中国科学院微生物研究所、中国微生物学会主办
文章信息
- 李伟娜, 蒋云云, 刘彦楠, 李春英, 范代娣
- Li Weina, Jiang Yunyun, Liu Yannan, Li Chunying, Fan Daidi
- 人参皂苷单体定向转化的生物催化及应用进展
- Biocatalytic strategies in producing ginsenoside by glycosidase-a review
- 生物工程学报, 2019, 35(9): 1590-1606
- Chinese Journal of Biotechnology, 2019, 35(9): 1590-1606
- 10.13345/j.cjb.190054
-
文章历史
- Received: January 31, 2019
- Accepted: April 2, 2019
- Published: April 11, 2019
2. 西北大学 化工学院 陕西省生物材料与发酵工程技术研究中心,陕西 西安 710069
2. Shaanxi R & D Center of Biomaterials and Fermentation Engineering, College of Chemical Engineering, Northwest University, Xi'an 710069, Shaanxi, China
肿瘤是全球致死率最高的恶性疾病,危害人类的生命与健康[1]。目前临床上肿瘤治疗药物很多来源于植物,如紫杉醇、雷公藤甲素等,而现代药理学研究表明,来源于人参Panax ginseng、西洋参American ginseng和三七Panax notoginseng中的重要次级代谢产物人参皂苷具显著抗癌效果,而尤以稀有人参皂苷及苷元的抗肿瘤、保护神经系统、保肝护肝等药理活性最为显著[2]。而人参皂苷、次级皂苷和皂苷元等成分在人参属植物中含量较少,体内转化量和生物利用度极低,须通过体外总皂苷降解获得。
通过多种技术手段去除骨架结构达玛烷四环三萜支链上所连糖基,定向获得人参皂苷单体成为研究的热点[3]。已经通过酸或热处理方法转化并分离出289种纯人参皂苷单体[4]。由国家药品监督管理局(SFDA)批准上市的抗癌新药参一胶囊(人参皂苷Rg3),成为我国首个实现人参皂苷工业化生产的一类中药单体抗癌药物。国家SFDA唯一认可的人参皂苷Rh2产品今幸胶囊,纯度98%的20(S)-Rh2经极其复杂的大孔树脂吸附、硅胶柱层析分离提取工艺获得,每斤价格高达100多万人民币[5]。
基于微生物及其酶的生物催化由于反应特异性高、条件温和、副产物少、后处理简单成为解决其瓶颈问题的最可行手段。原人参萜二醇(Protopanaxadiol,PPD)型及原人参萜三醇(Protopanaxatriol,PPT)型皂苷通过细胞转化或发酵以不同的水解途径转化为去糖基化(Deglycosylation)的人参皂苷[6]。实质为糖苷水解酶(Glycoside hydrolase,GH)对其侧链糖基(以1–4分子的D-葡萄糖、L-阿拉伯吡喃糖苷、L-阿拉伯呋喃糖苷、D-木糖和/或L-鼠李糖等组成)的特异性水解[7]。金凤燮课题组从微生物培养物、植物提取物得到人参皂苷糖苷酶(纤维素酶和糖苷酶新亚类),并依据底物糖基连接位置和内、外侧糖残基水解特异性进行了分类[8]。Shin等[7]对不同来源人参皂苷GH归属、分子量、最适反应pH、温度、比活等生化特性进行了总结。
微生物、酶法转化相比化学法在持续性、选择性和再生等方面的优势如表 1所示。相比化学法,微生物及其酶的生物转化仅存在溶剂耐受性和转化率方面的不足;相比微生物法,酶法主要存在成本和再生等问题[9]。本文综合人参皂苷生物转化最新进展的回顾,及目前广泛采用的生物催化方法的讨论,认为基于蛋白质工程的酶分子改造和绿色溶剂工程(以离子液体为主)的催化体系构建,在高效、定向转化人参皂苷单体方面有广阔的工业应用前景。
| Characteristics | Catalysis | ||
| Chemical | Enzymatic | Whole-cell | |
| Sustainability | Low | High | High |
| Cost | High | High | Low |
| Selectivity | Good | High | High |
| Self-replication | No | No | Yes |
| Solvent tolerance | High | Good | Low |
通常人参根可直接口服,或以粉末或提取物通过能量饮料、茶和功能性补充剂食用。然而,口服人参对主要人参皂苷的吸收来说是无效的[20]。因为糖基化人参皂苷在肠道中的生物利用率非常低(比如Rb1为0.1%–4.4%; Rb2为3.7%),且容易通过胆道或泌尿系统排出[11-12],需要通过肠道微生物群对其药物代谢动力学特性的改变来逆转低生物利用度,最终被位于肠壁的天然微生物群降解为真正容易被吸收并发挥生物活性作用的稀有人参皂苷(Rg2、Rg3、Compound K等)和苷元[13]。
稀有人参皂苷单体及其衍生物能够调控癌细胞炎症、氧化应激、血管生成、转移信号路径,单独或者结合其他药材治疗癌症[2]。基于糖基数量、位置和糖配基类型、双键位置和立体选择性,Quan等[14]通过酸转化快速制备了23种稀有人参皂苷,并基于它们对6种癌细胞(包括HCT-116、HepG2、MCF-7、Hela、PANC-1和A549)的细胞毒性作用分析了其皂苷结构-药理活性关系。Wei等[15]研究脂肪酸酯化法修饰Rh2,在体内抗肿瘤活性不变的同时,二辛酰酯(D-Rh2)体外对人肝细胞系QSG-7701毒性相比亲本Rh2显著降低。D-Rh2可能通过刺激淋巴细胞对肿瘤细胞产生细胞毒性而间接影响肿瘤生长,较低副作用的D-Rh2可用作抗肿瘤候选药物。
天然化合物人参皂苷的体外修饰与转化增加了人参皂苷结构多样性。为深入研发高抗癌活性药物的应用,本课题组在前期的研究工作中通过酸转化、柱分离制备了一系列稀有皂苷及其组合物,通过抗肿瘤活性分析及初步临床观察,发现PPD型人参皂苷双键异构体Rk1和Rg5 (即Rg3 C-20处脱羟基产物)因抗癌活性优异而极具开发潜力。皂苷单体杀伤肿瘤细胞作用的强度与苷元类型、糖链长短及C-20立体异构有关,改造单体结构可增强抗肿瘤活性[6, 14, 16]。
以酸水解、高温等改变皂苷结构的化学法,均存在价格高、选择性差、副产物多等问题,工业化生产不易实现,多数单体分子结构和抗肿瘤活性的构效关系仍不能被完全阐明[14]。通过微生物及其酶系的生物催化,由于反应特异性高、条件温和、副产物少、后处理简单,适于稀有人参皂苷Compound K、Rg3及其衍生物等转化制备。生物转化修饰结构已涉及羟基化、环氧化、甲基化、异构化、酯化、水解、醇和酮之间的氧化还原、脱氢等多种反应类型[17]。而人参皂苷结构修饰主要在于对特定位点的糖基进行水解,通过不同位置糖链结构的变化来改善化合物的生物活性。
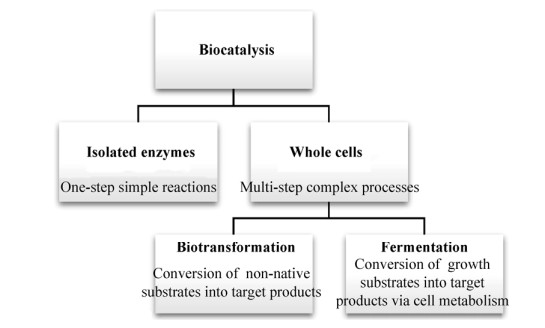
2 菌体及酶法转化人参皂苷单体生物催化剂应用形式(即细胞悬浮液、无细胞提取物和纯化酶)一般取决于下游加工及菌体固有特性[18] (图 1)。细胞为酶提供了天然环境并防止蛋白构象变化,多种胞内、胞外酶的产生取决于生长条件和细胞发育状况。全细胞催化有利于节约人力、生产成本、维护成本。微生物多样性是菌体对各类自然条件(例如温度、pH、压力和盐)长期适应的结果,极端微生物在实验室条件下通常很难生长。除常规的富集培养,还可通过宏基因组(Metagenome) DNA库筛选或序列搜索发现新的功能基因,再结合适当的克隆载体和宿主获得工程菌[19]。酶较全细胞对一步反应更有优势,负责生命过程所有必需代谢反应的催化,一般反应条件温和,pH、温度范围有限,体外仅在最佳pH和温度条件下发挥作用。纯酶反应特异性更高,而酶的分离和纯化过程耗时费钱。
微生物能够通过全细胞或发酵以不同代谢途径对人参皂苷进行去糖基化(Deglycosylation)[6]。例如,以人参皂苷Rb1为底物转化生产Compound K (已证明具诸如抗癌、抗炎、抗过敏、抗糖尿病、抗血管生成、抗衰老、神经保护和保肝作用等生物学功效[20]),枝孢霉菌Cladosporium cladosporioides[21]兼具两条水解途径:Rb1→G17→F2→Compound K→和Rb1→Rd→F2→Compound K;叶森金杆菌Chryseobacterium yeoncheonense和人体肠道菌群[22]兼具3条水解途径:Rb1→F2→Compound K、Rb1→G17→G75→Compound K或Rb1→G17→F2 → Compound K。
肠道菌群中的乳酸杆菌Lactobacillus和双歧杆菌属Bifidobacterium spp.可合成对人参皂苷去糖基化所必需的多种糖苷酶(EC 3.2.1),包括β-葡糖苷酶(EC 3.2.1.2)、纤维素酶(EC 3.2.1.4)、β-半乳糖苷酶(EC 3.2.1.23)、α-L-阿拉伯呋喃糖苷酶(EC 3.2.1.55)、α-L-阿拉伯糖苷酶(无EC编号)和β-木糖苷酶(EC 3.2.1.37)[10, 23]。肠道微生物组成及其糖苷酶活性对人参皂苷CK、Re等的代谢调控有重要作用[24]。而以人参皂苷为碳源进行的肠道细菌体外厌氧培养转化,存在培养基昂贵、产率低的问题[10]。
人参栽培土壤中可以分离用于人参皂苷转化的真菌[9]。曲霉属含β-葡萄糖苷酶约250种,在食品和饮料领域应用了1 500年,其中黑曲霉Aspergillus niger和米曲霉A. oryzae列为美国FDA通常被认为是安全的(GRAS)名单[25-26]。Lin等[27]直接使用杂色曲霉Aspergillus versicolor产孢子阶段分泌于固体培养物中的胞外β-葡萄糖苷酶进行人参皂苷Rb1到Rd的转化。孢子悬浮系统从摇瓶放大至2 L时的最大生物转化率85% (W/W)。因培养基便宜且生长较快,该类土壤真菌的转化较肠道细菌更经济可行,被指定为GRAS的土壤微生物能应用于食品领域。
预计到2021年益生菌市场总量增长至580亿美元[28]。以益生菌修饰人参皂苷糖基结构,有助于其保健品特性的强化和规范。Ku等[10, 29]分别用长双歧杆菌Bifidobacterium longum RD47和鼠李糖乳杆菌Lactobacillus rhamnosus GG将人参皂苷Rb2、Rc和Rb1水解为人参皂苷Rd。作为牛奶、蔬菜等食物发酵剂的乳酸菌(Lactic acid bacteria,LAB)在稀有人参皂苷的转化应用方面,Park等[30]首次通过食品级明串珠菌Leuconostocs和乳酸杆菌Lactobacilli全细胞转化生产Compound K。Huq等[31]以LAB在经济可食用培养基(有20 g/L萝卜,20 g/L葡萄糖和10 g/L酵母提取物)上经7 d发酵后,人参皂苷Rb1可转化Rg3,而MRS培养基中只能转化到Rd。作为表达外源基因高效生产酶蛋白的GRAS宿主,Li等[32]通过强化密码子,在乳酸乳球菌Lactococcus lactis中表达克隆自胶质类芽孢杆菌Paenibacillus mucilaginosus的重组β-葡萄糖苷酶,转化人参皂苷Rb1和Rd为F2。
在三萜合成途径构建方面,以酿酒酵母Saccharomyces cerevisiae为代表的微生物工程已被广泛用于人参皂苷的转化[33-34] (表 2)。经鉴定Cyt P450酶(CYP716A47)参与达玛烷型人参皂苷前体达玛烯二醇-Ⅱ C-12位羟基化,Han等[35]通过在S. cerevisiae中CYP716A47和达玛烯二醇合酶基因(PgDDS)的共表达,不添加达玛烯二醇-Ⅱ即可产生PPD。Dai等[36]在S. cerevisiae中引入达玛烯二醇-Ⅱ合成酶、PPD合成酶基因以及拟南芥NADPH-细胞色素P450还原酶(ATR1)基因,并通过过量表达截短(Truncation)的3-羟基-3-甲基戊二酰辅酶A还原酶、法呢基二磷酸合成酶、角鲨烯合成酶和2, 3-氧化角鲨烯合成酶基因,以及提高PPD合酶活性的密码子优化,最后通过双相萃取发酵获得8.40 mg/g DCW PPD (1 189 mg/L)。Zhao等[37]通过引入人参达玛烯二醇-Ⅱ合成酶、人参细胞色素P450型PPD合成酶(PPDS)和拟南芥ATR1基因,在S. cerevisiae中构建了PPD的生物合成途径,经细胞色素P450氧化系统优化,在5 L生物反应器中补料分批发酵,PPD产量达到1 436.6 mg/L。
| Strategies | Microbial hosts | Introduced gene and source | Type of ginsenosides | Amount of ginsenosides produced | Reference |
| Biosynthetic pathway constituted | Saccharomyces cerevisiae | CYP716A47 and PgDDS from P. ginseng | Galactose→→ Protopanaxadiol | / | [35] |
| PgDDS and PgPPDS genes from P. ginseng, NADPH-cytochrome P450 reductase gene of Arabidopsis thaliana (AtCPR1) | Glucose→→ Dammarenediol-Ⅱ→ Protopanaxadiol | 8.40 mg/g dry cell weight (DCW) protopanaxadiol (1 189 mg/L), together with 10.94 mg/g DCW dammarenediol-Ⅱ (1 548 mg/L) | [36] | ||
| PPDS modified through transmembrane domain truncation of P. ginseng+ ATR1of Arabidopsis thaliana | Glucose→→ Protopanaxadiol | 1 436.6 mg/L | [37] | ||
| UGTPg45 and UGTPg29 from P. ginseng+PPD-producing chassis | Glucose→→ Protopanaxadiol→ Rh2→Rg3 | 3.49 μmol/g DCW | [39] | ||
| UGTPg1/UGTPg100 from P. ginseng+PPD-producing chassis (PgDDS+ CYP716A47+CYP716A53v2+ PgCPR1 of P. ginseng) | Glucose→→Rh1, Glucose→→F1 | F1 and Rh1 measured as 42.1 mg/L and 92.8 mg/L, respectively | [40] | ||
| Semi-rationally designed UGT51 from S. cerevisiae+ PPD-producing chassis | Glucose→→Rh2 | 2.90 mg/g dry cell weight DCW, ~300 mg/L | [41] | ||
| Pichia pastoris | Self-assembly of ERG1 from P. pastoris and PgDDS from P. ginseng | Glucose→→ Dammarenediol-Ⅱ | 0.1 mg/g DCW | [42] | |
| Ectopic expression | Escherichia coli | SS, SE and CPR from S. cerevisiae+SE from Methylococcus capsulatus+ CPR from Arabidopsis thaliana | Isopentenyl pyrophosphate (IPP) and its isomer dimethylallyl pyrophosphate (DMAPP)→Dammarened iol-Ⅱ | 8.63 mg/L | [43] |
| UGT genes from P. notoginseng, Medicago sativa, and Bacillus subtilis | 20(R)PPD→Compound K→20(R)F2 | 0.06 mg/L | [44] | ||
| S. cerevisiae | UGTPg71A29, from P. ginseng | Rh1→Rg1, Rd→Rb1 | / | [45] | |
| “→” represents one-step reaction; “→→” represents more than one-step reactions; “/” represents data not mentioned. | |||||
通过在微生物底盘细胞中重构与优化天然化合物的生物合成途径, 实现目标化合物的从头合成。被首次表征的用于植物四环三萜底物糖基化的UDP-糖基转移酶(UGT) UGTPg1,转移葡糖基部分到PPD的C-20S-OH得到Compound K,是经一锅反应由简单糖生物合成Compound K成功的关键[38]。Wang等[39]从人参中克隆并鉴定了两种UGT基因:UGTPg45和UGTPg29,结合产PPD的酵母底盘细胞,成功构建了由葡萄糖生产Rh2和Rg3的酵母细胞工厂。Wei等[40]证明从人参分离的新型UGT基因UGTPg100和UGTPg101可特异糖基化PPT的C6-OH分别产生人参皂苷Rh1、F1,在酵母底盘细胞中构建了F1和Rh1的合成途径。Zhuang等[41]将用于PPD到人参皂苷Rh2转化的微生物糖基转移酶UGT51通过半理性设计,将突变糖基转移酶基因引入酵母并经代谢工程进一步优化后,在5 L生物反应器中补料分批发酵,Rh2产量达到约300 mg/L。
毕赤酵母Pichia pastoris与S. cerevisiae遗传操作相似且外源蛋白表达量更高,Zhao等[42]通过在P. pastoris中自组装达玛烯二醇-Ⅱ合酶和角鲨烯环氧酶促使达玛烯二醇-Ⅱ产量增加2.1倍。Li等[43]通过在大肠杆菌Escherichia coli中重建2, 3-氧化角鲨烯衍生的三萜类化合物生物合成途径,成功构建产达玛烯二醇-Ⅱ的E. coli底盘,达玛烯二醇-Ⅱ异源生物合成,发酵48 h产率为8.63 mg/L。但是这些工作目前还仅限于达玛烷型人参皂苷前体达玛烯二醇-Ⅱ的合成, 尚未真正意义上实现合成人参皂苷。
通过外源生物合成途径重建的工程菌直接从人参皂苷为底物合成稀有人参皂苷。Yu等[44]通过尿苷二磷酸糖基转移酶(UTG)基因工程化大肠杆菌分别由20(R)-PPD和20(R)-PPT转化生产20(R)-Compound K和20(R)-F1。Lu等[45]鉴定了参与人参皂苷Rg1和Rb1生物合成的UDP-糖基转移酶,并实现了S. cerevisiae中的全细胞催化。
2.2 酶催化微生物的代谢易受环境影响,转化人参皂苷的选择性差、产率低,参与反应的酶未确定。酶促转化由于操作方法简单且反应特异性强,被认为是结构修饰和代谢研究的有用工具。优化微生物产酶条件,实现生物量和产酶之间的平衡,尤以最大限度缩短处理时间、降低成本的重组酶转化最为显著[46]。
在E. coli中过表达各种重组β-葡糖苷酶以酶解糖基化的人参皂苷。Cui等[47]用重组酶在10 L发酵罐转化20 mg/mL Re得到113 g色谱纯(84.0±1.1)%的Rg2 (S),首次实现100 g级Rg2 (S)酶法生产。Kim等[48]补料分批培养强化表达β-D-糖苷酶和α-L-阿拉伯呋喃糖苷酶的E. coli,通过立体专一酶的级联转化,由人参叶提取物获得人参皂苷Compound K。Shin等[49]将能完全转化所有PPD型皂苷为Compound K的β-糖苷酶在E. coli重组表达,可将丢弃人参叶的PPD型皂苷转化为Compound K。Xie等[50]过表达来自嗜热酶α-丙氨酸呋喃糖苷酶,85 ℃、pH 5.0、1 h内转化25 g/L Rc为21.8 g/L Rd,Rd产率为21 800 mg/(L·h)。Quan等[51]首次报道了高热稳定性重组β-葡萄糖苷酶分别将人参皂苷Re和Rg1转化为人参皂苷Rg2和Rh1,在酶浓度1.36 U/mL、85 ℃、pH 5.5、1 h内转化10 g/L人参皂苷Re为8.02 g/L Rg2,2 g/L人参皂苷Rg1为1.56 g/L Rh1。
E. coli重组酶转化人参皂苷可能应用于医药或制药行业。然而在食品(尤其营养保健品)行业中,E. coli常被认为是不可食用细菌[52]。为提高用于人参皂苷微生物转化的糖苷酶产量,有必要在以下方面进一步改善:1)开发广泛的食品级宿主;2)提高细胞生物量和酶产量;3)确定用于酶诱导的最佳培养基组分[53]。
益生菌适合食品级糖基化酶的生产,其MRS培养基价格也仅为E. coli的3倍。以含外源基因重组质粒的益生菌系统替代E. coli,Youn等[54]在双歧杆菌B. bfidum中表达克隆自动物双歧杆菌Bifidobacterium animalis的重组β-葡萄糖苷酶以转化人参皂苷Rb1和Rb2。由于转基因应用于食品工业引发的争议,又转向以经典发酵法生产糖苷水解酶,筛选新的益生菌菌株并优化细胞培养条件。来源于蘑菇真菌Armillaria mellea的菌丝体酶制剂(菌体培养2周,25 ℃,经酶提取、硫酸铵沉淀、透析、冻干)转化Rb1到Compound K,最适培养条件为72–96 h、pH 4.0–4.5、温度45–55 ℃[55]。对于20 mg花费达163.2美元的98% F1,除了仅停留在确定微生物酶转化能力的小规模实验,Wang等[56]首次报道了以GRAS微生物食品级的商业酶Cellulase KN在48 h内完成对Re和Rg1 (源自10 mg/mL PPTGM)至F1的10 g级转化。
除了水解人参皂苷的糖基,微生物也能转化产生高生物活性的人参皂苷化学衍生物。Zhou等[57]以拟青霉Paecilomyces bainier sp. 229对人参皂苷Rb1的6 L发酵中,通过反复硅胶柱层析和高压液相色谱分离纯化出3-酮衍生物和两种新的脱氢代谢物。不同类别的酶能选择性修饰天然化合物的复杂反应性官能团而产生一系列衍生物,Teng等[58]以乙酸乙烯酯作为乙酰基供体,在有机溶剂中通过来自南极假丝酵母Candida Antarctica (Novozym 435)脂肪酶区域选择性地酰化人参皂苷,得到单酰基人参皂苷。Gebhardt等[59]通过来自牛初乳的β-1, 4-半乳糖基转移酶和Novozym 435脂肪酶的催化,制备获得了人参皂苷Rb1的一系列特异性衍生物。
2.3 糖苷酶固定化相对于游离酶,固定化酶为适于工业化应用的主要形式。基于吸附、包埋、交联、共价结合的酶的固定化方法的选择,以考察固定化酶操作稳定性为主,综合考虑工业放大时的技术可行性和固定化过程中涉及的酶、载体及试剂的费用。而提高时空产率高效酶反应器的开发,进一步推动了固定化酶技术在生物转化领域中的研究应用。而应用于人参皂苷转化的酶固定化报道仅有3篇。Zhang等[60]以交联-包埋法(交联3 h,戊二醛浓度0.1%,海藻酸钠浓度1%,CaCl2浓度2%)固定化酶转化人参皂苷Rg1为F1,3.76 U/g固定化酶载体,0.2 mg/mL底物,40 ℃转化2 d,4次平均转化率为80.49%。Yu等[61]以SiO2吸附蜗牛酶然后结合交联-包埋法(海藻酸钠质量浓度2%,CaCl2质量浓度2%,SiO2与蜗牛酶质量比为1:1)制备微球固定化蜗牛酶,转化人参皂苷Rb1为Compound K,55 ℃,1.0 mg/mL底物,转化36 h,5次平均转化率为36.79%。为解决酶与载体吸附力弱、与底物接触面积有限的问题,Yuan等[62]将纤维素酶固定在用聚乙烯亚胺和戊二醛活化的角叉菜胶珠表面,转化人参皂苷Rb1为Rd,同时测定了反应动力学参数Km和Vmax,在连续使用5次后,固定化酶可以保持初始活性的60%。
Graebin等[63]特别关注了糖苷酶家族GH1和GH3中β-葡萄糖苷酶的固定化方法。物理吸附、离子交换、疏水作用等增强了固定化体系酶的灵活性,相应载体包括土壤胶体颗粒、离子交换树脂、磁性Fe3O4纳米颗粒等。尤其丝瓜蔬菜海绵[64]、含氧化铁的细小土壤胶粒[65]等天然可降解、成本低廉载体的使用大大减少了化学载体丢弃时涉及的成本和环境问题。包埋固定化可改善酶的热稳定性、最佳使用温度和储存稳定性,但机械强度较低且酶渗漏导致固定化成本增加[66]。关于其相对活性和包封率,因纳米级聚合物材料(聚氨酯、乳胶和硅胶)代替传统藻酸盐珠的应用得到改善[67]。共价固定化可提高酶制剂稳定性,载体包括最常用壳聚糖及其他海绵、咖啡渣、硅胶、SiO2纳米颗粒、环氧树脂活化Eupergit C、胺琼脂糖凝胶等。
吸附或包埋固定β-葡糖苷酶由于酶逐渐释放导致催化剂半衰期有限;经化学反应的共价固定化引起酶活损失[68]。为克服这些问题,Mateo等[69]研究了酶物理聚集再交联(交联酶聚集体,CLEAs)制备固体生物催化剂的方法。通过形成纳米尺度CLEAs将β-葡糖苷酶固定于二氧化硅泡沫。高酶载量CLEAs在更广泛的温度和pH范围保持活性,且比游离酶Km低,可使用多达10个循环,残余活性超过85%[68]。近来GH1家族β-葡糖苷酶结构的解析有助于定点固定化工作的展开。经定点诱变后,酶与载体进行固定化,稳定性提高的同时获得产物抑制降低,活性、专一性提高等优势特性[63]。而基于重组DNA的新酶设计经过微生物的遗传修饰,验证食品安全性的同时,也应充分考虑酶及其载体释放到加工系统可能带来的安全隐患[70]。
3 糖苷酶转化分子机制的探究为解决微生物发酵及自身酶系对人参皂苷糖苷键催化存在的专一性差、效率低等问题,基于酶高级结构、分子识别机制改造酶结构,并通过基因工程菌表达以高效转化目的人参皂苷单体。Mak等[71]综合基因组挖掘(Integrative genomic mining approach)结合生物信息和分子建模采掘序列数据库(Mine sequence databases),使酶对目标反应专一性提高100倍,催化效率提高了75倍。因此除了针对特定产物挖掘专一性的新酶,研究工作还应集中在基于催化分子机制的酶分子改造。
糖苷酶家族活性结构域拓扑学构象有3种:口袋式(Pocket)、裂隙(Cleft或Groove)、隧道(Tunnel)[72]。基于构效关系水解酶定向转化与分子识别的报道较多[73-75]。基于高分辨高葡萄糖耐受β-葡糖苷酶(Bgl6)和三重突变体M3(随机诱变提高热稳定性)的晶体结构,Pang等[75]发现Bgl6形成的额外通道可作为葡萄糖二级结合位点,有助于葡萄糖耐量;三重突变增强酶内的疏水相互作用,可能是M3热稳定性增强的原因。Zhang等[76]基于活性位点比对和量子化学计算得出化学必需的相互作用,以验证催化机理假说揭示糖苷水解酶结构与功能的关系。
关于糖苷酶转化皂苷的催化机制的研究,如图 2所示,人粪便B. longum GH3β-葡萄糖苷酶基因BIBG3经克隆、结构分析,发现BIBG3络合D-葡萄糖的独特环状结构可参与底物结合口袋的形成,通过和底物的分子对接揭示了口袋的结合方式,找到关键酶活突变位点R484和H642[77]。而酶分子设计改造的报道迄今仅有3篇。Park等[78]分离出具广泛底物谱β-GH并测定其晶体结构,基于产物特性、底物对接,以在三萜类化合物特定糖基化位点优先水解聚糖的方式,重新设计了酶底物结合裂隙。使对Rb1的催化效率提高4–7倍,促进PPD型底物Rb1、Rb2和Rb3 (Rc)经过F2到C-K的继续转化(图 3)。Choi等[79]通过同源建模、分子对接、序列比对,确定参与α-L-阿拉伯呋喃糖苷酶活性的候选残基;经定点突变得到的L213Aβ-糖苷酶变体具有野生型没有的α-L-阿拉伯呋喃糖苷酶活性,促进Rc进一步水解转化为Rd,转化率比野生型酶高1.5倍(图 4)。在酵母底盘细胞重构与人参皂苷的生物合成途径优化方面,Zhuang等[41]通过同源建模、分子动力学和突变研究了UGT的催化分子机制[40-41, 45]。且经半理性设计的UGT51对PPD到Rh2的催化效率提高了约1 800倍。

|
| 图 2 BlBG整体结构及其催化Rb1反应活性位点几何结构分析(改编自参考文献[77]) Fig. 2 Schematic diagram of functional and structural characterization of a β-glucosidase involved in saponin metabolism from intestinal bacteria[77]. (A) Overall structure of BlBG and structural analysis on its active site geometry for transfer reaction of ginsenoside Rb1. (B) Structural comparison with other GH3 family members. (C) Compound protobioside (magenta) was docked to the active pocket of BlBG3. (D) Stereoview of the pocket with a van der Waals surface representation of BlBG3 with protobioside: glucose. |
| |

|
| 图 3 BGL167整体结构及其催化PPD型人参皂苷反应活性位点几何结构分析(改编自参考文献[78]) Fig. 3 Schematic diagram of rational design of a β-glycosidase with high regiospecificity for triterpenoid tailoring[78]. (A) Overall structure of the wild type enzyme (BGL167). (B) Structural analysis on its active site geometry for transfer reaction of PPD-type ginsenosides. (C) Structural comparison with other GH1 family members, substrate binding mode of the 3MT. (D) Biotransformation pathways of the major triterpenoids by 3MT. |
| |

|
| 图 4 来自S. solfataricus的β-糖苷酶配体对接和序列比对及其突变体催化人参皂苷Rc生物转化途径(改编自参考文献[79]) Fig. 4 Schematic diagram of rational design of a β-glycosidase with increased α-L-arabinofuranosidase activity for conversion of ginsenoside Rc to compound K[79]. (A) Construction of ligand docked pose, sequence alignment. (B) Biotransformation pathways from ginsenosides Rb1, Rb2, and Rc to C-K by the wild-type and L213A variant β-glycosidases from S. solfataricus. |
| |
蛋白质工程是提高酶对特定人参皂苷糖苷水解活性的有用工具。多种不同来源具人参皂苷转化活性的微生物糖苷酶(尤其β-葡萄糖苷酶)晶体结构已经被解析[7],因此可通过同源建模、分子对接设计、突变以改造酶分子,改善底物特异性和催化效率,产生高纯度的各种特殊三萜类化合物,实现目的人参皂苷专一、高效的酶法转化。
4 离子液体对生物催化性能的改善不同于分子组成的传统液体溶剂,大多常温下呈液态盐(由特定的有机阳离子与无机或有机阴离子构成)的离子液体(Ionic liquids,ILs),具饱和蒸气压低、不可燃、亲疏水、可设计等特性。研究最为广泛的是ILs及其水溶液中脂肪酶、蛋白酶和酯酶的催化,而对于糖苷酶水解作用的研究很少[80]。通常,疏水、低粘度、表面活性、亲电阴离子和离液阳离子(Chaotropic cation)的ILs增强酶活性和稳定性[80]。然而由于许多结果矛盾没有一般相关性规律,因此,以提高酶活性和稳定性的方法正在探索,如水中ILs微乳液、设计与酶相容的离子溶剂、酶电荷的修饰、酶的固定化等[81]。
4.1 离子液体参与水相转化应用概况全细胞催化存在催化剂不稳定、产物抑制、有毒副产物形成和质量传递等问题。在水-ILs的两相催化中,由于全细胞悬浮在水相而有机底物溶解在疏水ILs相(或贮存产物),从而避免了反应中低水溶性的底物(或产物)抑制。Chen等[82]在含ILs体系中全细胞催化水解甘草甜素制备单葡萄糖醛酸甘草次酸,发现在咪唑类ILs存在下,ILs提高了细胞膜通透性,且相比E. coli BL21和P. pastoris GS115,青霉Penicillium purpurogenum Li-3对ILs (主要为[Bmim] [PF6])耐受性最强,60 h收率达87.63%。ILs在生物催化中的应用需综合对所需化学反应(产物分离)的溶剂性质和对全细胞的溶剂毒性作用。而ILs对微生物细胞的毒性是其工业应用瓶颈之一,目前在含ILs系统中研究最多的微生物是E. coli和酿酒酵母S. cerevisiae。Egorova等[18]研究了ILs毒理学特性,从反应介质(溶剂)角度分析了全细胞在苛刻化学催化中的应用前景。
酶能够在体外不适合细胞生长的条件下进行催化,专一性强、催化方式简单。离子液体中阳离子或阴离子类型对酶的活性、稳定性和结构具重要影响。Yanhong等[83]对含C6MIm·BF4体系黑李种子β-糖苷酶糖基化红景天苷合成条件进行优化,发现阳离子咪唑环上的烷基取代基的最佳链长为C6。Ferdjani等[84]研究了在ILs/水不同比例时嗜热栖热菌Thermus thermophilus β-糖苷酶和两种分别来自海栖热袍菌Thermotoga maritima、嗜热脂肪芽孢杆菌Bacillus stearothermophilus的α-半乳糖苷酶的活性、稳定性,发现在适合水溶性ILs (Water-miscible ILs)中T. thermophilus β-糖苷酶热稳定性最高。Brakowski等[85]发现含ILs-水缓冲乳液中,[Bmim][Pf6]导致来自奥氏曲霉Aspergillus oryzae的β-半乳糖苷酶转糖基底物专一性的改变。Kudou等[86]基于咪唑ILs磷酸盐缓冲液,在乙酸1-丁基-3-甲基咪唑鎓[Bmim] [OAc],pH 7.0,葡糖苷酶水解活性最高,通过稳态发射光谱证明糖苷酶活性改善可能与酶构象的灵活性有关。
由于毒性低、生物可降解、易于制备、具100%原子经济等特点,基于含胆碱盐和三类氢键供体(酰胺、醇和糖)的低共熔溶剂(DESs)可作为传统离子液体的廉价替代品,成为β-葡萄糖苷酶催化的新型绿色溶剂,可拓展其在食品和医药领域方面的应用。以对硝基苯基-β-吡喃葡萄糖苷作为水解反应模型,Xu等[87]发现基于氯化胆碱的DESs (含6% (V/V)水的氯化胆碱/丙二醇)显著改善β-葡萄糖苷酶的活性和稳定性。在ILs中可通过酶表面电荷修饰和酶固定化来进行酶的稳定和激活,以增强在ILs中的耐受性。Zhao等[88]总结了固定化酶在ILs中被稳定和激活的实例,但大多数与脂肪酶有关。Nature Chemistry最新报道了Jason等[89]以阳离子化的葡萄糖苷酶降解溶于ILs的纤维素,极大提高了纤维素降解效率(100 ℃以上的催化效率是在水溶液时的30倍) (图 5)。表面阳离子化修饰的葡萄糖苷酶(以ILs代替水作溶剂,通过碳二酰亚胺介导N, N′-二(2-氨乙基)-1, 3-丙二胺定向偶联酶表面天冬氨酸和谷氨酸残基)在ILs中溶解度增加,在苛刻实验条件下依然能以恒定速率工作至少7 d。

|
| 图 5 离子液体体系纤维素酶非水均相转化多糖催化机理(改编自参考文献[89]) Fig. 5 Mechanisms of cellulase activity and structure on non-aqueous homogenous biocatalytic conversion of polysaccharides in ionic liquids[89]. (A) Cartoon detailing proposed mechanism for cellulose hydrolysis by β-glucosidase solubilized and stabilized in ionic liquids, showing dissolution of cellulose in ionic liquid. (B) Far-UV SRCD spectra showing secondary structure of C-Glu][S]. (C) SAXS profiles showing nanoconjugate dimensions for concentrated solutions of [C-Glu][S]. |
| |
ILs中酶以与偶联载体(聚合物、纳米微粒或碳纳米管)、被水凝胶包裹或以原始状态悬浮的方式进行固定化。无固相支撑的CLEAs有望提高ILs中酶稳定性,Toral等[90]发现CLEA和在聚丙烯上吸附交联的固定化脂肪酶能够在使游离酶失活的ILs (如[BMIM][NO3])中仍然保持催化活性。溶胶-凝胶基质具有防止反应过程中酶从载体泄漏的优点,而凝结和干燥过程中的凝胶收缩可能导致酶变性。通过在溶胶-凝胶固定化过程中添加离子液体,可提高包封酶的固定效率以及机械抗裂性,表明离子液体在酶性能中起着重要作用[91]。
4.2 在酶转化人参皂苷中的应用潜能在人参皂苷生物转化方面尚无ILs的应用,首先就人参皂苷溶解性进行分析。大多数人参皂苷在含水正丁醇(常作为皂苷萃取溶剂)中溶解度较大,而次级苷由于糖数目减少、极性减小、在水中溶解度降低,苷元则难溶于水。例如,Re不易溶解于水,而在DMSO中溶解度达200 mg/mL。Cui等[40]以20 mg/mL人参皂苷Re在含10% DMSO缓冲液中进行转化,实现了10 L发酵罐中100 g级Rg2(S)的酶法生产。来源于蘑菇真菌Armillaria mellea菌丝体的酶转化制备Compound K时,在底物Rb1中加入甲醇助溶[55]。
据此,有望以非挥发、环保的ILs代替DMSO和甲醇有机溶剂发挥对底物的助溶作用。以咪唑类ILs提取和富集三七中药材及其制剂中的微量人参皂苷20(S)-Rg3和Rk1[92-94],进一步证明ILs有助于增加稀有人参皂苷溶解度,可开发应用于人参皂苷的生物催化转化。结合以上ILs参与其他类型产物的水相反应的生物转化时对全细胞及酶催化性能的影响,可能获得比在有机溶剂时更高的催化活性、选择性和稳定性。因而,ILs参与的稀有人参皂苷生物催化体系的构建,在转化工艺中除ILs对底物溶解性作用外,还需要考虑作为反应介质ILs对菌体毒性或酶催化性能的影响,以及作为酶修饰剂,ILs的改性方法及酶稳定化的应用形式。
5 展望稀有人参皂苷生物催化转化的关键问题包括特异性酶活性不高、涉及酶及其催化机制不明确、结构生物信息学研究不系统,改变酶水解皂苷糖基专一性的研究尚处于起步阶段。益生菌及其酶的使用已在食品工业中的实用应用中显示出巨大潜力。而离子液体可以通过改变反应体系的极性增加糖类的溶解度,为合理设计糖基转化反应体系创造多种机会。因此,今后研究工作应集中在:1)基于同源建模、分子对接催化分子机制的揭示来改造酶分子,以改变酶专一性、提高酶活性和稳定性;2)基于ILs的溶剂工程在酶固定化及酶表面修饰的作用,改善酶转化人参皂苷的催化效率和稳定性。通过筛选及构建基因工程菌、发酵或胞外表达GH,以化学修饰并制备多种不同尺度、形态的益生菌酶制剂。同时,以光谱法、计算机模拟等可视化技术研究ILs-酶-底物相互作用,系统分析ILs反应体系中影响糖苷酶结构及其活性、专一性和稳定性的催化分子机制。最终实现对人参皂苷定向、高效的生物转化工艺,有助于深入探究并开发新癌症化学预防的药物化学和药理学方法。
| [1] |
Chen WQ, Zheng RS, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin, 2016, 66(2): 115-132. DOI:10.3322/caac.21338 |
| [2] |
Wong AS, Che CM, Leung KW. Recent advances in ginseng as cancer therapeutics: a functional and mechanistic overview. Nat Prod Rep, 2014, 32(2): 256-272. |
| [3] |
Lee CH, Kim JH. A review on the medicinal potentials of ginseng and ginsenosides on cardiovascular diseases. J Ginseng Res, 2015, 38(3): 161-166. |
| [4] |
Yang WZ, Hu Y, Wu WY, et al. Saponins in the genus Panax L. (Araliaceae): a systematic review of their chemical diversity. Phytochemistry, 2014, 106: 7-24. DOI:10.1016/j.phytochem.2014.07.012 |
| [5] |
Cheong JH, Kim H, Hong MJ, et al. Stereoisomer-specific anticancer activities of ginsenoside Rg3 and Rh2 in HepG2 cells: disparity in cytotoxicity and autophagy-inducing effects due to 20(S)-Epimers. Biol Pharm Bull, 2015, 38(1): 102-108. DOI:10.1248/bpb.b14-00603 |
| [6] |
Shin KC, Oh DK. Classification of glycosidases that hydrolyze the specific positions and types of sugar moieties in ginsenosides. Crit Rev Biotechnol, 2015, 36(6): 1036-1049. |
| [7] |
Park CS, Yoo MH, Noh KH, et al. Biotransformation of ginsenosides by hydrolyzing the sugar moieties of ginsenosides using microbial glycosidases. Appl Microbiol Biotechnol, 2010, 87(1): 9-19. DOI:10.1007/s00253-010-2567-6 |
| [8] |
Liu CY, Zhou RX, Sun CK, et al. Preparation of minor ginsenosides C-Mc, C-Y, F2, and C-K from American ginseng PPD-ginsenoside using special ginsenosidase type-Ⅰ from Aspergillus niger g. 848. J Gins Res, 2015, 39(3): 221-229. DOI:10.1016/j.jgr.2014.12.003 |
| [9] |
Guo CL, Cui XM, Yang XY, et al. Advances in studies on biotransformation of ginsensides. China J Chin Mater Med, 2014, 39(20): 3899-3904 (in Chinese). 郭从亮, 崔秀明, 杨晓艳, 等. 人参皂苷生物转化研究进展. 中国中药杂志, 2014, 39(20): 3899-3904. |
| [10] |
Ku S. Finding and producing probiotic glycosylases for the biocatalysis of ginsenosides: a mini review. Molecules, 2016, 21(5): 645. DOI:10.3390/molecules21050645 |
| [11] |
Hasegawa H. Proof of the mysterious efficacy of ginseng: basic and clinical trials: metabolic activation of ginsenoside: deglycosylation by intestinal bacteria and esterification with fatty acid. J Pharmacol Sci, 2004, 95(2): 153-157. |
| [12] |
Kim KA, Yoo HH, Gu W, et al. Effect of a soluble prebiotic fiber, NUTRIOSE, on the absorption of ginsenoside Rd in rats orally administered ginseng. J Ginseng Res, 2014, 38(3): 203-207. DOI:10.1016/j.jgr.2014.03.003 |
| [13] |
Wang JR, Yau LF, Zhang R, et al. Transformation of ginsenosides from Notoginseng by artificial gastric juice can increase cytotoxicity toward cancer cells. J Agric Food Chem, 2014, 62(12): 2558-2573. DOI:10.1021/jf405482s |
| [14] |
Quan K, Liu Q, Wan JY, et al. Rapid preparation of rare ginsenosides by acid transformation and their structure-activity relationships against cancer cells. Sci Rep, 2015, 5: 8598. DOI:10.1038/srep08598 |
| [15] |
Wei GQ, Zheng YN, Li W, et al. Structural modification of ginsenoside Rh2 by fatty acid esterification and its detoxification property in antitumor. Bioorg Med Chem Lett, 2012, 22(2): 1082-1085. DOI:10.1016/j.bmcl.2011.11.104 |
| [16] |
Cao JQ, Zhang XS, Qu FZ, et al. Dammarane triterpenoids for pharmaceutical use: a patent review (2005-2014). Expert Opin Ther Pat, 2015, 25(7): 805-817. DOI:10.1517/13543776.2015.1038239 |
| [17] |
He CA, Yu XY, Meng QX, et al. Research in Structural modification of biotransformation of natural drugs. Nat Prod Res Dev, 2012, 24(6): 843-847 (in Chinese). 贺赐安, 余旭亚, 孟庆雄, 等. 生物转化对天然药物进行结构修饰的研究进展. 天然产物研究与开发, 2012, 24(6): 843-847. DOI:10.3969/j.issn.1001-6880.2012.06.029 |
| [18] |
Egorova KS, Ananikov VP. Ionic liquids in whole-cell biocatalysis: a compromise between toxicity and efficiency. Biophys Rev, 2018, 10(3): 881-900. DOI:10.1007/s12551-017-0389-9 |
| [19] |
Ferrer M, Martinez-Martínez M, Bargiela R, et al. Estimating the success of enzyme bioprospecting through metagenomics: current status and future trends. Microb Biotechnol, 9(1): 22-34. DOI:10.1111/1751-7915.12309 |
| [20] |
Yang XD, Yang YY, Ouyang DS, et al. A review of biotransformation and pharmacology of ginsenoside compound K. Fitoterapia, 2015, 100: 208-220. DOI:10.1016/j.fitote.2014.11.019 |
| [21] |
Wu LP, Jin Y, Yin CR, et al. Co-transformation of Panax major ginsenosides Rb1 and Rg1 to minor ginsenosides C-K and F1 by Cladosporium cladosporioides. J Ind Microbiol Biotechnol, 2012, 39(4): 521-527. DOI:10.1007/s10295-011-1058-9 |
| [22] |
Hoang VA, Kim YJ, Nguyen NL, et al. Chryseobacterium yeoncheonense sp. nov., with ginsenoside converting activity isolated from soil of a ginseng field. Arch Microbiol, 2013, 195(7): 463-471. DOI:10.1007/s00203-013-0898-2 |
| [23] |
Rossi M, Amaretti A, Leonardi A, et al. Potential impact of probiotic consumption on the bioactivity of dietary phytochemicals. J Agric Food Chem, 2013, 61(40): 9551-9558. |
| [24] |
Zhang L, Li F, Qin WJ, et al. Changes in intestinal microbiota affect metabolism of ginsenoside Re. Biomed Chromatogr, 2018, 32(10): e4284. DOI:10.1002/bmc.v32.10 |
| [25] |
Molina G, Contesini FJ, De Melo RR, et al. β-Glucosidase from Aspergillus//Gupta VK, Ed. New and future developments in microbial biotechnology and bioengineering. Amsterdam: Elsevier, 2016: 155-169.
|
| [26] |
Ward OP. Production of recombinant proteins by filamentous fungi. Biotechnol Adv, 2012, 30(5): 1119-1139. DOI:10.1016/j.biotechadv.2011.09.012 |
| [27] |
Lin FJ, Guo XY, Lu WY. Efficient biotransformation of ginsenoside Rb1 to Rd by isolated Aspergillus versicolor, excreting β-glucosidase in the spore production phase of solid culture. Anton Leeuwenhoek, 2015, 108(5): 1117-1127. DOI:10.1007/s10482-015-0565-5 |
| [28] |
Mao KY, Chen DM, Fan YL, et al. Analysis on competitive situation of probiotics industry. Competitive Intelligence, 2018, 14(2): 30-34 (in Chinese). 毛开云, 陈大明, 范月蕾, 等. 益生菌产业竞争态势分析研究. 竞争情报, 2018, 14(2): 30-34. |
| [29] |
Ku S, You HJ, Park MS, et al. Whole-cell biocatalysis for producing ginsenoside Rd from Rb1 using Lactobacillus rhamnosus GG. J Microbiol Biotechnol, 2016, 26(7): 1206-1215. DOI:10.4014/jmb.1601.01002 |
| [30] |
Park SJ, Youn SY, Ji GE, et al. Whole cell biotransformation of major ginsenosides using Leuconostocs and Lactobacilli. Food Sci Biotechnol, 2012, 21(3): 839-844. DOI:10.1007/s10068-012-0108-z |
| [31] |
Huq MA, Akter SK, Kim YJ, et al. Biotransformation of major ginsenoside Rb1 to pharmacologically active ginsenoside Rg3 through fermentation by Weissella hellenica DC06 in newly developed medium. Bangladesh J Sci Indust Res, 2016, 51(4): 271-278. DOI:10.3329/bjsir.v51i4.30445 |
| [32] |
Li L, Shin SY, Lee SJ, et al. Production of ginsenoside F2 by using Lactococcus lactis with enhanced expression of β-glucosidase gene from Paenibacillus mucilaginosus. J Agric Food Chem, 2015, 64(12): 2506-2512. |
| [33] |
Kim YJ, Zhang DB, Yang DC. Biosynthesis and biotechnological production of ginsenosides. Biotechnol Adv, 2015, 33(6): 717-735. DOI:10.1016/j.biotechadv.2015.03.001 |
| [34] |
Yang JL, Hu ZF, Zhang TT, et al. Progress on the studies of the key enzymes of ginsenoside biosynthesis. Molecules, 2018, 23(3): 589. DOI:10.3390/molecules23030589 |
| [35] |
Han JY, Kim HJ, Kwon YS, et al. The Cyt P450 enzyme CYP716A47 catalyzes the formation of protopanaxadiol from dammarenediol-Ⅱ during ginsenoside biosynthesis in Panax ginseng. Plant Cell Physiol, 2011, 52(12): 2062-2073. DOI:10.1093/pcp/pcr150 |
| [36] |
Dai ZB, Liu Y, Zhang XA, et al. Metabolic engineering of Saccharomyces cerevisiae for production of ginsenosides. Metab Eng, 2013, 20: 146-156. DOI:10.1016/j.ymben.2013.10.004 |
| [37] |
Zhao FL, Bai P, Liu T, et al. Optimization of a cytochrome P450 oxidation system for enhancing protopanaxadiol production in Saccharomyces cerevisiae. Biotechnol Bioeng, 2016, 113(8): 1787-1795. DOI:10.1002/bit.v113.8 |
| [38] |
Yan X, Fan Y, Wei W, et al. Production of bioactive ginsenoside compound K in metabolically engineered yeast. Cell Res, 2014, 24(6): 770-773. DOI:10.1038/cr.2014.28 |
| [39] |
Wang PP, Wei YJ, Fan Y, et al. Production of bioactive ginsenosides Rh2 and Rg3 by metabolically engineered yeasts. Metab Eng, 2015, 29: 97-105. DOI:10.1016/j.ymben.2015.03.003 |
| [40] |
Wei W, Wang PP, Wei YJ, et al. Characterization of Panax ginseng UDP-glycosyltransferases catalyzing protopanaxatriol and biosyntheses of bioactive ginsenosides F1 and Rh1 in metabolically engineered yeasts. Mol Plant, 2015, 8(9): 1412-1424. DOI:10.1016/j.molp.2015.05.010 |
| [41] |
Zhuang Y, Yang GY, Chen XH, et al. Biosynthesis of plant-derived ginsenoside Rh2 in yeast via repurposing a key promiscuous microbial enzyme. Metab Eng, 2017, 42: 25-32. DOI:10.1016/j.ymben.2017.04.009 |
| [42] |
Zhao CC, Gao X, Liu XB, et al. Enhancing biosynthesis of a ginsenoside precursor by self-assembly of two key enzymes in Pichia pastoris. J Agric Food Chem, 2016, 64(17): 3380-3385. DOI:10.1021/acs.jafc.6b00650 |
| [43] |
Li DH, Zhang Q, Zhou ZJ, et al. Heterologous biosynthesis of triterpenoid dammarenediol-Ⅱ in engineered Escherichia coli. Biotechnol Lett, 2016, 38(4): 603-609. DOI:10.1007/s10529-015-2032-9 |
| [44] |
Yu L, Chen Y, Shi J, et al. Biosynthesis of rare 20(R)- protopanaxadiol/protopanaxatriol type ginsenosides through Escherichia coli engineered with UDP-glycosyltransferase genes. J Gins Res, 2019, 43: 116-124. DOI:10.1016/j.jgr.2017.09.005 |
| [45] |
Lu J, Yao L, Li JX, et al. Characterization of UDP-glycosyltransferase involved in biosynthesis of ginsenosides Rg1 and Rb1 and identification of critical conserved amino acid residues for its function. J Agric Food Chem, 2018, 66(36): 9446-9945. DOI:10.1021/acs.jafc.8b02544 |
| [46] |
Rakotoarivonina H, Hermant B, Monthe N, et al. The hemicellulolytic enzyme arsenal of Thermobacillus xylanilyticus depends on the composition of biomass used for growth. Microb Cell Fact, 2012, 11(1): 159. DOI:10.1186/1475-2859-11-159 |
| [47] |
Du J, Cui CH, Park SC, et al. Identification and characterization of a ginsenoside-transforming β-glucosidase from Pseudonocardia sp. Gsoil 1536 and its application for enhanced production of minor ginsenoside Rg2 (S). PLoS ONE, 2014, 9(6): e96914. DOI:10.1371/journal.pone.0096914 |
| [48] |
Kim TH, Yang EJ, Shin KC, et al. Enhanced Production of β-D-glycosidase and α-L-arabinofuranosidase in Recombinant Escherichia coli in fed-batch culture for the biotransformation of ginseng leaf extract to ginsenoside compound K. Biotechnol Bioproc Eng, 2018, 23(2): 183-193. DOI:10.1007/s12257-018-0027-9 |
| [49] |
Shin KC, Kim TH, Choi JH, et al. Complete biotransformation of protopanaxadiol-type ginsenosides to 20-O-β-glucopyranosyl-20(S)-protopanaxadiol using a novel and thermostable β-glucosidase. J Agric Food Chem, 2018, 66(11): 2822-2829. DOI:10.1021/acs.jafc.7b06108 |
| [50] |
Xie J, Zhao D, Zhao L, et al. Characterization of a novel arabinose-tolerant α-l-arabinofuranosidase with high ginsenoside Rc to ginsenoside Rd bioconversion productivity. J Appl Microbiol, 2016, 120(3): 647-660. DOI:10.1111/jam.2016.120.issue-3 |
| [51] |
Quan LH, Min JW, Sathiyamoorthy S, et al. Biotransformation of ginsenosides Re and Rg1 into ginsenosides Rg2 and Rh1 by recombinant β-glucosidase. Biotechnol Lett, 2012, 34(5): 913-917. DOI:10.1007/s10529-012-0849-z |
| [52] |
Pandey M, Verma RK, Saraf SA. Nutraceuticals: new era of medicine and health. Asian J Pharm Clin Res, 2010, 3: 11-15. |
| [53] |
Yi Z, He S, Simpson BK. Enzymes in food bioprocessing—novel food enzymes, applications, and related techniques. Curr Opin Food Sci, 2018, 19: 30-35. DOI:10.1016/j.cofs.2017.12.007 |
| [54] |
Youn SY, Park MS, Ji GE. Identification of the beta-glucosidase gene from Bifidobacterium animalis subsp. lactis and its expression in B. bifidum BGN4. J Microbiol Biotechnol, 2012, 22(12): 1714-1723. DOI:10.4014/jmb |
| [55] |
Upadhyaya J, Kim MJ, Kim YH, et al. Enzymatic formation of compound-K from ginsenoside Rb1 by enzyme preparation from cultured mycelia of Armillaria mellea. J Gins Res, 2016, 40(2): 105-112. DOI:10.1016/j.jgr.2015.05.007 |
| [56] |
Wang Y, Choi KD, Yu HS, et al. Production of ginsenoside F1 using commercial enzyme cellulase KN. J Gins Res, 2016, 40(2): 121-126. DOI:10.1016/j.jgr.2015.06.003 |
| [57] |
Zhou W, Huang H, Zhu HY, et al. New metabolites from the biotransformation of ginsenoside Rb1 by Paecilomyces bainier sp. 229 and activities in inducing osteogenic differentiation by Wnt/β-catenin signaling activation. J Gins Res, 2018, 42(2): 199-207. DOI:10.1016/j.jgr.2017.03.004 |
| [58] |
Teng R, Ang C, Mcmanus D, et al. Regioselective acylation of ginsenosides by Novozyme 435 to generate molecular diversity. Helv Chim Acta, 2010, 87(7): 1860-1872. |
| [59] |
Gebhardt S, Bihler S, Schubert-Zsilavecz M, et al. Biocatalytic generation of molecular diversity: modification of ginsenoside Rb1 by β-1, 4-galactosyltransferase and Candida antarctica Lipase, Part 4. Helv Chim Acta, 2015, 85(7): 1943-1959. |
| [60] |
Zhang Q, Zhao WQ, Meng F, et al. Transformation of ginsenoside F1 from ginsenoside Rg1 catalyzed by immobilized β-glycosidase. Chin J Antibiot, 2012, 37(1): 49-55 (in Chinese). 张琪, 赵文倩, 孟飞, 等. 固定化β-葡萄糖苷酶制备人参F1的研究. 中国抗生素杂志, 2012, 37(1): 49-55. DOI:10.3969/j.issn.1001-8689.2012.01.010 |
| [61] |
Yu ZH, Li QY, Cui L, et al. Transformation of rare ginsenoside Compound K from ginsenoside Rb1 catalyzed by snailase immobilization onto microspheres. Chin Tradit Herbal Drugs, 2014, 45(21): 3092-3097 (in Chinese). 于兆慧, 刘其媛, 崔莉, 等. 微球固定化蜗牛酶转化人参皂苷Rb1制备人参稀有皂苷Compound K研究. 中草药, 2014, 45(21): 3092-3097. DOI:10.7501/j.issn.0253-2670.2014.21.011 |
| [62] |
Yuan Y, Luan XN, Rana XK, et al. Covalent immobilization of cellulase in application of biotransformation of ginsenoside Rb1. J Mol Catal B Enzym, 2017, 133(S1): S525-S532. |
| [63] |
Graebin N, Schöffer J, Andrades D, et al. Immobilization of glycoside hydrolase families GH1, GH13, and GH70: state of the art and perspectives. Molecules, 2016, 21(8): 1074. DOI:10.3390/molecules21081074 |
| [64] |
Xue DS, Wang JB, Yao SJ. High production of β-glucosidase from a marine Aspergillus niger immobilized on towel gourd vegetable sponges. Chin Chem Lett, 2015, 26(8): 1011-1015. DOI:10.1016/j.cclet.2015.05.019 |
| [65] |
Yan JL, Pan GX, Li LQ, et al. Adsorption, immobilization, and activity of β-glucosidase on different soil colloids. J Colloid Interface Sci, 2010, 348(2): 565-570. DOI:10.1016/j.jcis.2010.04.044 |
| [66] |
de Alencar Figueira J, Dias FFG, Sato HH, et al. Screening of supports for the immobilization of β-Glucosidase. Enzyme Res, 2011, 2011: 642460. |
| [67] |
Javed MR, Buthe A, Rashid MH, et al. Cost-efficient entrapment of β-glucosidase in nanoscale latex and silicone polymeric thin films for use as stable biocatalysts. Food Chem, 2016, 190: 1078-1085. DOI:10.1016/j.foodchem.2015.06.040 |
| [68] |
Reshmi R, Sugunan S. Improved biochemical characteristics of crosslinked β-glucosidase on nanoporous silica foams. J Mol Catal B Enzym, 2013, 85-86: 111-118. DOI:10.1016/j.molcatb.2012.08.007 |
| [69] |
Mateo C, Palomo JM, Fernandez-Lorente G, et al. Improvement of enzyme activity, stability and selectivity via immobilization techniques. Enzyme Microb Technol, 2007, 40(6): 1451-1463. DOI:10.1016/j.enzmictec.2007.01.018 |
| [70] |
Zhang Y, He SD, Simpson BK. Enzymes in food bioprocessing-novel food enzymes, applications, and related techniques. Curr Opin Food Sci, 2018, 19: 30-35. DOI:10.1016/j.cofs.2017.12.007 |
| [71] |
Mak WS, Tran S, Marcheschi R, et al. Integrative genomic mining for enzyme function to enable engineering of a non-natural biosynthetic pathway. Nat Commun, 2016, 6: 10005. |
| [72] |
Samaei-Daryan S, Goliaei B, Ebrahim-Habibi A. characterization of surface binding sites in glycoside hydrolases: a computational study. J Mol Recognit, 2017, 30(9): e2624. DOI:10.1002/jmr.2624 |
| [73] |
Sainz-Polo MA, Valenzuela SV, González B, et al. Structural analysis of glucuronoxylan-specific Xyn30D and its attached CBM35 domain gives insights into the role of modularity in specificity. J Biol Chem, 2014, 289(45): 31088-31101. DOI:10.1074/jbc.M114.597732 |
| [74] |
Kong XJ, Yuan SG, Li L, et al. Engineering of an epoxide hydrolase for efficient bioresolution of bulky pharmaco substrates. Proc Natl Acad Sci USA, 2014, 111(44): 15717-15722. DOI:10.1073/pnas.1404915111 |
| [75] |
Pang PJ, Cao LC, Liu YH, et al. Structures of a glucose-tolerant β-glucosidase provide insights into its mechanism. J Struct Biol, 2017, 198(3): 154-162. DOI:10.1016/j.jsb.2017.02.001 |
| [76] |
Zhang YL, Zhao Z, Liu HY. Deriving chemically essential interactions based on active site alignments and quantum chemical calculations: a case study on glycoside hydrolases. ACS Catal, 2015, 5(4): 2559-2572. DOI:10.1021/cs501709d |
| [77] |
Yan S, Wei PC, Chen Q, et al. Functional and structural characterization of a β-glucosidase involved in saponin metabolism from intestinal bacteria. Biochem Biophys Res Commun, 2018, 496(4): 1349-1356. DOI:10.1016/j.bbrc.2018.02.018 |
| [78] |
Park SJ, Choi JM, Kyeong HH, et al. Rational design of a β-glycosidase with high regiospecificity for triterpenoid tailoring. ChemBioChem, 2015, 16(5): 854-860. DOI:10.1002/cbic.201500004 |
| [79] |
Choi JH, Shin KC, Oh DK. An L213A variant of β-glycosidase from Sulfolobus solfataricus with increased α-L-arabinofuranosidase activity converts ginsenoside Rc to compound K. PLoS ONE, 2018, 13(1): e0191018. DOI:10.1371/journal.pone.0191018 |
| [80] |
Ribeiro BD, Santos AG, Marrucho IM. CHAPTER 6: Biocatalysis in ionic liquids//Coelho MA, Ribeiro BD, Eds. White Biotechnology for Sustainable Chemistry. Cambridge, UK: Royal Society of Chemistry, 2015.
|
| [81] |
Gao WW, Zhang FX, Zhang GX, et al. Key factors affecting the activity and stability of enzymes in ionic liquids and novel applications in biocatalysis. Biochem Eng J, 2015, 99: 67-84. DOI:10.1016/j.bej.2015.03.005 |
| [82] |
Chen JY, Kaleem I, He DM, et al. Efficient production of glycyrrhetic acid 3-O-mono-β-D- glucuronide by whole-cell biocatalysis in an ionic liquid/buffer biphasic system. Process Biochem, 2012, 47(6): 908-913. DOI:10.1016/j.procbio.2011.10.024 |
| [83] |
Yanhong BI, Wang ZY, Mao YY, et al. Ionic Liquid effects on the activity of β-Glycosidase for the synthesis of salidroside in co-solvent systems. Chin J Catal, 2012, 33(7/8): 1161-1165. |
| [84] |
Ferdjani S, Ionita M, Roy B, et al. Correlation between thermostability and stability of glycosidases in ionic liquid. Biotechnol Lett, 2011, 33(6): 1215-1219. DOI:10.1007/s10529-011-0560-5 |
| [85] |
Brakowski R, Pontius K, Franzreb M. Investigation of the transglycosylation potential of ß-galactosidase from Aspergillus oryzae in the presence of the ionic liquid [Bmim][PF6]. J Mol Cat B Enzym, 2016, 130: 48-57. DOI:10.1016/j.molcatb.2016.05.006 |
| [86] |
Kudou M, Kubota Y, Nakashima N, et al. Improvement of enzymatic activity of β-glucosidase from thermotoga maritima by 1-butyl-3- methylimidazolium acetate. J Mol Cat B Enzym, 2014, 104: 17-22. DOI:10.1016/j.molcatb.2014.02.013 |
| [87] |
Xu WJ, Huang YK, Li F, et al. Improving β-glucosidase biocatalysis with deep eutectic solvents based on choline chloride. Biochem Eng J, 2018, 138: 37-46. DOI:10.1016/j.bej.2018.07.002 |
| [88] |
Zhao H. Methods for stabilizing and activating enzymes in ionic liquids-a review. J Chem Technol Biotech, 2010, 85(7): 891-907. DOI:10.1002/jctb.v85:7 |
| [89] |
Brogan APS, Bui-Le L, Hallett JP. Non-aqueous homogenous biocatalytic conversion of polysaccharides in ionic liquids using chemically modified glucosidase. Nat Chem, 2018, 10(8): 859-865. DOI:10.1038/s41557-018-0088-6 |
| [90] |
Toral AR, De Los Ríos AP, Hernández FJ, et al. Cross-linked Candida antarctica lipase B is active in denaturing ionic liquids. Enzyme Microb Technol, 2007, 40(5): 1095-1099. DOI:10.1016/j.enzmictec.2006.08.027 |
| [91] |
Venardos D, Klei HE, Sundstrom DW. Conversion of cellobiose to glucose using immobilized β-glucosidase reactors. Enzyme Microb Technol, 1980, 2(2): 112-116. DOI:10.1016/0141-0229(80)90065-4 |
| [92] |
Li LJ, Jin YR, Wang XZ, et al. Ionic liquid and aqueous two-phase extraction based on salting-out coupled with high-performance liquid chromatography for the determination of seven rare ginsenosides in Xue-Sai-Tong injection. J Sep Sci, 2015, 38(17): 3055-3062. DOI:10.1002/jssc.v38.17 |
| [93] |
Li FF, Li Q, Wu SG, et al. Salting-out extraction of sinomenine from Sinomenium acutum by an alcohol/salt aqueous two-phase system using ionic liquids as additives. J Chem Technol Biotechnol, 2017, 93(7): 1925-1930. DOI:10.1002/jctb.5318 |
| [94] |
Li LJ, Li XW, Ding J, et al. Ionic Liquid surfactant-mediated ultrasonic-assisted extraction coupled with HPLC for the determination of five rare ginsenosides in Panax notoginseng (Burk.) F.H.Chen. Chem J Chin Univ, 2016, 37(3): 454-459 (in Chinese). 李兰杰, 李绪文, 丁健, 等. 超声辅助结合离子液体双水相提取-高效液相色谱法测定三七中5种稀有人参皂苷的含量. 高等学校化学学报, 2016, 37(3): 454-459. |
 2019, Vol. 35
2019, Vol. 35