellobiose phosphorylase; 3: cellodextrin phosphorylase; 4: glucan phosphorylase; 5: laminaribiose phosphorylase; 6: D-tagatose-3-epimerase; 7: UDP-glucose-1-phosphate uridylyltransferase; 8: UDP-glucose-4-epimerase; 9: UDP-glucose-1-phosphate uridylyltransferase; 10: lacto-N-biose phosphorylase; 11: galacto-N-biose phosphorylase; 12: D-galactosyl-β1→4-L-rhamnose phosphorylase; 13: phosphoglucomutase; 14: inositol-1-phosphate synthase; 15: inositol monophosphatase. |
| |
(2) SPase分解Suc为反应提供能量。Myung等设计了LmSPase在内的包含15种生物催化剂的耦合酶系统,LmSPase作用于Suc为氢气生物合成过程中的水分解提供了能量[134]。
(3) 多酶联用能特异性移除SPase的非理想产物,驱动反应向底物消耗的方向进行,提高产率。在纤维二糖的合成过程中,葡萄糖异构酶能够将SPase的产物D-Fru转化为纤维二糖磷酸化酶的底物Glc,产量因此显著提高[121]。在氢气的合成过程中,磷酸盐葡萄糖激酶能够去除SPase的产物Glc,提高反应的总体经济[134]。
4 结论与展望综上所述,SPase是可作用于廉价供体的葡萄糖基转移酶,具有广泛的糖基受体底物特异性,在食品、化妆品、制药工业中具有广泛的应用。基于国内外科研工作的研究进展,SPase课题的深入开展可从以下4个方面出发:(1)筛选SPase的野生型高活性菌株,或利用基因工程、发酵工程、代谢工程等手段提高SPase产率和活力[81],丰富其来源。(2)许多蛋白质与机理研究强调了酶活性回环、底物进入通道对催化性质的重要性[135-136]。基于该理念以及改造目的灵活制定半理性、理性突变策略,结合高通量筛选方法实现SPase的定向进化,提高其应用潜力。(3)向催化体系中添加助溶剂能够成功改善疏水性糖基受体在催化体系中溶解度低的问题[137],需要综合考虑SPase的稳定性以及疏水性受体化合物的溶解度问题,设计理想的反应介质以拓展SPase的应用范围。(4)借助酶工程手段(酶固定化技术)实现SPase的重复使用,降低其在工业应用中的成本。
| [1] |
Desmet T, Soetaert W. Enzymatic glycosyl transfer: mechanisms and applications. Biocatal Biotransfor, 2011, 29(1): 1-18. DOI:10.3109/10242422.2010.548557 |
| [2] |
Xu LJ, Qi TT, Xu L, et al. Recent progress in the enzymatic glycosylation of phenolic compounds. J Carbohydr Chem, 2016, 35(1): 1-23. DOI:10.1080/07328303.2015.1137580 |
| [3] |
Bednarska NG, Wren BW, Willcocks SJ. The importance of the glycosylation of antimicrobial peptides: natural and synthetic approaches. Drug Discov Today, 2017, 22(6): 919-926. DOI:10.1016/j.drudis.2017.02.001 |
| [4] |
Wallace JE, Schroeder LR. Koenigs-Knorr reactions. Part 1. Effects of a 2-O-acetyl substituent, the promoter, and the alcohol concentration on the stereoselectivity of reactions of 1, 2-cis-glucopyranosyl bromide. J Chem Soc Perkin Trans 1, 1976, 18(18): 1938-1941. |
| [5] |
Wallace JE, Schroeder LR. Koenigs-Knorr reactions. Part Ⅱ. A mechanistic study of mercury(Ⅱ) cyanide-promoted reactions of 2, 3, 4, 6-tetra-O-methyl-α-D-glucopyranosyl bromide with cyclohexanol in benzene-nitromethane. J Chem Soc Perkin Trans 2, 1976, 8(14): 1632-1636. |
| [6] |
Wallace JE, Schroeder LR. Koenigs-Knorr reactions. Part 3. Mechanistic study of mercury(Ⅱ) cyanide promoted reactions of 2-O-acetyl-3, 4, 6-tri-O-methyl- α--glucopyranosyl bromide with cyclohexanol in benzene-nitromethane. J Chem Soc Perkin Trans 2, 1977, 8(6): 795-802. |
| [7] |
Schmidt RR, Michel J. Facile synthesis of α- and β-O-glycosyl imidates; preparation of glycosides and disaccharides. Angew Chem Int Ed Engl, 1980, 19(9): 731-732. DOI:10.1002/anie.198007311 |
| [8] |
Fügedi P, Garegg PJ. A novel promoter for the efficient construction of 1, 2-trans linkages in glycoside synthesis, using thioglycosides as glycosyl donor. Carbohydr Res, 1986, 149(1): C9-C12. DOI:10.1016/S0008-6215(00)90385-9 |
| [9] |
Mydock LK, Demchenko AV. Mechanism of chemical O-glycosylation: from early studies to recent discoveries. Org Biomol Chem, 2010, 8(3): 497-510. DOI:10.1039/B916088D |
| [10] |
Christensen HM, Oscarson S, Jensen HH. Common side reactions of the glycosyl donor in chemical glycosylation. Carbohydr Res, 2015, 408: 51-95. DOI:10.1016/j.carres.2015.02.007 |
| [11] |
De Roode BM, Franssen MCR, Van Der Padt A, et al. Perspectives for the industrial enzymatic production of glycosides. Biotechnol Prog, 2003, 19(5): 1391-1402. DOI:10.1021/bp030038q |
| [12] |
Coutinho PM, Stam M, Blanc E, et al. Why are there so many carbohydrate-active enzyme-related genes in plants?. Trends Plant Sci, 2003, 8(12): 563-565. DOI:10.1016/j.tplants.2003.10.002 |
| [13] |
Cantarel BL, Coutinho PM, Rancurel C, et al. The carbohydrate-active enzymes database (CAZy): an expert resource for glycogenomics. Nucleic Acids Res, 2009, 37(S1): D233-D238. |
| [14] |
Lombard V, Ramulu HG, Drula E, et al. The carbohydrate-active enzymes database (CAZy) in 2013. Nucleic Acids Res, 2014, 42(D1): D490-D495. DOI:10.1093/nar/gkt1178 |
| [15] |
De Bruyn F, Maertens J, Beauprez J, et al. Biotechnological advances in UDP-sugar based glycosylation of small molecules. Biotechnol Adv, 2015, 33(2): 288-302. DOI:10.1016/j.biotechadv.2015.02.005 |
| [16] |
Henrissat B, Davies G. Structural and sequence-based classification of glycoside hydrolases. Curr Opin Struct Biol, 1997, 7(5): 637-644. DOI:10.1016/S0959-440X(97)80072-3 |
| [17] |
Monchois V, Willemot RM, Monsan P. Glucansucrases: mechanism of action and structure–function relationships. FEMS Microbiol Rev, 1999, 23(2): 131-151. DOI:10.1016/S0168-6445(98)00041-2 |
| [18] |
Devlamynck T, Te Poele EM, Meng XF, et al. Glucansucrase Gtf180-△N of Lactobacillus reuteri 180: enzyme and reaction engineering for improved glycosylation of non-carbohydrate molecules. Appl Microbiol Biotechnol, 2016, 100(17): 7529-7539. DOI:10.1007/s00253-016-7476-x |
| [19] |
Wang R, Bae JS, Kim JH, et al. Development of an efficient bioprocess for turanose production by sucrose isomerisation reaction of amylosucrase. Food Chem, 2012, 132(2): 773-779. DOI:10.1016/j.foodchem.2011.11.035 |
| [20] |
Putaux JL, Potocki-Véronèse G, Remaud-Simeon M, et al. α-D-glucan-based dendritic nanoparticles prepared by in vitro enzymatic chain extension of glycogen. Biomacromolecules, 2006, 7(6): 1720-1728. DOI:10.1021/bm050988v |
| [21] |
Rolland-Sabaté A, Colonna P, Potocki-Véronèse G, et al. Elongation and insolubilisation of α-glucans by the action of Neisseria polysaccharea amylosucrase. J Cereal Sci, 2004, 40(1): 17-30. DOI:10.1016/j.jcs.2004.04.001 |
| [22] |
Overwin H, Wray V, Hofer B. Flavonoid glucosylation by non-Leloir glycosyltransferases: formation of multiple derivatives of 3, 5, 7, 3', 4'-pentahydroxyflavane stereoisomers. Appl Microbiol Biotechnol, 2015, 99(22): 9565-9576. DOI:10.1007/s00253-015-6760-5 |
| [23] |
Jeong JW, Seo DH, Jung JH, et al. Biosynthesis of glucosyl glycerol, a compatible solute, using intermolecular transglycosylation activity of amylosucrase from Methylobacillus flagellatus KT. Appl Biochem Biotechnol, 2014, 173(4): 904-917. DOI:10.1007/s12010-014-0889-z |
| [24] |
Tian YQ, Xu W, Zhang WL, et al. Amylosucrase as a transglucosylation tool: from molecular features to bioengineering applications. Biotechnol Adv, 2018, 36(5): 1540-1552. DOI:10.1016/j.biotechadv.2018.06.010 |
| [25] |
Kawasaki H, Nakamura N, Ohmori M, et al. Screening for bacteria producing sucrose phosphorylase and characterization of the enzymes. Biosci Biotechnol Biochem, 1996, 60(2): 319-321. DOI:10.1271/bbb.60.319 |
| [26] |
Mieyal JJ, Abeles RH. 17 Disaccharide phosphorylases. Enzymes, 1972, 7: 515-532. |
| [27] |
Goedl C, Sawangwan T, Wildberger P, et al. Sucrose phosphorylase: a powerful transglucosylation catalyst for synthesis of α-D-glucosides as industrial fine chemicals. Biocatal Biotransfor, 2010, 28(1): 10-21. DOI:10.3109/10242420903411595 |
| [28] |
Weimberg R, Doudoroff M. Studies with three bacterial sucrose phosphorylases. J Bacteriol, 1954, 68(3): 381-388. DOI:10.1128/JB.68.3.381-388.1954 |
| [29] |
Doudoroff M, Barker HA, Hassid WZ. Studies with bacterial sucrose phosphorylase; the mechanism of action of sucrose phosphorylase as a glucose-transferring enzyme (transglucosidase). J Biol Chem, 1947, 168(2): 725-732. DOI:10.1016/S0021-9258(17)30931-6 |
| [30] |
Kullin B, Abratt VR, Reid SJ. A functional analysis of the Bifidobacterium longum cscA and scrP genes in sucrose utilization. Appl Microbiol Biotechnol, 2006, 72(5): 975-981. DOI:10.1007/s00253-006-0358-x |
| [31] |
Kim M, Kwon T, Lee HJ, et al. Cloning and expression of sucrose phosphorylase gene from Bifidobacterium longum in E. coli and characterization of the recombinant enzyme. Biotechnol Lett, 2003, 25(15): 1211-1217. DOI:10.1023/A:1025035320983 |
| [32] |
Van Den Broek LA, Van Boxtel EL, Kievit RP, et al. Physico-chemical and transglucosylation properties of recombinant sucrose phosphorylase from Bifidobacterium adolescentis DSM20083. Appl Microbiol Biotechnol, 2004, 65(2): 219-227. |
| [33] |
Koga T, Nakamura K, Shirokane Y, et al. Purification and some properties of sucrose phosphorylase from Leuconostoc mesenteroides. J Agric Chem Soc Japan, 1991, 55(7): 1805-1810. |
| [34] |
Silverstein R, Voet J, Reed D, et al. Purification and mechanism of action of sucrose phosphorylase. J Biol Chem, 1967, 242(6): 1338-1346. DOI:10.1016/S0021-9258(18)96185-5 |
| [35] |
Robeson JP, Barletta RG, Curtiss R. Expression of a Streptococcus mutans glucosyltransferase gene in Escherichia coli. J Bacteriol, 1983, 153(1): 211-221. DOI:10.1128/JB.153.1.211-221.1983 |
| [36] |
Sprogøe D, Van Den Broek LAM, Mirza O, et al. Crystal structure of sucrose phosphorylase from Bifidobacterium adolescentis. Biochemistry, 2004, 43(5): 1156-1162. DOI:10.1021/bi0356395 |
| [37] |
Schwarz A, Nidetzky B. Asp-196→Ala mutant of Leuconostoc mesenteroides sucrose phosphorylase exhibits altered stereochemical course and kinetic mechanism of glycosyl transfer to and from phosphate. FEBS Lett, 2006, 580(16): 3905-3910. DOI:10.1016/j.febslet.2006.06.020 |
| [38] |
Mueller M, Nidetzky B. The role of Asp-295 in the catalytic mechanism of Leuconostoc mesenteroides sucrose phosphorylase probed with site-directed mutagenesis. FEBS Lett, 2007, 581(7): 1403-1408. DOI:10.1016/j.febslet.2007.02.060 |
| [39] |
Wiesbauer J, Goedl C, Schwarz A, et al. Substitution of the catalytic acid-base Glu237 by Gln suppresses hydrolysis during glucosylation of phenolic acceptors catalyzed by Leuconostoc mesenteroides sucrose phosphorylase. J Mol Catal B: Enzym, 2010, 65(1/4): 24-29. |
| [40] |
Mirza O, Skov LK, Sprogoe D, et al. Structural rearrangements of sucrose phosphorylase from Bifidobacterium adolescentis during sucrose conversion. J Biol Chem, 2006, 281(46): 35576-35584. DOI:10.1074/jbc.M605611200 |
| [41] |
Albenne C, Skov LK, Mirza O, et al. Molecular basis of the amylose-like polymer formation catalyzed by Neisseria polysaccharea amylosucrase. J Biol Chem, 2004, 279(1): 726-734. DOI:10.1074/jbc.M309891200 |
| [42] |
Doudoroff M, Hassid WZ, Barker HA. Studies with bacterial sucrose phosphorylase; enzymatic synthesis of a new reducing and of a new non-reducing disaccharide. J Biol Chem, 1947, 168(2): 733-746. DOI:10.1016/S0021-9258(17)30932-8 |
| [43] |
Guibert A, Monsan P. Production and purification of sucrose phosphorylase from Leuconostoc mesenteroides application to the production of glucose-1-phosphate. Ann New York Acad Sci, 1988, 542(1): 307-311. |
| [44] |
Wildberger P, Aish GA, Jakeman DL, et al. Interplay of catalytic subsite residues in the positioning of α-D-glucose 1-phosphate in sucrose phosphorylase. Biochem Biophys Rep, 2015, 2: 36-44. |
| [45] |
Wildberger P, Todea A, Nidetzky B. Probing enzyme-substrate interactions at the catalytic subsite of Leuconostoc mesenteroides sucrose phosphorylase with site-directed mutagenesis: the roles of Asp49 and Arg395. Biocatal Biotransfor, 2012, 30(3): 326-337. DOI:10.3109/10242422.2012.674720 |
| [46] |
Verhaeghe T, Diricks M, Aerts D, et al. Mapping the acceptor site of sucrose phosphorylase from Bifidobacterium adolescentis by alanine scanning. J Mol Catal B: Enzym, 2013, 96: 81-88. DOI:10.1016/j.molcatb.2013.06.014 |
| [47] |
Gold AM, Osber MP. α-D-Glucopyranosyl fluoride: a substrate of sucrose phosphorylase. Biochem Biophys Res Commun, 1971, 42(3): 469-474. DOI:10.1016/0006-291X(71)90394-9 |
| [48] |
Kitao S, Matsudo T, Saitoh M, et al. Enzymatic syntheses of two stable (-)-epigallocatechin gallate-glucosides by sucrose phosphorylase. Biosci Biotechnol Biochem, 1995, 59(11): 2167-2169. DOI:10.1271/bbb.59.2167 |
| [49] |
Kitao S, Sekine H. Transglucosylation catalyzed by sucrose phosphorylase from Leuconostoc mesenteroides and production of glucosyl-xylitol. Biosci Biotechnol Biochem, 1992, 56(12): 2011-2014. DOI:10.1271/bbb.56.2011 |
| [50] |
Kitao S, Sekine H. α-D-Glucosyl transfer to phenolic compounds by sucrose phosphorylase from Leuconostoc mesenteroides and production of α-arbutin. Biosci Biotechnol Biochem, 1994, 58(1): 38-42. DOI:10.1271/bbb.58.38 |
| [51] |
Kitao S, Serine H. Syntheses of two kojic acid glucosides with sucrose phosphorylase from Leuconostoc mesenteroides. Biosci Biotechnol Biochem, 1994, 58(2): 419-420. DOI:10.1271/bbb.58.419 |
| [52] |
Renirie R, Pukin A, Van Lagen B, et al. Regio- and stereoselective glucosylation of diols by sucrose phosphorylase using sucrose or glucose 1-phosphate as glucosyl donor. J Mol Catal B: Enzym, 2010, 67(3/4): 219-224. |
| [53] |
Luley-Goedl C, Sawangwan T, Brecker L, et al. Regioselective O-glucosylation by sucrose phosphorylase: a promising route for functional diversification of a range of 1, 2-propanediols. Carbohydr Res, 2010, 345(12): 1736-1740. DOI:10.1016/j.carres.2010.05.022 |
| [54] |
Wildberger P, Brecker L, Nidetzky B. Chiral resolution through stereoselective transglycosylation by sucrose phosphorylase: application to the synthesis of a new biomimetic compatible solute, (R)-2-O-α-D- glucopyranosyl glyceric acid amide. Chem Comm, 2014, 50(4): 436-438. DOI:10.1039/C3CC47249C |
| [55] |
Doudoroff M, Kaplan NO, Hassid WZ. Phosphorolysis and synthesis of sucrose with a bacterial preparation. J Biol Chem, 1943, 148(1): 67-75. DOI:10.1016/S0021-9258(18)72317-X |
| [56] |
Goedl C, Sawangwan T, Mueller M, et al. A high-yielding biocatalytic process for the production of 2-O-(a-D-glucopyranosyl)-sn-glycerol, a natural osmolyte and useful moisturizing ingredient. Angew Chem Int Ed, 2008, 47(52): 10086-10089. DOI:10.1002/anie.200803562 |
| [57] |
Chen HC, Yang SS, Xu AJ, et al. Insight into the glycosylation and hydrolysis kinetics of α-glucosidase in the synthesis of glycosides. Appl Microbiol Biotechnol, 2019, 103(23/24): 9423-9432. DOI:10.1007/s00253-019-10205-6 |
| [58] |
Desmet T, Soetaert W, Bojarová P, et al. Enzymatic glycosylation of small molecules: challenging substrates require tailored catalysts. Chem Eur J, 2012, 18(35): 10786-10801. DOI:10.1002/chem.201103069 |
| [59] |
Zhang K, Qu G, Liu WD, et al. Structure-function relationships of industrial enzymes. Chin J Biotechnol, 2019, 35(10): 1806-1818 (in Chinese). 张锟, 曲戈, 刘卫东, 等. 工业酶结构与功能的构效关系. 生物工程学报, 2019, 35(10): 1806-1818. |
| [60] |
Qu G, Zhu T, Jiang YY, et al. Protein engineering: from directed evolution to computational design. Chin J Biotech, 2019, 35(10): 1843-1856 (in Chinese). 曲戈, 朱彤, 蒋迎迎, 等. 蛋白质工程:从定向进化到计算设计. 生物工程学报, 2019, 35(10): 1843-1856. |
| [61] |
Hancock SM, Vaughan MD, Withers SG. Engineering of glycosidases and glycosyltransferases. Curr Opin Chem Biol, 2006, 10(5): 509-519. DOI:10.1016/j.cbpa.2006.07.015 |
| [62] |
Desmet T, Soetaert W. Broadening the synthetic potential of disaccharide phosphorylases through enzyme engineering. Process Biochem, 2012, 47(1): 11-17. DOI:10.1016/j.procbio.2011.10.039 |
| [63] |
Cerdobbel A, Desmet T, De Winter K, et al. Increasing the thermostability of sucrose phosphorylase by multipoint covalent immobilization. J Biotechnol, 2010, 150(1): 125-130. DOI:10.1016/j.jbiotec.2010.07.0299 |
| [64] |
De Winter K, Cerdobbel A, Soetaert W, et al. Operational stability of immobilized sucrose phosphorylase: Continuous production of α-glucose-1-phosphate at elevated temperatures. Process Biochem, 2011, 46(10): 2074-2078. DOI:10.1016/j.procbio.2011.08.002 |
| [65] |
Cerdobbel A, De Winter K, Desmet T, et al. Sucrose phosphorylase as cross-linked enzyme aggregate: improved thermal stability for industrial applications. Biotechnol J, 2010, 5(11): 1192-1197. DOI:10.1002/biot.201000202 |
| [66] |
Valikhani D, Bolivar JM, Pfeiffer M, et al. Multivalency effects on the immobilization of sucrose phosphorylase in flow microchannels and their use in the development of a high-performance biocatalytic microreactor. ChemCatChem, 2017, 9(1): 161-166. DOI:10.1002/cctc.201601019 |
| [67] |
Fujii K, Iiboshi M, Yanase M, et al. Enhancing the thermal stability of sucrose phosphorylase from Streptococcus mutans by random mutagenesis. J Appl Glycosci, 2006, 53(2): 91-97. DOI:10.5458/jag.53.91 |
| [68] |
Cerdobbel A, De Winter K, Aerts D, et al. Increasing the thermostability of sucrose phosphorylase by a combination of sequence- and structure-based mutagenesis. Protein Eng Des Select, 2011, 24(11): 829-834. DOI:10.1093/protein/gzr042 |
| [69] |
He HH, Lin HM, Kou LD, et al. Characterization of recombinant sucrose phosphorylase from Leuconostoc mesenteroides ATCC 12291 and its molecular modification for improved transglucoside activity. Food Sci, 2019, 12(2): 1-12 (in Chinese). 何贺贺, 林厚民, 寇力丹, 等. 肠膜明串珠菌ATCC 12291蔗糖磷酸化酶的酶学性质及转糖苷分子改造. 食品科学, 2019, 12(2): 1-12. |
| [70] |
Dirks-Hofmeister ME, Verhaeghe T, De Winter K, et al. Creating space for large acceptors: rational biocatalyst design for resveratrol glycosylation in an aqueous system. Angew Chem Int Ed, 2016, 54(32): 9289-9292. |
| [71] |
Kraus M, Grimm C, Seibel J. Redesign of the active site of sucrose phosphorylase through a clash-induced cascade of loop shifts. ChemBioChem, 2016, 17(1): 33-36. DOI:10.1002/cbic.201500514 |
| [72] |
Kraus M, Grimm C, Seibel J. Reversibility of a point mutation induced domain shift: expanding the conformational space of a sucrose phosphorylase. Sci Rep, 2018, 8(1): 10490. DOI:10.1038/s41598-018-28802-2 |
| [73] |
Kraus M, Grimm C, Seibel J. Switching enzyme specificity from phosphate to resveratrol glucosylation. Chem Comm, 2017, 53(90): 12181-12184. DOI:10.1039/C7CC05993K |
| [74] |
Kraus M, Gorl J, Timm M, et al. Synthesis of the rare disaccharide nigerose by structure-based design of a phosphorylase mutant with altered regioselectivity. Chem Comm, 2016, 52(25): 4625-4627. DOI:10.1039/C6CC00934D |
| [75] |
Verhaeghe T, De Winter K, Berland M, et al. Converting bulk sugars into prebiotics: semi-rational design of a transglucosylase with controlled selectivity. Chem Commun (Camb), 2016, 52(18): 3687-3689. DOI:10.1039/C5CC09940D |
| [76] |
Beerens K, De Winter K, Van De Walle D, et al. Biocatalytic synthesis of the rare sugar kojibiose: process scale-up and application testing. J Agric Food Chem, 2017, 65(29): 6030-6041. DOI:10.1021/acs.jafc.7b02258 |
| [77] |
Choi HC, Seo DH, Jung JH, et al. Development of new assay for sucrose phosphorylase and its application to the characterization of Bifidobacterium longum SJ32 sucrose phosphorylase. Food Sci Biotechnol, 2011, 20(2): 513-518. DOI:10.1007/s10068-011-0071-0 |
| [78] |
Macdonald SS, Armstrong Z, Morgan-Lang C, et al. Development and application of a high-throughput functional metagenomic screen for glycoside phosphorylases. Cell Chem Biol, 2019, 26(7): 1001-1012. DOI:10.1016/j.chembiol.2019.03.017 |
| [79] |
Vilozny B, Schiller A, Wessling RA, et al. Enzyme assays with boronic acid appended bipyridinium salts. Anal Chim Acta, 2009, 649(2): 246-251. DOI:10.1016/j.aca.2009.07.032 |
| [80] |
Aerts D, Verhaeghe T, De Mey M, et al. A constitutive expression system for high-throughput screening. Eng Life Sci, 2015, 11(1): 10-19. |
| [81] |
Wang MM, Wu J, Wu D. Cloning and expression of the sucrose phosphorylase gene in Bacillus subtilis and synthesis of kojibiose using the recombinant enzyme. Microb Cell Fact, 2018, 17(1): 23. DOI:10.1186/s12934-017-0842-2 |
| [82] |
Lee JH, Yoon SH, Nam SH, et al. Molecular cloning of a gene encoding the sucrose phosphorylase from Leuconostoc mesenteroides B-1149 and the expression in Escherichia coli. Enzyme Microb Technol, 2006, 39(4): 612-620. DOI:10.1016/j.enzmictec.2005.11.008 |
| [83] |
Li Y, Li Z, He XY, et al. Characterisation of a Thermobacillus sucrose phosphorylase and its utility in enzymatic synthesis of 2-O-α-D-glucopyranosyl-L- ascorbic acid. J Biotechnol, 2019, 305: 27-34. DOI:10.1016/j.jbiotec.2019.08.018 |
| [84] |
Zhang H, Sun X, Li WJ, et al. Expression and characterization of recombinant sucrose phosphorylase. Protein J, 2018, 37(1): 93-100. DOI:10.1007/s10930-017-9754-6 |
| [85] |
Su LQ, Wang T, Yu LG, et al. Highly efficient extracellular expression of naturally cytoplasmic Leuconostoc mesenteroides sucrose phosphorylase. J Chem Technol Biotechnol, 2018, 93(11): 3135-3142. DOI:10.1002/jctb.5665 |
| [86] |
Kitao S, Yoshida S, Horiuchi T, et al. Formation of kojibiose and nigerose by sucrose phosphorylase. Biosci Biotechnol Biochem, 1994, 58(4): 790-791. DOI:10.1271/bbb.58.790 |
| [87] |
Goedl C, Schwarz A, Minani A, et al. Recombinant sucrose phosphorylase from Leuconostoc mesenteroides: characterization, kinetic studies of transglucosylation, and application of immobilised enzyme for production of α-D-glucose 1-phosphate. J Biotechnol, 2007, 129(1): 77-86. DOI:10.1016/j.jbiotec.2006.11.019 |
| [88] |
Aerts D, Verhaeghe TF, Roman BI, et al. Transglucosylation potential of six sucrose phosphorylase toward different classes of acceptors. Carbohydr Res, 2011, 346(13): 1860-1867. DOI:10.1016/j.carres.2011.06.024 |
| [89] |
Yoon SH, Robyt JF. Study of the inhibition of four alpha amylases by acarbose and its 4IV-α-maltohexaosyl and 4IV-α-maltododecaosyl analogues. Carbohydr Res, 2003, 338(19): 1969-1980. DOI:10.1016/S0008-6215(03)00293-3 |
| [90] |
Schagen S, Overhagen S, Bilstein A. New data confirm skin revitalizing and stress protection by Glycoin® natural[EB/OL]. [2020-04-09]. https://www.researchgate.net/publication/315693856.
|
| [91] |
Takenaka F, Uchiyama H. Synthesis of α-D-glucosyl- glycerol by α-glucosidase and some of its characteristics. Biosci Biotechnol Biochem, 2000, 64(9): 1821-1826. DOI:10.1271/bbb.64.1821 |
| [92] |
Kwon T, Kim CT, Lee JH. Transglucosylation of ascorbic acid to ascorbic acid 2-glucoside by a recombinant sucrose phosphorylase from Bifidobacterium longum. Biotechnol Lett, 2007, 29(4): 611-615. DOI:10.1007/s10529-006-9285-2 |
| [93] |
Wakamiya H, Suzuki E, Yamamoto I, et al. In situ intestinal absorption of 2-O-α-D-glucopyranosyl-L- ascorbic acid in guinea pigs. J Nutr Sci Vitaminol, 1995, 41(2): 265-272. DOI:10.3177/jnsv.41.265 |
| [94] |
Gudiminchi RK, Nidetzky B. Walking a fine line with sucrose phosphorylase: efficient single-step biocatalytic production of L-ascorbic acid 2-glucoside from sucrose. ChemBioChem, 2017, 18(14): 1387-1390. DOI:10.1002/cbic.201700215 |
| [95] |
Saeedi M, Eslamifar M, Khezri K. Kojic acid applications in cosmetic and pharmaceutical preparations. Biomed Pharmacother, 2018, 110: 582-593. |
| [96] |
Nagahara A, Benjamin H, Storkson J, et al. Inhibition of benzo[a]pyrene-induced mouse forestomach neoplasia by a principal flavor component of Japanese-style fermented soy sauce. Cancer Res, 1992, 52(7): 1754-1756. |
| [97] |
Kataoka S, Liu W, Albright K, et al. Inhibition of benzo[a]pyrene-induced mouse forestomach neoplasia and reduction of H2O2 concentration in human polymorphonuclear leucocytes by flavour components of Japanese-style fermented soy sauce. Food Chem Toxicol, 1997, 35(5): 449-457. DOI:10.1016/S0278-6915(97)00009-4 |
| [98] |
Nunomura N, Sasaki M, Asao Y, et al. Isolation and identification of 4-hydroxy-2(or5)-ethyl-5(or 2)-methyl-3(2H)-furanone, as a flavor component in Shoyu (soy sauce). Agr Biol Chem, 1976, 40(3): 491-495. |
| [99] |
Kitao S, Matsudo T, Sasaki T, et al. Enzymatic synthesis of stable, odorless, and powdered furanone glucosides by sucrose phosphorylase. Biosci Biotechnol Biochem, 2000, 64(1): 134-141. DOI:10.1271/bbb.64.134 |
| [100] |
Decuyper L, Franceus J, Dhaene S, et al. Chemoenzymatic approach toward the synthesis of 3-O-(α/β)-glucosylated 3-hydroxy-β-lactams. ACS Omega, 2018, 3(11): 15235-15245. DOI:10.1021/acsomega.8b01969 |
| [101] |
Sun Q, Heilmann J, König B. Natural phenolic metabolites with anti-angiogenic properties – a review from the chemical point of view. Beilstein J Org Chem, 2015, 11: 249-264. DOI:10.3762/bjoc.11.28 |
| [102] |
Torres P, Poveda A, Jimenez-Barbero J, et al. Enzymatic synthesis of α-glucosides of resveratrol with surfactant activity. Adv Synth Catal, 2011, 353(7): 1077-1086. DOI:10.1002/adsc.201000968 |
| [103] |
Bungaruang L, Gutmann A, Nidetzky B. Leloir glycosyltransferases and natural product glycosylation: biocatalytic synthesis of the C-glucoside nothofagin, a major antioxidant of redbush herbal tea. Adv Synth Catal, 2013, 355(14/15): 2757-2763. |
| [104] |
Sugimoto K, Nishimura T, Nomura K, et al. Inhibitory effects of a-arbutin on melanin synthesis in cultured human melanoma cells and a three-dimensional human skin model. Biol Pharm Bull, 2004, 27(4): 510-514. DOI:10.1248/bpb.27.510 |
| [105] |
Sugimoto K, Nishimura T, Kuriki T. Development of α-arbutin: production at industrial scale and application for a skin lightening cosmetic ingredient. Trends Glycosci Glyc, 2007, 19(110): 235-246. DOI:10.4052/tigg.19.235 |
| [106] |
De Winter K, Dewitte G, Dirks-Hofmeister ME, et al. Enzymatic glycosylation of phenolic antioxidants: phosphorylase-mediated synthesis and characterization. J Agric Food Chem, 2015, 63(46): 10131-10139. DOI:10.1021/acs.jafc.5b04380 |
| [107] |
De Winter K, Desmet T, Devlamynck T, et al. Biphasic catalysis with disaccharide phosphorylases: chemoenzymatic synthesis of α-D-glucosides using sucrose phosphorylase. Org Process Res Dev, 2014, 18(6): 781-787. DOI:10.1021/op400302b |
| [108] |
Poulsen MM, Jørgensen JOL, Jessen N, et al. Resveratrol in metabolic health: an overview of the current evidence and perspectives. Ann New York Acad Sci, 2013, 1290(1): 74-82. DOI:10.1111/nyas.12141 |
| [109] |
Kraft TE, Parisotto D, Schempp C, et al. Fighting cancer with red wine? Molecular mechanisms of resveratrol. Crit Rev Food Sci Nutrit, 2009, 49(9): 782-799. DOI:10.1080/10408390802248627 |
| [110] |
Sato D, Shimizu N, Shimizu Y, et al. Synthesis of glycosides of resveratrol, pterostilbene, and piceatannol, and their anti-oxidant, anti-allergic, and neuroprotective activities. Biosci Biotechnol Biochem, 2014, 78(7): 1123-1128. DOI:10.1080/09168451.2014.921551 |
| [111] |
Sutherland BA, Rahman RMA, Appleton I. Mechanisms of action of green tea catechins, with a focus on ischemia-induced neurodegeneration. J Nutr Biochem, 2006, 17(5): 291-306. DOI:10.1016/j.jnutbio.2005.10.005 |
| [112] |
Zheng G, Sayama K, Okubo T, et al. Anti-obesity effects of three major components of green tea, catechins, caffeine and theanine, in mice. In Vivo, 2004, 18(1): 55-62. |
| [113] |
Byun EB, Kim WS, Sung NY, et al. Epigallocatechin- 3-gallate regulates anti-inflammatory action through 67-kDa laminin receptor-mediated tollip signaling induction in lipopolysaccharide-stimulated human intestinal epithelial cells. Cell Physiol Biochem, 2018, 46(5): 2072-2081. DOI:10.1159/000489447 |
| [114] |
Kitao S, Ariga T, Matsudo T, et al. The syntheses of catechin-glucosides by transglycosylation with Leuconostoc mesenteroides sucrose phosphorylase. Biosci Biotechnol Biochem, 1993, 57(12): 2010-2015. DOI:10.1271/bbb.57.2010 |
| [115] |
Scheppach W, Sommer H, Kirchner T, et al. Effect of butyrate enemas on the colonic mucosa in distal ulcerative colitis. Gastroenterology, 1992, 103(1): 51-56. DOI:10.1016/0016-5085(92)91094-K |
| [116] |
Kim SJ, Won Y. The effect of glycolic acid on cultured human skin fibroblasts: cell proliferative effect and increased collagen synthesis. J Dermatol, 1998, 25(2): 85-89. |
| [117] |
Sugimoto K, Nomura K, Nishiura H, et al. Novel transglucosylating reaction of sucrose phosphorylase to carboxylic compounds such as benzoic acid. J Biosci Bioeng, 2007, 104(1): 22-29. DOI:10.1263/jbb.104.22 |
| [118] |
Nomura K, Sugimoto K, Nishiura H, et al. Glucosylation of acetic acid by sucrose phosphorylase. Biosci Biotechnol Biochem, 2008, 72(1): 82-87. DOI:10.1271/bbb.70429 |
| [119] |
Meyer AS, Heinonen M, Frankel EN. Antioxidant interactions of catechin, cyanidin, caffeic acid, quercetin, and ellagic acid on human LDL oxidation. Food Chem, 1998, 61(1/2): 71-75. |
| [120] |
Shin MH, Cheong NY, Lee JH, et al. Transglucosylation of caffeic acid by a recombinant sucrose phosphorylase in aqueous buffer and aqueous -supercritical CO2 media. Food Chem, 2009, 115(3): 1028-1033. DOI:10.1016/j.foodchem.2009.01.013 |
| [121] |
Kitaoka M, Sasaki T, Taniguchi H. Conversion of sucrose into cellobiose using sucrose phosphorylase, xylose isomerase and cellobiose phosphorylase. Denpun Kagaku, 1992, 39(4): 281-283. |
| [122] |
Hou CT, Shaw JF. Biocatalysis and biotechnology for functional foods and industrial products. NewYork: Crc Press, 2007, 10: 1-2. |
| [123] |
Zhong C, Nidetzky B. Three-enzyme phosphorylase cascade for integrated production of short-chain cellodextrins. Biotechnol J, 2019, 15(3): 1900349. |
| [124] |
Ohdan K, Fujii K, Yanase M, et al. Enzymatic synthesis of amylose. Biocatal Biotransfor, 2006, 24(1/2): 77-81. |
| [125] |
Qi P, You C, Zhang YHP. One-pot enzymatic conversion of sucrose to synthetic amylose by using enzyme cascades. Acs Catal, 2014, 4(5): 1311-1317. DOI:10.1021/cs400961a |
| [126] |
Li T, Zhou X, Xu J, et al. Enzymatic synthesis of amylodextrin from sucrose. Food Ferment Ind, 2012, 38(10): 29-34 (in Chinese). 李恬, 周星, 徐进, 等. 以蔗糖为底物双酶法合成直链糊精. 食品与发酵工业, 2012, 38(10): 29-34. |
| [127] |
Kitaoka M, Sasaki T, Taniguchi H. Purification and properties of laminaribiose phosphorylase (EC 2.4.1.31) from Euglena gracilis Z. Arch Biochem Biophys, 1993, 304(2): 508-514. DOI:10.1006/abbi.1993.1383 |
| [128] |
贺海涛, 张洪涛, 詹晓北.双酶耦合催化法规模合成β-1, 3-葡寡糖研究//第十届全国化学生物学学术会议论文摘要集(墙报).武汉: 中国化学会, 2017.
|
| [129] |
Morimoto K, Yoshihara A, Furumoto T, et al. Production and application of a rare disaccharide using sucrose phosphorylase from Leuconostoc mesenteroides. J Biosci Bioeng, 2015, 119(6): 652-656. DOI:10.1016/j.jbiosc.2014.11.011 |
| [130] |
Nishimoto M, Kitaoka M. Practical preparation of lacto-N-biose Ⅰ, a candidate for the bifidus factor in human milk. Biosci Biotechnol Biochem, 2007, 71(8): 2101-2104. DOI:10.1271/bbb.70320 |
| [131] |
Nishimoto M, Kitaoka M. One-pot enzymatic production of β-D-galactopyranosyl-(1→3)-2- acetamido-2-deoxy-D-galactose (galacto-N-biose) from sucrose and 2-acetamido-2-deoxy-D-galactose (N-acetylgalactosamine). Carbohydr Res, 2009, 344(18): 2573-2576. DOI:10.1016/j.carres.2009.09.031 |
| [132] |
Nakajima M, Nishimoto M, Kitaoka M. Practical preparation of D-galactosyl-β1→4-L-rhamnose employing the combined action of phosphorylases. Biosci Biotechnol Biochem, 2010, 74(8): 1652-1655. DOI:10.1271/bbb.100263 |
| [133] |
Zhong C, You C, Wei P, et al. Thermal cycling cascade biocatalysis of myo-inositol synthesis from sucrose. ACS Catal, 2017, 7(9): 5992-5999. DOI:10.1021/acscatal.7b01929 |
| [134] |
Myung S, Rollin J, You C, et al. In vitro metabolic engineering of hydrogen production at theoretical yield from sucrose. Metab Eng, 2014, 24: 70-77. DOI:10.1016/j.ymben.2014.05.006 |
| [135] |
Nestl BM, Hauer B. Engineering of flexible loops in enzymes. ACS Catal, 2014, 4(9): 3201-3211. DOI:10.1021/cs500325p |
| [136] |
Kingsley LJ, Lill MA. Substrate tunnels in enzymes: structure-function relationships and computational methodology. Proteins: Struct Funct Bioinf, 2015, 83(4): 599-611. DOI:10.1002/prot.24772 |
| [137] |
De Winter K, Verlinden K, Křen V, et al. Ionic liquids as cosolvents for glycosylation by sucrose phosphorylase: balancing acceptor solubility and enzyme stability. Green Chem, 2013, 15(7): 1949-1955. DOI:10.1039/c3gc40449h |
 2021, Vol. 37
2021, Vol. 37