中国科学院微生物研究所、中国微生物学会主办
文章信息
- 姜思, 佟少明
- Jiang Si, Tong Shaoming
- 雨生红球藻虾青素合成研究进展
- Advances in astaxanthin biosynthesis in Haematococcus pluvialis
- 生物工程学报, 2019, 35(6): 988-997
- Chinese Journal of Biotechnology, 2019, 35(6): 988-997
- 10.13345/j.cjb.180450
-
文章历史
- Received: November 1, 2018
- Accepted: December 4, 2018
- Published: January 8, 2019
虾青素(Astaxanthin)是一种次级类胡萝卜素,由2个紫罗酮环通过多烯链相连构成,紫罗酮环的3和3′位置分别具有1个手性碳原子,因此存在3种可能形成的对映异构体:3S、3′S,3R、3′S和3R、3′R,人工合成的虾青素是这3种异构体的混合物[1]。虾青素具有强大的抗氧化能力,但不具备其他类胡萝素所具有的典型的助氧化作用[2-3]。另外,虾青素不是维生素的前体,过量摄入也不会使生物体遭受维生素过多症的威胁[4]。因此,基于虾青素的安全性及其对人类和动物健康的有益效果,已经成为最重要的类胡萝卜素生产对象之一,并被广泛应用于食品保健、医药、安全着色和水产养殖等领域[5-6]。
在自然界中,某些微藻、细菌和真菌以及一些食用类海产品都可以合成虾青素,但微藻合成虾青素的能力是最强的,雨生红球藻Haematococcus pluvialis更是其中的佼佼者[1]。雨生红球藻隶属于绿藻纲Chlorophyceae、团藻目Volvocales,是一种广泛分布于自然界中的单细胞淡水绿藻[7],在高光、氮缺失等胁迫条件下可以大量积累虾青素[8-10]。雨生红球藻累积的虾青素可占其干重的2.7%–3.8%,且雨生红球藻生成的虾青素是比人工合成虾青素生物活性更高的纯3S、3′S构象的虾青素[1]。基于雨生红球藻能够大量积累高活性虾青素这一特点,加之天然虾青素的生产需求及成本的实际要求,越来越多的研究集中到雨生红球藻的虾青素生物合成上[7]。
目前,对于雨生红球藻的虾青素合成和积累的研究主要集中在以下几个方面:1)影响雨生红球藻虾青素合成和积累的培养条件和培养方式的研究;2)虾青素在雨生红球藻中的代谢合成途径及相关分子调控机制的研究;3)雨生红球藻中虾青素合成与其他代谢合成之间的联系的研究;4)虾青素在雨生红球藻胁迫耐受中所扮演的角色的研究等。这些研究结果为了解雨生红球藻虾青素积累过程提供了大量的实验数据,同时也为在雨生红球藻中商业化生产虾青素提供了理论依据和技术支持。
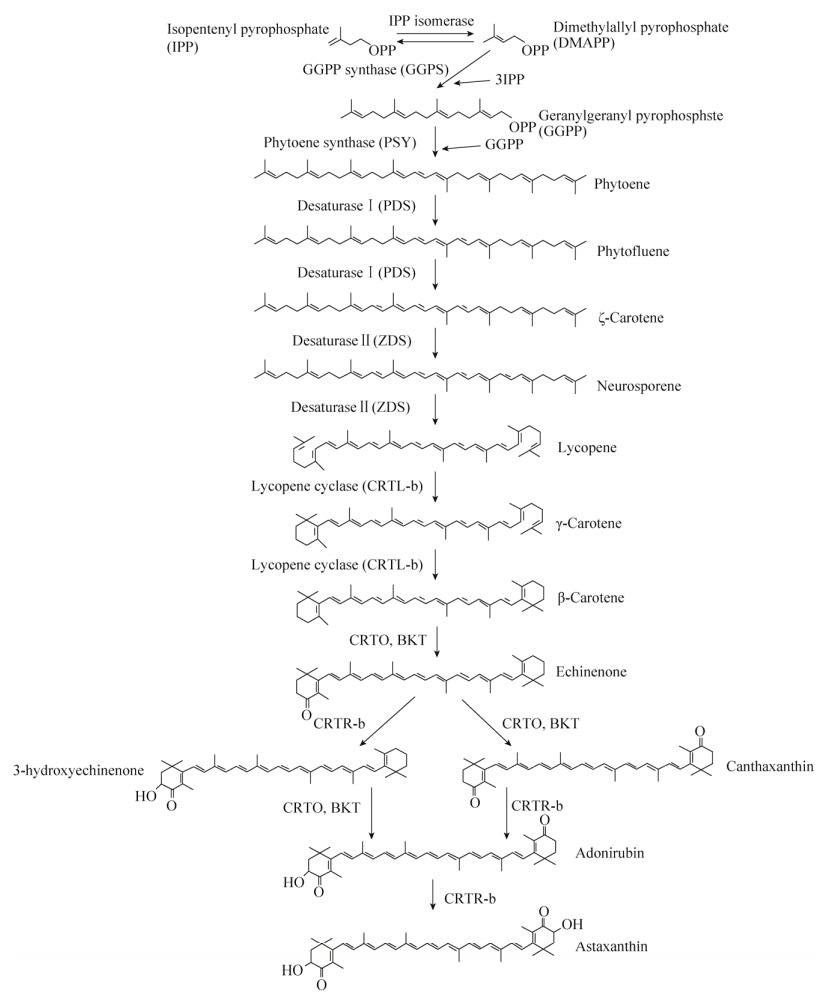
1 雨生红球藻中虾青素的生物合成自1884年首次报道雨生红球藻能够积累虾青素以来,虾青素生物合成的生物化学、酶学以及与此相关的一些基因的分离得到了较多的研究,对虾青素合成的整个代谢过程也有了充分的了解(图 1)。虾青素合成需要以初级类胡萝卜素的β-胡萝卜素(β-Carotene)为前体[11],β-胡萝卜素合成的前体物质为异戊烯焦磷酸(Isopentenyl pyrophosphate,IPP)和二甲基烯丙基焦磷酸(Dimethylallyl pyrophosphate,DMAPP),其中雨生红球藻中的IPP是通过非甲羟戊酸途径合成的[7],DMAPP是由IPP异构酶(IPI)催化IPP生成的[12-13]。首先,3个IPP分子在牻牛儿牻牛儿焦磷酸合成酶(GGPP synthase,GGPS)的作用下被依次添加到DMAPP中产生C20牻牛儿牻牛儿焦磷酸(Geranylgeranyl pyrophosphate,GGPP) [14]。两个GGPP分子的头对尾缩合在八氢番茄红素合成酶(Phytoene synthase,PSY)催化下得到第一个在紫外光谱区有吸收的C40的前体物质八氢番茄红素(Phytoene) [15-17]。接下来的4步反应是由八氢番茄红素去饱和酶(Phytoene desaturaseⅠ,PDS)和ζ-胡萝卜素去饱和酶(ζ-Carotene desaturase Ⅱ,ZDS)催化的八氢番茄红素的连续去饱和反应,依次生成六氢番茄红素(Phytofluene)、ζ-胡萝卜素(ζ-Carotene)、链孢红素(Neurosporene)、番茄红素(Lycopene),其中由PDS催化的反应是该途径的限速步骤,通过这些反应增加了共轭碳-碳双键的数量[15, 18]。最后由定位在叶绿体膜上的番茄红素β-环化酶(Lycopene β-cyclase,LCYB/CRTL-b)催化番茄红素环化成含有2个β-紫罗兰酮环的β-胡萝卜素[19]。从IPP起始的β-胡萝卜素的合成过程被认为发生在叶绿体中,并运输到油脂滴(Lipid droplets,LD)中储存下来,但β-胡萝卜素运输到油脂滴中的机制仍然不清楚[11, 20]。
以β-胡萝卜素为底物的虾青素的形成需要通过3, 3′-羟化酶(3, 3′-hydroxylase,CRTR-b/CHY/ CRTZ)及4, 4′-酮化酶(4, 4′-ketolase,CRTO/BKT)催化在紫罗酮环的C3和C3′处及C4和C4′处分别引入两个羟基和两个酮基[21-23]。虾青素的合成是以β-胡萝卜素酮基化起始的,由于BKT和CRTR-b作用顺序的不同,共有3条潜在虾青素合成路径,目前被研究者广泛接受的路径有2条:其中一条代谢中间体依次为海胆酮(Echinenone)、角黄素(Canthaxanthin)和金盏花红素(Adonirubin),并涉及了两次连续的酮化和两次连续的羟基化;另一条通路的代谢中间体依次为海胆酮、3-羟基海胆酮(3-hydroxyechinenone)和金盏花红素(Adonirubin),涉及两次交替的酮化和羟基化[24]。雨生红球藻产生的虾青素主要以酯化的形式存在,酯化的过程被证实发生在内质网中,此外,由于CRTO蛋白在叶绿体中酶活性受到抑制,所以虾青素合成的过程极有可能也发生在内质网中[20, 25-26]。
2 雨生红球藻中虾青素合成的诱导与调控当处于正常培养条件下的雨生红球藻细胞暴露于胁迫环境中时,藻细胞会由绿色的具鞭毛游动细胞逐渐向红色无鞭毛不动的包囊细胞转变[7]。雨生红球藻的这种响应胁迫条件的变化过程是极其复杂的,可能伴随着其他代谢事件的发生[27]。目前,大量的研究结果表明,诸多胁迫条件均可以诱导雨生红球藻的虾青素积累,例如高光、营养缺乏、极端温度、高盐度等[28-31]。
转crtR-b和bkt两种酶基因的酵母可以生成虾青素以及在雨生红球藻的野生型和虾青素超积累突变体MT 2877中crtR-b转录水平与虾青素的积累呈线性相关的事实都证实了bkt和crtR基因表达对于生物体的虾青素合成至关重要[32-33]。另外,将一定浓度的环己酰亚胺添加到处于胁迫处理早期阶段(约6 h)的雨生红球藻中,会使其细胞中虾青素的积累受到抑制,而在胁迫处理早期之后添加环己酰亚胺则不会抑制虾青素的产生,这说明虾青素的合成可能受到诸如bkt和crtR-b等基因的转录水平的调控[24]。此外,研究发现胁迫条件下BKT蛋白的表达量仅在胁迫后的一段时间内随bkt转录本的积累平行地增加,随后酶的积累将不伴随mRNA量的成比例增加,这表明虾青素的合成调控可能受到翻译水平的调控[34]。一定量的硫酸亚铁和乙酸钠的加入可以在高光胁迫的基础上进一步促进虾青素的积累,而不会使类胡萝卜素合成相关基因表达进一步上调,此结果则暗示着翻译后水平调控的存在[35-38]。此外,本实验室的研究发现在高光缺氮胁迫下,一些与胁迫有关的转录因子包括Myb转录因子、WRKY family等的转录表达上调,也暗示虾青素合成的转录水平调控是重要的调控方式之一。总之,通过虾青素关键酶基因的转录和翻译水平的调控及酶蛋白的翻译后活性调控是实现雨生红球藻虾青素的诱导合成的主要机制。
高光是诱导雨生红球藻虾青素合成的胁迫条件之一,越来越多的研究表明光合作用与虾青素合成两者之间关系紧密。一方面,高光胁迫能够使光合系统产生活性氧簇(Reactive oxygen species,ROS),ROS成员之一H2O2的处理可以使正常培养条件下的雨生红球藻积累虾青素,而ROS清除剂处理会抑制胁迫条件下虾青素的大量积累,甚至有研究者认为ROS作为第二受体参与诱导虾青素的积累[4, 39-40]。另一方面,Steinbrenner和Linden用光合抑制剂的实验发现虾青素合成关键酶基因的光诱导可能受光合作用控制,其中涉及作为氧化还原传感器的质醌库(Plastoquinone,PQ)可用于在转录水平上调节叶绿体和核编码的光合相关基因的表达,以及通过LHC Ⅱ调节两个光系统之间的激发能量分布,从而控制红球藻中虾青素上游类胡萝卜素的生物合成[41]。此外,先前已报道质体末端氧化酶(Plastid terminal oxidase of chlororespiration,PTOX)的活性的增加可防止电子传递链饱和,同时其活性高低对于胡萝卜素生成也至关重要,涉及八氢番茄红素去饱和酶和ζ-胡萝卜素去饱和酶的氧化还原反应,这也表明光合作用可能通过PTOX对虾青素合成过程进行调控[31]。
虾青素被发现可以结合到光系统Ⅰ (Photosystem Ⅰ complex,PSⅠ)和光系统Ⅱ (Photosystem Ⅱ complex,PSⅡ)上,替代部分β-胡萝卜素,但虾青素与光系统的结合并没有提高光保护能力,反而降低了激发能向反应中心转移的效率,因此,研究者认为虾青素结合会部分破坏PSⅠ和PSⅡ的稳定性,相关的研究也证实在强光下生长的雨生红球藻细胞具有较高的PSⅡ不稳定性的特征,这表明虾青素可能通过这种方式反过来调控光合作用,并减少线性光合电子传递而增加环式电子传递来增强微藻应对强光的抵抗力[42-44]。在本实验室的研究中也发现在高光及缺氮的胁迫下,编码捕光天线蛋白复合体LHC的基因所有成员都出现了上调或下调表达的变化,但光系统中的LCH基因表达的变化与虾青素结合到光系统之间的关系有待进一步确认。
3 虾青素的酯化及其在油脂滴中的积累在雨生红球藻中合成的虾青素并不全部以游离的形式存在,尤其是红色包囊时期的雨生红球藻中积累的大部分虾青素是以与棕榈酸、油酸或亚油酸这些脂肪酸(Fatty acid,FA)脱水后形成的单酯和二酯的形式存在,其中有70%单酯、25%二酯而仅有5%游离的虾青素,用于虾青素酯化的二酰基甘油酰基转移酶已经被分离得到,同时酯化后的虾青素也被证明比游离的虾青素具有更高的稳定性[26, 45-47],酯化后的虾青素被储存在LD中,三酰基甘油(Triacylglycerol,TAG)这种中性脂质是细胞质中LD的主要成分[48-49]。通过抑制脂质生物合成可以消除虾青素的积累,而阻断虾青素生物合成不能阻止中性脂质的积累和LD的形成,这表现出虾青素的积累对酯化过程和在LD中储藏的依赖,Chen等获得的结果也证实这种相互作用是在代谢物水平上与反馈相关[26, 50]。总之,虾青素的酯化及其在LD中的积累的过程进一步协助雨生红球藻在胁迫条件下大量积累虾青素。
虾青素的酯化及油脂滴中主要的中性油脂的形成均需要脂肪酸(Fatty acids,FA)的参与,大量的虾青素的酯化和其在LD的储藏就意味着需要大量的脂肪酸,在虾青素积累的包囊细胞中也确实存在着大量的FA[51-52]。在虾青素积累过程中还涉及FA合成的关键前体乙酰辅酶A池的增加,与FA合成相关的其他基因如烷烃1-单加氧酶、醇脱氢酶和三酰基甘油脂肪酶的上调表达[53-54]。由此,虾青素的积累与FA的积累表现出了积累上的同步性,即趋向于FA合成和虾青素合成的碳分配,本实验室的转录组数据也证实了这一点,但这种同步性的表现是否存在共同的调控方式,有待进一步探究。
4 其他影响虾青素积累的因素一些外源物质的添加会对雨生红球藻虾青素的产量有显著影响。如Lu等发现了外源甲基茉莉酮酸酯和赤霉素增加了雨生红球藻藻株WB-1中虾青素的产量,并在bkt1基因的5′侧翼区域发现了典型的赤霉素A3响应顺式元件,这暗示着虾青素的合成调控非常有可能受到内源激素的调节[55]。Ding等则利用褪黑素证实了cAMP信号传导途径与微藻虾青素生物合成正相关,另外,一氧化氮(NO)依赖性MAPK信号传导级联被激活,证实MAPK是生理过程中NO作用的靶标[56]。在高光和缺氮条件下添加二丁基羟基甲苯(Butylated hydroxytoluene,BHT)的实验证实,作为非生物胁迫物质BHT的外源添加不仅增加了虾青素和脂质的积累,同时使内源NO的产生量增加,由此,结合Ding等[56]的实验可以推测,NO可能参与了虾青素合成的诱导调控[57]。
5 总结与展望就目前的研究结果来看,虾青素的合成受到胁迫条件的诱导,其中最主要的是高光和氮饥饿胁迫;虾青素合成的代谢途径基本明确,存在着以β-胡萝卜素为前体起始于酮化作用的两条合成路线;参与虾青素合成的酮化酶和羟化酶,受到多水平的调控;光合作用、ROS的积累、虾青素的酯化以及其在油脂滴中的积累,均与虾青素的合成积累过程有着密切的关系。此外,近年来在雨生红球藻的研究中广泛应用的转录组技术所得的数据突出了虾青素合成关键酶基因表达对于虾青素合成的重要性,也证实了胁迫条件下碳分配趋向于虾青素合成和脂肪酸合成[58]。代谢组学的研究在雨生红球藻虾青素积累中也得到了应用,在代谢水平上证实了胁迫条件下碳分配趋向于虾青素合成和脂肪酸合成的事实[54, 59]。总之,在胁迫条件下,雨生红球藻代谢活动总体趋向于虾青素的合成和积累有利的方向进行(图 2)。
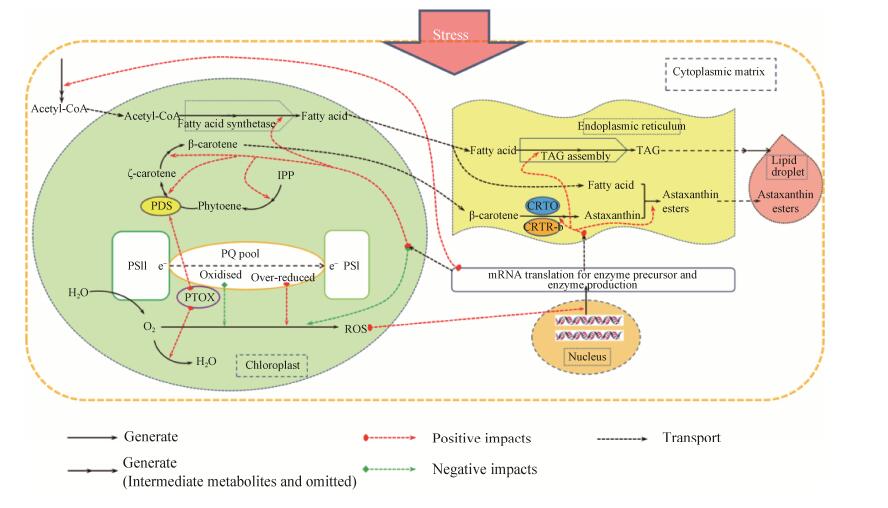
|
| 图 2 胁迫条件下雨生红球藻中虾青素的积累 Fig. 2 Accumulation of astaxanthin in H. pluvialis under stress conditions. |
| |
尽管我们已经掌握了许多雨生红球藻虾青素合成的知识,但事实上许多方面的研究仍处于探索阶段,比如,虾青素合成过程中所涉及的推测的中间代谢体仍然没能测定完全,虾青素生物合成途径中相关酶的表达调控还不清晰,以及胁迫诱导引起的信号传导通路还未知;ROS和PTOX被认为可能参与到了虾青素的合成诱导调控中,但仍缺乏直接的证据;虾青素积累极其依赖于酯化过程和其在油脂滴中的积累,但这三者之间的更为深入的关系仍然是未知的。此外,我们对于雨生红球藻在耐受胁迫过程中虾青素所扮演的角色了解仍然是不完全的,尤其是那些发生在虾青素大量积累和其他类胡萝卜素合成之前的早期事件更是了解甚少,这些早期事件可能涉及保护细胞免受活性氧积累的损伤、呼吸的顺势变化、光合固定以及不同代谢物池和细胞区室之间碳的分配等不同的生理过程。虽然,近年来的转录组和代谢组的研究为我们提供了更为全面的转录和代谢水平上的认识,帮助找寻与虾青素合成有关的线索,但值得注意的是,利用胁迫处理所得的线索只能表明其与胁迫处理具有直接的相关性,而不能证明其与虾青素积累的相关性,所以仍然需要其他的研究证实这些线索与虾青素积累的相关性。总的来看,当前的研究受限于藻细胞应对胁迫的复杂响应以及虾青素合成的复杂调控,因此亟需有效的手段和方法找出藻细胞应对胁迫的复杂响应中与虾青素积累相关的部分,并分水平地逐步明确虾青素合成的复杂调控。
| [1] | Ambati RR, Phang SM, Ravi S, et al. Astaxanthin: sources, extraction, stability, biological activities and its commercial applications-a review. Mar Drugs, 2014, 12(1): 128–152. DOI: 10.3390/md12010128 |
| [2] | Higuera-Ciapara I, Felix-Valenzuela L, Goycoolea FM. Astaxanthin: a review of its chemistry and applications. Crit Rev Food Sci Nutr, 2006, 46(2): 185–196. |
| [3] | Otani H. Site-specific antioxidative therapy for prevention of atherosclerosis and cardiovascular disease. Oxid Med Cell Longev, 2013, 2013: 796891. |
| [4] | Solovchenko AE. Recent breakthroughs in the biology of astaxanthin accumulation by microalgal cell. Photosynth Res, 2015, 125(3): 437–449. DOI: 10.1007/s11120-015-0156-3 |
| [5] | Yuan JP, Peng J, Yin K, et al. Potential health-promoting effects of astaxanthin: a high-value carotenoid mostly from microalgae. Mol Nutr Food Res, 2011, 55(1): 150–165. DOI: 10.1002/mnfr.v55.1 |
| [6] | Davinelli S, Nielsen ME, Scapagnini G. Astaxanthin in skin health, repair, and disease: a comprehensive review. Nutrients, 2018, 10(4): 522. DOI: 10.3390/nu10040522 |
| [7] | Shah MM, Liang YM, Cheng JJ, et al. Astaxanthin-producing green microalga Haematococcus pluvialis: from single cell to high value commercial products. Front Plant Sci, 2016, 7: 531. |
| [8] | Harker M, Tsavalos AJ, Young AJ. Factors responsible for astaxanthin formation in the Chlorophyte Haematococcus pluvialis. Bioresour Technol, 1996, 55(3): 207–214. DOI: 10.1016/0960-8524(95)00002-X |
| [9] | Orosa M, Valero JF, Herrero C, et al. Comparison of the accumulation of astaxanthin in Haematococcus pluvialis and other green microalgae under N-starvation and high light conditions. Biotechnol Lett, 2001, 23(13): 1079–1085. DOI: 10.1023/A:1010510508384 |
| [10] | Domínguez-Bocanegra AR, Legarreta IG, Jeronimo FM, et al. Influence of environmental and nutritional factors in the production of astaxanthin from Haematococcus pluvialis. Bioresour Technol, 2004, 92(2): 209–214. DOI: 10.1016/j.biortech.2003.04.001 |
| [11] | Grünewald K, Hagen C. β-carotene is the intermediate exported from the chloroplast during accumulation of secondary carotenoids in Haematococcus pluvialis. J Appl Phycol, 2001, 13(1): 89–93. DOI: 10.1023/A:1008183328839 |
| [12] | Sun ZR, Cunningham FX Jr, Gantt E. Differential expression of two isopentenyl pyrophosphate isomerases and enhanced carotenoid accumulation in a unicellular chlorophyte. Proc Natl Acad Sci USA, 1998, 95(19): 11482–11488. DOI: 10.1073/pnas.95.19.11482 |
| [13] | Gwak Y, Hwang YS, Wang BB, et al. Comparative analyses of lipidomes and transcriptomes reveal a concerted action of multiple defensive systems against photooxidative stress in Haematococcus pluvialis. J Exp Bot, 2014, 65(15): 4317–4334. DOI: 10.1093/jxb/eru206 |
| [14] | Britton G. Biosynthesis of carotenoids//Young AJ, Britton G, Eds. Dordrecht: Springer, 1993: 133–182. |
| [15] | Cunningham FX, Gantt E. Genes and enzymes of carotenoid biosynthesis in plants. Annu Rev Plant Physiol Plant Mol Biol, 1998, 49(1): 557–583. DOI: 10.1146/annurev.arplant.49.1.557 |
| [16] | Steinbrenner J, Linden H. Regulation of two carotenoid biosynthesis genes coding for phytoene synthase and carotenoid hydroxylase during stress-induced Astaxanthin formation in the green alga Haematococcus pluvialis. Plant Physiol, 2001, 125(2): 810–817. DOI: 10.1104/pp.125.2.810 |
| [17] | Vidhyavathi R, Venkatachalam L, Sarada R, et al. Regulation of carotenoid biosynthetic genes expression and carotenoid accumulation in the green alga Haematococcus pluvialis under nutrient stress conditions. J Exp Bot, 2008, 59(6): 1409–1418. DOI: 10.1093/jxb/ern048 |
| [18] | Li YT, Sommerfeld M, Chen F, et al. Effect of photon flux densities on regulation of carotenogenesis and cell viability of Haematococcus pluvialis (Chlorophyceae). J Appl Phycol, 2010, 22(3): 253–263. DOI: 10.1007/s10811-009-9453-6 |
| [19] | Chamovitz D, Sandmann G, Hirschberg J. Molecular and biochemical characterization of herbicide-resistant mutants of cyanobacteria reveals that phytoene desaturation is a rate-limiting step in carotenoid biosynthesis. J Biol Chem, 1993, 268(23): 17348–17353. |
| [20] | Collins AM, Jones HD, Han DX, et al. Carotenoid distribution in living cells of Haematococcus pluvialis (Chlorophyceae). PLoS ONE, 2011, 6(9): e24302. DOI: 10.1371/journal.pone.0024302 |
| [21] | Kajiwara S, Kakizono T, Saito T, et al. Isolation and functional identification of a novel cDNA for astaxanthin biosynthesis from Haematococcus pluvialis, and astaxanthin synthesis in Escherichia coli. Plant Mol Biol, 1995, 29(2): 343–352. DOI: 10.1007/BF00043657 |
| [22] | Lotan T, Hirschberg J. Cloning and expression in Escherichia coli of the gene encoding β-C-4-oxygenase, that converts β-carotene to the ketocarotenoid canthaxanthin in Haematococcus pluvialis. FEBS Lett, 1995, 364(2): 125–128. DOI: 10.1016/0014-5793(95)00368-J |
| [23] | Misawa N, Satomi Y, Kondo K, et al. Structure and functional analysis of a marine bacterial carotenoid biosynthesis gene cluster and astaxanthin biosynthetic pathway proposed at the gene level. J Bacteriol, 1995, 177(22): 6575–6584. DOI: 10.1128/jb.177.22.6575-6584.1995 |
| [24] | Lemoine Y, Schoefs B. Secondary ketocarotenoid astaxanthin biosynthesis in algae: a multifunctional response to stress. Photosynth Res, 2010, 106(1/2): 155–177. |
| [25] | Grünewald K, Hirschberg J, Hagen C. Ketocarotenoid biosynthesis outside of plastids in the unicellular green alga Haematococcus pluvialis. J Biol Chem, 2001, 276(8): 6023–6029. DOI: 10.1074/jbc.M006400200 |
| [26] | Chen GQ, Wang BB, Han DX, et al. Molecular mechanisms of the coordination between astaxanthin and fatty acid biosynthesis in Haematococcus pluvialis (Chlorophyceae). Plant J, 2015, 81(1): 95–107. DOI: 10.1111/tpj.12713 |
| [27] | Leu S, Boussiba S. Advances in the production of high-value products by microalgae. Ind Biotechnol, 2014, 10(3): 169–183. DOI: 10.1089/ind.2013.0039 |
| [28] | Sarada R, Tripathi U, Ravishankar GA. Influence of stress on astaxanthin production in Haematococcus pluvialis grown under different culture conditions. Proc Biochem, 2002, 37(6): 623–627. DOI: 10.1016/S0032-9592(01)00246-1 |
| [29] | Pelah D, Marton I, Wang W, et al. Accumulation and protection activity of protease-resistant heat-stable proteins in Haematococcus pluvialis during high light and nitrogen starvation. J Appl Phycol, 2004, 16(2): 153–156. DOI: 10.1023/B:JAPH.0000044944.53478.b2 |
| [30] | Giannelli L, Yamada H, Katsuda T, et al. Effects of temperature on the astaxanthin productivity and light harvesting characteristics of the green alga Haematococcus pluvialis. J Biosci Bioeng, 2015, 119(3): 345–350. DOI: 10.1016/j.jbiosc.2014.09.002 |
| [31] | Li YT, Sommerfeld M, Chen F, et al. Consumption of oxygen by astaxanthin biosynthesis: a protective mechanism against oxidative stress in Haematococcus pluvialis (Chlorophyceae). J Plant Physiol, 2008, 165(17): 1783–1797. DOI: 10.1016/j.jplph.2007.12.007 |
| [32] | Lin YJ, Chang JJ, Lin HY, et al. Metabolic engineering a yeast to produce astaxanthin. Bioresour Technol, 2017, 245: 899–905. DOI: 10.1016/j.biortech.2017.07.116 |
| [33] | Hu ZY, Li YT, Sommerfeld M, et al. Enhanced protection against oxidative stress in an astaxanthin-overproduction Haematococcus mutant (Chlorophyceae). Eur J Phycol, 2008, 43(4): 365–376. DOI: 10.1080/09670260802227736 |
| [34] | Meng CX, Teng CY, Jiang P, et al. Cloning and characterization of beta-carotene ketolase gene promoter in Haematococcus pluvialis. Acta Biochim Biophys Sin, 2005, 37(4): 270–275. DOI: 10.1111/abbs.2005.37.issue-4 |
| [35] | Kobayashi M, Kakizono T, Nagai S. Enhanced carotenoid biosynthesis by oxidative stress in acetate-induced cyst cells of a green unicellular alga, Haematococcus pluvialis. Appl Environ Microbiol, 1993, 59(3): 867–873. |
| [36] | Jeon YC, Cho CW, Yun YS. Combined effects of light intensity and acetate concentration on the growth of unicellular microalga Haematococcus pluvialis. Enzyme Microb Technol, 2006, 39(3): 490–495. DOI: 10.1016/j.enzmictec.2005.12.021 |
| [37] | Hong ME, Choi YY, Sim SJ. Effect of red cyst cell inoculation and iron (Ⅱ) supplementation on autotrophic astaxanthin production by Haematococcus pluvialis under outdoor summer conditions. J Biotechnol, 2016, 218: 25–33. DOI: 10.1016/j.jbiotec.2015.11.019 |
| [38] | He BX, Hou LL, Dong MM, et al. Transcriptome analysis in Haematococcus pluvialis: astaxanthin induction by high light with acetate and Fe2+. Int J Mol Sci, 2018, 19(1): E175. DOI: 10.3390/ijms19010175 |
| [39] | Yong YYR, Lee YK. Do carotenoids play a photoprotective role in the cytoplasm of Haematococcus lacustris (Chlorophyta)?. Phycologia, 1991, 30(3): 257–261. DOI: 10.2216/i0031-8884-30-3-257.1 |
| [40] | Kobayashi M, Kakizono T, Nishio N, et al. Antioxidant role of astaxanthin in the green alga Haematococcus pluvialis. Appl Microbiol Biotechnol, 1997, 48(3): 351–356. |
| [41] | Steinbrenner J, Linden H. Light induction of carotenoid biosynthesis genes in the green alga Haematococcus pluvialis: regulation by photosynthetic redox control. Plant Mol Biol, 2003, 52(2): 343–356. DOI: 10.1023/A:1023948929665 |
| [42] | Wang BB, Zhang Z, Hu Q, et al. Cellular capacities for high-light acclimation and changing lipid profiles across life cycle stages of the green alga Haematococcus pluvialis. PLoS ONE, 2014, 9(9): e106679. DOI: 10.1371/journal.pone.0106679 |
| [43] | Mascia F, Girolomoni L, Alcocer MJP, et al. Functional analysis of photosynthetic pigment binding complexes in the green alga Haematococcus pluvialis reveals distribution of astaxanthin in photosystems. Sci Rep, 2017, 7: 16319. DOI: 10.1038/s41598-017-16641-6 |
| [44] | Scibilia L, Girolomoni L, Berteotti S, et al. Photosynthetic response to nitrogen starvation and high light in Haematococcus pluvialis. Algal Res, 2015, 12: 170–181. DOI: 10.1016/j.algal.2015.08.024 |
| [45] | Hagen C, Braune W, Birckner E, et al. Functional aspects of secondary carotenoids in Haematococcus lacustris (Girod) Rostafinski (Volvocales). I. The accumulation period as an active metabolic process. The New Phytol, 1993, 125(3): 625–633. DOI: 10.1111/nph.1993.125.issue-3 |
| [46] | Kobayashi M, Kurimura Y, Kakizono T, et al. Morphological changes in the life cycle of the green alga Haematococcus pluvialis. J Fermentat Bioeng, 1997, 84(1): 94–97. DOI: 10.1016/S0922-338X(97)82794-8 |
| [47] | Kobayashi M, Sakamoto Y. Singlet oxygen quenching ability of astaxanthin esters from the green alga Haematococcus pluvialis. Biotechnol Lett, 1999, 21(4): 265–269. DOI: 10.1023/A:1005445927433 |
| [48] | Murphy DJ. The biogenesis and functions of lipid bodies in animals, plants and microorganisms. Prog Lipid Res, 2001, 40(5): 325–438. DOI: 10.1016/S0163-7827(01)00013-3 |
| [49] | Guo Y, Cordes KR, Farese RV Jr, et al. Lipid droplets at a glance. J Cell Sci, 2009, 122(6): 749–752. DOI: 10.1242/jcs.037630 |
| [50] | Zhekisheva M, Zarka A, Khozin-Goldberg I, et al. Inhibition of astaxanthin synthesis under high irradiance does not abolish triacylglycerol accumulation in the green alga Haematococcus pluvialis (Chlorophyceae). J Phycol, 2005, 41(4): 819–826. DOI: 10.1111/jpy.2005.41.issue-4 |
| [51] | Damiani MC, Popovich CA, Constenla D, et al. Lipid analysis in Haematococcus pluvialis to assess its potential use as a biodiesel feedstock. Bioresour Technol, 2010, 101(11): 3801–3807. DOI: 10.1016/j.biortech.2009.12.136 |
| [52] | Goncalves EC, Johnson JV, Rathinasabapathi B. Conversion of membrane lipid acyl groups to triacylglycerol and formation of lipid bodies upon nitrogen starvation in biofuel green algae Chlorella UTEX29. Planta, 2013, 238(5): 895–906. DOI: 10.1007/s00425-013-1946-5 |
| [53] | Kim DK, Hong SJ, Bae JH, et al. Transcriptomic analysis of Haematococcus lacustris during astaxanthin accumulation under high irradiance and nutrient starvation. Biotechnol Bioproc Eng, 2011, 16(4): 698–705. DOI: 10.1007/s12257-011-0081-z |
| [54] | Su YX, Wang JX, Shi ML, et al. Metabolomic and network analysis of astaxanthin-producing Haematococcus pluvialis under various stress conditions. Bioresour Technol, 2014, 170: 522–529. DOI: 10.1016/j.biortech.2014.08.018 |
| [55] | Lu YD, Jiang P, Liu SF, et al. Methyl jasmonate- or gibberellins A3-induced astaxanthin accumulation is associated with up-regulation of transcription of β-carotene ketolase genes (bkts) in microalga Haematococcus pluvialis. Bioresour Technol, 2010, 101(16): 6468–6474. DOI: 10.1016/j.biortech.2010.03.072 |
| [56] | Ding W, Zhao YT, Xu JW, et al. Melatonin: A multifunctional molecule that triggers defense responses against high light and nitrogen starvation stress in Haematococcus pluvialis. J Agric Food Chem, 2018, 66(29): 7701–7711. DOI: 10.1021/acs.jafc.8b02178 |
| [57] | Zhao YT, Yue CC, Ding W, et al. Butylated hydroxytoluene induces astaxanthin and lipid production in Haematococcus pluvialis under high-light and nitrogen-deficiency conditions. Bioresour Technol, 2018, 266: 315–321. DOI: 10.1016/j.biortech.2018.06.111 |
| [58] | Li QQ, Zhang LT, Liu JG. Examination of carbohydrate and lipid metabolic changes during Haematococcus pluvialis non-motile cell germination using transcriptome analysis. J Appl Phycol, 2018. DOI: 10.1007/s10811-018-1524-0 |
| [59] | Recht L, Töpfer N, Batushansky A, et al. Metabolite profiling and integrative modeling reveal metabolic constraints for carbon partitioning under nitrogen starvation in the green algae Haematococcus pluvialis. J Biol Chem, 2014, 289(44): 30387–30403. DOI: 10.1074/jbc.M114.555144 |
 2019, Vol. 35
2019, Vol. 35