中国科学院微生物研究所、中国微生物学会主办
文章信息
- 宋云皓, 满都拉, 王瑞刚, 万永青, 郜晋楠, 田瑞华, 段开红
- Song Yunhao, Mandlaa, Wang Ruigang, Wan Yongqing, Gao Jinnan, Tian Ruihua, Duan Kaihong
- 微贮用植物乳杆菌固定化菌剂的制备
- Preparation of immobilized Lactobacillus plantarum agent for silage
- 生物工程学报, 2018, 34(9): 1518-1527
- Chinese Journal of Biotechnology, 2018, 34(9): 1518-1527
- 10.13345/j.cjb.180006
-
文章历史
- Received: January 4, 2018
- Accepted: June 7, 2018
2 内蒙古农业大学 食品科学与工程学院,内蒙古 呼和浩特 010018
2 College of Food Science and Engineering, Inner Mongolia Agricultural University, Hohhot 010018, Inner Mongolia, China
近年来,如何高效环保地资源化利用作为副产物的秸秆受到了国内外学者的广泛关注[1]。微贮是一种可以将秸秆转化为反刍动物饲料的处理方式,以其操作简单、利用率高等优点已被逐步推广应用。在秸杆饲料自然微贮过程中,容易受其他杂菌的影响而使微贮饲料腐败变质[2-3],所以需要额外添加乳酸菌[4-6]使其更快地成为优势菌群,抑制其他杂菌的生长,改善微贮饲料的品质及适口性,提高牛羊的采食量[7]。由于微生物在保存的过程中比较容易受到外界因素的影响,利用包埋技术将微生物进行制剂或制粒可以有效提高微生物的存活率。
固定化技术由于其表面富含微孔被广泛利用于液体体系中[8-11]用以微生物的连续发酵。其中,对污水的治理[12]以及固定化乳酸菌[13-15]的研究越来越受到重视。在包埋微乳酸菌的过程中,使用壳聚糖与海藻酸钠(SA)进行包埋是最常用的方法[16-18],然而一些研究表明,使用与环境适应性更好的载体可以更好地提升使用效果[19-21]。交联法同样用于乳酸菌的固定化中,聚乙烯醇(PVA)是最常见的交联剂[22-24],使用交联法获得的固定化小球具有较高的机械强度。Selvarajan等[25]和Zhao等[26]的研究,先将微生物吸附于载体上再进行交联或包埋,其活力均有较大提升,而交联的过程中加入SA进行包埋-交联,同样可以提升微生物的活性[27]。
利用多种微生物共同处理秸秆的报道较多[28-30],但当不同种的微生物同时处理秸秆有可能会引起营养竞争,尤其是乳酸菌在厌氧发酵过程中会产生大量乳酸,这会降低与乳酸菌同时释放到体系中的纤维素降解菌处理秸秆的效率,故两类微生物需要分为不同时间释放到环境中。由于固定化技术可通过破裂载体的方式将微生物缓释到发酵体系中,受到了本研究的关注。
虽然有关包埋固定化技术在微生物领域的研究较多,但是有关在固定化发酵微贮技术中的研究较为少见,且相关研究主要集中在如何利用固定化技术提高活菌数和发酵时间等领域[31]。
因此,本研究创新性地使用包埋技术用于固态微贮发酵,通过在载体中加入易被降解的材料,从而使微生物缓释于发酵体系中,为开发缓释包埋微贮菌剂奠定基础。
1 材料与方法 1.1 菌种本实验室低温保存的植物乳杆菌属S1 Lactobacillus plantarum,经NCBI序列比对,与Lactobacillus plantarum LC016617.1相似度为99%。
1.2 培养基MRS培养基:蛋白胨10 g,牛肉膏8 g,酵母提取物6 g,K2HPO4 2.5 g,柠檬酸二铵2.5 g,醋酸钠6 g,葡萄糖20 g,MgSO4 0.5 g,MnSO4 0.25 g,琼脂粉15 g,溶于1 000 mL蒸馏水中调pH值为6.2–6.4,121 ℃灭菌20 min。
MRS液体培养基:蛋白胨10 g,牛肉膏8 g,酵母提取物6 g,K2HPO4 2.5 g,柠檬酸二铵2.5 g,醋酸钠6 g,葡萄糖20 g,MgSO4 0.5 g,MnSO4 0.25 g,溶于1 000 mL蒸馏水中调pH值为6.2–6.4,121 ℃灭菌20 min。
无机盐(MSM)培养基按照Tao等[34]的方式制备。
1.3 菌悬液的制备实验室低温保藏的植物乳杆菌S1涂布于MRS培养基,置于37 ℃厌氧培养3 d,连续培养3代以上后使用。
将活化好的植物乳杆菌属S1接种于MRS液体培养基中,37 ℃下振荡3 d,所得发酵液用无机盐培养基调为OD600=0.5的菌悬液置于4 ℃备用。
1.4 固定化小球的制备 1.4.1 交联-包埋固定化小球的制备PVA、SA和CMC-Na的混合载体具有强度高、稳定性强等优点,但是其浓度过少或过多均会影响成球效果和作用性能。为了确定最佳组合比例,将PVA、SA和CMC-Na按不同比例混合,水浴加热(90 ℃左右)溶解,冷却后(40 ℃左右)得混合胶体。一边振荡一边用50 mL注射器将混合液逐滴加入到5% Ca(NO3)2溶液中浸泡1 h后,将小球在–20 ℃下保存24 h,最后在室温解冻。取出固定化小球用蒸馏水冲洗3次,存放备用。
1.4.2 包埋固定化小球的制备将SA与CMC-Na按照最佳比例混合,在室温下溶解得到混合胶体。在振荡的条件下,用50 mL注射器将混合液逐滴加入到4% CaCl2溶液中。包埋24 h后,取出固定化小球用蒸馏水冲洗3次,存放备用。将未经吸附的微生物固定在成球效果较好的小球中测定包埋率。
1.5 复合固定化方法吸附:把玉米秸秆用去离子水冲洗后,在105 ℃条件下烘干至恒重,然后粉碎过40目筛。称取干重0.5 g玉米秸秆吸附载体倒入锥形瓶,加入50 mL无机盐培养基灭菌后备用,冷却至室温后加入适量菌悬液,振荡培养8 h[8]。所得培养物在5 000 r/min条件下离心清洗得吸附载体,加入无菌水定容至10 mL备用[8]。
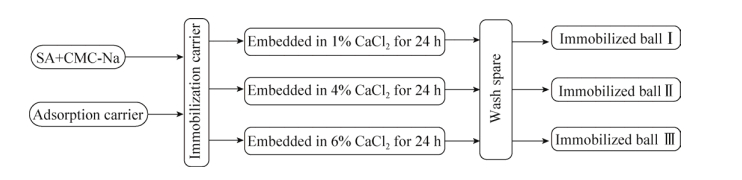
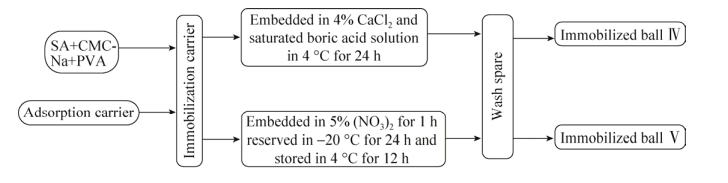
固定化采用两种方式进行:一种为包埋法,一种为包埋-交联法。包埋:在以上试验确定SA+CMC-Na的最佳配比后,将SA+CMC-Na加30 mL蒸馏水浸泡过夜,次日高温灭菌,冷却至40 ℃左右。加入10 mL吸附载体用无菌水定容至100 mL后充分混匀。混匀后用注射器分别滴入1% CaCl2、4% CaCl2、6% CaCl2溶液中,具体方法见图 1。包埋-交联法[35-36]:在以上试验确定PVA+SA+CMC-Na的最佳配比后,将PVA+SA+ CMC-Na加入60 mL蒸馏水浸泡过夜,经高温灭菌后,冷却至40 ℃左右。此时加入10 mL上述吸附载体,加入无菌水定容至50 mL后充分混匀,包埋微生物量为10%然后进行交联:第一种交联方法是混匀后用注射器滴入含4% CaCl2的饱和硼酸溶液中(NaOH调pH值为6.7),在4 ℃下静置24 h,制成球。第二种交联方法是将混合物逐滴滴入5% Ca(NO3)2溶液中浸泡1 h后,将小球在–20 ℃下保存24 h,最后在室温解冻,具体方法见图 2。
|
| 图 1 固定化吸附-包埋工艺流程图 Figure 1 The flowchart of immobilization adsorption-embedding process. |
| |
|
| 图 2 固定化吸附-包埋-交联工艺流程图 Figure 2 The flowchart of immobilization adsorption-embedding-crosslinking process. |
| |
密度的比较:用电子天平称取量筒的质量为m量筒,把制作的固定化小球倒入量筒得m总,m颗粒的质量为:m总–m量筒。再加入一定量的蒸馏水(V)至完全覆盖住固定化小球,测得总体积V总,固定化颗粒的体积V颗粒=V总–V水,则固定化微生物小球的密度ρ颗粒=m颗粒/V颗粒。
直径的比较:随机选取10个大小均匀的固定化小球,用游标卡尺测量其直径,计算其平均直径,即为固定化小球的直径D。
膨胀率的测试:选取固定化小球100个浸泡在去离子水中12 h后测其直径为D12。膨胀率系数X为:D12/D。
强度系数的测定:将固定化小球100颗放入150 mL锥形瓶中,加入75 mL去离子水,于100 r/min、30 ℃恒温振荡24 h后完好小球占原小球总数的比率。
固定化小球包埋率的测定:固定化小球包埋率为固定化小球中的活菌数占包埋前活菌数总数的百分比。统计包埋前菌落总数A,称得所制成的固定化小球总质量m1,取出1 g左右固定化小球,精确测量其质量m2,充分研磨后用无菌水溶解计数,得固定化后小球包埋菌数为B。
包埋率(Y) %=(B×m1)/(A×m2)×100。
1.7 数据分析每个处理设3个重复,当P < 0.05时,视为处理之间差异显著。数据分析及绘图利用Microsoft Office Excel 2015完成。
2 结果与分析 2.1 包埋-交联法中载体的最佳组合配比PVA的浓度过高时,由于混合物粘度较高使得凝珠不易成球,PVA的浓度过低时,混合物粘度不高,成球率较低;SA的浓度过高时,会影响混合物的粘度,容易造成拖尾现象。SA的浓度过低时,形成的固定化小球强度较低,成球率不高。羧甲基纤维素钠作为赋形剂也同样影响固定化小球的成球效果(表 1)。
| Ratio of immobilization carrier | Balling effects | Mechanical strength (%) | Yield (%) |
| 5% PVA+0.3% SA+0.2% CMC-Na | – | – – | – – |
| 5% PVA+0.3% SA+0.3% CMC-Na | – | – – | – – |
| 5% PVA+0.3% SA+0.4% CMC-Na | – | – – | – – |
| 5% PVA+0.4% SA+0.2% CMC-Na | – | – – | – – |
| 5% PVA+0.4% SA+0.3% CMC-Na | – | – – | – – |
| 5% PVA+0.4% SA+0.4% CMC-Na | – | – – | – – |
| 5% PVA+0.5% SA+0.2% CMC-Na | + | 20 | – – |
| 5% PVA+0.5% SA+0.3% CMC-Na | + | 30 | – – |
| 5% PVA+0.5% SA+0.4% CMC-Na | + | 50 | – – |
| 6% PVA+0.3% SA+0.2% CMC-Na | + | 50 | – – |
| 6% PVA+0.3% SA+0.3% CMC-Na | + | 70 | 37.6±1.5 |
| 6% PVA+0.3% SA+0.4% CMC-Na | ++ | 95 | 78.7±2.7 |
| 6% PVA+0.4% SA+0.2% CMC-Na | ++ | 97 | 79.0±2.1 |
| 6% PVA+0.4% SA+0.3% CMC-Na | ++ | 99 | 79.2±1.9 |
| 6% PVA+0.4% SA+0.4% CMC-Na | ++ | 96 | 78.5±1.1 |
| 6% PVA+0.5% SA+0.2% CMC-Na | ++ | 90 | 71.0±1.7 |
| 6% PVA+0.5% SA+0.3% CMC-Na | ++ | 92 | 72.2±1.0 |
| 6% PVA+0.5% SA+0.4% CMC-Na | + | 50 | – – |
| 7% PVA+0.3% SA+0.2% CMC-Na | + | 80 | 56.5±2.5 |
| 7% PVA+0.3% SA+0.3% CMC-Na | + | 75% | 55.4±3.7 |
| 7% PVA+0.3% SA+0.4% CMC-Na | + | 71 | 53.2±1.8 |
| 7% PVA+0.4% SA+0.2% CMC-Na | + | 76 | 56.7±1.2 |
| 7% PVA+0.4% SA+0.3% CMC-Na | + | 69 | 35.7±2.0 |
| 7% PVA+0.4% SA+0.4% CMC-Na | + | 40 | – – |
| 7% PVA+0.5% SA+0.2% CMC-Na | + | 62 | 31.1±1.5 |
| 7% PVA+0.5% SA+0.3% CMC-Na | + | 58 | – – |
| 7% PVA+0.5% SA+0.4% CMC-Na | – | – – | – – |
| 8% PVA+0.3% SA+0.2% CMC-Na | + | 30 | – – |
| 8% PVA+0.3% SA+0.3% CMC-Na | + | 20 | – – |
| 8% PVA+0.3% SA+0.4% CMC-Na | – | – – | – – |
| 8% PVA+0.4% SA+0.2% CMC-Na | – – | – – | – – |
| 8% PVA+0.4% SA+0.3% CMC-Na | – – | – – | – – |
| 8% PVA+0.4% SA+0.4% CMC-Na | – – | – – | – – |
| 8% PVA+0.5% SA+0.2% CMC-Na | – – | – – | – – |
| 8% PVA+0.5% SA+0.3% CMC-Na | – – | – – | – – |
| 8% PVA+0.5% SA+0.4% CMC-Na | – – | – – | – – |
| –: not balling; – –: severe tailing; +: balling; ++: better balling. | |||
从结果中可以得出PVA的合理浓度为6%–8%,SA的合理浓度为0.3%–0.5%,CMC-Na的合理浓度为0.3%,这一组合制成的固定化小球机械强度和包埋率等因素的效果较好,固定化小球性能最优。
根据结果,最终确定聚乙烯醇和海藻酸钠与羧甲基纤维素钠作为固定化微生物的包埋载体的最佳配比为6% PVA+0.4% SA+0.3% CMC-Na,此结果将用于后续试验。
2.2 包埋法中载体的最佳组合配比当SA的浓度过低时,形成的固定化小球机械强度较低,成球率不高。当SA的浓度过高时,由于混合物粘度较高使得固定化小球不易成型,而且容易造成拖尾或者团聚现象。当CMC-Na的浓度过高时也会使混合物粘度增大,从而造成成球效果不佳等现象;当CMC-Na的浓度过低时,会使固定化小球的机械强度降低,包埋率降低(表 2)。
| Ratio of immobilization carrier | Balling effects | Mechanical strength (%) | Yield (%) |
| 0.8% SA+0.3% CMC-Na | – | – – | – – |
| 0.8% SA+0.4% CMC-Na | – | – – | – – |
| 0.8% SA+0.5% CMC-Na | – | – – | – – |
| 0.8% SA+0.6% CMC-Na | – | – – | – – |
| 0.8% SA+0.7% CMC-Na | – | – – | – – |
| 1% SA+0.3% CMC-Na | + | 20 | – – |
| 1% SA+0.4% CMC-Na | + | 50 | – – |
| 1% SA+0.5% CMC-Na | ++ | 80 | 73.9±2.3 |
| 1% SA+0.6% CMC-Na | ++ | 70 | 38.2±2.7 |
| 1% SA+0.7% CMC-Na | – – | – – | – – |
| 1.2% SA+0.3% CMC-Na | ++ | 90 | 80.1±3.4 |
| 1.2% SA+0.4% CMC-Na | ++ | 93 | 83.2±2.1 |
| 1.2% SA+0.5% CMC-Na | ++ | 99 | 87.3±2.8 |
| 1.2% SA+0.6% CMC-Na | ++ | 95 | 85.2±1.9 |
| 1.2% SA+0.7% CMC-Na | + | 80 | 73.8±2.0 |
| 1.4% SA+0.3% CMC-Na | ++ | 85 | 78.2±1.5 |
| 1.4% SA+0.4% CMC-Na | + | 80 | 73.5±2.1 |
| 1.4% SA+0.5% CMC-Na | + | 50 | – – |
| 1.4% SA+0.6% CMC-Na | – – | – – | – – |
| 1.4% SA+0.7% CMC-Na | – – | – – | – – |
| –: not balling; – –: severe tailing; +: balling; ++: better balling. | |||
从表 2可以得出SA的合理浓度为1%–1.2%,CMC-Na的合理浓度为0.3%–0.4%,这一组合制成的固定化小球在成球效果、机械强度和包埋率等因素的效果较好,固定化小球性能最优。
最终SA和CMC-Na的最佳配比为1.2% SA+0.5%CMC-Na,使用此的比例混合制备的固定化小球在成球效果、机械强度和包埋率等因素综合表现最好。同时,这种方法制备的固定化小球较为经济,此结果将用于后续的试验。
从图 3可以清楚发现两类固定化小球质地均匀,机械强度较好,而且具有较好的弹性。本试验所得的两类固定化小球在4%的CaCl2溶液中能保持30 d以上外形的完整性和性能的稳定性。

|
| 图 3 不同固定化小球外观效果图 Figure 3 Appearance of different immobilized microbial pellets. (A) The appearance of embedded balls. (B) The appearance of embedded-crosslinked balls. |
| |
如表 3所示。兼顾处理效果和经济性两方面因素,复合固定化微生物小球Ⅱ (CMIB Ⅱ)实用性能强,包埋率最高,而且制备的胶体在成球效果、机械强度和传质性的综合表现最好,在后续的试验中我们将使用此方法对植物乳杆菌S1进行固定化,用于微贮饲料的发酵。
| Immobilized ball | Density (g/cm3) | Diameter (mm) | Mechanical strength (%) | Expansion rate (%) | Embedded rate (%) | Bacteria No. (×108 CFU/mL) |
| MIB Ⅰ Immobilized ball Ⅰ |
0.608 9 | 4.25 | 80 | 1.9±0.22 | 73.2±3.2 | 1.43±0.045 |
| MIB Ⅱ | 0.607 9 | 4.41 | 96 | 2.2±0.17 | 85.5±1.6 | 1.96±0.031 |
| MIB Ⅲ | 0.612 0 | 4.55 | 98 | 1.3±0.14 | 75.1±2.1 | 1.56±0.033 |
| MIB Ⅳ | 0.773 1 | 4.45 | 98 | 2.0±0.30 | 71.5±2.3 | 1.37±0.031 |
| MIB Ⅴ | 0.782 0 | 4.90 | 99 | 2.0±0.25 | 72.3±1.3 | 1.50±0.032 |
| CMIB Ⅰ | 0.615 6 | 4.47 | 77 | 2.1±0.21 | 75.5±3.2 | 1.69±0.054 |
| CMIB Ⅱ | 0.612 3 | 4.55 | 95 | 2.5±0.19 | 88.3±2.6 | 2.20±0.057 |
| CMIB Ⅲ | 0.623 5 | 4.56 | 96 | 1.6±0.28 | 76.2±1.8 | 1.73±0.031 |
| CMIB Ⅳ | 0.704 2 | 5.10 | 99 | 0.4±0.08 | 70.7±0.9 | 1.29±0.012 |
| CMIB Ⅴ | 0.877 9 | 5.70 | 98 | 1.2±0.13 | 73.5±2.2 | 1.46±0.032 |
| MIB: immobilized microorganism balls; CMIB: composite immobilized microorganism balls. | ||||||
采用将混合物逐滴滴入4%氯化钙中包埋24 h的方法既可以避免饱和硼酸溶液对微生物的毒性,又可以使固定化小球表面积增大、机械强度变高(表 3)。这种方法制作的固定化小球形状均匀、机械强度高,且活菌存活率较多,是理想的固定化方法。
3 讨论与结论 3.1 吸附法对固定化微生物的影响通过对比固定化微生物小球(MIB)和复合固定化微生物小球(CMIB)的物理参数,发现添加适量的吸附载体,增加固定化小球内部空间骨架结构,有利于提高固定化小球的内部空间结构体积,使得微生物与基质接触机会更大,并增大了其生长空间,从而起到了改善小球物理性能的效果,该结果与刘金苓等[37]的结果相吻合。
本研究选择粉碎的玉米秸秆为吸附载体的原因为:第一,玉米秸秆自身含有营养物质可以供植物乳杆菌S1利用,从而延长固定化小球的保存时间。第二,此固定化小球用于玉米秸秆的微贮,在固定化时就开始利用玉米秸秆中的营养物质,在后期释放到微贮体系中时,不需要再去重新适应环境即可快速处理秸秆。另外,纤维素降解菌能够缓慢降解小球,使乳酸菌缓慢释放到发酵体系中。
3.2 包埋-交联法对固定化微生物的影响本试验讨论了两种包埋-交联方法对植物乳杆菌S1包埋率的影响,经过交联处理的固定化小球的机械强度普遍高于未经交联处理组,与此同时其包埋率却低于未经交联处理组。这有可能是在交联过程中聚乙烯醇(PVA)在载体中起到了很强的支撑作用,但其交联剂饱和硼酸溶液在交联时反应比较剧烈,有可能导致微生物损失了一部分的生物活性[38]。
在传统的包埋-交联法对固定化微生物的研究中,其基本所有的应用均为处理液体中的某种废物,所以机械强度与载体的通透性十分重要,但由于后续的研究需要将固定化小球中的微生物释放出来而不是包裹在载体中发挥作用,所以机械强度与载体的通透性在本研究中并不能成为评价固定化小球性能的决定性指标。
3.3 包埋法对固定化微生物的影响在使用海藻酸钠(SA)作为包埋剂时,其与CaCl2形成的海藻酸钙凝胶是可逆的[39],而且在低pH的条件下,凝胶会发生解聚现象[40],这几点在其作为人体益生菌包埋剂时是十分不理想的。然而,在本试验中凝胶在释放进固态发酵体系中必须在十几个小时内快速破裂,故上述几点成为了缓释植物乳杆菌S1的关键点,为下一步研究打下基础。
通过比较试验数据得出:兼顾处理效果和经济性两方面因素,我们采用第二种包埋方法的SA和CMC-Na的最佳配为1.2%SA+0.5%CMC-Na的玉米球实用性能强,包埋率最高,活菌数最高可达2.20×108 CFU/mL,而且制备的胶体在成球效果、机械强度和传质性的综合表现最好。
| [1] |
Zuo X, Wang HY, Wang YJ. Estimation and suitability evaluation of corn straw resources in China.
Chin J Agric Resour Reg Plann, 2015, 36(6): 5–10.
(in Chinese). 左旭, 王红彦, 王亚静. 中国玉米秸秆资源量估算及其自然适宜性评价. 中国农业资源与区划, 2015, 36(6): 5-10. |
| [2] | Duniere L, Xu SW, Long J, et al. Bacterial and fungal core microbiomes associated with small grain silages during ensiling and aerobic spoilage. BMC Microbiol, 2017, 17: 50. DOI: 10.1186/s12866-017-0947-0 |
| [3] | Xu ZS, He HY, Zhang SS, et al. Effects of inoculants Lactobacillus brevis and Lactobacillus parafarraginis on the fermentation characteristics and microbial communities of corn stover silage. Sci Rep, 2017, 7: 13614. DOI: 10.1038/s41598-017-14052-1 |
| [4] | Kim HS, Han OK, Kim SC, et al. Screening and investigation Lactobacillius spp. to improve Secale cereale silage quality. Anim Sci J, 2017, 88(10): 1538–1546. DOI: 10.1111/asj.2017.88.issue-10 |
| [5] | Chen XZ, Li WY, Gao CF, et al. Silage preparation and fermentation quality of kudzu, sugarcane top and their mixture treated with lactic acid bacteria, molasses and cellulase. Anim Sci J, 2017, 88(11): 1715–1721. DOI: 10.1111/asj.2017.88.issue-11 |
| [6] | Gharechahi J, Kharazian ZA, Sarikhan S, et al. The dynamics of the bacterial communities developed in maize silage. Microb Biotechnol, 2017, 10(6): 1663–1676. DOI: 10.1111/1751-7915.12751 |
| [7] | Ke WC, Ding WR, Xu DM, et al. Effects of addition of malic or citric acids on fermentation quality and chemical characteristics of alfalfa silage. J Dairy Sci, 2017, 100(11): 8958–8966. DOI: 10.3168/jds.2017-12875 |
| [8] |
Jiang LH, Wang M, Zhang WJ, et al. Study of immobilization technique of nitrogen-fixing bacteria, phosphobacteria and silicate bacteria used for microbiological fertilizer.
Chin Agric Sci Bull, 2010, 26(12): 18–21.
(in Chinese). 江丽华, 王梅, 张文君, 等. 固氮、解磷、解钾混合菌株协同固定化技术. 中国农学通报, 2010, 26(12): 18-21. DOI:10.3969/j.issn.1007-7774.2010.12.006 |
| [9] |
Yu FH, Ma YS, Liu SP, et al. Immobilization of high-temperature alkaline lipase by sodium alginate and carboxymethyl cellulose sodium.
China Brew, 2015, 34(5): 78–81.
(in Chinese). 虞凤慧, 马韵升, 刘圣鹏, 等. 海藻酸钠与羧甲基纤维素钠固定化高温碱性脂肪酶. 中国酿造, 2015, 34(5): 78-81. |
| [10] |
Zhu GL, Hu YY. Nitrogen removal with ANAMMOX mixed culture immobilized in different materials.
Acta Sci Circumst, 2008, 28(9): 1861–1866.
(in Chinese). 朱刚利, 胡勇有. 不同材料包埋固定化厌氧氨氧化混培物. 环境科学学报, 2008, 28(9): 1861-1866. DOI:10.3321/j.issn:0253-2468.2008.09.022 |
| [11] |
Zhang K, Liu FY, Li W, et al. Degradation of decabromodiphenyl ether by corncob-sodium alginate immobilized bacteria.
Chin J Appl Environ Biol, 2016, 22(5): 904–910.
(in Chinese). 张可, 刘福义, 陈伟, 等. 玉米芯吸附-海藻酸钠固定微生物对十溴联苯醚的降解. 应用与环境生物学报, 2016, 22(5): 904-910. |
| [12] |
Qu Y, Zhang PY, Guo SS, et al. Application of multi-technology of microorganisms immobilization for biological treatment of sewage.
Sichuan Environ, 2009, 28(3): 78–84.
(in Chinese). 曲洋, 张培玉, 郭沙沙, 等. 复合固定化法固定化微生物技术在污水生物处理中的研究应用. 四川环境, 2009, 28(3): 78-84. DOI:10.3969/j.issn.1001-3644.2009.03.020 |
| [13] |
Sun X. Immobilization of lactobacillus based on adsorption and microencapsulation technology and study of the properties of the immobilized lactobacillus[D]. Qingdao: Ocean University of China, 2007 (in Chinese). 孙欣.吸附与包埋技术固定化乳酸菌及其性质研究[D].青岛: 中国海洋大学, 2007. http://cdmd.cnki.com.cn/Article/CDMD-10423-2008012477.htm |
| [14] | Brachkova MI, Duarte MA, Pinto JF. Preservation of viability and antibacterial activity of Lactobacillus spp. in calcium alginate beads. Eur J Pharmaceut Sci, 2010, 41(5): 589–596. DOI: 10.1016/j.ejps.2010.08.008 |
| [15] | Brinques GB, Ayub MAZ. Effect of microencapsulation on survival of Lactobacillus plantarum in simulated gastrointestinal conditions, refrigeration, and yogurt. J Food Eng, 2011, 103(2): 123–128. DOI: 10.1016/j.jfoodeng.2010.10.006 |
| [16] | Chen S, Cao Y, Ferguson LR, et al. The effect of immobilization of probiotic Lactobacillus reuteri DPC16 in sub-100 μm microcapsule on food-borne pathogens. World J Microbiol Biotechnol, 2012, 28(6): 2447–2452. DOI: 10.1007/s11274-012-1046-x |
| [17] | Krunić TŽ, Bulatović ML, Obradović NS, et al. Effect of immobilisation materials on viability and fermentation activity of dairy starter culture in whey-based substrate. J Sci Food Agric, 2016, 96(5): 1723–1729. DOI: 10.1002/jsfa.2016.96.issue-5 |
| [18] | Trabelsi I, Ayadi D, Bejar W, et al. Effects of Lactobacillus plantarum immobilization in alginate coated with chitosan and gelatin on antibacterial activity. Int J Biol Macromol, 2014, 64: 84–89. DOI: 10.1016/j.ijbiomac.2013.11.031 |
| [19] | Mu RJ, Yuan Y, Wang L, et al. Microencapsulation of Lactobacillus acidophilus with konjac glucomannan hydrogel. Food Hydrocoll, 2018, 76: 42–48. DOI: 10.1016/j.foodhyd.2017.07.009 |
| [20] | Schoina V, Terpou A, Angelika-Ioanna G, et al. Use of Pistacia terebinthus resin as immobilization support for Lactobacillus casei cells and application in selected dairy products. J Food Sci Technol, 2015, 52(9): 5700–5708. DOI: 10.1007/s13197-014-1627-9 |
| [21] | Shaharuddin S, Muhamad Ⅱ. Microencapsulation of alginate-immobilized bagasse with Lactobacillus rhamnosus NRRL 442: enhancement of survivability and thermotolerance. Carbohydr Polym, 2015, 119: 173–181. DOI: 10.1016/j.carbpol.2014.11.045 |
| [22] |
Li F, Lü XW, Yan W. Study on Polyvinyl Alcohol (PVA) as entrapping agents for immobilizing microbial cell.
China Water Wastewat, 2000, 16(12): 14–17.
(in Chinese). 李峰, 吕锡武, 严伟. 聚乙烯醇作为固定化细胞包埋剂的研究. 中国给水排水, 2000, 16(12): 14-17. DOI:10.3321/j.issn:1000-4602.2000.12.004 |
| [23] |
Mao YH, Wang JL. Immobilization of activated sludge in PVA matrix using innovative methods.
Acta Sci Circumstant, 2013, 33(2): 370–376.
(in Chinese). 茆云汉, 王建龙. 聚乙烯醇固定化微生物新方法的研究. 环境科学学报, 2013, 33(2): 370-376. |
| [24] | Zaushitsyna O, Dishisha T, Hatti-Kaul R, et al. Crosslinked, cryostructured Lactobacillus reuteri monoliths for production of 3-hydroxypropionaldehyde, 3-hydroxypropionic acid and 1, 3-propanediol from glycerol. J Biotechnol, 2017, 241: 22–32. DOI: 10.1016/j.jbiotec.2016.11.005 |
| [25] | Selvarajan E, Mohanasrinivasan V, Devi CS, et al. Immobilization of β-galactosidase from Lactobacillus plantarum HF571129 on ZnO nanoparticles: characterization and lactose hydrolysis. Bioproc Biosyst Eng, 2015, 38(9): 1655–1669. DOI: 10.1007/s00449-015-1407-6 |
| [26] | Zhao ZJ, Xie XN, Wang Z, et al. Immobilization of Lactobacillus rhamnosus in mesoporous silica-based material: An efficiency continuous cell-recycle fermentation system for lactic acid production. J Biosci Bioeng, 2016, 121(6): 645–651. DOI: 10.1016/j.jbiosc.2015.11.010 |
| [27] | Wang X, Yao MQ, Liu L, et al. Degradation of chlorpyrifos in contaminated soil by immobilized laccase. J Serb Chem Soci, 2016, 81(10): 1215–1224. DOI: 10.2298/JSC160128066W |
| [28] | De Boever JL, Dupon E, Wambacq E, et al. The effect of a mixture of Lactobacillus strains on silage quality and nutritive value of grass harvested at four growth stages and ensiled for two periods. Agric Food Sci, 2013, 22(1): 115–126. DOI: 10.23986/afsci.6709 |
| [29] | Lara EC, Basso FC, De Assis FB, et al. Changes in the nutritive value and aerobic stability of corn silages inoculated with Bacillus subtilis alone or combined with Lactobacillus plantarum. Anim Product Sci, 2016, 56(11): 1867–1874. DOI: 10.1071/AN14686 |
| [30] | Zielińska K, Fabiszewska A, Stefańska I. Different aspects of Lactobacillus inoculants on the improvement of quality and safety of alfalfa silage. Chil J Agric Res, 2015, 75(3): 298–306. DOI: 10.4067/S0718-58392015000400005 |
| [31] | Clarke P. Development of microbial inocula for silage fermentations[D]. Dublin: Dublin Institute of Technology, 2001. https://www.researchgate.net/publication/241802054_Development_of_microbial_inocula_for_silage_fermentations |
| [32] | Tao XQ, Lu GN, Dang Z, et al. A phenanthrene-degrading strain Sphingomonas sp. GY2B isolated from contaminated soils. Proc Biochem, 2007, 42(3): 401–408. DOI: 10.1016/j.procbio.2006.09.018 |
| [33] | Wang YJ, Yang XJ, Li HY, et al. Immobilization of Acidithiobacillus ferrooxidans with complex of PVA and sodium alginate. Polym Degradat Stabil, 2006, 91(10): 2408–2414. DOI: 10.1016/j.polymdegradstab.2006.03.015 |
| [34] | Wang YJ, Yang XJ, Wei T, et al. High-rate ferrous iron oxidation by immobilized Acidithiobacillus ferrooxidans with complex of PVA and sodium alginate. J Microbiol Methods, 2007, 68(2): 212–217. DOI: 10.1016/j.mimet.2006.07.013 |
| [35] |
Liu JL, Zhong YM, Wang LJ, et al. Preliminary study on nitrogen removal with anaerobic ammonium oxidizing (ANAMMOX) bacteria immobilized in absorbent and entrapping materials.
Acta Sci Circumstant, 2010, 30(3): 470–476.
(in Chinese). 刘金苓, 钟玉鸣, 王丽娇, 等. 厌氧氨氧化微生物的吸附、包埋固定化效果初探. 环境科学学报, 2010, 30(3): 470-476. |
| [36] |
Liu YK, Mao YH, Wang JL. Methods and application of immobilized microorganism in PVA.
Sci Technol Rev, 2016, 34(22): 56–61.
(in Chinese). 刘元坤, 茆云汉, 王建龙. PVA固定化微生物方法及应用. 科技导报, 2016, 34(22): 56-61. |
| [37] | Smidsrød O, Skja˚k-Brk G, et al. Alginate as immobilization matrix for cells. Trends Biotechnol, 1990, 8: 71–78. DOI: 10.1016/0167-7799(90)90139-O |
| [38] |
Wang QW. Study on the bifidabacterium-loaded microcapsule prepared by internal emulsiifcation method[D]. Wuxi: Jiangnan University, 2014 (in Chinese). 王庆卫.内源乳化法制备双歧杆菌微胶囊的研究[D].无锡: 江南大学, 2014. http://cdmd.cnki.com.cn/Article/CDMD-10295-1014370784.htm |
 2018, Vol. 34
2018, Vol. 34