中国科学院微生物研究所、中国微生物学会主办
文章信息
- 王翌, 李淼, 孙元, 仇华吉
- Wang Yi, Li Miao, Sun Yuan, Qiu Hua-Ji
- 靶向M细胞的抗原递送——增强黏膜免疫应答的关键策略
- Microfold cells-targeting antigen delivery: a promising strategy to enhance the efficacy of mucosal vaccines
- 生物工程学报, 2019, 35(2): 216-225
- Chinese Journal of Biotechnology, 2019, 35(2): 216-225
- 10.13345/j.cjb.180234
-
文章历史
- Received: June 7, 2018
- Accepted: September 12, 2018
- Published: September 30, 2018
临床中,大多数病原微生物是通过黏膜入侵机体的,黏膜构成了机体抵抗病原入侵的第一道防线。黏膜免疫系统既是机体整个免疫网络的重要组成部分,又是具有独特结构和功能的独立免疫系统。黏膜疫苗不仅能诱导黏膜免疫应答,还能诱导全身免疫应答,且能在病原体入侵的早期阶段发挥免疫保护作用,有效克服现有疫苗的局限性[1]。同时,黏膜疫苗免疫操作简便,非专业人员也能随时随地完成疫苗接种,尤其对于发展中国家而言,十分经济、可行。20世纪50年代问世的Sabin株脊髓灰质炎口服减毒活疫苗是黏膜疫苗的首个成功范例[2]。尽管黏膜免疫具有很多优势,但到目前为止,商品化的黏膜疫苗数量有限[3],这主要是由于黏膜免疫系统复杂的结构和特殊的理化特性制约了黏膜疫苗的发展。为克服现有障碍,研究者们采用不同的抗原递送策略以期将疫苗抗原精准递送至特定黏膜免疫诱导位点,实现黏膜免疫的高效应答[4]。其中,将疫苗抗原与黏膜免疫细胞配体融合表达后可借助配体与受体的结合,实现抗原的靶向递送。M细胞(Microfold cells)是黏膜免疫系统中抗原进入黏膜相关淋巴组织的主要门户,利用靶向M细胞的抗原递送策略作为突破口,可诱导高效的黏膜免疫应答,突破黏膜疫苗研制的现有瓶颈。
1 黏膜免疫系统是机体抵御病原入侵的第一道免疫屏障人和动物机体的黏膜在与病原体接触的过程中逐渐形成具备特有淋巴组织结构的黏膜免疫系统,其在抵抗病原微生物入侵中起着至关重要的作用,是机体抵御感染的第一道免疫屏障。黏膜广泛存在于胃肠道、呼吸道和泌尿生殖道,其中胃肠道黏膜作为黏膜免疫系统的重要组成部分,特有的淋巴组织结构特点尤为显著,因此本研究将主要以胃肠道为例介绍其在黏膜免疫系统中的重要作用。胃肠道黏膜免疫系统主要是指胃肠道相关的淋巴样组织(Gut-associated lymphoid tissues,GALTs),可根据形态结构、分布及功能将GALTs分为淋巴滤泡和黏膜固有层中的弥散淋巴组织两部分,分别为免疫应答诱导部位和效应部位,二者共同发挥黏膜免疫屏障的作用[4]。其中,诱导部位包括Peyer氏结(Peyer’s patches,PPs)、M细胞和树突状细胞(Dendritic cells,DCs)等,主要负责对抗原进行捕获、处理和递呈,是免疫反应起始的部位。PPs是典型的黏膜相关淋巴组织,由小肠中多个淋巴滤泡聚集组合而成,是能够执行免疫应答全过程的场所,在诱导肠道免疫的过程中发挥重要作用[5]。M细胞能够将肠腔内抗原输送至淋巴滤泡,进一步由抗原递呈细胞(Antigen presenting cells,APCs) (如DCs、B淋巴细胞和巨噬细胞等)处理[6]。而在免疫应答效应部位,特异性免疫反应所分泌的抗原特异性抗体如分泌型免疫球蛋白A (Secretory immunoglobulin A,sIgA)及分化成熟的免疫细胞执行特定的功能[7-8]。其中,幼稚B细胞和IgA+ B细胞从PPs中快速转移至肠系膜淋巴结,进一步发育成熟。最后,抗原特异性的CD4+ T细胞和IgA+ B细胞通过优先归巢机制经由胸导管和血液循环迁移到远距离的效应部位,如肠黏膜固有层、鼻黏膜、肺等发挥抗感染作用[9]。
2 M细胞在黏膜免疫应答中发挥重要作用1965年,研究者在兔阑尾中首次发现了M细胞,最初称其为淋巴上皮细胞。后来,由于观察到人M细胞顶部的“微皱褶”结构而将其改名为微皱褶细胞[10]。M细胞能够捕获腔内抗原并将其转运到淋巴组织,在诱导黏膜免疫的过程中发挥重要作用,而利用靶向M细胞的特异性分子(配体)与抗原结合形成复合物可以增强抗原入侵能力,有效启动免疫反应,进而发挥黏膜免疫保护作用。
靶向M细胞的抗原递送策略具有极大的应用潜力,深入了解M细胞的基本特征与功能有助于更好地利用这一策略。M细胞具有独特的形态特征,比如短而不规则的微绒毛使得M细胞的顶部呈凹陷状[11],M细胞的糖萼层薄使其成为腔内抗原内流和细菌附着的理想靶标[12]。同时,M细胞的形态在各种动物之间存在较大的差异,即使在同一物种,不同解剖位置上也有差异[13]。此外,M细胞具有强大的内吞活性,用以行使捕获腔内抗原的功能。尽管M细胞的结构特征与其他上皮细胞不同,但紧密连接和桥粒使M细胞与相邻的柱状细胞和交错的侧膜接触,同样可以维持屏障功能[14]。M细胞的基底膜内陷,形成许多“小囊”,可供淋巴细胞驻留。这些“小囊”的形成大大减少了胞内距离,使M细胞能够在10–15 min内快速地将抗原物质运送到基底膜[15]。这些特点促使M细胞极易内化腔内大分子物质,包括蛋白质和微粒,如病毒、细菌甚至小型寄生虫,并将其输送到淋巴组织(图 1)[16]。

|
| 图 1 细胞捕获腔内抗原启动黏膜免疫 Fig. 1 M cells capture luminal antigens to initiate mucosal immune responses. M cells have a strong ability to capture antigens. M cells take up luminal antigens and deliver them to antigen presenting cells, such as dendritic cells, thereby activating T and B cells for initiating immune responses |
| |
M细胞摄取抗原不会导致其降解,而是将完整的抗原递呈给下层的淋巴组织,发挥起始免疫的作用[17]。对M细胞的研究显示,M细胞具有强大的吞噬活性和抗原捕获能力,其摄取腔内抗原有3种途径:非特异性的细胞内吞作用、特异性受体介导的内吞作用和DCs通过M细胞将树突延伸至腔内捕获抗原[17]。首先,一些肠道致病菌利用M细胞作为感染入侵位点,通过与M细胞表面的特定受体相互作用而与M细胞结合。而其他上皮细胞也可表达类似受体,但这些受体被其毛刷边界和较厚的黏液层所掩盖,比滤泡相关上皮(Follicle-associated epithelium,FAE)中M细胞的受体更难接触到抗原[17]。最先发现与M细胞特异性结合的病原体的例子是假结核耶尔森菌侵袭素结合M细胞的整合素β1[18]。其次,微生物入侵的刺激不仅能促进M细胞介导的抗原摄取,而且还能使DCs进入FAE,这些DCs可以调节M细胞的活性或直接捕获抗原,此类现象是在接种呼肠孤病毒后发现的[19-21]。另外,sIgA可以作为一个桥梁在FAE中完成M细胞对抗原的间接摄取,从而实现针对sIgA免疫复合物的M细胞胞吞转运途径[22]。M细胞可摄取与sIgA结合的可溶性抗原以及sIgA包裹的细菌等大分子抗原[23]。sIgA与抗原的结合会引起自身分子的构象变化,这可使抗原-sIgA复合物优先于游离的sIgA与M细胞结合[22]。
3 利用M细胞的表面标志可实现抗原的靶向递送尽管至今还没有通用的M细胞表面标志,但在过去的几年中,已有关于具有物种和组织特异性M细胞表面标志的相关研究[24-25]。M细胞游离面的表面标志与摄取抗原和病原微生物的入侵密切相关,利用其特殊的表面标志可实现抗原的靶向递送(图 2)。
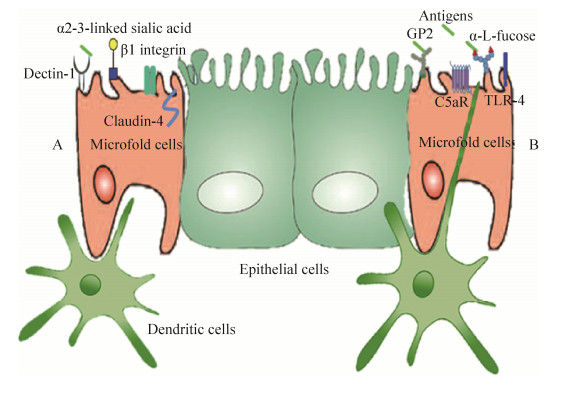
|
| 图 2 细胞利用不同受体捕获抗原模式图 Fig. 2 M cell receptors used for targeting strategies in mucosal vaccination. (A) M cell receptors and ligands: Dectin-1 can be targeted by secretory immunoglobulin A (sIgA) which transport antigen as a carrier. α2-3-linked sialic acid is recognized by the protein σ1 expressed by reoviruses. The C-terminal domain of Clostridium perfringens enterotoxin can target claudin-4 receptor. The invasin of Yersinia enterocolitica and the Arg-Gly-Asp (RGD) peptide can be used to target the β1 integrin. (B) M cell receptors and ligands: FimH expressed by E. coli and Salmonella and anti-glycoprotein 2 (GP2) monoclonal antibody can interact with GP2 on M cells. C5aR is able to be targeted by outer membrane protein H (OmpH) and Co1 ligands. Lipopolysaccharide (LPS) can recognize Toll-like receptor 4 (TLR-4) on M cells. Ulex europaeus agglutinin-1 (UEA-1) reacts with α-L-fucose to achieve targeting M cells |
| |
凝集素是研究最多的生物粘附剂之一,由蛋白质和糖蛋白组成,可与特定的碳水化合物残基结合。M细胞表达独特的碳水化合物受体,可与凝集素特异性结合,为黏膜疫苗的递送提供靶标,这使得凝集素成为黏膜靶向递送策略的理想选择[26]。其中,研究最为广泛的植物凝集素之一是与小鼠M细胞上α-L-岩藻糖残基结合的植物凝集素-1 (Ulex europaeus agglutinin 1,UEA-1) [4]。其次,许多肠道病原体通过M细胞入侵宿主,可利用细菌粘附素、毒素和病毒蛋白实现靶向M细胞的抗原递送[27]。例如,糖蛋白2 (Glycoprotein 2,GP2)是一种在人和小鼠中表达的M细胞受体,可通过识别细菌外膜上Ⅰ型菌毛的成分FimH,与大肠杆菌和沙门氏菌等结合[28];伊尔森氏鼠疫杆菌外膜蛋白H (Outer membrane protein H,OmpH)通过与M细胞表面受体C5aR的结合,完成靶向M细胞的抗原递送[29]。精氨酸-甘氨酸-天冬氨酸肽(Arg-Gly-Asp peptide,RGD肽)是一种普遍存在的细胞粘附肽,可与整合素β1结合,而由于整合素β1在M细胞表面超表达,所以利用RGD肽可完成对M细胞的靶向作用[30]。此外,M细胞表面的其他蛋白受体也已用于抗原的靶向递送[31-32]。目前,经研究证实可实现抗原靶向递送的M细胞表面标志及其相应配体如表 1所示。
| Ligands | M cell-specific molecules (Receptors on M cell) | References |
| UEA-1 | α-L-fucose | [33-34] |
| Co1 | C5aR | [35-36] |
| mAb NKM 16-2-4 | α(1, 2)-fucose-containing carbohydrate | [37] |
| FimH (E. coli and Salmonella) | GP2 | [28, 38-39] |
| Invasin | β1 integrin | [18] |
| Protein σ1 (reovirus) | α2-3-linked sialic acid | [40-41] |
| LPS | TLR-4 | [42] |
| OmpH | C5aR | [29, 43] |
| RGD peptide | β1 integrin | [30] |
| C-terminal domain of the Clostridium perfringens | Claudin-4 | [44-46] |
| Anti-glycoprotein 2 | GP2 | [47] |
| sIgA | Dectin-1 | [48-50] |
| UEA-1: Ulex europaeus agglutinin-1; GP2: glycoprotein 2; LPS: lipopolysaccharide; TLR-4: Toll-like receptor 4; OmpH: outer membrane protein H; RGD peptide: Arg-Gly-Asp peptide; mAb NKM 16-2-4: a novel M cell-specific monoclonal antibody. | ||
摄取抗原是启动黏膜免疫反应的首要步骤,M细胞作为病原入侵机体的主要门户在病原微生物入侵和抗原递呈中发挥重要作用。增加M细胞数量有助于吸收更多的腔内抗原,但除了目标抗原,还包括其他无关抗原,这会增加诱发食物过敏和炎症疾病的可能性。因此,靶向M细胞的抗原递送是增强黏膜免疫应答的上佳策略[6]。
长期以来,由于对M细胞表面分子知之甚少,缺乏关于M细胞特异性表面标志的足够信息,研制以M细胞为靶标的黏膜疫苗一直较为困难[51],直到建立人M细胞体外培养模型才克服这一难题[37, 52]。研究表明,M细胞捕获抗原是由细胞表面与致病感染密切相关的特异性受体所介导的[6]。以M细胞为靶标,设计靶向M细胞配体介导的抗原递送系统,可以特异性增强抗原的摄取,进而增强黏膜免疫效力,推动黏膜疫苗发展。病原体可利用M细胞表达的表面标志作为入侵机体的门户,一些病原体通过与M细胞表面标志结合而起始感染。大量研究证实,抗原-M细胞配体复合物能通过配体与M细胞特异性表面标志的相互作用实现抗原的靶向递送。例如,当将识别M细胞表面标志α(1, 2)岩藻糖的特异性抗体NKM 16-2-4应用于肉毒毒素口服疫苗模型时,NKM 16-2-4结合抗原后进行免疫接种诱导了高水平的抗原特异性sIgA[37]。以M细胞为靶标的配体Co1和侵袭蛋白OmpH已成功应用于登革热病毒口服疫苗的研制,登革热病毒EDIII蛋白与Co1或OmpH融合表达后免疫动物,通过与M细胞受体C5aR的相互作用,不仅增强了EDIII蛋白的M细胞靶向性,同时诱导了全身和局部黏膜免疫反应[29, 35, 53]。我们同样选择OmpH构建了其与传染性法氏囊病病毒的主要抗原VP2蛋白融合表达的重组乳酸菌,口服免疫后诱导了有效的黏膜和全身免疫应答,提供了良好的免疫保护[54]。负载乙型肝炎病毒表面抗原的PLGA纳米颗粒与UEA-1共轭结合后免疫小鼠,在黏膜分泌物中可观察到增强的抗原特异性sIgA反应,而未结合UEA-1的抗原免疫小鼠则无明显变化[55]。小鼠和人的M细胞均表达封闭蛋白4 (Claudin-4),产气荚膜梭菌肠毒素的羧基端结构域能与其结合,与单独表达流感病毒血凝素(Hemagglutinin,HA)相比,融合羧基端结构域的HA滴鼻免疫小鼠后,能诱导更强的HA特异性体液和黏膜免疫反应[44]。GP2是M细胞表面特异性表达的抗原捕获受体,抗GP2单克隆抗体与卵清蛋白(Ovalbumin,OVA)融合表达后能有效增强对M细胞的靶向性,将该融合蛋白与单独的OVA抗原分别口服免疫小鼠后,发现融合蛋白免疫组小鼠的粪便中OVA特异性黏膜免疫反应明显强于对照组[47]。此外,研究发现sIgA能与M细胞表面受体dectin-1结合,将人类免疫缺陷病毒(Human immune deficiency virus,HIV)抗原p24与sIgA化学结合后口服免疫小鼠,可将抗原快速递送至肠道组织并进一步靶向M细胞,并且在局部黏膜和全身均诱导了有效的免疫反应,使用表达p24的重组痘苗病毒攻毒后,免疫小鼠的病毒载量和症状明显减少[48],这表明sIgA-抗原复合物的黏膜递送策略用于肠道病原体的疫苗研制具有很大的潜力。
5 黏膜疫苗的机遇与挑战目前市场上已批准使用的疫苗多为肌肉或皮下注射,与其相比,黏膜疫苗具有众多优势[56]。黏膜疫苗可以模拟自然感染途径,直接刺激黏膜下丰富的淋巴组织产生大量免疫活性细胞和抗体,切断病原微生物入侵机体的途径,同时诱导局部黏膜免疫和全身免疫反应。而且,黏膜疫苗免疫操作简便,对机体刺激性小,可降低发生不良反应的风险,也可避免通过受污染的注射器等传播疾病的可能,这比现有的许多疫苗更具吸引力[3]。此外,肠道环境特殊,存在大量细菌等微生物,对通过口服免疫的黏膜疫苗纯度要求较为宽松,例如在内毒素相关限定方面,口服疫苗更易满足要求[57]。在经济上,黏膜疫苗适合于贫穷地区的大规模疫苗接种,减少了对训练有素的医务人员的需求,使普通饲养人员自主免疫成为可能[58]。黏膜疫苗成功研制的关键是设计不同的抗原递送策略将疫苗抗原稳定递送至特定黏膜免疫诱导位点,以诱导高水平的免疫应答。而分别以M细胞表面标志α(1, 2)岩藻糖和M细胞表面标志GP2为靶标诱导的黏膜免疫反应表明,M细胞是从肠腔内摄取抗原而诱导黏膜免疫的关键[28, 37]。此外,M细胞具有较强的跨细胞转运能力,摄取抗原后可将其转移至基底外侧囊内高度分布的APCs,促进黏膜免疫反应的发生,这使其成为构建黏膜疫苗的理想靶标。同时,细胞共培养系统的发展及其模型的改进促进了M细胞相关受体的研究,所获进展已有效地应用于黏膜靶向药物和黏膜疫苗佐剂的研发[59]。
虽然M细胞是通过黏膜接种途径诱导抗原特异性免疫反应的理想细胞,但使用M细胞作为抗原递送的靶标极具挑战性。首先M细胞仅占人类和小鼠等肠道淋巴滤泡表面积的10%,且M细胞的分离鉴定十分困难,这就导致对其特异性表面标志的研究进展有限[60]。其次,M细胞捕获腔内抗原的具体机制仍不清楚。因此,为了在黏膜疫苗中应用靶向M细胞策略,需要进一步理解黏膜免疫学和M细胞生物学特性。另外,简单地将抗原靶向到M细胞并不能保证产生有效的保护性免疫,为了设计有效的靶向M细胞的黏膜疫苗,必须考虑配体和载体的性质、结合的方法以及抗原-配体复合物的大小,还需要使用相关的动物模型来检测抗原-配体复合物的免疫效力。鉴于此,研究者应该关注配体的研究,同时选用适当的载体将抗原呈递至M细胞,从而诱导高效的免疫应答。目前,大多数的动物试验都是在啮齿动物上进行的,而糖基化模式和受体可能具有物种特异性。因此,研制抗原-配体复合物介导的黏膜疫苗需要使用靶动物进行免疫效力评价。此外,在黏膜疫苗中仍存在一些不容忽视的局限性,例如,实验动物反复大剂量口服抗原易诱导黏膜免疫耐受[61]。接种疫苗的最终目的是产生安全高效的保护性免疫。目前正在研发能够保护抗原不被胃肠道降解的递送系统,从而诱导高效的免疫保护。黏膜疫苗需要抵抗恶劣的酸性条件以及多种消化酶,才能到达黏膜免疫组织。为了解决这个问题,减毒活菌可以用作黏膜疫苗载体,因为它们可以在肠道内传播,即使只将少量抗原递呈给免疫组织也能激发足够的免疫应答[62-64]。然而,弱毒病原体存在毒力返强的潜在风险,而乳酸菌等益生菌可以克服毒力返强和酸性条件的双重挑战完成抗原向黏膜免疫系统的递送。因此,可以同时应用靶向M细胞和益生菌载体递送抗原的双重策略[65-66],发挥集成优势以减少其单独使用的局限性,以期为黏膜疫苗的成功研制提供一系列有效的策略和平台。
| [1] | Stary G, Olive A, Radovic-Moreno AF, et al. A mucosal vaccine against Chlamydia trachomatis generates two waves of protective memory T cells. Science, 2015, 348(6241): aaa8205. DOI: 10.1126/science.aaa8205 |
| [2] | Payne AM. Oral immunization against poliomyelitis. Bull World Health Organ, 1960, 23(6): 695–703. |
| [3] | Kim SH, Jang YS. The development of mucosal vaccines for both mucosal and systemic immune induction and the roles played by adjuvants. Clin Exp Vaccine Res, 2017, 6(1): 15–21. DOI: 10.7774/cevr.2017.6.1.15 |
| [4] | Longet S, Lundahl MLE, Lavelle EC. Targeted strategies for mucosal vaccination. Bioconjugate Chem, 2018, 29(3): 613–623. DOI: 10.1021/acs.bioconjchem.7b00738 |
| [5] | Martens EC, Neumann M, Desai MS. Interactions of commensal and pathogenic microorganisms with the intestinal mucosal barrier. Nat Rev Microbiol, 2018, 16: 457–470. DOI: 10.1038/s41579-018-0036-x |
| [6] | Azizi A, Kumar A, Diaz-Mitoma F, et al. Enhancing oral vaccine potency by targeting intestinal M cells. PLoS Pathog, 2010, 6(11): e1001147. DOI: 10.1371/journal.ppat.1001147 |
| [7] | Macpherson AJ, McCoy KD, Johansen FE, et al. The immune geography of IgA induction and function. Mucosal Immunol, 2008, 1(1): 11–22. DOI: 10.1038/mi.2007.6 |
| [8] | Pabst R, Russell MW, Brandtzaeg P. Tissue distribution of lymphocytes and plasma cells and the role of the gut. Trends Immunol, 2008, 29(5): 206–208. DOI: 10.1016/j.it.2008.02.006 |
| [9] | Brandtzaeg P. Induction of secretory immunity and memory at mucosal surfaces. Vaccine, 2007, 25(30): 5467–5484. DOI: 10.1016/j.vaccine.2006.12.001 |
| [10] | Owen RL, Jones AL. Epithelial cell specialization within human Peyer's patches: an ultrastructural study of intestinal lymphoid follicles. Gastroenterology, 1974, 66(2): 189–203. |
| [11] | Corr SC, Gahan CCGM, Hill C. M-cells: origin, morphology and role in mucosal immunity and microbial pathogenesis. FEMS Immunol Med Microbiol, 2008, 52(1): 2–12. DOI: 10.1111/j.1574-695X.2007.00359.x |
| [12] | Mabbott NA, Donaldson DS, Ohno H, et al. Microfold (M) cells: important immunosurveillance posts in the intestinal epithelium. Mucosal Immunol, 2013, 6(4): 666–677. DOI: 10.1038/mi.2013.30 |
| [13] | Miller H, Zhang J, Kuolee R, et al. Intestinal M cells: the fallible sentinels?. World J Gastroenterol, 2007, 13(10): 1477–1486. DOI: 10.3748/wjg.v13.i10.1477 |
| [14] | Clark MA, Hirst BH. Expression of junction-associated proteins differentiates mouse intestinal M cells from enterocytes. Histochem Cell Biol, 2002, 118(2): 137–147. |
| [15] | Neutra MR, Mantis NJ, Kraehenbuhl JP. Collaboration of epithelial cells with organized mucosal lymphoid tissues. Nat Immunol, 2001, 2(11): 1004–1009. DOI: 10.1038/ni1101-1004 |
| [16] | Roy U, Ding H, Pilakka-Kanthikeel S, et al. Preparation and characterization of anti-HIV nanodrug targeted to microfold cell of gut-associated lymphoid tissue. Int J Nanomedicine, 2015, 10: 5819–5835. |
| [17] | Schulz O, Pabst O. Antigen sampling in the small intestine. Trends Immunol, 2013, 34(4): 155–161. DOI: 10.1016/j.it.2012.09.006 |
| [18] | Kishikawa S, Sato S, Kaneto S, et al. Allograft inflammatory factor 1 is a regulator of transcytosis in M cells. Nat Commun, 2017, 8: 14509. DOI: 10.1038/ncomms14509 |
| [19] | Anosova NG, Chabot S, Shreedhar V, et al. Cholera toxin, E. coli heat-labile toxin, and non-toxic derivatives induce dendritic cell migration into the follicle-associated epithelium of Peyer's patches. Mucosal Immunol, 2008, 1(1): 59–67. DOI: 10.1038/mi.2007.7 |
| [20] | Chabot S, Wagner JS, Farrant S, et al. TLRs regulate the gatekeeping functions of the intestinal follicle-associated epithelium. J Immunol, 2006, 176(7): 4275–4283. DOI: 10.4049/jimmunol.176.7.4275 |
| [21] | Man AL, Lodi F, Bertelli E, et al. Macrophage migration inhibitory factor plays a role in the regulation of microfold (M) cell-mediated transport in the gut. J Immunol, 2008, 181(8): 5673–5680. DOI: 10.4049/jimmunol.181.8.5673 |
| [22] | Mantis NJ, Rol N, Corthésy B. Secretory IgA's complex roles in immunity and mucosal homeostasis in the gut. Mucosal Immunol, 2011, 4(6): 603–611. DOI: 10.1038/mi.2011.41 |
| [23] | Kadaoui KA, Corthésy B. Secretory IgA mediates bacterial translocation to dendritic cells in mouse Peyer's patches with restriction to mucosal compartment. J Immunol, 2007, 179(11): 7751–7757. DOI: 10.4049/jimmunol.179.11.7751 |
| [24] | Gicheva N, Macauley MS, Arlian BM, et al. Siglec-F is a novel intestinal M cell marker. Biochem Biophys Res Commun, 2016, 479(1): 1–4. DOI: 10.1016/j.bbrc.2016.08.055 |
| [25] | Zhao JY, Li XY, Luo QF, et al. Screening of surface markers on rat intestinal mucosa microfold cells by using laser capture microdissection combined with protein chip technology. Int J Clin Exp Med, 2014, 7(4): 932–939. |
| [26] | Bies C, Lehr CM, Woodley JF. Lectin-mediated drug targeting: history and applications. Adv Drug Deliv Rev, 2004, 56(4): 425–435. DOI: 10.1016/j.addr.2003.10.030 |
| [27] | Ramirez JEV, Sharpe LA, Peppas NA. Current state and challenges in developing oral vaccines. Adv Drug Deliv Rev, 2017, 114: 116–131. DOI: 10.1016/j.addr.2017.04.008 |
| [28] | Hase K, Kawano K, Nochi T, et al. Uptake through glycoprotein 2 of FimH+ bacteria by M cells initiates mucosal immune response. Nature, 2009, 462(7270): 226–230. DOI: 10.1038/nature08529 |
| [29] | Kim SH, Jung DI, Yang IY, et al. M cells expressing the complement C5a receptor are efficient targets for mucosal vaccine delivery. Eur J Immunol, 2011, 41(11): 3219–3229. DOI: 10.1002/eji.201141592 |
| [30] | Garinot M, Fiévez V, Pourcelle V, et al. PEGylated PLGA-based nanoparticles targeting M cells for oral vaccination. J Control Release, 2007, 120(3): 195–204. DOI: 10.1016/j.jconrel.2007.04.021 |
| [31] | Yoo MK, Kang SK, Choi JH, et al. Targeted delivery of chitosan nanoparticles to Peyer's patch using M cell-homing peptide selected by phage display technique. Biomaterials, 2010, 31(30): 7738–7747. DOI: 10.1016/j.biomaterials.2010.06.059 |
| [32] | Singh B, Maharjan S, Jiang T, et al. Combinatorial approach of antigen delivery using M cell-homing peptide and mucoadhesive vehicle to enhance the efficacy of oral vaccine. Mol Pharm, 2015, 12(11): 3816–3828. |
| [33] | Misstear K, McNeela EA, Murphy AG, et al. Targeted nasal vaccination provides antibody-independent protection against Staphylococcus aureus. J Infect Dis, 2014, 209(9): 1479–1484. DOI: 10.1093/infdis/jit636 |
| [34] | Du LP, Yu ZY, Pang FJ, et al. Targeted delivery of GP5 antigen of PRRSV to M Cells enhances the antigen-specific systemic and mucosal immune responses. Front Cell Infect Microbiol, 2018, 8: 7. DOI: 10.3389/fcimb.2018.00007 |
| [35] | Kim SH, Seo KW, Kim J, et al. The M cell-targeting ligand promotes antigen delivery and induces antigen-specific immune responses in mucosal vaccination. J Immunol, 2010, 185(10): 5787–5795. DOI: 10.4049/jimmunol.0903184 |
| [36] | Kim SH, Kim YN, Kim J, et al. C5a receptor targeting of partial non-structural protein 3 of dengue virus promotes antigen-specific IFN-γ-producing T-cell responses in a mucosal dengue vaccine model. Cell Immunol, 2018, 325: 41–47. DOI: 10.1016/j.cellimm.2018.01.016 |
| [37] | Nochi T, Yuki Y, Matsumura A, et al. A novel M cell-specific carbohydrate-targeted mucosal vaccine effectively induces antigen-specific immune responses. J Exp Med, 2007, 204(12): 2789–2796. DOI: 10.1084/jem.20070607 |
| [38] | Sato S, Kaneto S, Shibata N, et al. Transcription factor Spi-B-dependent and -independent pathways for the development of Peyer's patch M cells. Mucosal Immunol, 2013, 6(4): 838–846. DOI: 10.1038/mi.2012.122 |
| [39] | Fukuda S, Hase K, Ohno H. Application of a mouse ligated Peyer's patch intestinal loop assay to evaluate bacterial uptake by M cells. J Vis Exp, 2011(58): 3225. |
| [40] | Rynda A, Maddaloni M, Mierzejewska D, et al. Low-dose tolerance is mediated by the microfold cell ligand, reovirus protein σ1. J Immunol, 2008, 180(8): 5187–5200. DOI: 10.4049/jimmunol.180.8.5187 |
| [41] | Helander A, Silvey KJ, Mantis NJ, et al. The viral σ1 protein and glycoconjugates containing α2-3-linked sialic acid are involved in type 1 reovirus adherence to M cell apical surfaces. J Virol, 2003, 77(14): 7964–7977. DOI: 10.1128/JVI.77.14.7964-7977.2003 |
| [42] | Keely S, Glover LE, Weissmueller T, et al. Hypoxia-inducible factor-dependent regulation of platelet-activating factor receptor as a route for gram-positive bacterial translocation across epithelia. Mol Biol Cell, 2010, 21(4): 538–546. DOI: 10.1091/mbc.e09-07-0573 |
| [43] | Kim SH, Yang IY, Jang SH, et al. C5a receptor-targeting ligand-mediated delivery of dengue virus antigen to M cells evokes antigen-specific systemic and mucosal immune responses in oral immunization. Microbes Infect, 2013, 15(13): 895–902. DOI: 10.1016/j.micinf.2013.07.006 |
| [44] | Lo DD, Ling J, Eckelhoefer AH. M cell targeting by a Claudin 4 targeting peptide can enhance mucosal IgA responses. BMC Biotechnol, 2012, 12: 7. DOI: 10.1186/1472-6750-12-7 |
| [45] | Suzuki H, Nagatake T, Nasu A, et al. Impaired airway mucociliary function reduces antigen-specific IgA immune response to immunization with a claudin-4-targeting nasal vaccine in mice. Sci Rep, 2018, 8: 2904. DOI: 10.1038/s41598-018-21120-7 |
| [46] | Suzuki H, Watari A, Hashimoto E, et al. C-terminal Clostridium perfringens enterotoxin-mediated antigen delivery for nasal pneumococcal vaccine. PLoS ONE, 2015, 10(5): e0126352. DOI: 10.1371/journal.pone.0126352 |
| [47] | Shima H, Watanabe T, Fukuda S, et al. A novel mucosal vaccine targeting Peyer's patch M cells induces protective antigen-specific IgA responses. Int Immunol, 2014, 26(11): 619–625. DOI: 10.1093/intimm/dxu061 |
| [48] | Rochereau N, Pavot V, Verrier B, et al. Secretory IgA as a vaccine carrier for delivery of HIV antigen to M cells. Eur J Immunol, 2015, 45(3): 773–779. DOI: 10.1002/eji.201444816 |
| [49] | Mikulic J, Bioley G, Corthésy B. SIgA-Shigella immune complexes interact with dectin-1 and SIGNR3 to differentially regulate mouse Peyer's patch and mesenteric lymph node dendritic cell's responsiveness. J Mol Biol, 2017, 429(15): 2387–2400. DOI: 10.1016/j.jmb.2017.05.024 |
| [50] | Rochereau N, Drocourt D, Perouzel E, et al. Dectin-1 is essential for reverse transcytosis of glycosylated SIgA-antigen complexes by intestinal M cells. PLoS Biol, 2013, 11(9): e1001658. DOI: 10.1371/journal.pbio.1001658 |
| [51] | Kuolee R, Chen WX. M cell-targeted delivery of vaccines and therapeutics. Expert Opin Drug Deliv, 2008, 5(6): 693–702. DOI: 10.1517/17425247.5.6.693 |
| [52] | Schimpel C, Teubl B, Absenger M, et al. Development of an advanced intestinal in vitro triple culture permeability model to study transport of nanoparticles. Mol Pharmaceutics, 2014, 11(3): 808–818. DOI: 10.1021/mp400507g |
| [53] | Kim SH, Jung DI, Yang IY, et al. Application of an M-cell-targeting ligand for oral vaccination induces efficient systemic and mucosal immune responses against a viral antigen. Int Immunol, 2013, 25(11): 623–632. DOI: 10.1093/intimm/dxt029 |
| [54] | Liu L, Zhang W, Song Y, et al. Recombinant Lactococcus lactis co-expressing OmpH of an M cell-targeting ligand and IBDV-VP2 protein provide immunological protection in chickens. Vaccine, 2018, 36(5): 729–735. DOI: 10.1016/j.vaccine.2017.12.027 |
| [55] | Gupta PN, Khatri K, Goyal AK, et al. M-cell targeted biodegradable PLGA nanoparticles for oral immunization against hepatitis B. J Drug Target, 2007, 15(10): 701–713. DOI: 10.1080/10611860701637982 |
| [56] | Daifalla N, Cayabyab MJ, Xie E, et al. Commensal Streptococcus mitis is a unique vector for oral mucosal vaccination. Microbes Infect, 2015, 17(3): 237–242. DOI: 10.1016/j.micinf.2014.11.002 |
| [57] | Lycke N. Recent progress in mucosal vaccine development: potential and limitations. Nat Rev Immunol, 2012, 12(8): 592–605. |
| [58] | Davitt CJH, Lavelle EC. Delivery strategies to enhance oral vaccination against enteric infections. Adv Drug Deliv Rev, 2015, 91: 52–69. DOI: 10.1016/j.addr.2015.03.007 |
| [59] | Gullberg E, Keita V, Salim SY, et al. Identification of cell adhesion molecules in the human follicle-associated epithelium that improve nanoparticle uptake into the Peyer's patches. J Pharmacol Exp Ther, 2006, 319(2): 632–639. DOI: 10.1124/jpet.106.107847 |
| [60] | Tyrer PC, Ruth Foxwell A, Kyd JM, et al. Receptor mediated targeting of M-cells. Vaccine, 2007, 25(16): 3204–3209. DOI: 10.1016/j.vaccine.2007.01.028 |
| [61] | Ashour HM. Immune tolerance elicited via unique ocular and oral routes. Curr Mol Med, 2015, 15(1): 78–81. |
| [62] | Liu XH, Wu HZ, Chang XY, et al. Notable mucosal immune responses induced in the intestine of zebrafish (Danio rerio) bath-vaccinated with a live attenuated Vibrio anguillarum vaccine. Fish Shellfish Immunol, 2014, 40(1): 99–108. DOI: 10.1016/j.fsi.2014.06.030 |
| [63] | Canadian Paediatric Society, Infectious Diseases and Immunization Committee. FluMist vaccine: questions and answers - summary. Paediatr Child Health, 2011, 16(1): 31. DOI: 10.1093/pch/16.1.31 |
| [64] | Li QH, Jin G, Wang JY, et al. Live attenuated Salmonella displaying HIV-110E8 epitope on fimbriae: systemic and mucosal immune responses in BALB/c mice by mucosal administration. Sci Rep, 2016, 6: 29556. DOI: 10.1038/srep29556 |
| [65] | Ma ST, Wang L, Huang XW, et al. Oral recombinant Lactobacillus vaccine targeting the intestinal microfold cells and dendritic cells for delivering the core neutralizing epitope of porcine epidemic diarrhea virus. Microb Cell Fact, 2018, 17: 20. DOI: 10.1186/s12934-018-0861-7 |
| [66] | Wang XN, Wang L, Zheng DZ, et al. Oral immunization with a Lactobacillus casei-based anti-porcine epidemic diarrhoea virus (PEDV) vaccine expressing microfold cell-targeting peptide Co1 fused with the COE antigen of PEDV. J Appl Microbiol, 2018, 124(2): 368–378. DOI: 10.1111/jam.2018.124.issue-2 |
 2019, Vol. 35
2019, Vol. 35




