中国科学院微生物研究所、中国微生物学会主办
文章信息
- 符文慧, 侯敢, 黄迪南
- Fu Wenhui, Hou Gan, Huang Dinan
- 长链非编码RNA与宫颈癌
- Long non-coding RNA and cervical cancer
- 生物工程学报, 2019, 35(4): 598-606
- Chinese Journal of Biotechnology, 2019, 35(4): 598-606
- 10.13345/j.cjb.180493
-
文章历史
- Received: November 28, 2018
- Accepted: January 29, 2019
宫颈癌是严重威胁妇女健康的第2位恶性肿瘤,每年全球新增宫颈癌病例约49万多例。近年来我国宫颈癌的发生有明显上升和年轻化趋势[1]。宫颈癌是由多种因素、多个基因以及多个环节共同相互作用形成复杂分子调控机制的疾病。早期宫颈癌以手术切除为主,预后良好,而中晚期宫颈癌以手术联合放化疗为主,预后较差[2]。宫颈癌的诊断和治疗缺乏监测肿瘤转移、判断预后、复发以及指导个体化治疗的特异性指标,所以,寻找理想有效的肿瘤分子标志物,对于提高宫颈癌的诊断和治疗具有重要意义。目前生命科学领域研究热门的肿瘤分子标志物是lncRNAs。陈干涛等学者应用高通量lncRNA芯片技术检测宫颈癌组织和正常宫颈组织lncRNA表达谱的变化,共检测出30 586条lncRNA,进行聚类分析和比较后,发现差异性表达的lncRNA共22 043条,其中11 545条表达上调,10 498条表达下调[3]。表明lncRNA在宫颈癌中扮演着重要的角色,而其在宫颈癌中具有什么功能和如何发挥作用值得探讨。
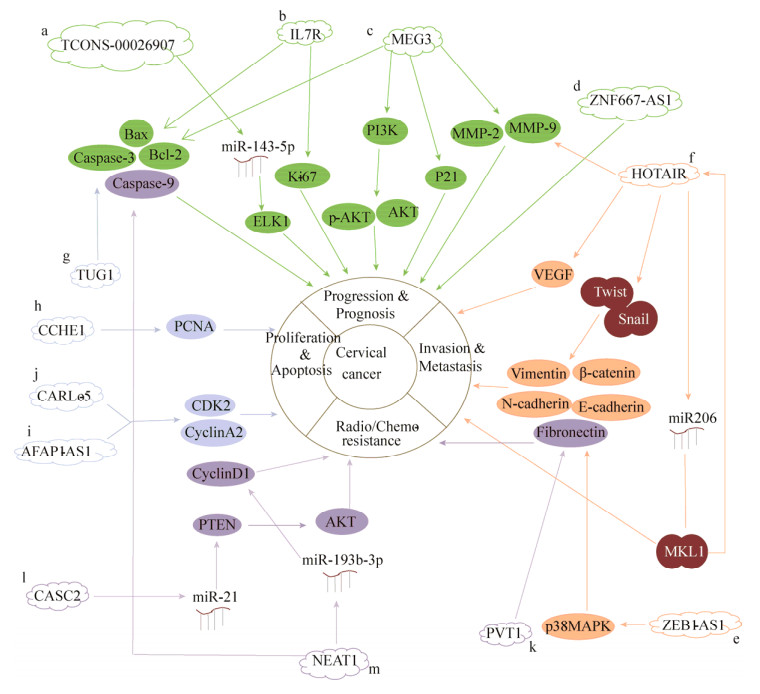
绝大多数人类基因组都能被转录,但仅有少于2%的基因组为蛋白质编码基因,其余基因组转录生成非编码RNA (Non-coding RNA,ncRNAs)。ncRNAs根据分子长度可分成两大类,即长度小于200 nt的短链非编码RNA (Small non-coding RNA,sncRNAs)和长度大于200 nt的长链非编码RNA (Long non-coding RNA,lncRNAs)。根据邻近蛋白编码转录本的相对位置,lncRNAs可分为正义lncRNAs、反义lncRNAs、双向lncRNAs、基因内lncRNAs、基因间lncRNAs[4]。随着二代测序技术的发展,lncRNAs在真核生物的生物学功能和行为机制逐渐被阐明。研究表明,lncRNAs可与DNA、RNA以及蛋白质相互作用,通过对DNA甲基化、组蛋白修饰和miRNA竞争抑制作用的不同分子机制调控细胞的生物学过程[5-7]。lncRNAs不仅广泛参与机体正常的生长发育过程,而且与人类疾病的发生发展密切相关。研究表明多种lncRNAs在宫颈癌的发生发展及宫颈癌治疗后表达变化显著[3, 8]。因此,深入研究lncRNAs与宫颈癌之间的关系,有望为宫颈癌的临床诊断和有效治疗提供新的依据。lncRNAs在宫颈癌的异常调节详见图 1。
|
| 图 1 lncRNAs在宫颈癌的异常调节 Fig. 1 Abnormal regulation of lncRNAs in cervical cancer. a: TCONS_00026907 regulated ELK1 to promote cervical cancer progression by inhibiting miR-143-5p[12]. b: IL7R was an independent prognostic factor for cervical cancer via regulating Bcl-2, caspase-3 and Ki-67[13]; c: MEG3 inhibited cervical cancer progression by modulating PI3K/AKT/Bcl-2/Bax/P21 and PI3K/AKT/MMP-2/9 signaling pathways[15]; d: ZNF667-AS1 was associated with overall survival, tumor size, and FIGO stage of cervical cancer and inhibited cervical cancer cell proliferation and cloning[16]; e:ZEB1-AS1 regulated EMT-related protein E-cadherin to promote invasion and migration of cervical cancer cells via promoting p38MAPK signaling pathway[18]; f: HOTAIR promoted invasion and migration of cervical cancer cells by regulating VEGF, MMP-9, EMT-related proteins E-cadherin, β-catenin and vimentin, transcription factors Snail and Twist, and regulating MKL1 through inhibiting miR206[20-21]; g: TUG1 inhibited apoptosis of cervical cancer cells by regulating Bcl-2 and caspase-3[23]; h: CCHE1 promoted proliferation of cervical cancer cells via regulating PCNA[24]; i, j: AFAP1-AS1 and CARLo-5 promoted proliferation of cervical cancer cells by regulating cell cycle-associated proteins CDK2 and CyclinA2[25]; k: PVT1 promoted chemotherapy resistance of cervical cancer via regulating EMT-related proteins E-cadherin, Fibronectin and Vimentin[31]; l: Low expression of CASC2 regulated PTEN to promote chemotherapy resistance of cervical cancer by promoting miR-21[32]; m: NEAT1 regulated cyclin D1, caspase-3 and caspase-9 to promote radiotherapy resistance of cervical cancer by inhibiting miR-193b-3p[33]. |
| |
LncRNAs在细胞的多个生物学进程中发挥功能,研究表明多种lncRNAs在宫颈癌中存在差异性表达,并且可作为抑癌基因或促癌基因参与宫颈癌细胞的生长、分化、迁移、侵袭以及凋亡等过程,从而影响宫颈癌的发生与发展进程[3, 9]。
1.1 lncRNAs影响宫颈癌的进程和预后宫颈癌是由多种因素、多个基因以及多个环节通过复杂的分子调节机制共同相互作用而引发的疾病。越来越多的研究表明lncRNAs可用于肿瘤的诊断和预后评估[10-11]。Jin等通过lncRNA微阵列芯片发现TCONS_00026907异常表达于宫颈癌。TCONS_00026907在宫颈癌组织表达显著升高,且其可促进细胞周期、增殖、迁移和侵袭的过程,以及抑制细胞凋亡。机制研究表明沉默TCONS_00026907后,miR-143-5p表达显著升高,而miR-143-5p下游的靶基因ELK1表达显著降低,进而抑制宫颈癌的发展进程[12] (图 1a)。另外,从正常宫颈组织、宫颈上皮内瘤变(Cervical intraepithelial neoplasia,CIN)至宫颈癌组织,Fan等[13]发现炎症相关lncRNA白介素7受体(Interleukin 7 receptor,IL7R)表达呈递增趋势。高表达IL7R与肿瘤的大小、FIGO分期、淋巴结转移呈正相关,且其高表达的患者整体生存率缩短,具有不良的预后。Cox回归分析表明IL7R可作为宫颈癌独立的预后因子。功能实验显示干扰IL7R表达可抑制宫颈癌的生长,体外实验显示Bcl-2表达降低,caspase-3表达升高,通过促进细胞凋亡进而抑制宫颈癌的生长;并且体内实验也显示肿瘤增殖标志物Ki-67表达降低而抑制宫颈癌的生长(图 1b)。
与此相反,长链非编码MEG3、ZNF667-AS1在宫颈癌中低表达。母系表达基因3 (Materally expressed gene 3,MEG3)是首个被发现的印记lncRNA,具有抑制肿瘤的作用,其定位于染色体14q32。MEG3低表达于宫颈癌且与FIGO分期、肿瘤大小和淋巴结转移呈负相关[14]。Wang等[15]通过RT-PCR和Western blotting分析显示过表达MEG3后,PI3K、AKT、MMP-2、MMP-9和Bcl-2基因和蛋白表达都降低,而Bax和P21基因和蛋白表达都升高。这表明在宫颈癌中MEG3通过调节PI3K/AKT/Bcl-2/Bax/P21和PI3K/AKT/MMP- 2/9信号通路抑制宫颈癌的进程(图 1c)。另外,转录因子锌指蛋白667 (Zinc finger protein 667, ZNF667-AS1)也被称为lncRNA MORT。其在宫颈癌中显著低表达,且表达量与整体生存率、肿瘤大小和FIGO分期呈负相关,而高表达ZNF667-AS1可降低宫颈癌细胞增殖和克隆能力[16](图 1d)。
1.2 lncRNAs影响宫颈癌的侵袭和迁移侵袭是肿瘤细胞转移过程中最关键的步骤,其包括细胞基质的降解、肿瘤细胞动力分子通路的激活、细胞间链接的转变等[17]。lncRNAs对促进细胞的生长必不可少,其异常表达有助于肿瘤细胞的生长存活。长链非编码ZEB1反义1 (LncRNA ZEB1 Antisense 1,ZEB1-AS1)在宫颈癌中表达上调,且其与宫颈癌侵袭和迁移的临床特性相关。Gan等[18]在实验中发现通过干扰ZEB1-AS1表达可显著降低p-p38表达,表明沉默ZEB1-AS1可有效抑制p38MAPK信号通路。进一步实验发现相对于对照组,共转染ZEB1-AS1siRNA和p38MAPK通路的抑制剂SB203580可使抑制上皮-间质转化(Epithelial-to-mesenchymal transition,EMT)相关的蛋白E-钙粘蛋白(E-cadherin)表达显著上调,而促进EMT转化的波形蛋白(Vimentin)和N-钙粘蛋白(N-cadherin)没有明显变化。此外,干扰ZEB1-AS1后,抑制HeLa细胞EMT转化过程可被p38MAPK激活剂茴香霉素逆转,表明低表达ZEB1-AS1可通过阻断p38MAPK信号通路抑制HeLa细胞EMT转化,进而抑制HeLa细胞侵袭和迁移(图 1e)。另外,Huang等发现同源盒基因转录物反义RNA (Homeobox gene transcript antisense RNA,HOTAIR),一种高表达于宫颈癌的lncRNA,其与宫颈癌的不良预后相关[19]。Kim等[20]发现沉默HOTAIR后,VEGF和MMP-9表达显著降低,E-cadherin表达升高,而β-catenin和Vimentin表达降低,促进EMT转化的转录因子Snail和Twist表达降低,从而抑制宫颈癌侵袭和迁移。这些标志物和转录因子都是肿瘤侵袭和迁移的重要参与者(图 1f)。另外,在HOTAIR沉默后,miR206表达上调,而miR206下游靶蛋白巨核细胞白血病因子1 (Megakaryoblastic leukemia 1,MKL1)表达下调,并且可增加MKL1在胞质的分布,表明HOTAIR通过抑制miR206表达进而促进MKL1表达和改变MKL1细胞内的分布从而促进宫颈癌侵袭和迁移。然而,MKL1可结合HOTAIR启动子CArG盒活化HOTAIR转录,并与HOTAIR形成正反馈而促进HOTAIR表达。总而言之,MKL1是HOTAIR在宫颈癌侵袭和迁移的重要促发者[21] (图 1f)。
1.3 lncRNAs影响宫颈癌的凋亡和增殖lncRNAs通过调控肿瘤细胞的增殖和凋亡,进而影响肿瘤细胞的转归。牛磺酸上调基因1 (Taurine upregulated gene 1,TUG1)是在宫颈癌表达上调的lncRNA,其与宫颈癌细胞的生物学特性和不良预后密切相关[22]。Hu等研究发现,通过实验敲除TUG1,细胞凋亡的线粒体通路相关蛋白Bcl-2表达显著降低,caspase-3表达显著升高,从而促进宫颈癌细胞凋亡[23] (图 1g)。此外,宫颈癌高表达lncRNA 1 (Cervical carcinoma high- expressed lncRNA 1,CCHE1)其过表达可促进宫颈癌细胞增殖,而敲除CCHE1则抑制细胞增殖[24]。RNA pull-down分析显示CCHE1生理上与PCNA mRNA结合,两者之间相互作用,导致肿瘤增殖标志物增殖细胞核抗原(Proliferating cell nuclear antigen,PCNA)表达量上调,从而促进宫颈癌细胞增殖(图 1h)。另外,下调肌动蛋白纤维相关蛋白1-反义RNA 1 (Actin filament-associated protein 1-antisense RNA1,AFAP1-AS1)和癌症相关区域长非编码RNA (Cancer-associated region long non-coding RNA,CARLo-5)可使细胞发生S期阻滞,S期相关蛋白CDK2和Cyclin A2表达量出现不同程度的下调,进而抑制HeLa细胞增殖能力。表明AFAP1-AS1和CARLo-5通过调节细胞周期而影响宫颈癌增殖[25] (图 1i和1j)。
1.4 lncRNAs影响宫颈癌的放化疗抵抗作用越来越多的研究表明,lncRNAs可通过调节细胞周期、凋亡以及DNA损伤修复等方面发挥放化疗抵抗的功能[26-29]。Iden等发现人浆细胞瘤转化迁移基因1 (Plasmacytoma variant translocation 1,PVT1),一种显著高表达于宫颈癌的lncRNA,其高表达与化疗药物顺铂抵抗相关[30]。同时,Shen等[31]也发现抑制PVT1表达可使CaSki细胞中E-cadherin表达显著升高,而Fibronectin和Vimentin表达显著降低,从而增加CaSki细胞对紫杉醇敏感性,而高表达PVT1可诱导EMT转化而使宫颈癌细胞对紫杉醇产生抵抗。这表明PVT1通过促进EMT转化而促进宫颈癌细胞对紫杉醇产生抵抗(图 1k)。此外,易感候选基因2 (Cancer susceptibility candidate 2,CASC2)是低表达于宫颈癌的lncRNA。沉默CASC2表达可显著减弱顺铂抑制宫颈癌细胞增殖和提高半数致死量(IC50)值,而过表达则可增加顺铂抑制宫颈癌细胞增殖和降低IC50值。这表明低表达CASC2促进宫颈癌对顺铂抵抗。机制研究显示CASC2可竞争性抑制miR-21,从而使miR-21下游靶蛋白PTEN (肿瘤抑制子)表达上调,PTEN通过调控AKT信号通路而促进宫颈癌细胞对顺铂化学敏感性[32] (图 1l)。另外,核富集丰富转录本1 (Nuclear enriched abundant transcript 1,NEAT1)是高表达于放疗抵抗宫颈癌细胞的lncRNA[33]。沉默NEAT1表达可减弱放疗抵抗细胞增殖和减少电离辐射剂量,而过表达NEAT1则相反。这表明高表达NEAT1与宫颈癌放疗抵抗密切相关。机制研究发现沉默NEAT1后,miR-193b-3p表达上调,cyclin D1表达下调而使细胞周期阻滞于G0/G1期,并且caspase-3和caspase-9表达上调而诱导细胞凋亡。这表明高表达NEAT1通过调节细胞周期和凋亡促进宫颈癌细胞对放疗抵抗(图 1m)。综上所述,在宫颈癌放化疗中,lncRNAs可通过一定的分子调节机制发挥放化疗抵抗功能。因此,对宫颈癌患者的治疗,尤其是放疗或化疗抵抗的患者,通过调节lncRNAs可能是一个良好的选择。
2 lncRNAs在宫颈癌的分子调节机制 2.1 lncRNAs与蛋白质/mRNAs的相互作用lncRNAs可与蛋白质、mRNA或miRNA相互作用,通过调节基因表达参与生命体基本的生物学功能,例如基因印记、组蛋白修饰、mRNA剪接等[34]。宫颈癌相关lncRNAs可直接结合蛋白质或mRNA在转录后水平发挥调控作用。LINC00473是高表达于宫颈癌的lncRNA,其高表达可促进宫颈癌细胞增殖和抑制细胞凋亡[35]。机制研究显示LINC00473可直接与细胞增殖相关转录因子白介素结合因子2 (Interleukin enhancer binding factor 2,ILF2)结合,这对ILF2 mRNA水平无影响,但ILF2蛋白质水平发生了显著变化:沉默LINC00473后,ILF2蛋白半衰期缩短,而过表达LINC00473后,ILF2蛋白半衰期延长。这表明LINC00473可抑制ILF2蛋白降解,从而促进宫颈癌细胞增殖和抑制细胞凋亡。此外,宫颈癌高表达CCHE1可结合PCNA的mRNA使PCNA表达上调,进而促进宫颈癌细胞增殖[24]。这些研究表明在宫颈癌发展的过程中,lncRNAs与蛋白质或mRNA相互作用发挥着关键的作用。
2.2 lncRNAs与miRNAs的相互作用竞争内源RNA (Competing endogenous RNA, ceRNA)假说是ncRNA家族中的lncRNAs、mRNA、假基因和环状RNA通过其miRNA反应元件(MiRNA response elements,MRE)竞争性地结合miRNA,从而调控基因的表达。也就是说lncRNAs可充当ceRNA在转录后水平上抑制miRNA表达和活性[36-38]。Gao等报道PVT1表达与miR-424呈负相关,表明PVT1可通过负性调节miR-424表达而促进宫颈癌细胞增殖、迁移和侵袭,从而促进宫颈癌的发展[39]。另外,CASC2可竞争性结合miR-21,使miR-21下游靶蛋白PTEN表达上调,从而促进宫颈癌细胞对顺铂化学敏感性而抑制宫颈癌的发展[32]。此外,NEAT1也可作为ceRNA抑制miR-193b-3p表达,从而使miR-193b-3p下游靶标cyclin D1表达上调,加速细胞周期而促进宫颈癌的发展[33]。综上所述,lncRNA可充当“miRNA海绵”抑制miRNA的表达,使miRNA下游靶标表达上调或下调,从而促进肿瘤或者抑制肿瘤的发展。
2.3 lncRNAs的单核苷酸多态性(Single nucleotide polymorphisms, SNPs)全基因组关联研究(Genome-wide association studies,GWAS)揭示大量与疾病和性状相关的遗传变异关系密切,其中至少有三分之一已识别的变异不在蛋白质编码基因内,而是映射到非编码区间[40]。Verhaegh等首次报道H19基因的单核苷酸多态性与膀胱癌形成风险密切相关,从此拉开了lncRNA的单核苷酸与肿瘤研究的序幕[41]。多项研究表明抑癌基因或促癌基因单核苷酸多态性等体细胞突变在宫颈癌遗传易感性中起重要作用[42-44]。Guo等[45]通过对510名宫颈癌患者与713名正常人进行病例对照分析发现HOTAIR内3个单倍体标签SNPs (rs920778,rs1899663和rs4759314)与宫颈癌形成风险关系紧密。其中在HOTAIR增强子基因内的SNP rs920778与宫颈癌有强关联性。相对于野生型rs920778 CC基因型,携带rs920778 (CT+TT)突变基因型的患者形成宫颈癌的风险增加2.17倍。HOTAIR rs920778 SNP T变异等位基因定位于HOTAIR内含子2区,可增强位于HOTAIR内含子2区增强子的活性。并且相对于rs920778 CC者,携带rs920778 CT或TT基因型的宫颈癌患者HOTAIR mRNA表达显著升高。这表明风险相关等位基因T与HOTAIR表达息息相关,并且SNP rs920778可促进HOTAIR表达,进而促进宫颈癌的遗传易感性。同时,Jin等也报道HOTAIR rs7958904通过调节宫颈癌细胞增殖影响宫颈癌的遗传易感性[46]。此外,有研究表明TNF和HNRNPL相关免疫调节lncRNA (TNF and HNRNPL related immunoregulatory lncRNA,THRIL)基因的rs7133268可减少宫颈癌前病变的遗传易感性[47]。这些研究表明lncRNA的单核苷酸多态性在宫颈癌的发生与发展中发挥重要作用。
3 lncRNAs在宫颈癌的潜在临床应用转移和复发是宫颈癌临床治疗的最大障碍。所以,寻找有效的肿瘤标志物对提高宫颈癌的预后具有重要价值。lncRNAs可用于宫颈癌的诊断和预后评估。例如,受试者工作特征曲线(Receiver operating characteristic curve,ROC)分析显示SPRY4内含子转录本1 (SPRY4 intronic transcript 1,SPRY4-IT1)的表达是区分宫颈癌组织和正常组织的良好候选物(灵敏度:78.3%,特异性:63.6%),ROC曲线下的面积(Area under ROC curve,AUC)为0.741(95%CI:0.632–0.849, P < 0.001),表明SPRY4用于宫颈癌的诊断准确性较高[48]。此外,HOTAIR也可用于区分宫颈癌组
织与正常组织(敏感性:60.6%,特异性:87.2%)以及淋巴结是否发生转移(敏感度:85.1%,特异性:64.9%),并且多因素Cox回归模型显示FIGO分期(P < 0.000 1,HR=1.994,95%CI: 1.359–2.927)、淋巴结转移(P=0.005,HR=2.636,95% CI: 1.348– 5.156)和HOTAIR表达水平(P=0.012,HR=2.863,95% CI: 1.263–6.490),表明HOTAIR对宫颈癌诊断准确性高,可作为宫颈癌预后评估的独立预测因子[19]。综上所述,lncRNAs中的SPRY4和HOTAIR是宫颈癌有前途的标志物,可作为宫颈癌诊断指标和预后判断的良好标志物。
4 结语与展望lncRNAs贯穿于生物学过程的始终,其通过各种不同的分子调节机制参与调控宫颈癌细胞的增殖、抗凋亡、迁移、放化疗抵抗等过程,并且在宫颈癌发生发展过程中发挥着关键的作用。因此,这些lncRNAs都是宫颈癌理想的分子标志物,有望成为宫颈癌治疗的有效靶点。虽然在宫颈癌病理发展的机制中,lncRNAs的研究已经取得了一定成就,但宫颈癌的发生发展并非单因素决定,lncRNAs参与宫颈癌发病具体的调控机制及功能模式有待进一步的深入研究,有待与影响宫颈癌发病的其他因素相结合。例如:lncRNAs与表观遗传学的研究,包括DNA甲基化、组蛋白修饰、基因印迹、染色体重塑等方面;lncRNAs与miRNA调控网络的研究以及lncRNAs与细胞信号转导通路的研究等。后续研究的工作重心应以现有的研究成果为切入点,进一步深化lncRNAs调控宫颈癌发生发展机制的研究,全面揭示其中的奥秘。lncRNAs作为临床宫颈癌诊治的有效标志物必将具有广阔的应用前景。
| [1] |
Xia Y, Zhang T, Zhang ZX, et al. Gene, human papillomavirus and serum related protein detection in New progress of the application of cervical cancer diagnosis.
J Chin Pract Diagn Ther, 2008, 22(12): 924–927.
(in Chinese). 夏燕, 张钿, 张朝霞, 等. 基因、人乳头瘤病毒和血清蛋白相关检测在宫颈癌诊断中的应用新进展. 中华实用诊断与治疗杂志, 2008, 22(12): 924-927. |
| [2] |
Wang RG, Shen Y, Li Q, et al. DNA damage-induced lncRNA-UCA1 promotes HeLa cell proliferation.
Chin J Biochem Mol Biol, 2015, 31(5): 505–513.
(in Chinese). 王瑞国, 沈远, 李清, 等. DNA损伤诱导的长链非编码RNA-UCA1促进HeLa细胞增殖. 中国生物化学与分子生物学报, 2015, 31(5): 505-513. |
| [3] |
Chen GT, Yang X, Cheng YX. Different expression profiles of lncRNA in cervical cancer tissues and its significance.
Pract J Cancer, 2017, 32(4): 523–527.
(in Chinese). 陈干涛, 杨潇, 程艳香. LncRNA在宫颈癌组织中的表达谱变化及意义. 实用癌症杂志, 2017, 32(4): 523-527. DOI:10.3969/j.issn.1001-5930.2017.04.001 |
| [4] | Esteller M. Non-coding RNAs in human disease. Nat Rev Genet, 2011, 12(12): 861–874. DOI: 10.1038/nrg3074 |
| [5] | Lu HZ, He Y, Lin L, et al. Long non-coding RNA MALAT1 modulates radiosensitivity of HR-HPV+ cervical cancer via sponging miR-145. Tumour Biol, 2016, 37(2): 1683–1691. DOI: 10.1007/s13277-015-3946-5 |
| [6] | Heilmann K, Toth R, Bossmann C, et al. Genome-wide screen for differentially methylated long noncoding RNAs identifies Esrp2 and lncRNA Esrp2-as regulated by enhancer DNA methylation with prognostic relevance for human breast cancer. Oncogene, 2017, 36(46): 6446–6461. DOI: 10.1038/onc.2017.246 |
| [7] | Ma ZH, Huang H, Wang JR, et al. Long non-coding RNA SNHG15 inhibits P15 and KLF2 expression to promote pancreatic cancer proliferation through EZH2-mediated H3K27me3. Oncotarget, 2017, 8(48): 84153–84167. |
| [8] |
Cheng YX, Yang X, Chen GT. Significance of different expression profiles of LncRNA in cervical cancer before and after radiotherapy and chemotherapy.
Med J Wuhan Univ, 2017, 38(2): 241–246, 333.
(in Chinese). 程艳香, 杨潇, 陈干涛. 宫颈癌放化疗前后LncRNA的表达谱变化及意义. 武汉大学学报:医学版, 2017, 38(2): 241-246, 333. |
| [9] | Gibb EA, Becker-Santos DD, Enfield KSS, et al. Aberrant expression of long noncoding RNAs in cervical intraepithelial neoplasia. Int J Gynecol Cancer, 2012, 22(9): 1557–1563. DOI: 10.1097/IGC.0b013e318272f2c9 |
| [10] | Kunej T, Obsteter J, Pogacar Z, et al. The decalog of long non-coding RNA involvement in cancer diagnosis and monitoring. Crit Rev Clin Lab Sci, 2014, 51(6): 344–357. DOI: 10.3109/10408363.2014.944299 |
| [11] | Dai MY, Chen SY, Wei XM, et al. Diagnosis, prognosis and bioinformatics analysis of lncRNAs in hepatocellular carcinoma. Oncotarget, 2017, 8(56): 95799–95809. |
| [12] | Jin XJ, Chen XJ, Hu Y, et al. LncRNA-TCONS_00026907 is involved in the progression and prognosis of cervical cancer through inhibiting miR-143-5p. Cancer Med, 2017, 6(6): 1409–1423. DOI: 10.1002/cam4.2017.6.issue-6 |
| [13] | Fan YY, Nan Y, Huang JJ, et al. Up-regulation of inflammation-related LncRNA-IL7R predicts poor clinical outcome in patients with cervical cancer. Biosci Rep, 2018, 38(3): BSR20180483. DOI: 10.1042/BSR20180483 |
| [14] | Zhang J, Yao TT, Wang YX, et al. Long noncoding RNA MEG3 is downregulated in cervical cancer and affects cell proliferation and apoptosis by regulating miR-21. Cancer Biol Ther, 2016, 17(1): 104–113. DOI: 10.1080/15384047.2015.1108496 |
| [15] | Wang XG, Wang ZH, Wang JC, et al. LncRNA MEG3 has anti-activity effects of cervical cancer. Biomed Pharmacother, 2017, 94: 636–643. DOI: 10.1016/j.biopha.2017.07.056 |
| [16] | Zhao LP, Li RH, Han DM, et al. Independent prognostic Factor of low-expressed LncRNA ZNF667-AS1 for cervical cancer and inhibitory function on the proliferation of cervical cancer. Eur Rev Med Pharmacol Sci, 2017, 21(23): 5353–5360. |
| [17] | Friedl P, Wolf K. Tumour-cell invasion and migration: diversity and escape mechanisms. Nat Rev Cancer, 2003, 3(5): 362–374. DOI: 10.1038/nrc1075 |
| [18] | Gan L, Chen Y, Liu H, et al. Long non-coding RNA ZEB1-antisense 1 affects cell migration and invasion of cervical cancer by regulating epithelial- mesenchymal transition via the p38MAPK signaling pathway. Gynecol Obstet Invest, 2018: 1–9. |
| [19] | Huang L, Liao LM, Liu AW, et al. Overexpression of long noncoding RNA HOTAIR predicts a poor prognosis in patients with cervical cancer. Arch Gynecol Obstet, 2014, 290(4): 717–723. DOI: 10.1007/s00404-014-3236-2 |
| [20] | Kim HJ, Lee DW, Yim GW, et al. Long non-coding RNA HOTAIR is associated with human cervical cancer progression. Int J Oncol, 2015, 46(2): 521–530. DOI: 10.3892/ijo.2014.2758 |
| [21] | Zheng P, Yin Z, Wu Y, et al. LncRNA HOTAIR promotes cell migration and invasion by regulating MKL1 via inhibition miR206 expression in HeLa cells. Cell Commun Signal, 2018, 16: 5. DOI: 10.1186/s12964-018-0216-3 |
| [22] | Zhu J, Shi HR, Liu HN, et al. Long non-coding RNA TUG1 promotes cervical cancer progression by regulating the miR-138-5p-SIRT1 axis. Oncotarget, 2017, 8(39): 65253–65264. |
| [23] | Hu YY, Sun XW, Mao CC, et al. Upregulation of long noncoding RNA TUG1 promotes cervical cancer cell proliferation and migration. Cancer Med, 2017, 6(2): 471–482. DOI: 10.1002/cam4.2017.6.issue-2 |
| [24] | Yang M, Zhai X, Xia BR, et al. Long noncoding RNA CCHE1 promotes cervical cancer cell proliferation via upregulating PCNA. Tumour Biol, 2015, 36(10): 7615–7622. DOI: 10.1007/s13277-015-3465-4 |
| [25] |
Li XR. Effects of cell proliferation, cycle, apoptosis and the expression of survivin gene after down-regulating the expression of lncRNA in Hela[D]. Zhanjiang: Guangdong Medical University, 2018 (in Chinese). 李相如. lncRNA沉默对Hela细胞survivin基因表达以及细胞增殖、周期和凋亡的影响[D].湛江: 广东医科大学, 2018. |
| [26] | Jing L, Yuan W, Dong RF, et al. HOTAIR enhanced aggressive biological behaviors and induced radio-resistance via inhibiting p21 in cervical cancer. Tumour Biol, 2015, 36(5): 3611–3619. DOI: 10.1007/s13277-014-2998-2 |
| [27] | Harries LW. Long non-coding RNAs and human disease. Biochem Soc Trans, 2012, 40(4): 902–906. DOI: 10.1042/BST20120020 |
| [28] | Lipovich L, Johnson R, Lin CY. MacroRNA underdogs in a microRNA world: evolutionary, regulatory, and biomedical significance of mammalian long non-protein-coding RNA. Biochim Biophys Acta, 2010, 1799(9): 597–615. DOI: 10.1016/j.bbagrm.2010.10.001 |
| [29] | Hu XG, Jiang HJ, Jiang XJ. Downregulation of lncRNA ANRIL inhibits proliferation, induces apoptosis, and enhances radiosensitivity in nasopharyngeal carcinoma cells through regulating miR-125a. Cancer Biol Ther, 2017, 18(5): 331–338. DOI: 10.1080/15384047.2017.1310348 |
| [30] | Iden M, Fye S, Li KG, et al. The lncRNA PVT1 contributes to the cervical cancer phenotype and associates with poor patient prognosis. PLoS ONE, 2016, 11(5): e0156274. DOI: 10.1371/journal.pone.0156274 |
| [31] | Shen CJ, Cheng YM, Wang CL. LncRNA PVT1 epigenetically silences miR-195 and modulates EMT and chemoresistance in cervical cancer cells. J Drug Target, 2017, 25(7): 637–644. DOI: 10.1080/1061186X.2017.1307379 |
| [32] | Feng YQ, Zou W, Hu CH, et al. Modulation of CASC2/miR-21/PTEN pathway sensitizes cervical cancer to cisplatin. Arch Biochem Biophys, 2017, 623-624: 20–30. DOI: 10.1016/j.abb.2017.05.001 |
| [33] | Han DM, Wang JF, Cheng GH. LncRNA NEAT1 enhances the radio-resistance of cervical cancer via miR-193b-3p/CCND1 axis. Oncotarget, 2018, 9(2): 2395–2409. |
| [34] | Maass PG, Luft FC, Bähring S. Long non-coding RNA in health and disease. J Mol Med, 2014, 92(4): 337–346. DOI: 10.1007/s00109-014-1131-8 |
| [35] | Shi C, Yang YJ, Yu JP, et al. The long noncoding RNA LINC00473, a target of microRNA 34a, promotes tumorigenesis by inhibiting ILF2 degradation in cervical cancer. Am J Cancer Res, 2017, 7(11): 2157–2168. |
| [36] | Hendrickson DG, Hogan DJ, McCullough HL, et al. Concordant regulation of translation and mRNA abundance for hundreds of targets of a human microRNA. PLoS Biol, 2009, 7(11): e1000238. DOI: 10.1371/journal.pbio.1000238 |
| [37] | Salmena L, Poliseno L, Tay Y, et al. A ceRNA hypothesis: the Rosetta Stone of a hidden RNA language?. Cell, 2011, 146(3): 353–358. |
| [38] | Guo HL, Ingolia NT, Weissman JS, et al. Mammalian microRNAs predominantly act to decrease target mRNA levels. Nature, 2010, 466(7308): 835–840. DOI: 10.1038/nature09267 |
| [39] | Gao YL, Zhao ZS, Zhang MY, et al. Long noncoding RNA PVT1 facilitates cervical cancer progression via negative regulating of miR-424. Oncol Res, 2017, 25(8): 1391–1398. DOI: 10.3727/096504017X14881559833562 |
| [40] | Hindorff LA, Sethupathy P, Junkins HA, et al. Potential etiologic and functional implications of genome-wide association loci for human diseases and traits. Proc Natl Acad Sci USA, 2009, 106(23): 9362–9367. DOI: 10.1073/pnas.0903103106 |
| [41] | Verhaegh GW, Verkleij L, Vermeulen SHHM, et al. Polymorphisms in the H19 gene and the risk of bladder cancer. Eur Urol, 2008, 54(5): 1118–1126. DOI: 10.1016/j.eururo.2008.01.060 |
| [42] | Yang YJ, Ren J, Zhang QZ. Distribution of human papilloma virus type 16 E6/E7 gene mutation in cervical precancer or cancer: a case control study in Guizhou Province, China. J Med Virol, 2016, 88(2): 345–350. DOI: 10.1002/jmv.24333 |
| [43] | Pu Y, Zhang Z, Zhou B, et al. Association of an insertion/deletion polymorphism in IL1A 3ʹ-UTR with risk for cervical carcinoma in Chinese Han Women. Hum Immunol, 2014, 75(8): 740–744. DOI: 10.1016/j.humimm.2014.05.004 |
| [44] | Zhang W, Jiang YH, Yu QM, et al. EGFR promoter methylation, EGFR Mutation, and HPV infection in Chinese cervical squamous cell carcinoma. Appl Immunohistochem Mol Morphol, 2015, 23(9): 661–666. DOI: 10.1097/PAI.0000000000000128 |
| [45] | Guo LS, Lu XG, Zheng LJ, et al. Association of long non-coding RNA HOTAIR polymorphisms with cervical cancer risk in a Chinese population. PLoS ONE, 2016, 11(7): e0160039. DOI: 10.1371/journal.pone.0160039 |
| [46] | Jin H, Lu XY, Ni J, et al. HOTAIR rs7958904 polymorphism is associated with increased cervical cancer risk in a Chinese population. Sci Rep, 2017, 7(1): 3144. DOI: 10.1038/s41598-017-03174-1 |
| [47] | Wang Y, Liu Y, Li ZY, et al. Association between MALAT1 and THRIL polymorphisms and precancerous cervical lesions. Genet Test Mol Biomarkers, 2018, 22(9): 509–517. DOI: 10.1089/gtmb.2018.0097 |
| [48] | Cao Y, Liu YL, Lu XY, et al. Upregulation of long noncoding RNA SPRY4-IT1 correlates with tumor progression and poor prognosis in cervical cancer. FEBS Open Bio, 2016, 6(9): 954–960. DOI: 10.1002/2211-5463.12102 |
 2019, Vol. 35
2019, Vol. 35