中国科学院微生物研究所、中国微生物学会主办
文章信息
- 薛飞燕, 杨明峰, 马兰青
- Xue Feiyan, Yang Mingfeng, Ma Lanqing
- 微生物法合成红景天苷
- Microbial synthesis of salidroside
- 生物工程学报, 2019, 35(7): 1184-1192
- Chinese Journal of Biotechnology, 2019, 35(7): 1184-1192
- 10.13345/j.cjb.180449
-
文章历史
- Received: November 1, 2018
- Accepted: February 27, 2019
红景天苷(Salidroside)亦称红景天甙,其化学结构式为酪醇8-O-β-D-葡萄糖苷(C14H20O7),是以酪醇(4-羟基苯乙醇,Tyrosol,C8H10O2)为苷元的醇羟基与尿苷二磷酸葡萄糖(Uridine diphosphate glucose, UDP-glucose, C15H24N2O17P2)半缩醛羟基脱水后形成的糖苷(图 1)[1-2]。作为红景天属药用植物的主要活性成分,红景天苷被证实具有耐缺氧、抗辐射、抗疲劳、抗肿瘤、降血糖、提高免疫力和记忆力等重要生理功效[1, 3]。随着人们对红景天苷药理作用认识的不断深入,其需求量与日俱增。
最初人们依靠野生红景天属植物提取红景天苷,深受资源有限和含量低的约束[4];后来利用组织培养和细胞悬浮培养等技术克服了野生资源的不足[5-6],可仍然存在生产周期长、产量低等问题;近些年,相关研究者通过不断尝试和比较各种方法,普遍认为微生物法合成红景天苷具有潜在工业化应用价值[7-8]。
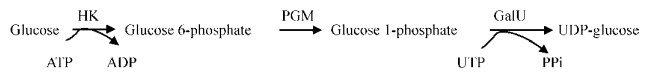
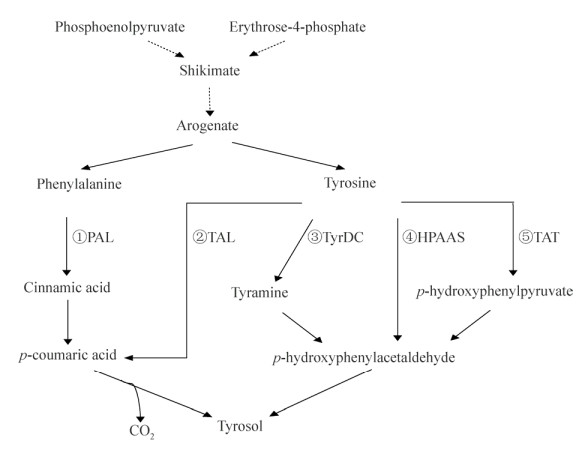
1 红景天苷生物合成途径微生物法合成植物天然产物在实现产业化的进程中面临的挑战之一就是理清其生物合成途径[7]。如图 1所示,红景天苷生物合成途径的最后一步反应已经被证实为尿苷二磷酸葡萄糖基转移酶(Uridine diphosphate glucosyltransferase, UGT)催化底物酪醇与尿苷二磷酸葡萄糖合成红景天苷[6-7]。反应中涉及的尿苷二磷酸葡萄糖(UDP-glucose)其生物合成途径研究较成熟,属于初级代谢范畴,微生物合成UDP-glucose通常选择如图 2的代谢途径,通过弱化竞争途径或过表达关键酶(如PGM和GalU等)保障其供应量[9-11]。因此,关于红景天苷生物合成途径的研究,人们一方面致力于途径中催化反应的关键酶UGT相关的研究:如本课题组率先获得催化活性较高的植物UGT73B6[12]、UGT72B14[2];随后通过调研植物UGT超家族晶体结构,介绍了UGTs的整体结构特点(如保守的PSPG结构单元)以及蛋白与底物相互作用的细节(如口袋结构结合糖基供体)[13];Fan等根据植物UGTs的PGSG结构特征进一步筛选获得高活性微生物UGT[14]。另一方面,研究者还重点关注了底物酪醇(Tyrosol)生成途径,研究表明其来源于莽草酸途径(Shikimate pathway)所生成的阿罗酸(Arogenate),阿罗酸合成酪醇的生物途径因体系差异而有所不同[15-18]:根据植物芳香族天然产物代谢特点和规律,研究者最初认为酪醇来源于苯丙氨酸解氨(PAL)途径(图 3中支路①)和酪氨酸脱羧(TyrDC)途径(图 3中支路③)[18],如Keski-Saaris等和Hu等证明了PAL的活性对红景天苷积累有重要影响[19-20],Landtag等和Lan等研究均表明TyrDC的活性对红景天苷的积累很重要[21-22],本课题组前期工作确认高山红景天Rhodiola sachalinensis植物中其苷元酪醇的主要合成途径为酪氨酸脱羧途径,其中TyrDC为关键酶和限速酶[23];最近,Michael等发现红景天植物中含有一种依赖于磷酸吡哆醛的羟基苯乙醛合酶(HPAAS)可直接催化酪氨酸获得4-羟基苯乙醛然后还原生成酪醇(图 3中支路④)[24];另外,酿酒酵母Saccharomyces cerevisiae以酪氨酸为底物经过转氨脱羧反应合成酪醇机制很早就有报道,符合图 3中支路⑤TAT途径[25],Satoh等则在大肠杆菌Escherichia coli中同时构建了图 3中支路③和⑤实现了酪醇的有效合成[26];按照图 3中支路②TAL途径合成酪醇的案例暂未见报道,但利用TAL催化活性实现由酪氨酸(Tyrosine)合成4-香豆酸/对羟基肉桂酸/对羟基苯丙烯酸(p-coumaric acid)已有研究,如Kim等在阐述类苯乙醇合成现状时介绍了TAL催化合成4-香豆酸进而生产咖啡酸苯乙酯的途径[8];Rodriguez等研究表明在酿酒酵母中超量表达约氏黄杆菌Flavobacterium johnsoniaeu的TAL能够增加4-香豆酸合成量[27];Vannelli等和Vargas-Tah等研究均表明利用粘红酵母Rhodotorula glutinis双功能酶PAL/TAL可以实现对羟基肉桂酸的有效合成[28-29]。为此,本课题组在发现粘红酵母代谢产物中有酪醇组分后已经开展了其酪醇合成途径包括图 3中支路①和②的验证工作,以期更进一步阐述酪醇的生物合成途径,为建立新的微生物法合成红景天苷体系提供基础。
|
| 图 3 酪醇可能的生物合成途径 Fig. 3 Proposed pathways of tyrosol biosynthsis. PAL: phenylalanine ammonia lyase; TAL: tyrosine ammonia lyase; TyrDC: tyrosine decarboxylase; HPAAS: hydroxyphenylacetaldehyde synthase; TAT: tyrosine aminotransferase. The dotted arrow represents the multistep reaction. The solid arrow represents one-step reaction. |
| |
利用野生菌或天然酶合成红景天苷的文献报道中涉及的微生物以真菌为主(表 1)。最初的研究思路是以红景天植物浸出物为培养基成分发酵获得微生物菌体后提取酶液进行体外催化酪醇合成红景天苷:如贾艳萍等利用犁头霉Absidia sp.的粗提酶液催化15 g/L的酪醇合成约1.5 g/L的红景天苷[30];Zhang等利用黑曲霉Aspergillus niger提纯酶催化1.5%的酪醇合成红景天苷可达10%[31];王梦亮等从红景天植物根系土壤中筛选获得微生物菌株米曲霉Aspergillus oryzae能够利用5 g/L的酪醇合成0.7 g/L的红景天苷[32]。后来研究者发现可以利用双菌株共培养(Coculture)的协同效应或微生物细胞融合(Cell fusion)的方式来提高红景天苷的含量:宋伟舟等利用双菌株协同液体发酵红景天使其红景天苷含量提高86.29%[33];冯敏等利用细胞融合双亲菌株固体发酵大花红景天粉末,使其红景天苷含量提高140%[34]。近期有利用植物内生真菌合成红景天苷的报道:如曲霉Aspergillus和镰刀霉Fusarium[35],深色有隔内生真菌Phialocephala fortinii培养7 d后可将红景天苷产量提高到2.339 g/L[36]。
| Microorganisms | Substrate | Time | Yield | Highlights | Reference |
| Absidia sp. | 15 g/L tyrosol | 6 h | ~1.5 g/L | Crude enzyme, enzymic synthesis | [30] |
| Aspergillus niger | 1.5% tyrosol | 6 h | 10% | Purified enzyme, enzymic synthesis |
[31] |
| Aspergillus oryzae | 5 g/L tyrosol | 48 h | 0.7 g/L | Whole cell catalyst | [32] |
| Aspergillus niger-Aspergillus niger |
Rhodiala crenulata | 2–4 d | 0.630 4% | Two strains, coculture | [33] |
| Aspergillus niger | Rhodiala crenulata | 3–4 d | 1.019% | Cell fusion, solid fermentation | [34] |
| Aspergillus, Fusarium | PDB culture medium | 8 d | No data | Endophytic fungus of Rhodiola | [35] |
| Phialocephala fortinii | Czapek-Dox culture medium | 7 d | 2.339 g/L | Endophytic fungus of Rhodiola | [36] |
| PDB: Potato dextrose broth. | |||||
随着基因工程技术的发展、红景天苷生物合成途径的不断明晰和代谢途径中限速酶的逐步明确,利用微生物工程菌或重组酶合成红景天苷的研究取得了突破性进展,特别是以利用模式微生物(如大肠杆菌和酿酒酵母)为宿主的研究发展迅速(表 2)。2011年Yu等报道了3个高山红景天UGTs在大肠杆菌中实现了重组表达,重组酶体外酶促反应均获得了红景天苷产物[2]。2016年,Xue等利用密码子优化(Codon optimization)的办法实现了UGT72B14在大肠杆菌的高效表达,并利用分批-补料(Fed-batch)的策略得到微生物红景天苷产量为6.7 mg/L[37]。2017年,Fan等通过基因挖掘进一步获得高活性的地衣芽胞杆菌UGTBL1并构建了重组大肠杆菌,工程菌全细胞催化24 h后可将红景天苷的产量提高到1.04 g/L[14]。
| Microorganisms | Genes introduced | Substrate and concentration |
Time | Yield | Highlights | Reference |
| Escherichia coli | RsUGT73B6, RsUGT72B14, RsUGT74R1 | Tyrosol, 250 μmol/L | 30 min | No data | Enzymatic reaction |
[2] |
| Escherichia coli | RsUGT72B14 | Tyrosol, 50 mg/L | 9 h | 6.7 mg/L | Fed-batch cultivation, codon optimization |
[37] |
| Escherichia coli | BlUGTBL1 | Tyrosol, 1 g/L | 24 h | 1.04 g/L | Microbial UGT used |
[14] |
| Escherichia coli | ScARO10, RsUGT73B6 | Glucose, 2% | 48 h | 56.9 mg/L | Growth in LB, synthesis in M9Y |
[38] |
| Escherichia coli | PcAAS, AtUGT85A1 | Glucose, 2% | 48 h | 288 mg/L | Growth in LB, synthesis in M9 |
[39] |
| Escherichia coli- Escherichia coli |
PpKDC4, AtUGT85A1, ScARO10 | Glucose, 8 g/L+ Xylose, 2g/L+ |
129 h | 6.03 g/L | Two strains, coculture, fed-batch fermentation |
[40] |
| Saccharomyces cerevisiae |
Rr4HPAAS, RrT8GT | Glucose, 4% | 48 h | 1.5 mg/L | Pathway elucidation, codon optimization |
[24] |
| Saccharomyces cerevisiae |
PcAAS, AtUGT85A1 | Glucose, 20 g/L+ | 168 h | 732.5 mg/L |
Plasmid-free strain, fed-batch fermentation |
[41] |
| Rs: Rhodiola sachalinensis; UGT: Uridine diphosphate dependent glycosyltransferase; Pc: Petroselinum crispum; AAS: Aromatic aldehyde synthase; At: Arabidopsis thaliana; Bl: Bacillus licheniformis; Pp: Pichia pastoris; Sc: Saccharomyces cerevisiae; ARO10: Pyruvate decarboxylase; KDC: Decarboxylase; Rr: Rhodiola rosea; 4HPAAS: 4-hydroxyphenylacetaldehyde synthase; T8GT: Tyrosol:UDP-glucose 8-O-glucosyltransferase; “+”: Substrate was fed during fermentation, but the feeding titer was not reported. | ||||||
为了进一步增强微生物合成红景天苷的应用可行性,人们充分利用基因工程、代谢工程和发酵工程技术发展了红景天苷以葡萄糖为底物的从头合成(de novo)技术。2014年,Bai等通过引入酿酒酵母ARO10和高山红景天UGT73B6等关键酶,采用菌体生长和产物合成在不同培养基分段发酵重组大肠杆菌的策略,以葡萄糖为底物可得红景天苷产量为56.9 mg/L[38]。2017年,Chung等通过引入香芹AAS和拟南芥UGT85A1,以葡萄糖为底物同样采用分段培养重组大肠杆菌可使红景天苷的产量提高到288 mg/L[39]。2018年,Liu等通过引入酿酒酵母ARO10、毕赤酵母KDC4和拟南芥UGT85A1等关键基因构建两株大肠杆菌,采用两株菌的共培养方式和分批-补料的调控策略,以葡萄糖和木糖为底物发酵129 h可以将红景天苷的产量进一步提高到6.03 g/L[40]。
随着大肠杆菌合成红景天苷的技术不断成熟,人们又开始致力于酿酒酵母合成红景天苷体系的研发。Torrens-Spence等通过引入红景天4HPAAS和T8GT,采用密码子优化策略在酿酒酵母中构建了红景天苷合成途径,获得产量为1.5 mg/L[24]。Jiang等利用基因整合技术在酿酒酵母中引入香芹AAS和拟南芥UGT85A1,通过分批-补料的发酵调控策略发酵168 h同样可实现红景天苷的产量为732.5 mg/L[41]。
3 展望红景天苷作为红景天的有效成分,其抗缺氧、抗疲劳、抗衰老、防辐射、增强心血管系统功能及对肿瘤的抑制等功效越来越受到关注。国内外相关知识产权也由初期集中在植物红景天苷提取方法及综合应用[42-43],逐渐延伸至红景天苷相关产品制备方法及针对性应用效果[44-46],并进一步扩展至生物酶催化法[47-49]及微生物发酵法[50-51]合成红景天苷的技术研发。我国植物红景天苷提取及制剂开发技术已经迈向产业发展阶段(如公安部昆明警犬基地公布了利用红景天苷制备工作犬用抗高原反应药物组合物的方法与应用,四川康美保宁制药有限公司公布了一种乙醇快速提取红景天的方法,西安惠博生物科技有限公司公布了固定化酶催化制备红景天苷的方法等)[52-54],而微生物法合成红景天苷目前还处于基础研发阶段,虽然国内在“高活性酶和潜力宿主菌的筛选”、“关键基因和代谢途径改造”、“培养方式和调控模式设计”等方面的研究处于领先地位[36, 40-41],但需要继续攻克高效合成及高效利用等瓶颈技术。
一方面要充分发掘利用更多生物资源如植物基因资源构建类似于图 3中支路②或④的高效途径;或者同时启动多条途径如图 3中支路①和②提高工作效率;或者借鉴UDP-葡萄糖原位再生体系增强糖苷合成思路提高红景天苷合成效率[55]。
另一方面要充分利用现代生物学技术如Liu等利用基因组分析和合成生物学手段构建了酵母高效合成灯盏花素[56],同理可以充分利用基因编辑技术、合成生物学和生物信息学手段增强红景天苷微生物细胞工厂运行效率;或者利用代谢组学和微生物发酵联产技术实现红景天苷和其他活性成分(如络缌及其衍生物[6]、香豆素[16]、羟基酪醇[39]或淫羊藿次苷D2[24, 38, 51]等)联产以提高红景天苷生产和应用效率。
综上,微生物法合成红景天苷已有良好基础,相信通过相关领域研究者深度挖掘丰富的生物资源和充分利用现代生物学技术,能够早日实现产业化。
| [1] | Zhong ZF, Han J, Zhang JZ, et al. Pharmacological activities, mechanisms of action, and safety of salidroside in the central nervous system. Drug Des Devel Ther, 2018, 12: 1479–1489. DOI: 10.2147/DDDT |
| [2] | Yu HS, Ma LQ, Zhang JX, et al. Characterization of glycosyltransferases responsible for salidroside biosynthesis in Rhodiola sachalinensis. Phytochemistry, 2011, 72(9): 862–870. DOI: 10.1016/j.phytochem.2011.03.020 |
| [3] | Dimpfel W, Schombert L, Panossian AG. Assessing the quality and potential efficacy of commercial extracts of Rhodiola rosea L. by analyzing the salidroside and rosavin content and the electrophysiological activity in hippocampal long-term potentiation, a synaptic model of memory. Front Pharmacol, 2018, 9: 425. DOI: 10.3389/fphar.2018.00425 |
| [4] |
Wu XW, Peng YS, Wang RF. Research progress of alternative production approaches of salidroside.
Chin J Chin Mater Med, 2013, 38(21): 3656–3660.
(in Chinese). 吴秀稳, 彭玉帅, 王如峰. 红景天苷的替代生产方法研究概况. 中国中药杂志, 2013, 38(21): 3656-3660. |
| [5] | Wu SX, Zu YG, Wu M. High yield production of salidroside in the suspension culture of Rhodiola sachalinensis. J Biotechnol, 2003, 106(1): 33–43. DOI: 10.1016/j.jbiotec.2003.07.009 |
| [6] | Grech-Baran M, Sykłowska-Baranek K, Pietrosiuk A. Biotechnological approaches to enhance salidroside, rosin and its derivatives production in selected Rhodiola spp.in vitro cultures. Phytochem Rev, 2015, 14(4): 657–674. DOI: 10.1007/s11101-014-9368-y |
| [7] | Liu XN, Ding WT, Jiang HF. Engineering microbial cell factories for the production of plant natural products: from design principles to industrial-scale production. Microb Cell Fact, 2017, 16: 125. DOI: 10.1186/s12934-017-0732-7 |
| [8] | Kim SY, Song MK, Jeon JH, et al. Current status of microbial phenylethanoid biosynthesis. J Microbiol Biotechnol, 2018, 28(8): 1225–1232. DOI: 10.4014/jmb.1805.05021 |
| [9] |
Chen S, Li Y, Liu H, et al. Research advances in biosynthesis of UDPG.
Chin Biotechnol, 2012, 32(9): 125–130.
(in Chinese). 陈圣, 李艳, 刘欢, 等. 生物法合成尿苷二磷酸葡萄糖的研究进展. 中国生物工程杂志, 2012, 32(9): 125-130. |
| [10] | Watanabe D, Zhou Y, Hirata A, et al. Inhibitory role of Greatwall-like protein kinase Rim15p in alcoholic fermentation via upregulating the UDP-glucose synthesis pathway in Saccharomyces cerevisiae. Appl Environ Microbiol, 2016, 82(1): 340–351. DOI: 10.1128/AEM.02977-15 |
| [11] | Padilla L, Morbach S, Krämer R, et al. Impact of heterologous expression of Escherichia coli UDP-glucose pyrophosphorylase on trehalose and glycogen synthesis in Corynebacterium glutamicum. Appl Environ Microbiol, 2004, 70(7): 3845–3854. DOI: 10.1128/AEM.70.7.3845-3854.2004 |
| [12] | Ma LQ, Liu BY, Gao DY, et al. Molecular cloning and overexpression of a novel UDP-glucosyltransferase elevating salidroside levels in Rhodiola sachalinensis. Plant Cell Rep, 2007, 26(7): 989–999. DOI: 10.1007/s00299-007-0317-8 |
| [13] |
Lü HS, Xue FY, Liu CM, et al. Crystal structures of plant uridine diphosphate-dependent glycosyltransferases.
Chin J Biotech, 2014, 30(6): 838–847.
(in Chinese). 吕鹤书, 薛飞燕, 柳春梅, 等. 植物尿苷二磷酸糖基转移酶超家族晶体结构. 生物工程学报, 2014, 30(6): 838-847. |
| [14] | Fan B, Chen TY, Zhang S, et al. Mining of efficient microbial UDP-glycosyltransferases by motif evolution cross plant kingdom for application in biosynthesis of salidroside. Sci Rep, 2017, 7: 463. DOI: 10.1038/s41598-017-00568-z |
| [15] |
Cui JL, Fu SB, Wang ML. Advance in studies on biosynthesis, chemical synthesis and biocatalysis synthesis of salidroside.
Nat Prod Res Dev, 2013, 25(6): 851–840.
(in Chinese). 崔晋龙, 付少彬, 王梦亮. 红景天苷生物、化学和生物催化合成的分子理论及应用. 天然产物研究与开发, 2013, 25(6): 851-840. DOI:10.3969/j.issn.1001-6880.2013.06.028 |
| [16] | Schenck CA, Maeda HA. Tyrosine biosynthesis, metabolism, and catabolism in plants. Phytochemistry, 2018, 149: 82–102. DOI: 10.1016/j.phytochem.2018.02.003 |
| [17] | Shi LL, Wang L, Zhang YX, et al. Approaches to biosynthesis of salidroside and its key metabolic enzymes. Forest Stud China, 2007, 9(4): 295–299. DOI: 10.1007/s11632-007-0047-6 |
| [18] |
Ma LQ, Liu CM, Yu HS, et al. Salidroside biosynthesis pathway: the initial reaction and glycosylation of tyrosol.
Chin J Biotech, 2012, 28(3): 282–294.
(in Chinese). 马兰青, 柳春梅, 于寒松, 等. 红景天甙生物合成途径:酪醇合成的起始反应及其糖基化. 生物工程学报, 2012, 28(3): 282-294. |
| [19] | Keski-Saari S, Falck M, Heinonen J, et al. Phenolics during early development of Betula pubescens seedlings: inhibition of phenylalanine ammonia lyase. Trees, 2007, 21(3): 263–272. DOI: 10.1007/s00468-006-0117-8 |
| [20] | Hu GS, Hur YJ, Jia JM, et al. Effects of 2-aminoindan-2-phosphonic acid treatment on the accumulation of salidroside and four phenylethanoid glycosides in suspension cell culture of Cistanche deserticola. Plant Cell Rep, 2011, 30(4): 665–674. DOI: 10.1007/s00299-010-0997-3 |
| [21] | Landtag J, Baumert A, Degenkolb T, et al. Accumulation of tyrosol glucoside in transgenic potato plants expressing a parsley tyrosine decarboxylase. Phytochemistry, 2002, 60(7): 683–689. DOI: 10.1016/S0031-9422(02)00161-9 |
| [22] | Lan XZ, Chang K, Zeng LJ, et al. Engineering salidroside biosynthetic pathway in hairy root cultures of Rhodiola crenulata based on metabolic characterization of tyrosine decarboxylase. PLoS ONE, 2013, 8(10): e75459. DOI: 10.1371/journal.pone.0075459 |
| [23] | Zhang JX, Ma LQ, Yu HS, et al. A tyrosine decarboxylase catalyzes the initial reaction of the salidroside biosynthesis pathway in Rhodiola sachalinensis. Plant Cell Rep, 2011, 30(8): 1443–1453. DOI: 10.1007/s00299-011-1053-7 |
| [24] | Torrens-Spence MP, Pluskal T, Li FS, et al. Complete pathway elucidation and heterologous reconstitution of Rhodiola salidroside biosynthesis. Mol Plant, 2018, 11(1): 205–217. DOI: 10.1016/j.molp.2017.12.007 |
| [25] | Sentheshanmuganathan S, Elsden SR. The mechanism of the formation of tyrosol by Saccharomyces cerevisiae. Biochem J, 1958, 69(2): 210–218. DOI: 10.1042/bj0690210 |
| [26] | Satoh Y, Tajima K, Munekata M, et al. Engineering of a tyrosol-producing pathway, utilizing simple sugar and the central metabolic tyrosine, in Escherichia coli. J Agric Food Chem, 2012, 60(4): 979–984. DOI: 10.1021/jf203256f |
| [27] | Rodriguez A, Kildegaard KR, Li MJ, et al. Establishment of a yeast platform strain for production of p-coumaric acid through metabolic engineering of aromatic amino acid biosynthesis. Metab Eng, 2015, 31: 181–188. DOI: 10.1016/j.ymben.2015.08.003 |
| [28] | Vannelli T, Qi WW, Sweigard J, et al. Production of p-hydroxycinnamic acid from glucose in Saccharomyces cerevisiae and Escherichia coli by expression of heterologous genes from plants and fungi. Metab Eng, 2007, 9(2): 142–151. DOI: 10.1016/j.ymben.2006.11.001 |
| [29] | Vargas-Tah A, Martínez LM, Hernández-Chávez G, et al. Production of cinnamic and p-hydroxycinnamic acid from sugar mixtures with engineered Escherichia coli. Microb Cell Fact, 2015, 14: 6. DOI: 10.1186/s12934-014-0185-1 |
| [30] |
Jia YP, Guo HY, Zhang CZ, et al. Optimal condition of enzyme reaction for salidroside synthesis.
J Dalian Inst Light Ind, 2004, 23(2): 97–99.
(in Chinese). 贾艳萍, 郭宏艳, 张春枝, 等. 微生物酶合成红景天苷的条件优化. 大连轻工业学院学报, 2004, 23(2): 97-99. DOI:10.3969/j.issn.1674-1404.2004.02.006 |
| [31] | Zhang CZ, Yu HS, Lu MC, et al. Enzymic synthesis of salidroside: purification and characterization of salidrosidase from Aspergillas niger. Proc Biochem, 2005, 40(9): 3143–3147. DOI: 10.1016/j.procbio.2005.03.043 |
| [32] |
Wang ML, Zhang F, Liu DS. Preliminary study on synthesis of salidroside through glucosylation of D-glucose and tyrosol catalyzed by microorganism.
Chin J Catalysis, 2006, 27(3): 233–236.
(in Chinese). 王梦亮, 张芳, 刘滇生. 微生物催化D-葡萄糖与酪醇葡糖基转移合成红景天甙的初步研究. 催化学报, 2006, 27(3): 233-236. DOI:10.3321/j.issn:0253-9837.2006.03.009 |
| [33] |
Song WZ, Sun JF, Liu YY, et al. Raising the yield of salidroside and tyrosol by double strains syngistic fermentation.
Lishizhen Med Matr Res, 2010, 21(1): 156–159.
(in Chinese). 宋伟舟, 孙剑峰, 刘玉应, 等. 双菌株协同发酵提高红景天中苷和酪醇含量的研究. 时珍国医国药, 2010, 21(1): 156-159. DOI:10.3969/j.issn.1008-0805.2010.01.077 |
| [34] |
Feng M, Jiang C, Gao XH, et al. Construction of strain with high salidroside production by cell fusion and optimization of its solid-state fermentation.
Lishizhen Med Mater Res, 2010, 21(11): 2868–2870.
(in Chinese). 冯敏, 蒋春, 高雪华, 等. 细胞融合构建红景天苷高转化菌株及固态发酵工艺优化. 时珍国医国药, 2010, 21(11): 2868-2870. DOI:10.3969/j.issn.1008-0805.2010.11.070 |
| [35] |
Wu XM, Ren WM, Yang XD, et al. Isolation of endophytic fungi from Rhodiola sachalinensis and screening of salidroside producing-strains.
Lishizhen Med Mater Res, 2014, 25(11): 2769–2772.
(in Chinese). 吴晓民, 任谓明, 杨信东, 等. 高山红景天内生真菌的分离及产红景天苷菌株的筛选. 时珍国医国药, 2014, 25(11): 2769-2772. |
| [36] | Cui JL, Guo TT, Chao JB, et al. Potential of the endophytic fungus Phialocephala fortinii Rac56 found in Rhodiola plants to produce salidroside and p-tyrosol. Molecules, 2016, 21(4): 502. DOI: 10.3390/molecules21040502 |
| [37] | Xue FY, Guo HL, Hu YY, et al. Expression of codon-optimized plant glycosyltransferase UGT72B14 in Escherichia coli enhances salidroside production. Biomed Res Int, 2016, 2016: 9845927. |
| [38] | Bai YF, Bi HP, Zhuang YB, et al. Production of salidroside in metabolically engineered Escherichia coli. Sci Rep, 2014, 4: 6640. |
| [39] | Chung D, Kim SY, Ahn JH. Production of three phenylethanoids, tyrosol, hydroxytyrosol, and salidroside, using plant genes expressing in Escherichia coli. Sci Rep, 2017, 7: 2578. DOI: 10.1038/s41598-017-02042-2 |
| [40] | Liu X, Li XB, Jiang JL, et al. Convergent engineering of syntrophic Escherichia coli coculture for efficient production of glycosides. Metab Eng, 2018, 47: 243–253. DOI: 10.1016/j.ymben.2018.03.016 |
| [41] | Jiang JJ, Yin H, Wang S, et al. Metabolic engineering of Saccharomyces cerevisiae for high-level production of salidroside from glucose. J Agric Food Chem, 2018, 66(17): 4431–4438. DOI: 10.1021/acs.jafc.8b01272 |
| [42] | Xiu RL. Rhodiola and uses thereof: US, 20020127285A1. 2002-09-12. |
| [43] |
Shi LF, Cai Q, Yao B. Rhodiola sacra glycoside derivative and its preparation method and use: CN, 1235905C. 2006-01-11 (in Chinese). 石力夫, 蔡溱, 姚斌.红景天苷衍生物及其制备方法和用途: 中国CN, 1235905C. 2006-01-11. |
| [44] | Gottfried K. Composition and method for treating patients with high neurotransmitter levels: US, 20070292536A1. 2007-12-20. |
| [45] | Coleman HD, Sudol RN, Sapone WJ. Dietary supplement for promoting removal of heavy metals from the body: US, 20090011048A1. 2009-01-08. |
| [46] | Nancy YY, Fanny CF, Fu GM, et al. Rhodiola rosea extracts and isolated compounds and uses thereof for treating neurodegenerative diseases: HK, WO2013/020368A1. 2013-02-14. |
| [47] |
Wang YN, Ma LQ, Shi GL, et al. Method for preparing salidroside by utilizing UGT72B14: CN, 101914120B. 2013-02-06 (in Chinese). 王有年, 马兰青, 师光禄, 等.一种利用UGT72B14制备红景天甙的方法: 中国, 101914120B. 2013-02-06. |
| [48] |
Ma LQ, Shi GL, Wang YN, et al. Method for preparing salidroside by utilizing RsTyrDC: CN, 101914121B. 2013-02-06 (in Chinese). 马兰青, 师光禄, 王有年, 等.利用RsTyrDC制备红景天甙的方法: 中国, 101914121B. 2013-02-06. |
| [49] |
Zhang ZS. Abalone viscera[beta]-glucosidase and method for synthesizing rhodioloside thereby: CN, 104401013B. 2017-04-12 (in Chinese). 张志山.一种鲍鱼内脏β-葡萄糖苷酶及其用于合成红景天苷的方法: 中国, 104401013B. 2017-04-12. |
| [50] |
Cui JL, Wang ML. Method for producing salidroside and tyrosol employing phialocephala fortinii: CN, 104388497B. 2017-04-26 (in Chinese). 崔晋龙, 王梦亮.利用红松头霉菌生产红景天苷和酪醇的方法: 中国, 104388497B. 2017-04-26. |
| [51] |
Bi HP, Bai YF, Zhuang YB, et al. Escherichia coli expression strain for high production of tyrosol and/or salidroside and icarisid D2 and application of Escherichia coli expression strain: CN, 104946575B. 2017-11-14 (in Chinese). 毕慧萍, 白艳芬, 庄以彬, 等.一种高产酪醇和/或红景天苷和淫羊藿次苷D2的大肠杆菌表达菌株及其应用: 中国, 104946575B. 2017-11-14. |
| [52] |
Chen FL, Chen C, Li J, et al. Preparation and application of anti-altitude reaction drugs for working dogs: CN, 109091475A. 2018-12-28 (in Chinese). 陈方良, 陈超, 李静, 等.工作犬用抗高原反应药物组合物及其制备方法与应用: 中国, 109091475A. 2018-12-28. |
| [53] |
Xu DJ, Chi HC, Feng ZW, et al. A method of Rhodiola extraction: CN, 109232680A. 2019-01-18 (in Chinese). 许冬瑾, 迟慧春, 冯志伟, 等.一种红景天的提取方法: 中国, 109232680A. 2019-01-18. |
| [54] |
Zhao CH, Gui JC, Liu MD, et al. A method of salidroside preparation: CN, 109280681A. 2019-01-29 (in Chinese). 赵彩红, 桂计成, 刘毛东, 等.一种红景天苷的制备方法: 中国, 109280681A. 2019-01-29. |
| [55] | Huang FC, Hinkelmann J, Hermenau A, et al. Enhanced production of β-glucosides by in-situ UDP-glucose regeneration. J Biotechnol, 2016, 224: 35–44. DOI: 10.1016/j.jbiotec.2016.02.022 |
| [56] | Liu XN, Cheng J, Zhang GH, et al. Engineering yeast for the production of breviscapine by genomic analysis and synthetic biology approaches. Nat Commun, 2018, 9: 448. DOI: 10.1038/s41467-018-02883-z |
 2019, Vol. 35
2019, Vol. 35