中国科学院微生物研究所、中国微生物学会主办
文章信息
- 梁启星, 石竟成, 金学荣, 堵国成, 康振
- Liang Qixing, Shi Jingcheng, Jin Xuerong, Du Guocheng, Kang Zhen
- 肠激酶在毕赤酵母中的分泌表达优化
- Optimization of enterokinase secretion in Pichia pastoris
- 生物工程学报, 2020, 36(8): 1689-1698
- Chinese Journal of Biotechnology, 2020, 36(8): 1689-1698
- 10.13345/j.cjb.190577
-
文章历史
- Received: December 23, 2019
- Accepted: March 3, 2020
2. 江南大学 生物工程学院 糖化学与生物技术教育部重点实验室,江苏 无锡 214122
2. Key Laboratory of Carbohydrate Chemistry and Biotechnology, Ministry of Education, School of Biotechnology, Jiangnan University, Wuxi 214122, Jiangsu, China
肠激酶(EC 3.4.21.9,EK)是一种丝氨酸蛋白水解酶,广泛存在于哺乳动物的消化系统中,作为体内部分酶原的激活剂。EK由重链和轻链构成,单独表达EK的轻链具有生物活性(文中提到的EK均为轻链)。EK特异性识别DDDDK序列,作用于赖氨酸羧基末端的肽键,产生的目标蛋白首位氨基酸忠实于天然蛋白[1],不会在其N端引入额外氨基酸残基。这种酶切特点使EK广泛应用于生物医药领域,成为融合表达肽类药物下游纯化时的首选工具酶。天然纯化的牛源EK中掺杂其他蛋白水解酶,利用基因工程生产的重组EK很好地避免了这一问题[2]。
近年来,巴斯德毕赤酵母作为异源蛋白表达的宿主非常流行,已成功表达了200多种异源蛋白[3]。与传统的原核表达系统相比,巴斯德毕赤酵母具有翻译后修饰功能,包括信号肽序列加工、促进二硫键形成、糖基化修饰等。以大肠杆菌为宿主表达的EK多以包涵体的形式存在,需体外复性,操作复杂[4-6]。毕赤酵母可以直接表达具有活性的EK,这使毕赤酵母成为表达EK的最佳选择宿主[7]。然而,EK在毕赤酵母中存在着表达量低的问题,据报道表达量一般不超过10 mg/L,这限制了EK的生产应用[7-8]。
Ewelina等从解脂耶氏酵母中找到了一些新的信号肽应用于外源蛋白的表达[9]。利用这些信号肽,改造酿酒酵母α-factor信号肽的pre-region区,用6个不同的信号肽序列替换原来的MRFPSIFTAVLFAASSALA序列来提高EK的胞外分泌水平。在得到分泌能力较强的信号肽后,又在此基础上分别共表达了毕赤酵母内源蛋白BiP、PDI和Kex2。在相关报道中,BiP作为内质网驻留蛋白,有指导蛋白质折叠组装,将错误折叠的蛋白引向降解途径的作用[10],BiP蛋白的共表达增加了单链抗体片段在毕赤酵母中的表达水平[11]。二硫键异构酶(PDI)有打开错配二硫键、促进新键形成的作用。Kex2特异性识别KR这两个氨基酸,对成熟的α-factor信号肽进行加工,Kex2的共表达有效地切除α-factor信号肽序列,有助于脂肪酶的胞外表达[12]。Chun等在大肠杆菌中将EK与PDI融合表达,提高了EK的活性[13]。Wang等将EK第64位上的氨基酸由天冬酰胺突变成谷氨酰胺,EK比酶活达到约250 000 U/mg[14]。张云丰等通过N端改造工程提高了胰蛋白酶的比酶活[15]。考虑到EK和胰蛋白酶同属于丝氨酸蛋白酶家族,据此,本研究在EK的改造上,从N端随机引入3个氨基酸序列,提高了EK的比酶活,成功地优化了EK在毕赤酵母中的分泌表达,为未来相关的生产应用奠定了基础。
1 材料与方法 1.1 菌株与质粒实验中用到的菌株毕赤酵母Pichia pastoris GS115和大肠杆菌Escherichia coli JM109以及质粒pPIC9K、pGAPZαA、pGAPZB购于Invitrogen公司。E. coli JM109作为克隆宿主,P. pastoris GS115作为构建的出发菌株。
1.2 培养基组分毕赤酵母重组菌在YPD中培养,成分如下(g/L):酵母提取物10、胰蛋白胨20、葡萄糖20。毕赤酵母筛选转化子时根据抗性不同可在YPD中添加相应抗生素(博莱霉素100 μg/mL或潮霉素B 200 μg/mL)混合。毕赤酵母组氨酸营养缺陷型的筛选用MD培养基,成分如下(g/L):葡萄糖20、YNB 13.4、生物素0.000 4、琼脂糖20。大肠杆菌在带有抗生素的LB培养基中培养,成分如下(g/L):酵母提取物5、胰蛋白胨10、氯化钠10,并将pH调至7.4。根据抗性添加相应抗生素浓度为:博莱霉素50 μg/mL或潮霉素B 50 μg/mL。
1.3 菌株的构建构建的菌株和质粒列于表 1中,用到的引物罗列在表 2中。bEKL的基因序列(GenBank登录号282009)由GENEWIZ合成并插入到载体pGAPZαA表达框中得到GPEK1菌株。用6种不同的信号肽序列替换α-factor信号肽的pre-region区,将构建好的质粒电转至P. pastoris GS115获得含有不同信号肽的重组毕赤酵母菌。以毕赤酵母P. pastoris GS115基因组为模板,扩增3种内源蛋白BiP、PDI和Kex2基因,插入到质粒pTEF7K (基于pPIC9K质粒,该质粒除去了氨苄青霉素抗性,并用组成型启动子TEF1p代替AOX1p启动子)的表达框中。在pGAPZB质粒的基础上,用潮霉素B代替了博莱霉素的抗性基因,得到了质粒pGZBH。将PDI和Kex2分别插入pTEF7K和pGZBH载体中,共同电转化到重组菌GKSP1,获得菌株GP1KePd。在EK的N端改造中,以pGAPZαA-SP1-bEKL为模板,设计含有简并性碱基的上游引物Forward (引物中N代表A/T/C/G四种碱基,K代表G/T两种碱基),下游引物Reverse。经PCR反向扩增后,磷酸化连接并克隆至E. coli JM109,转化培养后收集平板上全部菌体,抽提质粒。将抽提好的质粒电转至P. pastoris GS115,得到的转化子逐个挑入48孔板中,培养3 d后测定上清液酶活,选取酶活较高的菌株提取基因组作为模板,PCR扩增目的片段后进行测序,确定引入的氨基酸组合,最终得到N端随机引入3个氨基酸的突变体菌株。
| Strains or plasmids | Description | References |
| Strains | ||
| Escherichia coli JM109 | Cloned host | Invitrogen |
| Pichia pastoris GS115 | Expression host | Invitrogen |
| GPEK1 | P. pastoris GS115 harbring pGAPZαA-bEKL | This work |
| GKSP1 | P. pastoris GS115 harbring pGAPZαA-SP1-bEKL | This work |
| GKSP2 | P. pastoris GS115 harbring pGAPZαA-SP2-bEKL | This work |
| GKSP3 | P. pastoris GS115 harbring pGAPZαA-SP3-bEKL | This work |
| GKSP4 | P. pastoris GS115 harbring pGAPZαA-SP4-bEKL | This work |
| GKSP7 | P. pastoris GS115 harbring pGAPZαA-SP7-bEKL | This work |
| GKSP8 | P. pastoris GS115 harbring pGAPZαA-SP8-bEKL | This work |
| GP1KeK | P. pastoris GS115 harbring pGAPZαA-SP1-bEKL and pTEF7K-Kex2 | This work |
| GP1PdK | P. pastoris GS115 harbring pGAPZαA-SP1-bEKL and pTEF7K-PDI | This work |
| GP1BiK | P. pastoris GS115 harbring pGAPZαA-SP1-bEKL and pTEF7K-BiP | This work |
| GP1KePd | P. pastoris GS115 harbring pGAPZαA-SP1-bEKL, pTEF7K-Kex2 and pGZBH-PDI | This work |
| GP1EFM | P. pastoris GS115 harbring pGAPZαA- SP1-EFM-bEKL | This work |
| GP1RNL | P. pastoris GS115 harbring pGAPZαA- SP1-RNL-bEKL | This work |
| GP1LKR | P. pastoris GS115 harbring pGAPZαA- SP1-LKR-bEKL | This work |
| GP1WLR | P. pastoris GS115 harbring pGAPZαA- SP1-WLR-bEKL | This work |
| Plasmids | ||
| pGAPZαA | pUC ori, BleoR, GAP promoter, α-factor | Invitrogen |
| pGAPZB | pUC ori, BleoR, GAP promoter | Invitrogen |
| pPIC9K | pUC ori, KanR, AmpR, AOX1 promoter, α-factor | Invitrogen |
| pTEF7K | pUC ori, KanR, TEF1 promoter | This work |
| pGZBH | pUC ori, HygR, GAP promoter | This work |
| pTEF7K-PDI | pUC ori, KanR, TEF1 promoter, containing PDI | This work |
| pTEF7K-BiP | pUC ori, KanR, TEF1 promoter, containing BiP | This work |
| pTEF7K-Kex2 | pUC ori, KanR, TEF1 promoter, containing Kex2 | This work |
| pGZBH-PDI | pUC ori, HygR, GAP promoter, containing PDI | This work |
| pGAPZαA-bEKL | pUC ori, BleoR, GAP promoter, containing α-factor and bEKL | This work |
| pGAPZαA-SP1-bEKL | pUC ori, BleoR, GAP promoter, containing SP1 and bEKL | This work |
| pGAPZαA-SP2-bEKL | pUC ori, BleoR, GAP promoter, containing SP2 and bEKL | This work |
| pGAPZαA-SP3-bEKL | pUC ori, BleoR, GAP promoter, containing SP3 and bEKL | This work |
| pGAPZαA-SP4-bEKL | pUC ori, BleoR, GAP promoter, containing SP4 and bEKL | This work |
| pGAPZαA-SP7-bEKL | pUC ori, BleoR, GAP promoter, containing SP7 and bEKL | This work |
| pGAPZαA-SP8-bEKL | pUC ori, BleoR, GAP promoter, containing SP8 and bEKL | This work |
| pGAPZαA-SP1-EFM-bEKL | pUC ori, BleoR, GAP promoter, containing SP1 and bEKL | This work |
| pGAPZαA-SP1-RNL-bEKL | pUC ori, BleoR, GAP promoter, containing SP1 and bEKL | This work |
| pGAPZαA-SP1-LKR-bEKL | pUC ori, BleoR, GAP promoter, containing SP1 and bEKL | This work |
| pGAPZαA-SP1-WLR-bEKL | pUC ori, BleoR, GAP promoter, containing SP1 and bEKL | This work |
| Name | Primer sequence (5′–3′) |
| SP1(ZαA)-F | GTTACCGCCGCGCTGGCCTCGTCCGCCATGGCCGCTCCAGTCAACACTACAACAGAAGA |
| SP1(ZαA)-R | CAGCGCGGCGGTAACGGCAGCAAATGTGAACTTCATCGTTTCGAAATAGTTGTTCAATT |
| SP2(ZαA)-F | CTTCTGGCTCTGGCCGCCGTCGCCACCGCCGCTCCAGTCAACACTACAACAGAAGA |
| SP2(ZαA)-R | GGCCAGAGCCAGAAGGGCGGTGGAGAACTTCATCGTTTCGAAATAGTTGTTCAATT |
| SP3(ZαA)-F | GCTGTCGCTGCTGGCGGTCCCGGCCACCGCCGCTCCAGTCAACACTACAACAGAAGA |
| SP3(ZαA)-R | GCCAGCAGCGACAGCAATAGAGATTTCATCGTTTCGAAATAGTTGTTCAATT |
| SP4(ZαA)-F | ATCGCTGCTGCCCTGGCCTCGCTGGTGGCAGCAGCTCCAGTCAACACTACAACAGAAGA |
| SP4(ZαA)-R | CAGGGCAGCAGCGATTGAGACCGCTGAGAACTTCATCGTTTCGAAATAGTTGTTCAATT |
| SP7(ZαA)-F | TTACCGCTTGTGCTACTCTGGCTCTCGCTCTGGCTGCTCCAGTCAACACTACAACAGAAG |
| SP7(ZαA)-R | TAGCACAAGCGGTAAACAGAATGGTAGACAGCTTCATCGTTTCGAAATAGTTGTTCAATT |
| SP8(ZαA)-F | GTTACTGTCTGCTTTTCCGTTGCCTCGGCTGCTCCAGTCAACACTACAACAGAAGA |
| SP8(ZαA)-R | AAAGCAGACAGTAACCAGCAGGGCGAGCACCTTCATCGTTTCGAAATAGTTGTTCAATT |
| pTEF7K-F | GCGGCCGCGAATTAATTCGCCTTAGACA |
| pTEF7K-R | GAATTCTACGTATTAGATTAG |
| Kex2(7K)-F | CTAATCTAATACGTAGAATTCATGTATTTGCCAGCACTTCGC |
| Kex2(7K)-R | TGTCTAAGGCGAATTAATTCGCGGCCGCTTACAATGCCGCACGTTTGGGAT |
| PDI(7K)-F | GCAATCTAATCTAATACGTAGAATTCATGCAATTCAACTGGAATATTAAAAC |
| PDI(7K)-R | TGTCTAAGGCGAATTAATTCGCGGCCGCTTAAAGCTCGTCGTGAGCGT |
| BiP(7K)-F | GCAATCTAATCTAATACGTAGAATTCATGCTGTCGTTAAAACCATCTTG |
| BiP(7K)-R | TGTCTAAGGCGAATTAATTCGCGGCCGCCTACAACTCATCATGATCATAGTC |
| pGZBH-F | GGTGGCCTCGTTTCGAAATAGT |
| pGZBH-R | CAGCTTGTTTTAGCCTTAGACATGAC |
| PDI(ZBH)-F | ACTATTTCGAAACGAGGCCACCATGCAATTCAACTGGAATATTAAAACTGTG |
| PDI(ZBH)-R | GTCATGTCTAAGGCTAAAACAAGCTGTTAAAGCTCGTCGTGAGCGTCT |
| Forward | NNKNNKNNKATAGTTGGTGGTTCTGATTCC |
| Reverse | AGCTTCAGCCTCTCTTTTCTC |
| Underlines represent the homologous segment. | |
从冷冻的甘油管中挑取菌液,YPD平板上划线,在30 ℃条件下培养3 d后挑选平板上的单菌落于摇菌管中继续培养24 h后作为种子液,将种子液接种至250 mL挡板摇瓶中发酵培养。
1.5 EK的纯化及蛋白浓度测定EK采用镍柱纯化的方式,平衡缓冲液:20 mmol/L磷酸钠、500 mmol/L氯化钠、20 mmol/L咪唑,洗脱缓冲液:20 mmol/L磷酸钠、500 mmol/L氯化钠、250 mmol/L咪唑。操作步骤参考HisTrapTM FF的使用说明书,蛋白浓度的测定按照Bradford试剂盒说明进行操作。
1.6 EK的酶活测定和底物动力学分析EK的底物GD4K-β-Na由南京肽业有限公司合成。0.25 mmol/L的GD4K-β-Na溶解在底物缓冲液中(含有10% DMSO、25 mmol/L Tris-HCl (pH 8.0)和10 mmol/L CaCl2的)。发酵上清液与底物缓冲液混合,测定37 ℃条件下7 min内的荧光变化率(λex=337 nm,λem=420 nm)[16]。上述条件下,将1 min内荧光值增加1 (1 U=1 abs/min)定义为1 U[14]。设定不同的底物浓度,测定各底物浓度下EK的酶活,并采用Hanes-Woolf作图法计算Km值和Vmax。测定EK的底物动力学。
1.7 SDS-PAGE与Western blottingSDS-PAGE所用的凝胶以及缓冲液购买于赛默飞世尔科技有限公司。纯化样品经预处理后加样,设定电压120 V进行电泳。电泳完成后,考马斯亮蓝R-250染色30 min,洗脱液洗去背景色后成像。Western blotting需要将凝胶上的蛋白质样转至聚偏二氟乙烯膜(PVDF)上,用20 mL含有5% (W/V)脱脂牛奶的TBST (10 mmol/L Tris-HCl、150 mmol/L NaCl,pH调至7.5)在室温下浸泡孵育膜1 h,随后将膜浸入用封闭溶液稀释的一抗(购买于Beyotime的His抗体)中,4 ℃孵育过夜,再用封闭溶液稀释的二抗(稀释比为1︰5 000,HRP标记的山羊抗小鼠IgG (H+L))浸泡孵育1 h,最后显色。
1.8 信号肽的二级结构预测对改造后信号肽的全长进行了二级结构预测(https://npsa-prabi.ibcp.fr/NPSA/npsasopma.html),氨基酸序列及信号肽的二级结构信息仅展示了pre-region区(图 1)。

|
| 图 1 pre-region区氨基酸序列与二级结构信息预测 Fig. 1 Prediction of amino acids sequence and secondary structure information of pre-region. c: coil; h: helix; e: sheet. |
| |
为分析突变体菌株对EK酶活性的影响,蛋白质的空间结构预测在在线服务器上进行(http://new.robetta.org/submit.php),将预测好的蛋白模型进行在线质量评估(https://servicesn.mbi.ucla.edu/SAVES/),根据评估的结果再进行相应的优化。在得到较为可信的蛋白结构后,又比对分析了蛋白质的二级结构序列,该过程由ESPript3.0完成(http://espript.ibcp.fr/ESPript/ESPript/)。
2 结果与分析 2.1 信号肽改造提高EK的表达为提高毕赤酵母分泌表达EK的能力,出发菌株GPEK1的α-factor信号肽的pre-region区被6个不同信号肽序列替换,结果表明当α-factor信号肽改造成SP1和SP2时,毕赤酵母胞外上清中EK的活性有显著提高(图 2A),对应的菌株GKSP1和GKSP2的酶活分别是(5 235±389) U/mL和(5 173±333) U/mL。为比较SP1和SP2信号肽的分泌效率,将胞外酶活与胞内酶活的比值作为分泌效率的指标,测定单位OD600内菌体胞外和胞内的EK的酶活性,结果如图 2B所示,SP1拥有更强的分泌能力,分泌效率达到41%,将EK的酶活提高了2.1倍。在外源蛋白的分泌过程中,信号肽引导外源蛋白通过共翻译转运途径或翻译后转运途径运输,具有分泌特征的信号肽引导新生肽定位于内质网,新生肽初步加工后再由内质网转运到高尔基体,最后由高尔基体分泌到胞外[17-18]。信号肽的疏水性以及空间构象影响信号肽与信号肽识别颗粒(SRP)的结合[19],SP1信号肽可能与SRP有更好的识别结合作用,引导更多的蛋白通过共翻译转运途径分泌到胞外,进而提高了EK的分泌水平。SP3信号肽序列疏水中心α-螺旋结构被破坏,这将导致信号肽失去其功能[9, 20],影响外源蛋白的分泌表达。SDS-PAGE结果见图 2C。EK的理论相对分子量为28 kDa,由于毕赤酵母存在糖基化修饰,EK的实际大小并不确定,Western blotting鉴定EK相对分子量(图 2D)。结果表明,毕赤酵母中EK的表达被糖基化修饰,相对分子量大致集中在38–62 kDa间,该范围内的条带呈现出弥散拖尾的特征。

|
| 图 2 不同信号肽菌株EK的酶活测定及蛋白电泳结果 Fig. 2 Determination of enterokinase activity of strains with different signal peptides and results of SDS-PAGE. (A) Comparison of enterokinase activity among different strains. GPEK1 containing α-factor, GKSP1 containing SP1, GKSP2 containing SP2, GKSP3 containing SP3, GKSP4 containing SP4, GKSP7 containing SP7, GKSP8 containing SP8. (B) Comparison of secretory efficiency of signal peptides. (C) SDS-PAGE analysis of enterokinase secreted by different signal peptides. M: marker; 1: supernatant of GPEK1; 2: supernatant of GKSP1; 3: supernatant of GKSP2; 4: supernatant of GKSP3; 5: supernatant of GKSP4; 6: supernatant of GKSP7; 7: supernatant of GKSP8. (D) Western blotting analysis of enterokinase. M: marker; 1: supernatant of GKSP1. |
| |
在菌株GKSP1的基础上,为进一步提高EK的胞外酶活,共表达了毕赤酵母中内源蛋白BiP、PDI和Kex2,从结果来看单独共表达BiP对EK的酶活并没有提高,分别共表达PDI和Kex2可将EK酶活提高至(6 178±432) U/mL和(7 219±489) U/mL。然而,当PDI和Kex2组合一起同时共表达后,EK的酶活并未再次提升,酶活只有(5 616±320) U/mL (图 3A)。SDS-PAGE结果见图 3B。内质网驻留蛋白BiP的共表达对毕酵母分泌外源蛋白的能力存在较大的差异,过表达BiP甚至可能对外源蛋白分泌造成负面影响[21-22]。PDI可以打开内质网上新生肽错配的二硫键,促进二硫键的再次形成[23],错误折叠的蛋白或聚集体则通过内质网的响应系统进入相关的降解途径(ERAD)[24]。推测PDI的共表达减少错误折叠蛋白的形成,有利于EK的正确折叠,同时缓解了降解途径的代谢压力。信号肽将外源蛋白引导至内质网后,pre-region区在内质网上移除,pro-region区在高尔基体上被Kex2切除,至此,信号肽全长被完整除去。然而,毕赤酵母自身表达的Kex2可能不足,外源分泌蛋白的N端往往还含有未完全移除的信号肽[25]。Kex2酶的共表达有助于信号肽pro-region区的移除,利于外源蛋白的活性表达。另外,来不及移除pro-region区的EK很可能会影响到酶的最终空间构象。分析共表达Kex2后GP1KeK胞外上清与胞内EK的酶活发现GP1KeK菌株的分泌效率与GKSP1菌株相比并没有明显的差异(数据未展示)。PDI的氨基酸序列存在Kex2的识别位点KR (这两个碱性氨基酸处于Loop环上),当同时共表达PDI和Kex2时,可能导致过表达的Kex2以PDI为底物进行了酶切,减弱了Kex2的作用。

|
| 图 3 共表达内源蛋白EK的酶活测定及蛋白电泳结果 Fig. 3 Determination of enterokinase activity of co-expressed endogenous protein and results of SDS-PAGE. (A) Determination of enterokinase activity of co-expressing endogenous protein strains. (B) SDS-PAGE analysis of enterokinase by co-expressing endogenous protein. M: marker; 1: supernatant of GKSP1; 2: supernatant of GP1BiK; 3: supernatant of GP1PdK; 4: supernatant of GP1KeK; 5: supernatant of GP1KePd. The upper and lower arrows indicate the maximum and minimum values of EK relative molecular weight. |
| |
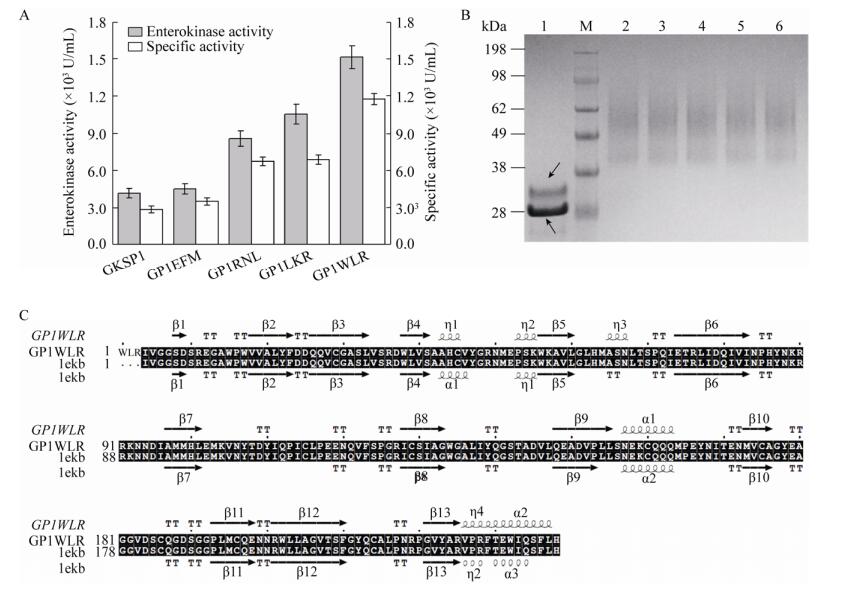
EK的N端随机引入3个氨基酸组合,通过48孔板筛选最终得到了4个突变体菌株:GP1EFM、GP1RNL、GP1LKR和GP1WLR。摇瓶培养后测定上清液中酶活,随后蛋白纯化,测定纯化后蛋白样品活性,并用Bradford试剂盒检测纯化样品蛋白浓度,计算其比酶活(图 4A)。纯化样品去糖基化分析和SDS-PAGE结果如图 4B。EK的N端引入EFM、RNL、LKR和WLR这4种氨基酸组合后,与GKSP1菌株相比,引入WLR这3个氨基酸的GP1WLR菌株酶活有显著的提高,酶活可达(15 145±920) U/mL,比酶活为(1 174 600±53 100) U/mg,EK的表达量为12.9 mg/L。蛋白电泳结果显示EK的电泳条带与Western blotting鉴定结果一致,用EndoH酶去糖基化后,可得到单一的蛋白条带其大小在33 kDa附近,高于EK的理论相对分子量28 kDa。这可能因为EK不仅存在N-型糖基化,还存在O-型糖基化的结果,与Wang等结果一致[14]。为分析EK的N端引入WLR这3个氨基酸后酶本身的结构变化,在线服务器上预测该突变体EK的空间构象,随后通过序列比对分析其二级结构的变化(图 4C)。结果表明,与EK (PDB ID:1ekb)的晶体结构相比,突变体GP1WLR具有以下变化:氨基酸43–45位从α-螺旋变为310-螺旋,氨基酸65–67位从TT (β转角)变为310-螺旋,氨基酸109位和110位从Loop环变为TT,氨基酸235–238位从Loop环变为α-螺旋。推测这些结构的改变可能导致EK空间构象变化或表面的疏水性变化,进而影响EK的催化能力以及对底物的亲和能力。
|
| 图 4 N端突变体EK的比酶活测定及蛋白纯化样品电泳结果 Fig. 4 Determination of enterokinase specific activity of N-terminal mutants and SDS-PAGE results of purified protein samples. (A) Determination of enterokinase activity and specific activity. (B) SDS-PAGE analysis of enterokinase purified. M: marker; 1: purified sample of de-glycosylated EK, upper black arrow indicates de-glycosylated EK, the black arrow below indicates the EndoH enzyme. 2: purified sample of GKSP1; 3: purified sample of GP1EFM; 4: purified sample of GP1RNL; 5: purified sample of GP1LKR; 6: purified sample of GP1WLR. (C) Protein secondary structure alignment with ESPript 3. |
| |
分析纯化后EK的底物动力学发现,EK在N端引入的3个氨基酸后降低了对底物的亲和力:Km值升高。但在催化能力上突变体的kcat值均有所提高(表 3)。与GKSP1菌株相比,突变体GP1WLR菌株kcat/Km值提高1.5倍。
| Mutants | Km (×10–4, mol/L) | kcat (s–1) | kcat/Km (L/(×105, mol·s)) |
| GKSP1 | 0.97±0.03 | 27.78±3.1 | 2.86±0.11 |
| GP1EFM | 1.19±0.27 | 45.56±4.8 | 3.81±0.18 |
| GP1LKR | 2.67±0.21 | 88.33±7.3 | 3.31±0.18 |
| GP1RNL | 1.31±0.12 | 52.56±3.9 | 4.01±0.23 |
| GP1WLR | 2.62±0.19 | 111.13±8.6 | 4.24±0.17 |
本研究通过对α-factor信号肽pre-region区序列的改造得到SP1信号肽提高了EK的分泌能力,分泌效率提高2.1倍,与出发菌株GPEK1相比,EK表达量从6.8 mg/L提高至14.3 mg/L。共表达毕赤酵母内源蛋白发现:Kex2和PDI的共表达对EK的酶活都有明显的提高,Kex2的共表达可将EK的酶活提高至(7 219±489) U/mL。然而,这两个内源蛋白同时共表达时效果减弱,并未达到预期结果。在EK的N端改造方面,引入WLR这3个氨基酸的突变体菌株GP1WLR与GKSP1菌株相比,酶活提高了近3倍,达到(15 145±920) U/mL,比酶活为(1 174 600±53 100) U/mg,远高于目前报道的250 000 U/mg[14]。以上研究为外源蛋白的表达优化提供一种思路,同时也为EK的未来应用奠定了基础。
| [1] |
Matsushima M, Ichinose M, Yahagi N, et al. Structural characterization of porcine enteropeptidase. J Biol Chem, 1994, 269(31): 19976-19982. |
| [2] |
Zhang XH, Tan SH, Li TM. Characterization of enterokinase and its research advances in genetic engineering. Pharmaceuti Biotechnol, 2005, 12(5): 347-350 (in Chinese). 张向辉, 谭树华, 李泰明. 肠激酶特点及其基因工程的研究进展. 药物生物技术, 2005, 12(5): 347-350. |
| [3] |
Ahmad M, Hirz M, Pichler H, et al. Protein expression in Pichia pastoris: recent achievements and perspectives for heterologous protein production. Appl Microbiol Biot, 2014, 98(12): 5301-5317. |
| [4] |
Yuan LD, Hua ZC. Expression, purification, and characterization of a biologically active bovine enterokinase catalytic subunit in Escherichia coli. Prot Express Purificat, 2002, 25(2): 300-304. |
| [5] |
Gasparian ME, Ostapchenko VG, Schulga AA, et al. Expression, purification, and characterization of human enteropeptidase catalytic subunit in Escherichia coli. Prot Expr Purificat, 2003, 31(1): 133-139. |
| [6] |
Huang L, Ruan H, Gu WY, et al. Functional expression and purification of bovine enterokinase light chain in recombinant Escherichia coli. Prep Biochem Biotechnol, 2007, 37(3): 205-217. |
| [7] |
Vozza LA, Wittwer L, Higgins DR, et al. Production of a recombinant bovine enterokinase catalytic subunit in the methylotrophic yeast Pichia pastoris. BioTechnology, 1996, 14(1): 77-81. |
| [8] |
Fang L, Sun QM, Hua ZC. Expression of recombinant Chinese bovine enterokinase catalytic subunit in P. pastoris and its purification and characterization. Acta Biochim Biophys Sin, 2004, 36(7): 513-517. |
| [9] |
Celińska E, Borkowska M, Bialas W, et al. Robust signal peptides for protein secretion in Yarrowia lipolytica: identification and characterization of novel secretory tags. Appl Microbiol Biotechnol, 2018, 102(12): 5221-5233. |
| [10] |
Hendershot LM. The ER function BiP is a master regulator of ER function. Mt Sinai J Med, 2004, 71(5): 289-297. |
| [11] |
Damasceno LM, Anderson KA, Ritter G, et al. Cooverexpression of chaperones for enhanced secretion of a single-chain antibody fragment in Pichia pastoris. Appl Microbiol Biotechnol, 2007, 74(2): 381-389. |
| [12] |
Takahashi S, Ueda M, Tanaka A. Effect of the truncation of the C-terminal region of Kex2 endoprotease on processing of the recombinant Rhizopus oryzae lipase precursor in the co-expression system in yeast. J Mol Catal B, 2000, 10(1/3): 233-240. |
| [13] |
Chun H, Joo K, Lee J, et al. Design and efficient production of bovine enterokinase light chain with higher specificity in E. coli. Biotechnol Lett, 2011, 33(6): 1227-1232. |
| [14] |
Wang ZY, Guo C, Liu L, et al. Effects of N-glycosylation on the biochemical properties of recombinant bEKL expressed in Pichia pastoris. Enzyme Microb Technol, 2018, 114: 40-47. |
| [15] |
Zhang YF, Huang H, Yao XH, et al. High-yield secretory production of stable, active trypsin through engineering of the N-terminal peptide and self-degradation sites in Pichia pastoris. Bioresour Technol, 2018, 247: 81-87. |
| [16] |
Grant DAW, Hermon-Taylor J. Hydrolysis of artificial substrates by enterokinase and trypsin and the development of a sensitive specific assay for enterokinase in serum. Biochim Biophys Acta (BBA) - Enzymol, 1979, 567(1): 207-215. |
| [17] |
Bowers K, Stevens TH. Protein transport from the late Golgi to the vacuole in the yeast Saccharomyces cerevisiae. Biochim Biophys Acta-Mol Cell Res, 2005, 1744(3): 438-454. |
| [18] |
Loureiro J, Lilley BN, Spooner E, et al. Signal peptide peptidase is required for dislocation from the endoplasmic reticulum. Nature, 2006, 441(7095): 18894-18897. |
| [19] |
Matoba S, Ogrydziak DM. Another factor besides hydrophobicity can affect signal peptide interaction with signal recognition particle. J Biol Chem, 1998, 273(30): 18841-18847. |
| [20] |
Yang J, Li CY, Wang YY, et al. Computational analysis of signal peptide-dependent secreted proteins in Saccharomyces cerevisiae. Agric Sci China, 2006, 5(3): 221-227. |
| [21] |
Guan B, Jin J, Li HZ. Genetic engineering of Pichia pastoris expression system for improved secretion of heterologous proteins-a review. Acta Microbiol Sin, 2011, 51(7): 851-857 (in Chinese). 关波, 金坚, 李华钟. 改良毕赤酵母分泌表达外源蛋白能力的研究进展. 微生物学报, 2011, 51(7): 851-857. |
| [22] |
van der Heide M, Hollenberg C, van der Klei I, et al. Overproduction of BiP negatively affects the secretion of Aspergillus niger glucose oxidase by the yeast Hansenula polymorpha. Appl Microbiol Biot, 2002, 58(4): 487-494. |
| [23] |
Freedman RB. Protein disulfide isomerase: multiple roles in the modification of nascent secretory proteins. Cell, 1989, 57(7): 1069-1072. |
| [24] |
Schröder M, Kaufman RJ. ER stress and the unfolded protein response. Mutat Res-Fundam Mol Mechan Mutagen, 2005, 569(1/2): 29-63. |
| [25] |
Elliott S, Giffin J, Suggs S, et al. Secretion of glycosylated human erythropoietin from yeast directed by the α-factor leader region. Gene, 1989, 79(1): 167-180. |
 2020, Vol. 36
2020, Vol. 36




