中国科学院微生物研究所、中国微生物学会主办
文章信息
- 刘凡铭, 王琪, 钱昱臻, 张叶军, 张炳强, 李洪艳, 邹伟
- Liu Fanming, Wang Qi, Qian Yuzhen, Zhang Yejun, Zhang Bingqiang, Li Hongyan, Zou Wei
- 腺苷酸活化蛋白激酶在糖脂代谢调控中的研究进展
- Research progress of Adenosine 5ʹ-monophosphate-activated protein kinase in the regulation of glycolipid metabolism
- 生物工程学报, 2019, 35(6): 1021-1028
- Chinese Journal of Biotechnology, 2019, 35(6): 1021-1028
- 10.13345/j.cjb.180529
-
文章历史
- Received: December 21, 2018
- Accepted: March 25, 2019
- Published: April 9, 2019
2. 辽宁省生物技术与分子药物研发重点实验室,辽宁 大连 116081;
3. 卫生部细胞移植重点实验室临床中心,山东 青岛 266000
2. Liaoning Key Laboratory of Biotechnology and Molecular Drug Development, Dalian 116081, Liaoning, China;
3. Key Laboratory of Cell Transplantation, Ministry of Health, Qingdao 266000, Shandong, China
国际糖尿病联盟(IDF)发布的数据显示,2010年全球约2.85亿人患有糖尿病。如果以当前速度增长而不加以控制的话,预计到2040年糖尿病患病总数将超过6.42亿,其中Ⅱ型糖尿病约占其中的90%[1]。目前我国已有超过9 000万的糖尿病患者,是全球糖尿病第一大国。值得人们关注的是,糖尿病的发病年龄越来越趋向年轻化,其引发的长期糖、脂等代谢紊乱会导致机体多器官损伤,进而产生人脑[2]、肾[3]等器官病变,具有很高的致残率和致死率。
腺苷酸活化蛋白激酶(Adenosine 5ʹ- monophosphate (AMP)-activated protein kinase,AMPK)是生物能量代谢调节的关键分子。研究表明,AMPK是机体保持葡萄糖平衡所必需的,其激活能改善由Ⅱ型糖尿病引起的代谢失衡;体内脂肪组织含量的变化与胰岛素敏感性关系密切,而AMPK能通过多种途径调控血脂代谢。因此,AMPK有望作为Ⅱ型糖尿病及肥胖症等疾病的潜在治疗靶标。
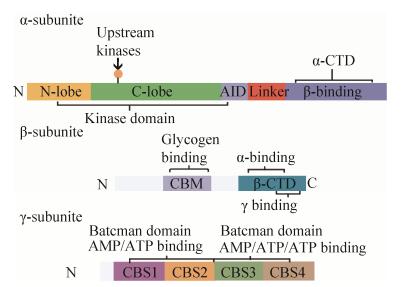
1 AMPK的结构与活性AMPK是由α、β和γ三个亚基按1︰1︰1比例组成的异源三聚体复合物,其中α亚基(63 kDa)是催化亚基,β (30 kDa)和γ (38-63 kDa)为调节亚基[4]。脯乳动物AMPK结构解析图如图 1所示。
|
| 图 1 哺乳动物AMPK结构解析图 Fig. 1 Domain map of typical mammalian AMPK. |
| |
AMPK α亚基肽链N端具有保守的丝氨酸/苏氨酸激酶结构域,肽链中段是激酶的自抑制区(Auto-inhibitory domain),而肽链C端包含与β和γ亚基结合的结构域[4]。
AMPK β亚基对异源三聚体的组装和AMPK复合物的细胞定位起着非常重要的作用。AMPK β亚基有两个特殊的结构域,分别是C端锚定α和γ亚基的结构域(Tethering domain),以及肽链中部与糖原结合的结构域(Glycogen binding domain, GBD)[4]。生理水平上,已有研究发现β亚基在AMPK活性调节中具有重要作用。一方面,β亚基豆蔻酰化是AMPK被上游激酶磷酸化所必需的[5]。另一方面,肌肉特异性β1和β2亚基双敲除的小鼠,由于不能形成AMPK三聚体,而彻底丧失了AMPK的活性;这些小鼠肌肉中生理性收缩引起的葡萄糖摄取作用显著减弱,小鼠表现出运动能力障碍[6]。
每个γ亚基具有4个由胱硫醚-β-合酶(Cystathionine-β-synthase,CBS)组成的结构高度保守的串联重复序列(这里简称为CBS1–4),每两个CBS组成一个贝特曼结构域(Bateman domains)。早期研究发现,AMPK γ3的225位精氨酸突变为谷氨酰胺(R225Q)可引起猪骨骼肌中糖原含量显著增加,原因是突变导致γ3亚基的CBS1不能与AMP结合从而阻碍了AMPK的激活[7]。研究发现AMP和ATP可与细菌表达的γ2亚基CBS区结合,参与AMPK的变构调节;而CBS区确实是AMPK复合物与AMP和ATP等腺嘌呤核苷酸结合的结构基础[8]。
组成AMPK的α、β、γ三个亚基各自具有不同亚型,包括α1、α2、β1、β2、γ1、γ2、γ3;这些亚型有的是由不同的基因编码,有的含有不同的剪接体(Splice variants);因此理论上来讲,AMPK三聚体复合物至少存在12种不同的结构。通过AMPK不同亚基间的组合,使得AMPK复合物的细胞定位和信号转导机制不尽相同,从而赋予了其不同的特性和功能[9]。
2 AMPK在糖代谢调控中的作用 2.1 AMPK在肝脏糖异生中的作用Ⅱ型糖尿病是机体内葡萄糖产生和摄取利用发生不平衡所导致的。肝脏是机体产生葡萄糖的主要场所,也是控制血糖稳态的重要器官。肝糖生成增加是空腹血糖升高主要原因[10]。糖异生是体内将丙酮酸、乳酸、甘油和生糖氨基酸等非糖物质转变为葡萄糖的代谢过程。在肝脏中,AMPK通过调节多个下游效应因子,抑制糖异生作用而控制肝糖产生。研究报道,给普通小鼠或肥胖的胰岛素抵抗小鼠静脉灌注AMPK激活剂AICAR (5-Aminoimidazole-4-carboxamide-1-β-D-ribofuranoside),都可以抑制肝糖的生成[11]。最近Hughey等对组织特异性α1/α2 KO小鼠的研究为AMPK和糖原的功能性相互作用提供了支持。具有肝脏特异性AMPKα1/α2的KO小鼠由于糖原分解减少导致肝葡萄糖输出减少,维持正常运动的能力受损[12]。具体表现为,在禁食和运动后,KO小鼠的肝糖原含量降低。当受到长期快速攻击时,这些小鼠的肝脏糖原分解减少,并且在没有AMPK活性的情况下无法维持肝脏ATP浓度,这支持了AMPK在肝脏中作为能量传感器的作用[13]。近年来有研究发现,AMPK磷酸化并抑制一种转录辅助激活因子TORC2 (Transducer of regulated CREB activity 2),使其停留在胞浆,不能转位到细胞核与环磷腺苷效应元件结合蛋白CREB (cAMP-response element binding protein)共同作用而转录表达过氧化物酶体增殖物激活受体γ (Peroxisome proliferator activated receptorγ, PPARγ)辅助激活因子-1α (PPARγcoactivator-1α,PGC1α),阻碍了PGC1α对磷酸烯醇丙酮酸羧激酶(Phosphoenolpyruvate carboxykinase,PEPCK)和葡萄糖-6-磷酸酶(Glucose-6-phosphatase,G6Pase)的转录,最终导致肝糖异生受到抑制[14]。脂肪细胞因子脂联素可以抑制糖异生[15]。脂联素能够激活AMPK,抑制G6Pase的活性,从而减少肝糖输出,起到降糖的功能[16]。本实验室前期研究发现,小檗碱(Berberine,BBR)的降糖作用与AMPK密切相关,BBR通过激活AMPK抑制细胞肝糖异生。我们选用小鼠原代肝实质细胞为研究对象,RNAi技术抑制AMPKα表达后,BBR抑制小鼠肝癌细胞糖生成的作用减弱(结果尚未发表)。这些实验证明,肝脏中AMPK通路能抑制糖异生,减少肝糖生成而降低血糖。
2.2 AMPK在骨骼肌糖代谢调控中的作用骨骼肌是机体内葡萄糖摄取和支配的主要场所,也是除肝脏外另一个维持血糖稳定的重要器官[17]。人们发现,体育锻炼时骨骼肌中的AMPK会被激活,从而增加了葡萄糖的摄取。这时人们提出用AMPK激活剂来治疗Ⅱ型糖尿病(T2DM)[18]。AMPK激活后,会刺激葡萄糖转运蛋白4型(GLUT4)易位至质膜,从而主动促进骨骼肌中葡萄糖的摄取增加,从而通过糖酵解产生ATP[19]。
Treebak等认为GLUT4从骨骼肌向质膜的易位是通过TBC1D1的磷酸化介导的[20],TBC1D1的磷酸化增加Rab家族G蛋白的活性并诱导GLUT4囊泡与质膜的融合。激活肌肉中的AMPK,增加葡萄糖摄取,弥补受损的胰岛素通路,为Ⅱ型糖尿病药物的研发提供了新思路。
以往的研究表明,胰岛素激活蛋白激酶B (Protein kinase B,Akt/PKB)信号通路的下游蛋白TBC1D4 (TBC (Tre-2/Bub2/Cdc16) 1 domain family member 4)能使GLUT4转位到细胞膜,增加肌肉对葡萄糖的摄取利用[21]。TBC1D4属于Rab-GTPase蛋白家族,可以发挥其GTP酶活性使Rab处于GDP结合状态,从而阻止GLUT4由细胞内囊泡向细胞膜转位。而TBC1D4特异位点磷酸化后失去GTP酶活性,GLUT4囊泡因此转位到细胞膜上发挥葡萄糖转运功能[22]。另外,TBC1D4被胰岛素介导的Akt磷酸化后与14-3-3蛋白结合,进一步帮助了GLUT4的释放与转位[23]。表达TBC1D4位点T649A突变的转基因小鼠表现出胰岛素敏感性下降和糖耐量异常,其分离的肌肉中,胰岛素刺激的糖摄取功能受损[24]。
迄今为止,AMPK通路调控葡萄糖转运的机理还没有完全搞清。研究显示,TBC1D1同样属于Rab-GTPase蛋白家族,与TBC1D4序列具有高度的同源性,但TBC1D4具有更多的Akt磷酸化位点,这提示我们胰岛素通路主要通过TBC1D4发挥糖转运作用。然而,TBC1D1有一个不存在于TBC1D4的重要的AMPK磷酸化位点Ser237;AMPK能诱导人TBC1D1上的Ser237磷酸化,以及TBX1D1与14-3-3结合。为了进一步证明AMPK的作用,Christian等敲除了小鼠AMPKα2基因,发现小鼠肌肉中的TBC1D1含量降低,Ser237磷酸化水平以及14-3-3蛋白结合能力均显著降低[25]。还有研究发现,在小鼠中把TBC1D1蛋白的4个位点突变(包括Ser231,相当于人TBC1D1的Ser237位点),则肌肉收缩引起的糖摄取减少[26]。这些实验说明,TBC1D1的Ser237位点磷酸化,可能对AMPK增加肌肉糖摄取具有重要作用;但目前还没有TBC1D1突变或敲除小鼠的研究证实,AMPK仅依赖于TBC1D1起糖摄取作用。有研究表明,除了GLUT4,AMPK也可以增加GLUT1对葡萄糖的摄取,机制与提高细胞膜上GLUT1的活性有关[27]。
AMPK通路是不是收缩引起的肌肉糖摄取所必需的通路?学界对这一问题存在过争议。AMPK α2敲除的小鼠AICAR介导的糖摄取被抑制,但收缩引起的糖摄取却完全正常;而AMPK α1敲除的小鼠,二者都没有受到影响[28]。但还有研究显示,LKB1敲除的小鼠中,肌肉收缩不能引起AMPK α1和α2的激活,且AICAR和收缩都不能增加其肌肉的糖摄取[29]。因此,人们猜测可能是AMPK α2敲除的小鼠中AMPK α1的表达代偿性增加,造成了研究结果的差异[30]。最新研究发现,肌肉特异性AMPK β1和β2亚基双敲除的小鼠中,不能检测到AMPK的活性,其肌肉收缩引起的糖摄取比正常小鼠明显减少;同时这些小鼠表现出奔跑速度和耐力下降等运动障碍[6]。这一研究成果有力地证明AMPK通路确是介导肌肉收缩引起糖摄取的主要信号通路。
3 AMPK在血脂代谢调控中的作用血脂异常是导致糖尿病和胰岛素抵抗患者发生心血管疾病的重要危险因素。血脂异常通常表现为,血液中能引起动脉粥样硬化的脂类(如胆固醇)和脂蛋白(如低密度脂蛋白)含量异常增高[31]。AMPK可通过多个途径调控血脂水平。首先,AMPK在肝脏中抑制胆固醇和脂肪酸的合成。AMPK可磷酸化并抑制这两个反应限速酶羟甲基戊二酸单酰辅酶A合成酶(3-hydroxy-3- methylglutaryl-coenzyme A reductase, HMG-CoA)和乙酰辅酶A羧激酶(Acetyl CoA carboxylase, ACC)的活性[32]。而长期的AMPK激活还可以通过抑制胆固醇调节元件结合蛋白1 (Sterol regulatory element-binding protein-1c,SREBP1)的表达,降低其转录活性进而下调抑制脂肪酸合酶(Fatty acid synthase,FAS)、丙酮酸激酶(Pyruvate kinase, PK)、HMG-CoA和ACC等脂质生成相关基因表达[33]。AMPK抑制ACC间接增加了脂肪酸的氧化,机制与ACC产物丙二酰辅酶A (Malonyl-CoA)的减少有关。Malonyl-CoA是肉毒碱棕榈酰转移酶1 (Carnitine palmitoyltransferase 1,CPT1)的抑制剂,malonyl-CoA浓度减少使CPT1活性增加,并将胞浆的长链脂肪酸通过脂酰肉毒碱穿梭机制转移进入线粒体,从而进行β氧化[34]。另外,AMPK也可以直接激活肝脏中丙二酰辅酶A脱羧酶(Malonyl-CoA decarboxylase,MCD)而进一步减少malonyl-CoA的水平[35]。
除了抑制脂肪酸的生成,AMPK也抑制甘油三酯的生成。小鼠全身和肝脏特异性敲除AMPK α2后,血液中甘油三酯水平异常升高。而给予AICAR、metformin和A-769662可以降低普通和肥胖小鼠的甘油三酯水平;同时,这些小鼠血液中的β-羟基丁酸含量增加,提示肝脏内的脂肪氧化加强[36]。与此一致,在肝脏中特异性敲除AMPK α2则小鼠的甘油三酯含量增加,脂肪生成加强[37]。机制上,AMPK抑制ChREBP (Carbohydrate response element-binding protein)转录活性进而减少糖类物质转化为脂肪,同时,AMPK还可以直接磷酸化ChREBP而降低其与DNA的结合能力[38]。另外,AMPK还可以通过磷酸化抑制肝脏线粒体的甘油三磷酸酰基转移酶(Glycerol-3- phosphate acyltransferase,GPAT)而减少甘油三酯的体内合成[39]。上述研究说明,AMPK通过减少脂肪(脂肪酸和甘油酯类)生成,促进脂肪氧化而调控肝脏的脂肪沉积,改善Ⅱ型糖尿病患者的血脂状况。
体内脂肪组织含量的变化与胰岛素敏感性关系密切。脂肪总量是脂肪组织内脂肪生成和脂肪水解两个过程的动态平衡,AMPK同时参与这两个过程的调控。上述研究表明,一方面,AMPK抑制白色脂肪组织中脂肪生成相关基因的表达,导致脂肪酸及甘油三酯合成减少;另一方面,AMPK调控脂肪水解过程,但作用机制还存在争议。研究表明,脂肪组织甘油三酯脂肪酶(Adipose triglyceride lipase,ATGL) Ser406可被AMPK磷酸化激活,引起脂肪细胞和动物体内脂肪水解的增加,而AMPK对脂肪水解酶(Hormone-sensitive lipase,HSL)的活性却起负调控作用[40]。以往的研究显示,儿茶酚胺与β肾上腺素受体结合后,通过G蛋白提高cAMP水平并激活PKA,引起脂肪水解。而在白色脂肪组织中,AICAR抑制β肾上腺素诱导的脂肪水解[41]。水解过程中HSL的Ser563、Ser659和Ser660被PKA磷酸化而激活[42];而AMPK可以通过磷酸化Ser565降低HSL的活性[43]。另有研究发现,PKA可以磷酸化AMPKα1的Ser173使其Thr172不能磷酸化从而抑制AMPK的激活,促进脂肪水解,因此,AMPK在脂肪水解过程中起负调控作用[44]。
上述研究表明,AMPK在脂肪水解的不同阶段似乎起着相反的调控作用。Ahmadian等认为,AMPK促进外周组织对脂肪酸的氧化作用,因此在脂肪动员过程中,AMPK起促进作用更合乎情理,这样才能为其他组织提供足够的游离脂肪酸[40]。但也有研究表明,AMPK激活会限制脂肪动员过程。AMPK在一定程度抑制HSL,保证了脂肪水解释放脂肪酸的速率不会超过骨骼肌、肝脏和心脏等组织摄取利用的能力和脂肪组织自身氧化的需要;否则血液中过多的游离脂肪酸(FFA)将给骨骼肌和肝脏等造成负担,脂肪组织也将浪费能量进行游离脂肪酸的酯化反应[45]。AMPK在脂肪动员中的作用尚需进一步研究予以证实。
4 展望许多研究已经证明,调控AMPK的激活可以抑制炎症过程,也可以改善由Ⅱ型糖尿病引起的代谢失衡,其机制部分是与其调节FA代谢(FAO↑/FAS↓)有关,为治疗这些疾病提供了一个策略[46]。但由于AMPK激活和调控的分子机制十分复杂,使用药物分子激活AMP治疗疾病是一个巨大的挑战。AMPK一方面可以通过调节FA代谢来抑制炎症,从而有益于预防糖尿病和癌症,另一方面,也可以通过调节肿瘤微环境的FA代谢诱导癌细胞存活的代谢适应从而促进肿瘤发生和发展。因此,AMPK活化可能是用于预防糖尿病和癌症的有前景的策略,而AMPK抑制是治疗已发生的癌症新型治疗策略。
由于体内AMP/ATP比例的升高能激活AMPK,任何通过干扰ATP合成来扰乱能量平衡的代谢压力都会激活AMPK。因此,AMPK活化在预防糖尿病和癌症中是具有前景的策略,AMPK有望成为Ⅱ型糖尿病、肥胖症和癌症的潜在治疗靶标。
| [1] | Reusch JEB, Manson JE. Management of type 2 diabetes in 2017: getting to goal. JAMA, 2017, 317(10): 1015–1016. DOI: 10.1001/jama.2017.0241 |
| [2] |
Chen DD. Oxovanadium complex intervention on learning and memory damage of diabetic mice and Caveolin-1 expression[D]. Dalian: Liaoning Normal University, 2009 (in Chinese). 陈冬冬.新型钒氧配合物对糖尿病小鼠学习记忆功能损伤的干预及与Caveolin-1表达的关系[D].大连: 辽宁师范大学, 2009. http://www.wanfangdata.com.cn/details/detail.do?_type=degree&id=Y1600733 |
| [3] | Liu Y, Chen DD, Xing YH, et al. A new oxovanadium complex enhances renal function by improving insulin signaling pathway in diabetic mice. J Diabetes Its Complicati, 2014, 28(3): 265–272. DOI: 10.1016/j.jdiacomp.2014.02.001 |
| [4] | Wang SB, Song P, Zou MH. AMP-activated protein kinase, stress responses and cardiovascular diseases. Clin Sci (Lond), 2012, 122(12): 555–573. DOI: 10.1042/CS20110625 |
| [5] | Ali N, Ling NM, Krishnamurthy S, et al. β-subunit myristoylation functions as an energy sensor by modulating the dynamics of AMP-activated protein kinase. Sci Rep, 2016, 6: 39417. DOI: 10.1038/srep39417 |
| [6] | O'Neill HM, Maarbjerg SJ, Crane JD, et al. AMP-activated protein kinase (AMPK) β1β2 muscle null mice reveal an essential role for AMPK in maintaining mitochondrial content and glucose uptake during exercise. Proc Natl Acad Sci USA, 2011, 108(38): 16092–16097. DOI: 10.1073/pnas.1105062108 |
| [7] | Granlund A, Jensen-Waern M, Essén-Gustavsson B. The influence of the PRKAG3 mutation on glycogen, enzyme activities and fibre types in different skeletal muscles of exercise trained pigs. Acta Vet Scand, 2011, 53: 20. DOI: 10.1186/1751-0147-53-20 |
| [8] | Hardie DG. AMP-activated protein kinase—an energy sensor that regulates all aspects of cell function. Genes Dev, 2011, 25(18): 1895–1908. DOI: 10.1101/gad.17420111 |
| [9] | Marín-Aguilar F, Pavillard LE, Giampieri F, et al. Adenosine monophosphate (AMP)-activated protein kinase: a new target for nutraceutical compounds. Int J Mol Sci, 2017, 18(2): 288. DOI: 10.3390/ijms18020288 |
| [10] | Saltiel AR, Kahn CR. Insulin signalling and the regulation of glucose and lipid metabolism. Nature, 2001, 414(6865): 799–806. DOI: 10.1038/414799a |
| [11] | Abdelazim A, Khater S, Ali H, et al. Panax ginseng improves glucose metabolism in streptozotocin-induced diabetic rats through 5ʹ Adenosine monophosphate kinase up-regulation. Saudi J Biol Sci, 2018. DOI: 10.1016/j.sjbs.2018.06.001 |
| [12] | Hughey CC, James FD, Bracy DP, et al. Loss of hepatic AMP-activated protein kinase impedes the rate of glycogenolysis but not gluconeogenic fluxes in exercising mice. J Biol Chem, 2017, 292(49): 20215–20140. |
| [13] | Hasenour CM, Ridley DE, James FD, et al. Liver AMP-activated protein kinase is unnecessary for gluconeogenesis but protects energy state during nutrient deprivation. PLoS ONE, 2017, 12(1). |
| [14] | Sato M, Dehvari N, Öberg AI, et al. Improving type 2 diabetes through a distinct adrenergic signaling pathway involving mTORC2 that mediates glucose uptake in skeletal muscle. Diabetes, 2014, 63(12): 4115–4129. DOI: 10.2337/db13-1860 |
| [15] | Akingbemi BT. Adiponectin receptors in energy homeostasis and obesity pathogenesis. Prog Mol Biol Transl Sci, 2013, 114: 317–342. DOI: 10.1016/B978-0-12-386933-3.00009-1 |
| [16] | Ma Y, Liu D. Hydrodynamic delivery of adiponectin and adiponectin receptor 2 gene blocks high-fat diet-induced obesity and insulin resistance. Gene Ther, 2013, 20(8): 846–852. DOI: 10.1038/gt.2013.8 |
| [17] | Kappel VD, Zanatta L, Postal BG, et al. Rutin potentiates calcium uptake via voltage-dependent calcium channel associated with stimulation of glucose uptake in skeletal muscle. Arch Biochem Biophys, 2013, 532(2): 55–60. DOI: 10.1016/j.abb.2013.01.008 |
| [18] | Friedrichsen M, Mortensen B, Pehmøller C, et al. Exercise-induced AMPK activity in skeletal muscle: role in glucose uptake and insulin sensitivity. Mol Cell Endocrinol, 2013, 366(2): 204–214. DOI: 10.1016/j.mce.2012.06.013 |
| [19] | Hunter RW, Treebak JT, Wojtaszewski JFP, et al. Molecular mechanism by which AMP-activated protein kinase activation promotes glycogen accumulation in muscle. Diabetes, 2011, 60(3): 766–774. DOI: 10.2337/db10-1148 |
| [20] | Treebak JT, Pehmøller C, Kristensen JM, et al. Acute exercise and physiological insulin induce distinct phosphorylation signatures on TBC1D1 and TBC1D4 proteins in human skeletal muscle. J Physiol, 2014, 592(2): 351–375. DOI: 10.1113/jphysiol.2013.266338 |
| [21] | O'Neill HM. AMPK and exercise: glucose uptake and insulin sensitivity. Diabetes Metab J, 2013, 37(1): 1–21. |
| [22] | Richter EA, Hargreaves M. Exercise, GLUT4, and skeletal muscle glucose uptake. Physiol Rev, 2013, 93(3): 993–1017. DOI: 10.1152/physrev.00038.2012 |
| [23] | Hardie DG, Ross FA, Hawley SA. AMPK: a nutrient and energy sensor that maintains energy homeostasis. Nat Rev Mol Cell Biol, 2012, 13(4): 251–262. |
| [24] | Hardie DG. AMPK: positive and negative regulation, and its role in whole-body energy homeostasis. Curr Opin Cell Biol, 2015, 33: 1–7. DOI: 10.1016/j.ceb.2014.09.004 |
| [25] | Frøsig C, Pehmøller C, Birk JB, et al. Exercise-induced TBC1D1 Ser237 phosphorylation and 14-3-3 protein binding capacity in human skeletal muscle. J Physiol, 2010, 588(22): 4539–4548. DOI: 10.1113/jphysiol.2010.194811 |
| [26] | An D, Toyoda T, Taylor EB, et al. TBC1D1 regulates insulin- and contraction-induced glucose transport in mouse skeletal muscle. Diabetes, 2010, 59(6): 1358–1365. DOI: 10.2337/db09-1266 |
| [27] | Naimi M, Vlavcheski F, Murphy B, et al. Carnosic acid as a component of rosemary extract stimulates skeletal muscle cell glucose uptake via AMPK activation. Clin Exp Pharmacol Physiol, 2017, 44(1): 94–102. DOI: 10.1111/1440-1681.12674 |
| [28] | Jørgensen SB, Viollet B, Andreelli F, et al. Knockout of the α2 but not α1 5'-AMP-activated protein kinase isoform abolishes 5-aminoimidazole-4-carboxamide- 1-β-4-ribofuranoside- but not contraction-induced glucose uptake in skeletal muscle. J Biol Chem, 2004, 279(2): 1070–1079. DOI: 10.1074/jbc.M306205200 |
| [29] | Brown JD, Hancock CR, Mongillo AD, et al. Effect of LKB1 deficiency on mitochondrial content, fibre type and muscle performance in the mouse diaphragm. Acta Physiol, 2011, 201(4): 457–466. DOI: 10.1111/apha.2011.201.issue-4 |
| [30] | Gong HJ, Zhang Y. GLUT4 is not essential for exercise-induced exaggerated muscle glycogen degradation in AMPKα2 knockout mice. J Exerc Sci Fit, 2012, 10(1): 16–22. DOI: 10.1016/j.jesf.2012.04.001 |
| [31] | Quehenberger O, Dennis EA. The human plasma lipidome. N Engl J Med, 2011, 365(19): 1812–1823. DOI: 10.1056/NEJMra1104901 |
| [32] | Han JS, Sung JH, Lee SK. Inhibition of cholesterol synthesis in HepG2 cells by GINST—Decreasing HMG-CoA reductase expression via AMP-activated protein kinase. J Food Sci, 2017, 82(11): 2700–2705. DOI: 10.1111/1750-3841.13828 |
| [33] | Stahmann N, Woods A, Carling D, et al. Thrombin activates AMP-activated protein kinase in endothelial cells via a pathway involving Ca2+/calmodulin- dependent protein kinase kinase β. Mol Cell Biol, 2006, 26(16): 5933–5945. DOI: 10.1128/MCB.00383-06 |
| [34] | Merrill GF, Kurth EJ, Hardie DG, et al. AICA riboside increases AMP-activated protein kinase, fatty acid oxidation, and glucose uptake in rat muscle. Am J Physiol, 1997, 273(6): E1107–E1112. |
| [35] | Paoli A, Bosco G, Camporesi EM, et al. Ketosis, ketogenic diet and food intake control: a complex relationship. Front Psychol, 2015, 6: 27. |
| [36] | Cool B, Zinker B, Chiou W, et al. Identification and characterization of a small molecule AMPK activator that treats key components of type 2 diabetes and the metabolic syndrome. Cell Metab, 2006, 3(6): 403–416. DOI: 10.1016/j.cmet.2006.05.005 |
| [37] | Lieberthal W, Tang MY, Zhang LQ, et al. Susceptibility to ATP depletion of primary proximal tubular cell cultures derived from mice lacking either the α1 or the α2 isoform of the catalytic domain of AMPK. BMC Nephrol, 2013, 14: 251. DOI: 10.1186/1471-2369-14-251 |
| [38] | Pinkosky SL, Filippov S, Srivastava RAK, et al. AMP-activated protein kinase and ATP-citrate lyase are two distinct molecular targets for ETC-1002, a novel small molecule regulator of lipid and carbohydrate metabolism. J Lipid Res, 2013, 54(1): 134–151. DOI: 10.1194/jlr.M030528 |
| [39] | Muoio DM, Seefeld K, Witters LA, et al. AMP-activated kinase reciprocally regulates triacylglycerol synthesis and fatty acid oxidation in liver and muscle: evidence that sn-glycerol-3- phosphate acyltransferase is a novel target. Biochem J, 1999, 338(3): 783–791. DOI: 10.1042/bj3380783 |
| [40] | Ahmadian M, Abbott MJ, Tang TY, et al. Desnutrin/ATGL is regulated by AMPK and is required for a brown adipose phenotype. Cell Metab, 2011, 13(6): 739–748. DOI: 10.1016/j.cmet.2011.05.002 |
| [41] | Gaidhu MP, Bikopoulos G, Ceddia RB. Chronic AICAR-induced AMP-kinase activation regulates adipocyte lipolysis in a time-dependent and fat depot-specific manner in rats. Am J Physiol Cell Physiol, 2012, 303(11): C1192–C1197. DOI: 10.1152/ajpcell.00159.2012 |
| [42] | McDonough PM, Maciejewski-Lenoir D, Hartig SM, et al. Differential phosphorylation of perilipin 1A at the initiation of lipolysis revealed by novel monoclonal antibodies and high content analysis. PLoS ONE, 2013, 8(2): e55511. DOI: 10.1371/journal.pone.0055511 |
| [43] | Gallo-Payet N. Adrenal and extra-adrenal functions of ACTH. J Mol Endocrinol, 2016, 56(4): T135–T156. DOI: 10.1530/JME-15-0257 |
| [44] | Yang RM, Chu XX, Sun L, et al. Hypolipidemic activity and mechanisms of the total phenylpropanoid glycosides from Ligustrum robustum (Roxb.) Blume by AMPK-SREBP-1c pathway in hamsters fed a high-fat diet. Phytother Res, 2018, 32(4): 715–722. DOI: 10.1002/ptr.v32.4 |
| [45] | Börner S, Albrecht E, Schäff C, et al. Reduced AgRP activation in the hypothalamus of cows with high extent of fat mobilization after parturition. Gen Comp Endocrinol, 2013, 193: 167–177. DOI: 10.1016/j.ygcen.2013.08.002 |
| [46] | Jeon SM. Regulation and function of AMPK in physiology and diseases. Exp Mol Med, 2016, 48(7): e245. DOI: 10.1038/emm.2016.81 |
 2019, Vol. 35
2019, Vol. 35




