中国科学院微生物研究所、中国微生物学会主办
文章信息
- 王文豪, 闻鹏飞, 许孔亮, 郑仁朝, 郑裕国
- Wang Wenhao, Wen Pengfei, Xu Kongliang, Zheng Renchao, Zheng Yuguo
- 工业环境下酶蛋白的催化行为与适应性改造研究进展
- Catalysis of enzymes under industrial environment and their adaptive modifications: a review
- 生物工程学报, 2019, 35(10): 1857-1869
- Chinese Journal of Biotechnology, 2019, 35(10): 1857-1869
- 10.13345/j.cjb.190258
-
文章历史
- Received: June 16, 2019
- Accepted: August 16, 2019
2. 浙江工业大学 生物工程学院 生物转化与生物净化教育部工程研究中心,浙江 杭州 310014
2. Engineering Research Center of Bioconversion and Biopurification of Ministry of Education, Zhejiang University of Technology, Hangzhou 310014, Zhejiang, China
随着人类社会的发展,生态恶化、环境污染和能源短缺已成为全球范围内不容忽视的重大课题。面对传统化学制造所带来的高污染、高能耗等问题,环境友好的生物制造技术正引起普遍关注,成为实现社会可持续发展的重要突破口[1]。酶是高效的生物催化剂,其反应条件温和,可生物降解以及高度立体、区域和化学选择性等特点在生物制造中具有显著优势[2]。工业酶的使用可减少原料、能源消耗以及废弃物的排放,具有绿色制造和可持续发展的典型特征。目前,酶已在化工、医药、食品、环保、制革、饲料、造纸和纺织等40多个行业广泛应用[3]。
现代生物制造业正朝着高强度、集约化、柔性化的方向快速发展,对反应过程、生产强度和操作柔性等提出了更高要求。具体而言,要求工业酶具有优异的酸碱、温度、离子强度、有机溶剂及底物耐受性能,能够在较宽的过程参数下发挥催化作用。然而,酶催化反应有赖于酶分子空间结构的稳定性与完整性,酶分子变性或亚基解聚均可导致酶活性丧失。因此,酶如何与工业生产环境匹配,发挥最大催化潜力,成为亟需破解的瓶颈[4]。研究酶在工业环境下的催化行为变化,寻找相应的适应性改造策略,可为工业生产提供稳定性更高、催化速度更快、耐受性更强的生物催化剂。本文对工业环境下酶蛋白催化行为的改变及其适应性改造作简要综述。
1 高温条件下酶蛋白的催化行为及适应性改造目前大多数酶都来源于中温微生物,其最佳酶活温度一般为20 ℃到45 ℃。但酶在加工及反应过程中常会遇到高温条件,高温会破坏酶蛋白中的氢键、盐桥、疏水相互作用和范德华力等非共价键,从而破坏酶空间结构,使其从折叠状态变为展开状态,酶活力降低,甚至失活[5]。因此,研究酶蛋白在高温条件下的催化行为,并对其进行适应性改造具有重要意义。
在分子水平上解释蛋白质的热稳定性或不稳定性是一个巨大的挑战,并且预测导致热稳定性增强的突变位点也很困难。大量研究表明,只有极少数序列的变化会显著影响酶蛋白的热稳定性[6-9]。能够提高蛋白质热稳定性的突变通常聚集在特定的区域(如蛋白质开始展开的位置),在其他区域引入类似的突变对酶蛋白热稳定性的影响不明显[10]。当突变位点位于动力学控制的展开区域中时,局部结构稳定性的提高可以改善蛋白质的整体稳定性[8]。然而,酶蛋白展开的途径通常是未知的,B因子-拟合法可通过酶蛋白的晶体结构信息识别目标位置,预测对热稳定性有影响的氨基酸位点[11]。高度灵活的残基在高温环境中会优先展开,选择B因子最高的残基进行突变,在这些位点上进行替换会有更多的机会获得正突变,并进一步稳定蛋白的折叠状态。
目前,可以从两个方面来增加蛋白质的热稳定性。一是通过增加有利的相互作用或减少不利的相互作用,稳定折叠状态;二是通过降低展开链的熵,破坏未折叠状态下蛋白质的稳定性[12]。在大多数情况下,引入更多二硫键、氢键、疏水相互作用等分子间相互作用,会使折叠起来的空间结构更加稳定[13-15],同时(β/α)8 -barrel折叠、表面电荷、短螺旋、盐桥也会使酶在高温下的构象变得更加稳定[16-18]。由此出发,对结构进行分析比对从而进行理性或者半理性的设计是提高工业酶热稳定性的一种有效策略(表 1)。
| Improvement method | Industrial enzyme | Modification description | Performance | Reference |
| Semi-rational design | Xylanase | Enforced hydrophobic interactions within N-terminal elements and between N-and C-terminal ends | 30-fold half-life improvement at 60 ℃ and a 17.4 ℃ increase in Tm | [19] |
| Rational design | Nattokinase | More rigid structure or stabilized folded state of the protein | QSN had 40% active residue at 55 ℃ for 50 min, while wild type is inactivated, and the half-life values were nearly 3 times longer than that of the wild type | [20] |
| Immobilization | Lipase PS | Immobilized into COFs | The free enzyme lost activity, while the COF biocomposite achieved a 37% and 48% conversion after 30 min and 1 h at 120 ℃ for 1 h, respectively | [21] |
Letian等[19]通过半理性设计提高了黑曲霉Aspergillus niger GH10木聚糖酶Xyn10A_ASPNG的热稳定性;他们首先通过计算分析确定了影响酶热稳定性的5个重要残基,随后通过轮次迭代饱和突变(ISM)进行随机突变,获得五重突变体4S1 (R25W/V29A/I31L/L43F/T58I)。与野生型相比,突变体4S1在60 ℃时的半衰期(t1/2)延长了30倍(图 1A),解链温度(Tm)增加了17.4 ℃ (图 1B)。同时,其在65 ℃加热15 min后仍能保持30%的初始酶活(野生型酶加热2 min后完全失活)。进一步分析表明,4S1中的每一个突变都有助于提高酶的热稳定性,5种突变的协同效应导致其热稳定性的显著改善。4S1与野生酶的结构分析及比对表明,N-末端之间以及N-和C-末端之间的疏水相互作用导致的N-末端卷曲,是其热稳定性提高的主要原因(图 1C-D)。
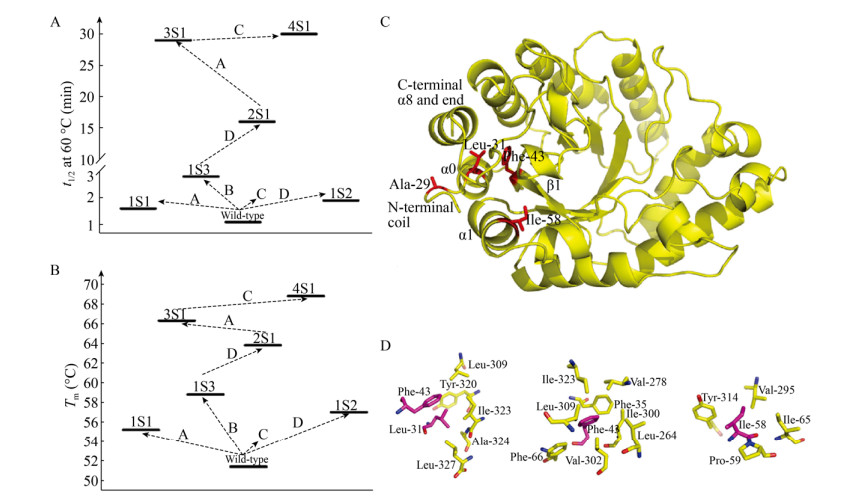
|
| 图 1 突变体4S1的热稳定性和结构示意图[19].以t1/2 60 ℃ (A)和Tm (B)来评价热稳定性(A、B、C和D分别对应于在25、29/31、43和58位点进行的随机饱和突变). (C)基于Swiss-mode的突变体4S1三级结构以及有益残基Ala-29、Leu-31、Phe-43和Ile-58分别在N末端卷曲、螺旋α0、链β1和螺旋α1的位置. (D)与Leu-31、Phe-43和Ile-58发生疏水相互作用的氨基酸残基示意图.此图已获John Wiley and Sons授权. Fig. 1 Thermal stability and structural schematic diagrams of mutant 4S1[19]. Evaluation of thermostability by (A) t1/2 60 ℃ and (B) Tm. The letters A, B, C, and D correspond to saturation mutagenesis randomized at position 25, 29/31, 43, and 58, respectively. (C) Tertiary structure of variant 4S1 Swiss-model and localization of beneficial residues Ala-29, Leu-31, Phe-43 and Ile-58 at N-terminal coil, helix α0, strand β1 and helix α1, respectively. (D) Schematic diagrams of residues involved in putative hydrophobic interactions with Leu-31, Phe-43 and Ile-58. Reproduced with permission from John Wiley and Sons. |
| |
Liu等[20]通过定点和组合突变提高纳豆激酶(NK)的热稳定性,在筛选得到11种突变体后,选择4种突变体进一步构建多点突变体。最后得到热稳定性最高的突变体QSN,在55 ℃反应50 min,仍有40%的活性残余,而野生型NK完全失活。突变体的t1/2为(33.37±0.27) min,是野生型的3倍。
Sun等[21]使用共价有机骨架(COFs)固定洋葱伯克霍尔德菌Burkholderia cepacia脂肪酶PS从而显著提高其热稳定性。在120 ℃高温条件下反应1 h (苯甲腈为溶剂),游离酶完全丧失活性,而固定化酶仍保留48%的活性(以转化率计)。这是由于载体增强了酶的刚性,有利于维持蛋白结构的折叠状态,防止酶在高温下发生构象转变,从而提高了其热稳定性。
2 强酸/碱条件下酶蛋白的催化行为及适应性改造酶是两性生物大分子,具有大量羧基和氨基等酸/碱性基团。酶蛋白只有在最佳pH条件下才能发挥最大的催化活性。然而,工业生产环境中存在的强酸或强碱条件会改变底物分子和酶分子的解离状态,从而影响酶和底物的结合,导致酶活降低,甚至会破坏酶的结构,使其无法满足工业需求,因此需要对酶进行适应性改造(表 2)。
| Improvement method | Industrial enzyme | Modification description | Performance | Reference |
| Semi-rational design | β-glucosidase | Reduced O-glycosylation | Mutants are stable over a wide pH range (3.0 to 10.0) | [24] |
| Rational design | Xylanase | Substituting base residues with acidic amino acids | XynR8 (N41D) retained 71.8%±2.2% of enzyme activity (wild-type enzyme was 30.6%±3.0%) | [26] |
| Directed evolution | Versatile peroxidase | More hydrogen bonds and salt bridges | The mutant had an initial enzyme activity of 61% after 24 hours at pH 3.5, while the native enzyme is completely inactivated | [29] |
| Immobilization | Glucose oxidase | Single-enzymenanogels (SENs) | Practically constant activity was observed over the pH range 3-9 with SENs11 | [30] |
pH稳定性是一种重要的酶学特性,与蛋白质折叠、化学物质干扰和翻译后修饰(PTMs)等因素密切相关[22]。其中PTMs发生在氨基酸侧链或末端,可通过引入新的官能团来提高其pH稳定性,例如磷酸盐(磷酸化)、乙酸盐(酰化)、酰胺基团(酰胺化)、甲基(甲基化)或碳水化合物分子(糖基化)等[23]。Wei等[24]在毕赤酵母GS115中表达了一种新鉴定的β-葡萄糖苷酶(GH3,Bgl3A),但Bgl3A的pH稳定性范围较窄(4.0-5.0),限制了其工业应用;他们通过对3个潜在N-糖基化位点(Asn23、Asn207和Asn278)和9个O-糖基化位点(残基313、417-421、424、425和429)进行定点突变,筛选得到去除了潜在O-糖基化位点的两个突变体,使其在较宽的pH范围内(3.0-10.0)均比较稳定。分析表明,O-糖基化是导致pH作用范围变窄的主要原因,从整体上看过量的O-糖基化对β-葡萄糖苷酶的pH稳定性具有负效应。
Fushinobu等提出酶催化位点附近的残基会影响催化残基的pKa值,进而影响酶的pH稳定性[25]。因此,以酸性氨基酸取代碱基残基是提高酶耐酸性的基本策略。Chen等[26]从瘤胃真菌培养物中克隆了糖基水解酶家族11木聚糖酶XynR8,并在大肠杆菌中表达获得低分子量的木聚糖酶。该酶在碱性条件下与氢键结合的残基为天冬酰胺,在酸性条件下起作用的残基则是天冬氨酸(酸性氨基酸)[27]。基于分子建模,将位于催化裂缝边缘的环-β-链区域内稳定XynR8结构的重要残基N41和N58突变为天冬氨酸,突变体在pH 2.0下显示出比野生型更好的耐酸性。Yang等[28]也通过将催化结构域上的4个碱基组氨酸残基His222、His275、His293和His310替换为天冬氨酸,突变后α-淀粉酶在酸性条件下的稳定性和催化活性均明显提高。
除此之外,氢键、盐桥、β-折叠以及暴露于溶剂中的带电残基也会对酶的pH稳定性造成影响。Saez-Jimenez等[29]通过定向进化对多功能过氧化物酶(VP)进行适应性改造,提高了低pH条件下的稳定性。基于VP晶体结构及与高pH稳定锰过氧化物酶(MnP4)的比较分析,通过定向改造将形成氢键、盐桥等相互作用的8个氨基酸残基和7个表面碱性残基引入VP中,使酸性和中性pH下的血红素口袋稳定。VP突变体在pH 3.5条件下保持24 h后,仍具有初始酶活61%的活力,而天然酶在同等条件下几乎完全失活。
Beloqui等[30]将单酶在纳米凝胶中(SENs)进行包埋提高了酶的pH稳定性。与之前两步丙烯酰化的方法不同(图 2A),该工作以蔗糖(5% W/V)为包埋启动子,然后使用丙烯酰胺、N, N′-亚甲基双丙烯酰胺、过硫酸铵、四甲基乙二胺一步形成纳米凝胶。此方法可以通过调整反应参数来精细调节水凝胶层的厚度。以黑曲霉Aspergillus niger葡萄糖氧化酶(GOx)作为模型酶,固定化得到酶-凝胶复合物(GOx_SENs)。研究结果表明,壳厚度会强烈影响酶的活性和稳定性,水凝胶层越厚,酶的稳定性越好(图 2B),但相应地酶活越低(图 2C)。固定化后形成的水凝胶层,使酶的构象更加稳定,从而导致宽的pH耐受性。厚度为0.8 nm的GOx_SENs11在pH 3.0-9.0范围内维持着几乎恒定的高活性(图 2D)。
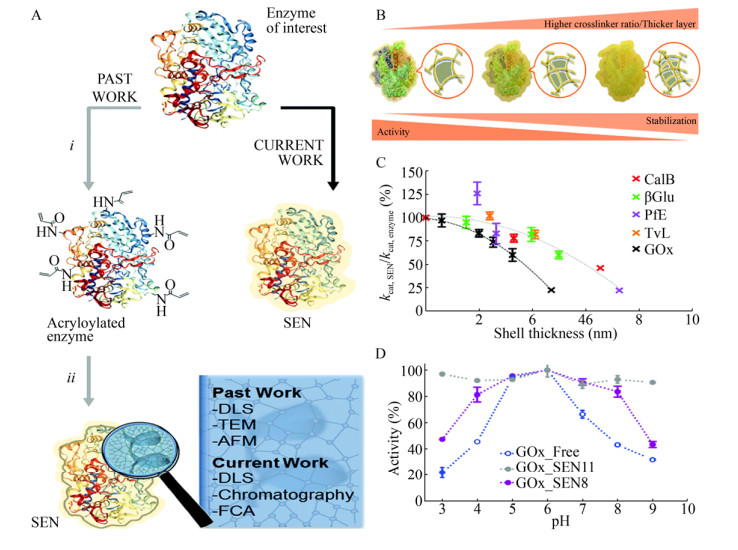
|
| 图 2 通过纳米凝胶包埋提高酶的pH稳定性[30]. (A) SEN的合成方案示意图. (B)聚丙烯酰胺层厚度和孔隙率对SEN活性和稳定性的影响. (C) kcat, SEN/kcat, enzyme与凝胶厚度的关系(根据9种不同蛋白的测量数据). (D)在不同pH值范围(从3.0到9.0)下GOx_SENs的相对活性曲线,其中GOx_SEN8的[AAm]/[MBAAm]比值为6︰1 (壳厚度为3.2 nm),GOx_SEN11的比值为2︰1 (0.8 nm).此图已获The Royal Society of Chemistry授权. Fig. 2 pH stability of the enzyme was improved by the entrapment in nanogels[30]. (A) Synthetic protocols to produce SENs. (B) Effect of the polyacrylamide layer thickness and porosity on the activity and stability of SENs. (C) Dependence of kcat, SEN/kcat, enzyme on the gel thickness, as determined for nine different proteins. (D) Relative activity profiles over a range of pH values (from 3.0 to 9.0) for GOx_SENs made with different [AAm]/[MBAAm]: 6:1 for GOx_SEN8 (shell thickness 3.2 nm), and 2:1 for GOx_SEN11 (0.8 nm). Reproduced with permission from The Royal Society of Chemistry. |
| |
对于大多数酶而言,只有在一定的盐度范围内才具有催化活性,超出这些范围会导致蛋白酶的快速变性与失活。在高盐环境中,高浓度盐离子会干扰氨基酸残基之间的静电相互作用,使蛋白质表面必需水分子层减少并导致其疏水相互作用增加,酶蛋白会因此聚集并发生变性[31]。但是,从高盐环境微生物中分离得到的嗜盐酶严格依赖体系中一定的盐离子浓度,可以在高盐环境中维持其结构稳定,在卤水、离子液体、离子洗涤剂等高盐工业环境中稳定存在,并展现出良好的催化活性[32]。
通过对嗜盐酶蛋白和非嗜盐酶蛋白的结构比对发现,嗜盐酶的特点在于盐桥和氢键明显增多,含有一些特殊的盐离子结合位点并且常以低聚体的形式存在,表面酸性氨基酸含量明显增多。嗜盐酶蛋白在高盐环境中更加稳定,这是因为:1)蛋白质由于羧基的水合而具有负电荷,负电荷会被高盐屏蔽,阻止了蛋白质的展开;2)酸性氨基酸提供了带负电荷的蛋白质表面,并且酸性残基位于蛋白质表面会参与形成较强水化壳层,从而维持蛋白质的稳定;3)嗜盐蛋白只含有少量非极性氨基酸,如半胱氨酸、异亮氨酸、赖氨酸等,这一特征也对蛋白的嗜盐性有一定影响;4)蛋白质需要“水合球”来抵抗蛋白质聚集体的形成[33],因此,其表面含有较少的疏水氨基酸和更多的亲水氨基酸[34-35]。这些研究结果给工业酶嗜盐性的适应性改造提供一定的指导,为开发高嗜盐性的工业酶指明了方向(表 3)。
| Improvement method | Industrial enzyme | Modification description | Performance | Reference |
| Rational design | Carbonic anhydrase II | Interactions with the highly ordered first Na+ hydration shell | Both the catalytic activity and the thermal expansion temperature are increased at high salt concentrations | [38] |
| Semi-rational design | Choline-phosphate cytidylyltransferase | Increased acidic amino acids on the surface of proteins | When the acetate concentration was increased, the response rate of the mutant was 2.2 times higher than that of the wild type CCT | [39] |
| Choline kinase | CKI mutants show high tolerance in over 1 200 mol/L acetate solutions | [40] |
从天然嗜盐微生物中克隆获得编码基因,并进行异源表达是目前获得高嗜盐性酶的主要方法。Zhang等[36]从地衣芽胞杆菌中克隆获得了一种冷适应、碱稳定和高盐耐受性的酯酶(Est700),该酶可被3.5 mol/L NaCl高度激活,并在5 mol/L NaCl中维持良好的稳定性,基本无活性损失。Gao等[37]从嗜盐古生菌中获得了一种耐碱嗜盐的蛋白酶,具有一定的碱稳定性(pH 7.0-10.0),并在较宽的盐度范围内(0-3 mol/L NaCl)中表现出催化能力。另外,为进一步提高天然嗜盐酶的催化活性,可采取理性设计方法对天然嗜盐酶进行改造。
另外,为进一步提高天然嗜盐酶的催化活性,可采取理性设计方法对天然嗜盐酶进行改造。Andrew等[38]对碳酸酐酶Ⅱ进行三步合理设计,共取代了18个表面残基,表面电荷发生了变化(图 3A-D)。与野生型相比,设计得到的突变体(M1-M4)的催化活性和热展开温度在高盐浓度下均有所提高(图 3E-F)。分子动力学计算表明,酶蛋白表面酸性氨基酸残基与阳离子形成的互补性螯合作用,以及由阳离子组成的高度有序的水化层作用是导致其嗜盐性形成的主要原因。

|
| 图 3 碳酸酐酶Ⅱ的表面电荷分布图及其在高盐条件下的活性[38]. (A-D)为WT和M4中表面电荷的分布图(蓝色代表正电荷,红色带代表负电荷). (E-F)不同酶在分别在不同浓度Na2SO4溶液(E)和NaCl溶液(F)中的活性与对照组的活性百分比.此图已获Springer Nature授权. Fig. 3 Surface charge distribution of carbonic anhydrase Ⅱ and the activity at high-salt conditions[38]. (A-D) is the distribution of surface charge in WT and M4 (blue for positive charges and red for negative charges). (E-F) Graphs indicate percentage of control activity±s. for enzymes in (E) Na2SO4 and (F) NaCl. Reproduced with permission from Springer Nature. |
| |
Wang等[39]通过定点突变提高了胆碱-磷酸胞苷酰转移酶(CCT)的嗜盐性。基于CCT三维结构模型,通过增加蛋白质表面酸性氨基酸的数量,减轻高乙酸盐浓度下的疏水效应,并对蛋白质表面变化最大的残基进行定点突变。获得的突变体在高盐条件下显示出良好的耐受能力,特别是当盐浓度(乙酸盐)超过1 200 mmol/L时仍保持较高的反应速率,比野生型CCT的反应速率高了2.2倍。Zheng等[40]也通过在蛋白质表面替换3-10个酸性氨基酸残基,减轻了高乙酸盐浓度下的疏水效应,提高了胆碱激酶(CKI)在高盐浓度下的催化活性和IC50值(活性抑制50%)。
4 有机溶剂中酶蛋白的催化行为及适应性改造有机相生物催化具有增加非极性底物溶解度、进行逆水解反应、抑制水依赖性副反应和利于酶与产品分离等优势,因此,有机溶剂中的酶促反应在工业上具有广阔的应用前景。但是,由于酶在有机溶剂中的活性较低且不稳定,蛋白酶在工业有机介质中的应用能力受到限制,需对酶进行适应性改造(表 4)。引起酶蛋白在有机相中活力下降及稳定性降低的原因较多[2, 17],包括:酶三级结构发生改变,导致蛋白质分子疏水核心的破坏;在水-有机溶剂两相体系中,酶蛋白分子与介质接触的界面处存在着大量的有机液体,使跨越两相界面的传质速率大幅度降低而导致蛋白质的静电、疏水相互作用和氢键作用力不稳定,进一步导致酶的不可逆变性。一般认为,完全脱水的蛋白质是无活性的,酶在有机溶剂中能保持活性和稳定性,与蛋白质分子和水分子的结合密不可分。作为润滑剂的水可促进酶催化所需的构象变化,使酶的稳定性提高[41]。
| Improvement method | Industrial enzyme | Modification description | Performance | Reference |
| Immobilization | Laccse | Immobilized on an enzymatic membrane reactors (EMRs) | The activity of immobilized laccase was up to 3.5 times greater than that of free one | [45] |
| Amidase | Immobilized into a magnetic hierarchically porous metal- organic framework | Immobilized amidase maintained at least 50% of the original activity, while free amidase lost more than 80% | [46] | |
| Semi-rational design | β-glucosidase I | Increased hydrogen bonds, intra-ionic interactions | Simultaneous enhanced organic solvent and thermal stability | [47] |
| Surface charge engineering | Lipase A | Mutant with highly negatively charged | The t1/2 of mutations in BsLA4M1 increasing from 56 to 474 min | [48] |
Mohtashami等[42]使用洋葱伯克霍尔德氏菌脂肪酶(BCL,PDB ID: 3lip)和变色栓菌漆酶(TVL,PDB ID: 1gyc)进行分子动力学模拟,其中BCL属于α/β水解酶折叠,β链形成酶的活性中心,而TVL是一种含3个结构域的单亚基酶。研究发现,相比之下TVL在甲醇溶液中的耐受度更好。另外,结构分析结果表明,有机溶剂与水相中BCL的构象发生了变化,导致其稳定性及酶活性的降低。构象变化是酶在有机溶剂存在下失活的最常见原因,特别是能够渗透到酶活性位点的亲水性溶剂能够诱导蛋白质二级和三级结构的变化[43]。
获得在有机介质中稳定的酶的策略可分为3大类:分离可在极端条件下起作用的新酶;修饰酶结构以增加其对有机溶剂的抗性;改变溶剂环境以降低其对酶的变性作用。
提高酶在有机溶剂中稳定性最简单的方法是酶固定化。Ruiz等[44]通过将变色栓菌漆酶吸附在玻璃、玻璃粉、硅胶等载体上,观察到漆酶在乙醚和乙酸乙酯中的活性和稳定性得到显著改善,而游离酶在这些溶剂中没有活性。同样,Liu等[45]开发出能够在有机溶剂中固定漆酶的膜,将漆酶与膜结合得到酶膜反应器(EMRs)。该研究中,漆酶通过共价键固定在有机溶剂抗性羟基化聚酮(PK-OH)膜上,酮基团通过氢键的方式促进了酶与膜的固定。在均相含水有机溶剂中,固定化漆酶对2, 2′-联氮双(3-乙基苯并噻唑啉-6-磺酸)的活性较游离酶提高了2.5倍,并且能够储存40 d,重复使用10次和连续反应50 h后保持其活性。Zheng等[46]首次将酰胺酶固定在磁性多级孔金属有机框架材料(MOF)中,该固定化酰胺酶显示出了优异的有机耐受性以及催化活性,固定化酰胺酶相互作用力以及结构刚性的增强使其在各有机溶剂(如乙醇、甲醇和DMSO)中孵育24 h后仍能保持50%以上的原始活性,而游离酶活性损失超过80%。另外,通过突变改变蛋白质一级序列也能够提高酶蛋白在有机介质中的催化稳定性。Batra等[47]使用随机与定点突变结合的方法开发了毕赤酵母有机耐受性β-葡萄糖苷酶Ι (BGLI),改造后编码基因有3个有益突变,即G414D、Y722H和N789D (图 4)。其中两个突变(Y722H和N789D)位于酶表面,一个(G414D)位于亚表面。突变残基之间的氢键数和离子相互作用增加,使酶的稳定性和对有机溶剂的耐受性提高。Zhou等[48]通过表面电荷工程修饰改变枯草芽胞杆菌脂肪酶A蛋白分子表面所带电荷。高度带负电的突变酶在高浓度离子液体中显示出较强的稳定性,因为表面负电荷的引入可以诱导酶蛋白从β折叠向α螺旋的转变,提高在有机溶剂中的耐受性。
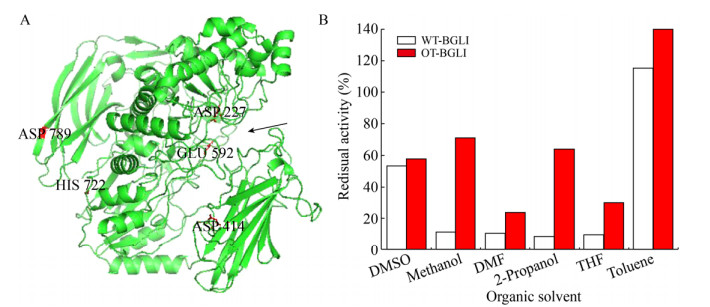
|
| 图 4 β-葡萄糖苷酶Ι的结构模型及其有机溶剂耐受性[47]. (A) OT-BGLI的模型结构,推定的活性位点残基(D227和E592)和突变的残基(D414,Y722和D789)在各位置标记,催化残基的活性位点用箭头标记. (B) OT-BGLI和WT-GBLI在不同有机溶剂中处理3 h后的残余活性,二甲基亚砜、甲醇、N, N-二甲基甲酰胺和四氢呋喃浓度为30%,2-丙醇浓度为20%,甲苯浓度为15% (以体积比计).此图已获Elsevier授权. Fig. 4 Structural model and organic tolerance of β-glucosidase oxime Ι[46]. (A) Modeled structure of OT-BGLI. Putative active site residues (D227 and E592) and mutated residues (D414, Y722 and D789) are labeled at their respective positions. Active site flanked by catalytic residues is marked with an arrow. (B) Stability of OT-BGLI in different organic solvents. The residual activity values were obtained after 3 h incubation in the solvents. WT-BGLI was taken as control. The concertration of DMSO, methanol, DMF and THF were used at 30% (V/V), while 2-propanol was at 20% and toluene was at 15%. Reproduced with permission from Elsevier. |
| |
在高强度工业生产中,往往要求较高的底物浓度,从而对酶产生特异或非特异性抑制。在高浓度底物条件下,酶会与底物结合形成酶-底物复合物,酶分子活性中心被底物覆盖使酶无法继续催化反应,需对酶蛋白进行适应性改造(表 5)。
| Improvement method | Industrial enzyme | Modification description | Performance | Reference |
| Rational design | 2-deoxyribose-5- phosphate aldolase (DERA) | Mutation of C47 gives rise to a fully acetaldehyde-resistant DERA; Short and flexible C-terminal region | Higher substrate tolerance of DERAs among class I aldolases | [49-50] |
| Directed evolution | Aminotransferase | Of the 17 noncatalytically essential amino acid spredicted to interact with the substrate, 10 were mutated | 100 g/L substrate was converted to sitagliptin at > 99.95% ee in 92% yield | [51-52] |
由于底物对酶的抑制作用机理因酶与底物的不同而有所差异,以下以2-脱氧核糖-5-磷酸醛缩酶(DERA)的底物抑制机理为主探讨酶在高浓度底物下的催化行为。DERA的应用受到对高浓度乙醛(其天然底物)耐受性差的阻碍。Markus等[49]提出了DERA在高浓度乙醛中失活的机理是由于两分子乙醛醛缩反应后生成的巴豆醛与蛋白质内的赖氨酸残基形成席夫碱,共价连接到附近的半胱氨酸残基,从而阻断DERA活性中心。为了证明机理的准确性,该团队通过将半胱氨酸替换成甲硫氨酸,新突变体能够对乙醛产生良好的抗性(图 5)。类似的底物抑制机理诊断机制和预防策略也适用于其他生物催化剂,即需要对酶改造以适应高底物浓度时,可以通过质谱、NMR和X射线晶体分析等方法的单独或组合使用,揭示底物与蛋白质的作用机制,进一步通过合理设计改善酶在高浓度底物下的稳定性。例如Cao等[50]通过截短DERA C-末端19个氨基酸提高对底物磷酸酯部分的识别灵活性,提高了对底物磷酸酯的耐受性。
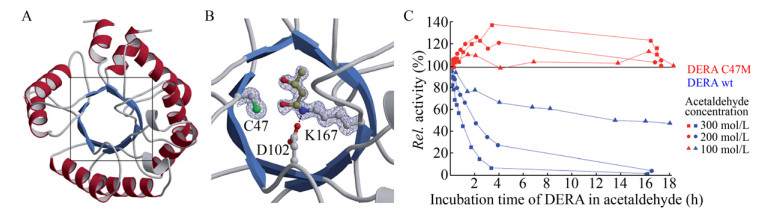
|
| 图 5 DERA的晶体结构及其底物耐受性[49]. (A)大肠杆菌DERA的晶体结构. (B) DERA的催化中心(用方框标出的部分),氨基酸侧链和底物的共价结合以球-棒模式展示(蓝色为氮,红色为氧,绿色为硫,浅灰色和黄色为碳). (C)野生型DERA与突变体C47M在不同乙醛浓度下的活性.此图已获The Royal Society of Chemistry授权. Fig. 5 The crystal structure and substrate tolerance of DERA[49]. (A) Crystal structures of monomerized E. coli DERA. (B) The boxed area containing the catalytic center is depicted in more detail before acetaldehyde incubation. Amino acid side chains as well as covalently bound substrates are displayed in ball-and-stick mode (nitrogen in blue, oxygen in red, sulfur in green, carbon in light gray and yellow, respectively). (C) Activity of wildtype vs. C47M DERA at different acetaldehyde concentrations. Reproduced with permission from The Royal Society of Chemistry. |
| |
使用上述策略改造酶提高底物耐受性的例子还有很多,如转氨酶催化前体酮生成手性胺制备西他列汀。Codexis和Merck公司的科学家[51-52]使用计算机辅助活性位点设计和定点饱和突变,得到含有27个突变位点的突变体。在预测能够与底物相互作用的17种非催化必需氨基酸中,10种被突变,突变体能在50%的DMSO中将100 g/L底物转化为西他列汀。
6 总结与展望生物催化技术诞生百余年来,已经发现的酶多达3 000余种,但商品酶只有200种左右,而工业上应用的酶仅有50多种,至于大量工业生产的酶只有10多种。目前,生物催化正处于以体外进化为标志的第三次浪潮,酶改造的进程大幅加快。可以预见,随着人工智能的迅速发展,酶的设计与合成将更为快速、理性、精准,酶催化功能改善的幅度和范围将进一步拓展。工业酶的使用将迎来新的浪潮,其绿色与可持续的特征将进一步凸显。
结合笔者在工业酶催化行为与适应性改造方面的研究实践,近期应着重在以下方面取得突破:1)利用蛋白质结构信息和不断进步的计算工具,对影响酶稳定性的机理进行深入探究,以期加强工业酶适应性改造的设计性和可预测性;2)加强产学研用的合作,了解工业环境对酶性能的需求,破解酶在工业应用中的瓶颈;3)加快酶的商品化速度,提高可及性,使品种丰富的酶像普通商品化试剂一样易于获得。
| [1] |
Schmid A, Dordick JS, Hauer B, et al. Industrial biocatalysis today and tomorrow. Nature, 2001, 409(6817): 258-268. DOI:10.1038/35051736 |
| [2] |
Luetz S, Giver L, Lalonde J. Engineered enzymes for chemical production. Biotechnol Bioeng, 2010, 101(4): 647-653. |
| [3] |
Zhang YF, Ge J, Zheng L. Enhanced activity of immobilized or chemically modified enzymes. ACS Catal, 2015, 5(8): 4503-4513. DOI:10.1021/acscatal.5b00996 |
| [4] |
Lee JW, Na D, Park JM, et al. Systems metabolic engineering of microorganisms for natural and non-natural chemicals. Nat Chem Biol, 2012, 8(6): 536-546. DOI:10.1038/nchembio.970 |
| [5] |
Atalah J, Cáceres-Moreno P, Espina G, et al. Thermophiles and the applications of their enzymes as new biocatalysts. Bioresour Technol, 2019, 280: 478-488. DOI:10.1016/j.biortech.2019.02.008 |
| [6] |
Eijsink VGH, Gåseidnes S, Borchert TV, et al. Directed evolution of enzyme stability. Biomol Eng, 2005, 22(1/3): 21-30. |
| [7] |
Bommarius AS, Broering JM. Established and novel tools to investigate biocatalyst stability. Biocatal Biotransformat, 2005, 23(3/4): 125-139. |
| [8] |
Wijma HJ, Floor RJ, Janssen DB. Structure- and sequence-analysis inspired engineering of proteins for enhanced thermostability. Curr Opin Struct Biol, 2013, 23(4): 588-594. DOI:10.1016/j.sbi.2013.04.008 |
| [9] |
Bommarius AS, Paye MF. Stabilizing biocatalysts. Chem Soc Rev, 2013, 42(15): 6534-6565. DOI:10.1039/c3cs60137d |
| [10] |
Eijsink VGH, Bjørk A, Gåseidnes S, et al. Rational engineering of enzyme stability. J Biotechnol, 2004, 113(1/3): 105-120. |
| [11] |
Reetz MT, Carballeira JD, Vogel A. Iterative saturation mutagenesis on the basis of B factors as a strategy for increasing protein thermostability. Angewand Chem Int Ed, 2006, 45(46): 7745-7751. DOI:10.1002/anie.200602795 |
| [12] |
Sun ZT, Liu Q, Qu G, et al. Utility of B-factors in protein science: Interpreting rigidity, flexibility, and internal motion and engineering thermostability. Chem Rev, 2019, 119(3): 1626-1665. DOI:10.1021/acs.chemrev.8b00290 |
| [13] |
Li WF, Zhou XX, Lu P. Structural features of thermozymes. Biotechnol Adv, 2005, 23(4): 271-281. DOI:10.1016/j.biotechadv.2005.01.002 |
| [14] |
Rakotoarivonina H, Hermant B, Aubry N, et al. Engineering the hydrophobic residues of a GH11 xylanase impacts its adsorption onto lignin and its thermostability. Enzyme Microb Technol, 2015, 81: 47-55. DOI:10.1016/j.enzmictec.2015.07.009 |
| [15] |
Wang YW, Fu Z, Huang HQ, et al. Improved thermal performance of Thermomyces lanuginosus GH11 xylanase by engineering of an n-terminal disulfide bridge. Bioresour Technol, 2012, 112(5): 275-279. |
| [16] |
Alponti JS, Maldonado RF, Ward RJ. Thermostabilization of Bacillus subtilis GH11 xylanase by surface charge engineering. Int J Biol Macromol, 2016, 87: 522-528. DOI:10.1016/j.ijbiomac.2016.03.003 |
| [17] |
Careri G. Cooperative charge fluctuations by migrating protons in globular proteins. Progr Biophys Mol Biol, 1998, 70(3): 223-249. DOI:10.1016/S0079-6107(98)00030-3 |
| [18] |
Schütte M, Fetzner S. EstA from Arthrobacter nitroguajacolicus Rü61a, a thermo- and solvent-tolerant carboxylesterase related to class C β-lactamases. Curr Microbiol, 2007, 54(3): 230-236. DOI:10.1007/s00284-006-0438-2 |
| [19] |
Song LT, Tsang A, Sylvestre M. Engineering a thermostable fungal GH10 xylanase, importance of N-terminal amino acids. Biotechnol Bioeng, 2015, 112(6): 1081-1091. DOI:10.1002/bit.25533 |
| [20] |
Liu ZM, Zhao H, Han LC, et al. Improvement of the acid resistance, catalytic efficiency, and thermostability of nattokinase by multisite-directed mutagenesis. Biotechnol Bioeng, 2019, 116(8): 1833-1843. DOI:10.1002/bit.26983 |
| [21] |
Sun Q, Fu CW, Aguila B, et al. Pore environment control and enhanced performance of enzymes infiltrated in covalent organic frameworks. J Am Chem Soc, 2018, 140(3): 984-992. DOI:10.1021/jacs.7b10642 |
| [22] |
Jung SK, Parisutham V, Jeong SH, et al. Heterologous expression of plant cell wall degrading enzymes for effective production of cellulosic biofuels. J Biomed Biotechnol, 2012, 2012: 405842. |
| [23] |
Wood EJ. Fundamentals of biochemistry: Life at the molecular level (Third Edition) by D. Voet, J. Voet, and C. W. Pratt. Biochem Mol Biol Educ, 2008, 36(4): 319-320. DOI:10.1002/bmb.20198 |
| [24] |
Wei X, Xu XX, Qian LC, et al. Engineering a highly active thermophilic β-glucosidase to enhance its pH stability and saccharification performance. Biotechnol Biofuels, 2016, 9: 147. DOI:10.1186/s13068-016-0560-8 |
| [25] |
Fushinobu S, Ito K, Konno M, et al. Crystallographic and mutational analyses of an extremely acidophilic and acid-stable xylanase: biased distribution of acidic residues and importance of Asp37 for catalysis at low pH. Protein Eng, 1998, 11(12): 1121-1128. DOI:10.1093/protein/11.12.1121 |
| [26] |
Chen Y C, Chiang YC, Hsu LC, et al. Structural modeling and further improvement in pH stability and activity of a highly-active xylanase from an uncultured rumen fungus. Bioresour Technol, 2012, 123: 125-134. DOI:10.1016/j.biortech.2012.05.142 |
| [27] |
Joshi MD, Sidhu G, Pot I, et al. Hydrogen bonding and catalysis: a novel explanation for how a single amino acid substitution can change the pH optimum of a glycosidase. J Mol Biol, 2000, 299(1): 255-279. DOI:10.1006/jmbi.2000.3722 |
| [28] |
Yang HQ, Liu L, Shin HD, et al. Structure-based engineering of histidine residues in the catalytic domain of α-amylase from Bacillus subtilis for improved protein stability and catalytic efficiency under acidic conditions. J Biotechnol, 2013, 164(1): 59-66. DOI:10.1016/j.jbiotec.2012.12.007 |
| [29] |
Sáez-Jiménez V, Fernández-Fueyo E, Medrano FJ, et al. Improving the pH-stability of versatile peroxidase by comparative structural analysis with a naturally-stable manganese peroxidase. PLoS ONE, 2015, 10(10): e0140984. DOI:10.1371/journal.pone.0140984 |
| [30] |
Beloqui A, Kobitski AY, Nienhaus GU, et al. A simple route to highly active single-enzyme nanogels. Chem Sci, 2018, 9(4): 1006-1013. DOI:10.1039/C7SC04438K |
| [31] |
Sinha R, Khare S K. Effect of organic solvents on the structure and activity of moderately halophilic Bacillus sp. EMB9 protease. Extremophiles, 2014, 18(6): 1057-1066. DOI:10.1007/s00792-014-0683-4 |
| [32] |
Munawar N, Engel PC. Prospects for robust biocatalysis: engineering of novel specificity in a halophilic amino acid dehydrogenase. Extremophiles, 2013, 17(1): 43-51. DOI:10.1007/s00792-012-0491-7 |
| [33] |
Paul S, Bag SK, Das S, et al. Molecular signature of hypersaline adaptation: insights from genome and proteome composition of halophilic prokaryotes. Genome Biol, 2008, 9: R70. DOI:10.1186/gb-2008-9-4-r70 |
| [34] |
Mokashe N, Chaudhari B, Patil U. Operative utility of salt-stable proteases of halophilic and halotolerant bacteria in the biotechnology sector. Int J Biol Macromol, 2018, 117: 493-522. DOI:10.1016/j.ijbiomac.2018.05.217 |
| [35] |
Yin J, Chen JC, Wu Q, et al. Halophiles, coming stars for industrial biotechnology. Biotechnol Adv, 2015, 33(7): 1433-1442. DOI:10.1016/j.biotechadv.2014.10.008 |
| [36] |
Zhang WJ, Xu H, Wu YQ, et al. A new cold-adapted, alkali-stable and highly salt-tolerant esterase from Bacillus licheniformis. Int J Biol Macromol, 2018, 111: 1183-1193. DOI:10.1016/j.ijbiomac.2018.01.152 |
| [37] |
Gao RC, Shi T, Liu XD, et al. Purification and Characterisation of a salt-stable protease from the halophilic archaeon Halogranum rubrum. J Sci Food Agric, 2017, 97(5): 1412-1419. DOI:10.1002/jsfa.7879 |
| [38] |
Warden AC, Williams M, Peat TS, et al. Rational engineering of a mesohalophilic carbonic anhydrase to an extreme halotolerant biocatalyst. Nat Commun, 2015, 6: 10278. DOI:10.1038/ncomms10278 |
| [39] |
Wang JZ, Zheng C, Cao Y, et al. Rational design of an efficient halotolerant enzymatic system for in vitro one-pot synthesis of cytidine diphosphate choline. Biotechnol J, 2018, 13(7): 1700577. DOI:10.1002/biot.201700577 |
| [40] |
Zheng C, Li ZJ, Yang HF, et al. Computation-aided rational design of a halophilic choline kinase for cytidine diphosphate choline production in high-salt condition. J Biotechnol, 2019, 290: 59-66. DOI:10.1016/j.jbiotec.2018.11.008 |
| [41] |
Stepankova V, Bidmanova S, Koudelakova T, et al. Strategies for stabilization of enzymes in organic solvents. ACS Catal, 2013, 3(12): 2823-2836. DOI:10.1021/cs400684x |
| [42] |
Mohtashami M, Fooladi J, Haddad-Mashadrizeh A, et al. Molecular mechanism of enzyme tolerance against organic solvents: insights from molecular dynamics simulation. Int J Biol Macromol, 2019, 122: 914-923. DOI:10.1016/j.ijbiomac.2018.10.172 |
| [43] |
Serdakowski AL, Dordick JS. Enzyme activation for organic solvents made easy. Trends Biotechnol, 2008, 26(1): 48-54. DOI:10.1016/j.tibtech.2007.10.007 |
| [44] |
Ruiz AI, Malavé AJ, Felby C, et al. Improved activity and stability of an immobilized recombinant laccase in organic solvents. Biotechnol Lett, 2000, 22(3): 229-233. DOI:10.1023/A:1005698301681 |
| [45] |
Liu CJ, Saeki D, Cheng L, et al. Polyketone-based membrane support improves the organic solvent resistance of laccase catalysis. J Colloid Interface Sci, 2019, 544: 230-240. DOI:10.1016/j.jcis.2019.03.003 |
| [46] |
Lin CP, Xu KL, Zheng RC, et al. Immobilization of amidase into a magnetic hierarchically porous metal-organic framework for efficient biocatalysis. Chem Commun, 2019, 55(40): 5697-5700. DOI:10.1039/C9CC02038A |
| [47] |
Batra J, Mishra S. Organic solvent tolerance and thermostability of a β-glucosidase co-engineered by random mutagenesis. J Mol Catal B: Enzym, 2013, 96: 61-66. DOI:10.1016/j.molcatb.2013.07.002 |
| [48] |
Zhou Y, Pérez B, Hao WW, et al. The additive mutational effects from surface charge engineering: A compromise between enzyme activity, thermostability and ionic liquid tolerance. Biochem Eng J, 2018, 148: 195-204. |
| [49] |
Dick M, Hartmann R, Weiergräeber OH, et al. Mechanism-based inhibition of an aldolase at high concentrations of its natural substrate acetaldehyde: structural insights and protective strategies. Chem Sci, 2016, 7(7): 4492-4502. DOI:10.1039/C5SC04574F |
| [50] |
Cao TP, Kim JS, Woo MH, et al. Structural insight for substrate tolerance to 2-deoxyribose-5-phosphate aldolase from the pathogen Streptococcus suis. J Microbiol, 2016, 54(4): 311-321. DOI:10.1007/s12275-016-6029-4 |
| [51] |
Sheldon RA, Woodley JM. Role of biocatalysis in sustainable chemistry. Chem Rev, 2017, 118(2): 801-838. |
| [52] |
Savile CK, Janey JM, Mundorff EC, et al. Biocatalytic asymmetric synthesis of chiral amines from ketones applied to sitagliptin manufacture. Science, 2010, 329(5989): 305-309. DOI:10.1126/science.1188934 |
 2019, Vol. 35
2019, Vol. 35




