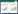中国科学院微生物研究所、中国微生物学会主办
文章信息
- 孙登岳, 程晓涛, 郭倩倩, 许盼盼, 朱张亮, 秦慧民, 路福平
- Sun Dengyue, Cheng Xiaotao, Guo Qianqian, Xu Panpan, Zhu Zhangliang, Qin Huimin, Lu Fuping
- 疏水性氨基酸的羟基化研究进展
- Advances in hydroxylation of hydrophobic amino acid
- 生物工程学报, 2018, 34(7): 1046-1056
- Chinese Journal of Biotechnology, 2018, 34(7): 1046-1056
- 10.13345/j.cjb.170509
-
文章历史
- Received: December 25, 2017
- Accepted: February 5, 2018
2 天津科技大学 工业发酵微生物教育部重点实验室,天津 300457;
3 工业酶国家工程实验室,天津 300457
2 Tianjin Key Laboratory of Industrial Microbiology, Tianjin 300457, China;
3 National Engineering Laboratory for Industrial Enzymes, Tianjin 300457, China
氨基酸是生命科学中普遍存在的重要化合物,在生物体中具有多种重要的功能。大多数氨基酸是蛋白质的主要组成部分,在维持基本机制的平衡中起着重要作用。其中,羟基氨基酸是糖肽抗生素、环肽和胶原蛋白的组成部分,具有多种生理活性[1]。研究表明,这些含有羟基氨基酸残基的肽,具有抗真菌、抗细菌、抗病毒和抗癌特性。此外,一些羟基氨基酸含有手性碳,是手性化合物的重要组成部分[2-3]。这种氨基酸由于能在多种化学材料、医学药物合成中作为手性前体、中间体和终产物,因此,广泛应用于多功能生物大分子、医药和精细化学品的合成[4-5]。羟基氨基酸除了可作为医药领域药物合成的中间体,还广泛用于饲料添加剂、食品强化剂、香料等多个领域,如L-β-羟基丙氨酸除用于食品领域中的氨基酸减肥饮料及运动饮料外,还可作为动物饲料添加剂;L-羟基脯氨酸在果汁、营养饮料、清凉饮料中常作为营养强化剂、增味剂;L-β-羟基丁氨酸也是一种添加剂,常用于食品和饲料领域。因此,羟基氨基酸的研究一直是一个不断发展且具有巨大潜力的研究领域[6]。
Fe(Ⅱ)和α-酮戊二酸依赖型羟化酶(Fe/αKGDs)是一类单核非血红素铁酶,在底物特定碳位具有羟基化、脱烷基化、脱饱和、环氧化、脱硫、卤化和环化形成过氧化物等催化特性[7-10]。非活性碳原子的不对称羟基化是工业上合成手性化合物重要方式,如药物和精细化学品的合成[11]。该类型羟化酶在辅因子存在下能够催化氨基酸生成羟化氨基酸。在催化反应过程中,Fe(Ⅱ)会形成催化活性中心,伴随羟化产物的生成,α-酮戊二酸经过氧化脱羧生成琥珀酸。Fe/αKGDs能发生多种类型的单加氧反应,一些催化反应涉及到次级代谢产物的合成途径,如β-内酰胺抗生素、类黄酮、生物碱、赤霉素等。Fe/αKGDs具有和各种亲水化合物反应的能力,氨基酸是其典型的底物,通常被催化为羟基氨基酸,Fe/αKGDs双加氧酶在催化氨基酸方面扮演着重要的角色,其中包括(2S, 3R, 4S)-4HIL、(2S, 4S)-5-羟基-亮氨酸、(2S, 3R)-3-羟基-亮氨酸、N-琥珀酰-(2S, 3R)-3-羟基-亮氨酸、cis-4-羟基-脯氨酸和4-羟基-缬氨酸等等。Fe/αKGDs作为自由氨基酸的生物催化剂,它的特点是能与氨基酸的特定碳位发生羟基化,如L-氨基酸的C3-、C4-和C5-位的催化反应。文中就疏水性氨基酸羟基化合成的最新研究进展进行了综述,详细阐述了羟化氨基酸的功能与应用,分析了Fe/αKGDs的结构特征及共性催化机制,对该类酶在生物工程领域的应用进行展望。
1 疏水性氨基酸羟基化合成及功能应用 1.1 异亮氨酸的羟基化合成及功能应用 1.1.1 (2S, 3R, 4S)-4-羟基-异亮氨酸(4-HIL)功能及应用4-HIL最初是在一年生草本植物葫芦巴的种子中发现的,它拥有80%的游离氨基酸,具有促胰岛素和抗肥胖的作用,是一种非常有前景的抗糖尿病药物[12-13]。在用于治疗Ⅱ型糖尿病的过程中,与普通化学药物相比,可以避免很多不良反应和副作用,如治疗Ⅱ型糖尿病时出现的低血糖现象[14-15]。此外,4-HIL对调节血脂和降低胆固醇水平也有很好的疗效,一方面,4-HIL在用于小鼠Ⅱ型糖尿病的模型中,具有抗高血糖和抗血脂异常的疗效[16-17],另一方面,它也可以显著降低血浆甘油三酯和总胆固醇的水平,分别降低了33%和22%[18]。综上,4-HIL在治疗慢性疾病方面是一种非常有前景的药物,其治疗功效主要包括抗感染、抗氧化、抗衰老、心脏保护和促进消化等[19-27]。因此,人们对4-HIL的需求越来越多,对其的研究越来越重视。
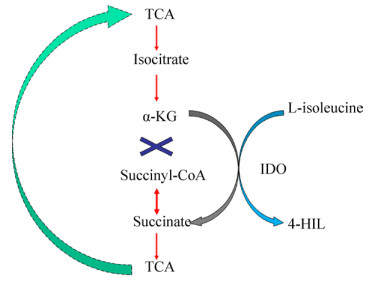
1.1.2 4-HIL的合成途径到目前为止,4-HIL的合成方法主要有3种:一是从葫芦巴中萃取分离获得4-HIL,二是化学合成,三是酶法生物合成(图 1A)。萃取分离法先用乙醇对葫芦巴进行处理,萃取原料中的氨基酸,之后用离子交换树脂对萃取物进一步洗脱纯化,得到目标产物4-HIL[28-29]。化学合成法通常用于化工产品和药物合成。Wang等[30]提供了4-HIL的8步高效合成方法,合成的关键步骤是将乙基-2-甲基乙酸乙酯转化为乙基(2S, 3S)-2-甲基-3-羟基丁二酸酯, 总收率达到了39%。而Rolland等[31]提出了一种4-HIL的六步化学合成,其收率高达40%。
|
| 图 1 典型氨基酸羟基化合成途径 Figure 1 Typical biocatalytic synthesis of hydroxylated L-amino acids by Fe/αKGDs. (A) The C-4 hydroxylation of L-isoleucine forms L-4-hydroxyisoleucine (4-HIL) (IDO: The stereo-specific L-isoleucine-4-hydroxylase). (B) The C-5 hydroxylation of L-leucine forms L-5-hydroxyisoleucine (LdoA: The stereo-specific L-leucine-5-hydroxylase). (C) The C-3 hydroxylation of L-leucine forms L-3-hydroxyleucine. (D) The C-4 hydroxylation of L-proline forms 4S and 4R-hydroxyproline, respectively. (E) The C-3 hydroxylation of L-proline forms 3S and 3R-hydroxyproline, respectively. |
| |
酶法生物合成4-HIL是目前研究最多的合成方法。酶法合成具有催化特异性强且简单高效的特点,因此,酶法生物合成4-HIL得到较多的研究和应用。Ogawa等[32]利用筛选到的一株简单节杆菌与含有支链氨基酸氨基转移酶基因的大肠杆菌菌体细胞在α-KG、乙醛和L-谷氨酸共同存在下静息培养,成功获得了4-HIL。Haefele等[33]研究结果表明,在Fe(Ⅱ)、α-KG、抗坏血酸和氧气的存在下,异亮氨酸可被异亮氨酸羟化酶催化形成4-HIL。Kodera等[12]通过筛选得到可把异亮氨酸催化生成4-HIL的苏云金杆菌菌株,并通过蛋白纯化得到了该羟化酶,该酶催化异亮氨酸具有高对映立体结构选择性,只产生(2S, 3R, 4S)-4- HIL。Smirnov等[34]基于4-羟基-异亮氨酸羟化酶(IDO)羟基化异亮氨酸过程中生成副产物琥珀酸的反应与TCA循环中α-KG氧化脱羧生成琥珀酸的反应相耦联的机理(图 2),通过改造菌株代谢途径和过表达羟化酶的方法使得L-Ile转化为4-HIL的产率高达82%。Shi等[35]对4-HIL生物合成过程中的关键酶4-HIL脱氢酶(HILDH)进行了改造,实现了一步催化反应高效生成(2S, 3R, 4S)-4-HIL,该酶具有严格的对映立体选择性,克服了4-HIL商业规模生产的最大局限性。
萃取分离和化学合成4-HIL需要复杂的多级反应,致使产品合成过程中含有较多复杂成分,单独采用上述两种方法很难分离出单一的4-HIL。而酶法生物催化合成能严格控制对映立体结构选择性,高效获得4-HIL。因此,酶法生物合成4-HIL必然将成为工业化应用的新方向。
1.2 脯氨酸的羟基化合成及功能应用 1.2.1 羟基脯氨酸的功能及应用羟基脯氨酸是较早发现的氨基酸类似物,而且是重要的药物合成中间体,它可用于制备各种手性药物和手性抗生素[36-37]。羟基脯氨酸有多种同分异构体,可形成顺式和反式构型,如cis-3-羟基-L-脯氨酸(C3LHyp)和trans-3-羟基-L-脯氨酸(T3LHyp) (图 1D),cis-4-羟基-L-脯氨酸(C4LHyp)和trans-4-羟基-L-脯氨酸(T4LHyp) (图 1E)。这些羟基脯氨酸常存在于一些蛋白结构中,包括胶原蛋白、植物细胞壁和多肽抗生素等,作用于多种自由氨基酸的Fe/αKGDs中,L-脯氨酸的羟化酶在工业中的应用研究较多。
1.2.2 C3LHyp和T3LHyp特性及合成途径T3LHyp在胶原蛋白中含量最丰富,由脯氨酰基-3-羟化酶特异性催化L-脯氨酸生成。而C3LHyp在治疗癌症和胶原蛋白代谢紊乱方面具有一定的疗效[38],是生物活性成分及手性药物合成的重要组成部分[39]。
T3LHyp主要来自于生物合成途径,但到目前为止,对脯氨酸-3-羟化酶的研究报道尚不多见,T3LHyp的生物合成途径仍然需要进一步的研究。在前期研究中,C3LHyp多是通过微生物发酵生产所得[40]。Johnston等[41]通过全细胞生物催化得到纯度极高的C3LHyp,他们将L-proline cis-3-羟化酶基因从链霉菌中克隆到大肠杆菌中,以L-脯氨酸为底物,连续发酵培养60 h,C3LHyp的收率高达99%。
1.2.3 C4LHyp和T4LHyp特性及合成途径T4LHyp是通过翻译后修饰形成,这是胶原蛋白合成最常见的方式。C4LHyp是由毒蘑菇毒伞蕈产生的,是毒蕈肽毒素的组成部分[42]。C4LHyp能够抑制细胞生长,常用于检测细胞在组织培养和体内的抗肿瘤活性[43]。目前,C4LHyp的生产主要用作一种胶原抗癌的药物[44-45]。
T4LHyp的合成仅有生物催化法被报道[46]。Shibasaki等[46]利用改造过的孢囊菌属菌株Dactylosporangium sp. RH1过表达trans-4-脯氨酸羟化酶,从而获得脯氨酸氧化缺陷细胞中的T4LHyp。在此方法的基础上,研究者又通过进一步筛选菌株和优化培养基,获得了高产T4LHyp的菌株[48-57]。Shibasaki等为了满足T4LHyp的工业需求,还尝试建立了满足商业规模的微生物发酵体系来生产T4LHyp[47]。
而Hara等[50]利用cis-4-脯氨酸羟化酶对游离的L-脯氨酸进行了催化合成,成功获得了C4Lhyp。此外,在cis-4-脯氨酸羟化酶和N-乙酰转移酶共同催化作用下也合成了N-乙酰- C4LHyp[51-52]。Bach等[51]设计使用来源于根瘤菌中的cis-4-脯氨酸羟化酶在体外催化L-脯氨酸成功得到C4LHyp,转化率为10%。现阶段C4LHyp的获得基本利用酶法进行生物合成。
综上,T4LHyp和C4LHyp的合成基本依赖4-脯氨酸羟化酶酶法生物催化,酶法生物催化法在合成羟脯氨酸方面具有一定优势。
1.3 色氨酸的羟基化合成及功能应用5-羟基-色氨酸(5-HTP)的合成是由L-色氨酸羟化酶催化底物L-色氨酸(L-tryptophan)产生的。5-HTP是血清素合成的中间体,常被用作治疗神经递质血清素的直接前体[52]。因此,5-HTP常被用于治疗一些神经方面的疾病,包括抑郁症、失眠、纤维肌痛、肥胖、慢性头痛等[53-57]。目前,从非洲加纳籽中进行提取和分离5-HTP是该物质的主要获取途径,该方法在工业上得到普遍应用[58]。Winnicka等[59]报道了利用关键中间体DL-丙氨酸采用化学和多酶联合反应合成5-HTP。Boroda等[60]则利用多酶反应以L-色氨酸为底物成功合成了5-HTP。然而,这些生物或化学合成5-HTP的方法存在诸多缺点,如收率低、纯度低、分离难度大等,因此5-HTP的合成及实现工业化还需要进一步的研究。
1.4 其他疏水性氨基酸的羟基化合成及功能应用除了上述重要的疏水性羟基氨基酸,还有其他研究相对较少的疏水氨基酸被报道。比如,3-羟基-缬氨酸是HIV蛋白酶抑制剂的合成中间体,在合成HIV蛋白酶抑制剂的过程中起重要作用[62];(2S, 3R)-3-羟基-亮氨酸常用于制备某些脱氢酶(图 1C),并且是血小板聚集抑制剂和抗生素的组成成分[61],而(2S, 4S)-5-羟基-亮氨酸(图 1B)则参与了(2S, 4S)-4-甲基脯氨酸的生物合成[62];而(2S, 3R)-3-羟基-苯丙氨酸是抗生素(Lysobactin)结构中的重要部分[61]。
2 Fe/αKGDs的结构特征单加氧酶,双加氧酶及Fe/αKGDs的发现和应用为工业合成羟化氨基酸提供了重要的生物催化剂。其中氨基酸是Fe/αKGDs典型底物,在催化氨基酸时具有很高的区域及对映立体选择性,此外,大量的Fe/αKGDs家族的酶晶体学研究也表明了这类酶都含有相似的保守序列及催化位点,具有共性结构特征[63-66]。
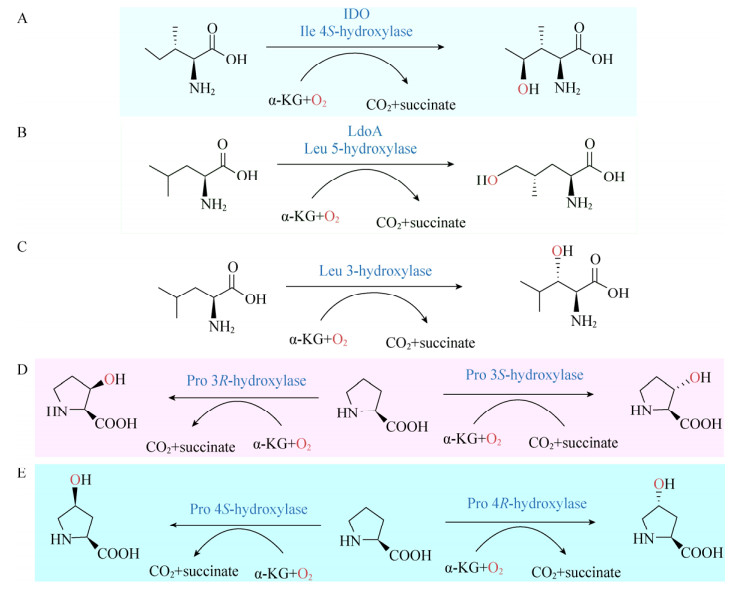
很多Fe/αKGDs家族的酶蛋白晶体结构研究揭示了该类酶都具有两种保守的结构特征[63]。首先,Fe(Ⅱ)和特定的氨基酸(两个组氨酸的残基和谷氨酸或天冬氨酸中的一个羧基)结合到一起;这个保守的金属结合位点被称为“2-His-1-carboxylate facial triad”[64]。其次,该保守的金属结合位点位于双链β螺旋(DSBH)折叠内,DSBH折叠结构被称为“果冻卷”折叠(Jelly-roll) (图 3),该结构在Fe/αKGDs这类酶中普遍存在[65]。DSBH结构是由8个反向β链形成的β-夹层结构构成,而β-夹层结构是由2个四链反向β折叠组成[65-66]。基于这种保守结构,有人推测DSBH折叠结构中的β链对底物的活性位点和特异性结合位点选择性都能提供支持和帮助[65]。另外,广泛的Fe/αKGDs家族的酶结构及光谱分析对深入了解该类酶的金属活性结合位点提供了更详细的信息。金属活性位点包含高度相似的保守序列HXD/EXnH motif,即金属结合位点(图 3)。
|
| 图 3 Fe/αKGDs家族典型酶TauD (PDB:1GY9)的蛋白结构 Figure 3 The structure of the typical archetype hydroxylase within this Fe/αKGDs family, TauD, is depicted as a graphic with α-KG and the coordinating side chains in stick mode and Fe as a sphere to illustrate the position of the metallocentre relative to the double-stranded β-helix fold (Protein Data Bank (PDB) access code 1GY9). |
| |
某些Fe/αKGDs是多结构域蛋白的组成部分,具有共性催化机制。研究最为深入的是Fe/αKGDs对底物的羟基化反应。羟基化可用于蛋白侧链的修饰,如修复烷基化的DNA/RNA,降解环境垃圾等[63-65]。羟基化过程需要氧气和辅因子,辅因子包括二价铁和α-KG。在催化过程中,α-KG被一个氧原子氧化形成琥珀酸和二氧化碳,而另一个氧原子攻击底物形成羟基氨基酸。Fe/αKGDs有一个高度相似的蛋白折叠结构,即DSBH折叠,作为蛋白催化反应结构的核心,HXD/EXnH催化位点结合到亚铁离子形成的活性中心上(图 4)[66-68]。α-KG与二价铁以双配位键的形式通过羧基和氧基团进行结合,该结合位点相对保守。但是,正是这种多变的底物结合位点,恰好导致了羟基化反应的底物特异性和高对映立体选择性。
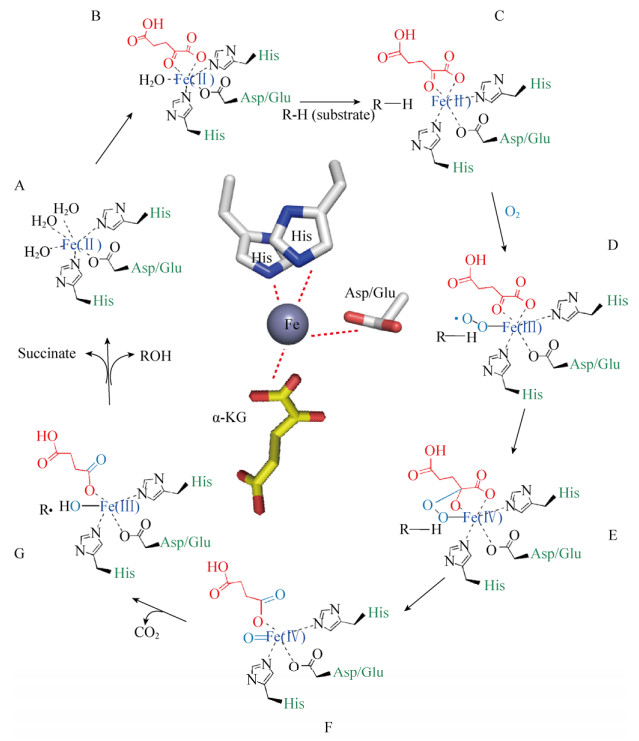
|
| 图 4 Fe/αKGDs共性催化机制示意图(示意图中间部分为Fe/αKGDs发生催化反应时金属活性中心和蛋白催化活性结合位点透视图) Figure 4 Proposed mechanism of reaction by Fe/αKGDs. Middle, an expandedview of the Fe/αKGDs metallocenter surrounded by species that are proposed to be formed during the enzymatic mechanism. The step A-G is the simplified hydroxylation mechanism. |
| |
在所有解析Fe/αKGDs酶复合物结构的报道中,Hanauske-Abel等首先提出了一种蛋白与金属活性中心共性结合机制,包含Fe(Ⅱ)-氨基酸侧链在金属活性中心以配位键的形式连接[72]。之后,多个Fe/αKGDs被发现且其结构被解析,进一步改进了该催化模型机制,提出了酶的共性催化机制,具体催化过程如图 4所示。首先催化起始阶段,亚铁离子形成一个活性中心,以八面配位体复合物的形式存在,蛋白中的催化活性位点即2个组氨酸残基与Asp/Glu的羧基(2-His-l- carboxylate),以3个配位键形式结合到亚铁活性中心[69],水分子占据了另外3个亚铁活性中心位点(图 4A)。α-KG的羰基和C-1位羧基取代了2个水分子,以二齿状配位键形式结合到金属活性中心亚铁上(图 4B)。在α-KG和蛋白催化位点与亚铁活性中心结合后,底物会取代第三个与亚铁活性中心结合的水分子,从而结合到亚铁活性中心(图 4C)。这时底物与活性中心的配位结合会削弱水分子与亚铁活性中心的作用力,因此,激发了一个氧分子发生氧化反应,同时产生三价铁-超氧中间体(Fe(Ⅲ)-superoxo species)[66] (图 4D)。Fe(Ⅲ)-superoxo species攻击α-KG C-2位的羰基,导致该三价铁复合体形成高价铁-超氧复合体(Fe(Ⅳ)=O),Fe(Ⅳ)=O是发生羟基化的关键复合体(图 4E)。之后,Fe(Ⅳ)=O激活α-KG使其发生氧化脱羧,释放二氧化碳[70],生成的琥珀酸依然结合在高价铁的活性中心上(图 4F)。这时Fe(Ⅳ)=O复合体夺取底物中的一个氢原子,生成Fe(Ⅲ)-OH复合体,底物脱氢后形成一个底物自由基(图 4G),最后羟基自由基反弹结合到底物的自由基上(或发生更复杂的化学反应)进而完成了底物羟基化,同时琥珀酸被释放出来,这时高价铁复原到初始二价铁的状态[71],从而完成了氨基酸羟基化过程。
上述Fe/αKGDs的共性催化机制中,Fe(Ⅲ)=O被确定为最关键的中间体,广泛的Fe/αKGDs类酶蛋白结构研究为这种共性催化机制提供了坚实的数据支持[72-74]。已经解析的一些该类酶的晶体结构也都揭示了这种共性催化机制,即二价铁活性中心形成的配位体复合物与Fe/αKGDs这类酶的3个氨基酸催化位点以配位键结合(图 4A-C)。当α-KG氧化脱羧反应发生时,底物并没有伴随发生氧化反应,这种反应机制在所有Fe/αKGDs发生羟基化时是一致的[72]。在某些情况下,这种反应特性可导致芳香族氨基酸残基在其活性位点上发生自动氧化,该过程可能和Fe(Ⅳ)=O复合中间体相关[75]。高价铁复合物能产生非耦合反应,Tuderman等[76]认为抗坏血酸具有减少高价铁生成的功能,从而使具有催化活性的二价铁复原到静止状态。
4 结语羟基氨基酸作为一类重要的非天然氨基酸,具有多种生物学功能,在生物技术中得到广泛应用。它不但具有抗真菌、抗菌、抗病毒和抗癌的作用,而且还可作为前体或者中间体用于药物的不对称合成,同时它还可作为手性化合物的组成部分。通过化学合成方法可以成功获得重要的羟基氨基酸,利用生物资源提取也可获得一些羟基氨基酸。但到目前为止,由于氨基酸羟化酶不断被发现,因此,采取生物催化方法获得特定羟基氨基酸的研究与日俱增,同时Fe/αKGDs这类加双氧酶共性催化机制的提出有利于为进一步提高其催化效率奠定基础,但是,一些其他氨基酸羟基化的生物催化机制还需要进一步的研究和探索。此外,Fe/αKGDs家族能催化合成羟基氨基酸的酶在未来一定会有更多被发现,羟基化氨基酸的研究和应用在今后也会越来越广泛。
| [1] | Choroba OW, Williams DH, Spencer JB. Biosynthesis of the vancomycin group of antibiotics:involvement of an unusual dioxygenase in the pathway to (S)-4-hydroxyphenylglycine. J Am Chem Soc, 2000, 122(22): 5389–5390. DOI: 10.1021/ja000076v |
| [2] | Higgins LJ, Yan F, Liu PH, et al. Structural insight into antibiotic fosfomycin biosynthesis by a mononuclear iron enzyme. Nature, 2005, 437(7060): 838–844. DOI: 10.1038/nature03924 |
| [3] | Bodner MJ, Phelan RM, Freeman MF, et al. Townsend, non-heme iron oxygenases generate natural structural diversity in carbapenem antibiotics. J Am Chem Soc, 2010, 132(1): 12. DOI: 10.1021/ja907320n |
| [4] | Blaskovich MA, Evindar G, Rose NG, et al. Stereoselective synthesis of threo and erythro β-hydroxy and β-disubsti-tuted-β-hydroxy α-amino acids. J Org Chem, 1998, 63(11): 3631–3646. DOI: 10.1021/jo972294l |
| [5] | Palomo C, Arrieta A, Cossío FP, et al. Highly stereoselective synthesis of α-hydroxy β-amino acids through β-lactams:application to the synthesis of the taxol and bestatin side chains and related systems. Tetrahedron Lett, 1990, 31(44): 6429–6432. DOI: 10.1016/S0040-4039(00)97083-7 |
| [6] | Hibi M, Kawashima T, Kodera T, et al. Characterization of Bacillus thuringiensis L-isoleucine dioxygenase for production of useful amino acids. Appl Environ Microb, 2011, 77(19): 6926–6930. DOI: 10.1128/AEM.05035-11 |
| [7] | Martinez S, Hausinger RP. Catalytic mechanisms of Fe(Ⅱ)-and 2-oxoglutaratedependent oxygenases. J Biol Chem, 2015, 290(34): 20702–20711. DOI: 10.1074/jbc.R115.648691 |
| [8] | Purpero V, Moran GR. The diverse and pervasive chemistries of the α-keto acid dependent enzymes, J Biol Inorg Chem. 2007, 12(5): 587-601. https://link.springer.com/article/10.1007%2Fs00775-007-0231-0 |
| [9] | Hausinger RP. Fe(Ⅱ)/α-ketoglutarate-dependent hydroxylases and related enzymes. Crit Rev Biochem Mol Biol, 2004, 39(1): 21–68. DOI: 10.1080/10409230490440541 |
| [10] | Prescott AG, Lloyd MD. The iron(Ⅱ) and 2-oxoacid-dependent dioxygenases and their role in metabolism. Nat Prod Rep, 2000, 17(4): 367–383. DOI: 10.1039/a902197c |
| [11] | Hibi M, Ogawa J. Characteristics and biotechnology applications of aliphatic amino acid hydroxylases belonging to the Fe(Ⅱ)/α-ketoglutarate-dependent dioxygenase superfamily. Appl Microbiol Biotechnol, 2014, 98(9): 3869–3876. DOI: 10.1007/s00253-014-5620-z |
| [12] | Kodera T, Smirnov SV, Samsonova NN, et al. A novel L-isoleucine hydroxylating enzyme, L-isoleucine dioxygenase from Bacillus thuringiensis, produces (2S, 3R, 4S)-4-hydroxyisoleucine. Biochem Biophys Res Commun, 2009, 390(3): 506–510. DOI: 10.1016/j.bbrc.2009.09.126 |
| [13] | Moorthy R, Prabhu KM, Murthy PS. Anti-hyperglycemic compound (GII) from fenugreek (Trigonella foenum-graecum Linn.) seeds, its purification and effect in diabetes mellitus. Indian J Exp Biol, 2010, 48(11): 1111–1118. |
| [14] | Jackson JE, Bressler R. Clinical pharmacology of sulfonylurea hypoglycemic agents:part 1. Drugs, 1981, 22(3): 211–245. DOI: 10.2165/00003495-198122030-00003 |
| [15] | Jennings AM, Wilson RM, Ward JD. Symptomatic hypoglycemia in NIDDM patients treated with oral hypoglycemic agents. Diab Care, 1989, 12(3): 203–208. DOI: 10.2337/diacare.12.3.203 |
| [16] | Singh AB, Tamarkar AK, Narender T, et al. Antihyperglycaemic effect of an unusual amino acid (4-hydroxyisoleucine) in C57BL/KsJ-db/db mice. Nat Prod Res, 2010, 24(3): 258–265. DOI: 10.1080/14786410902836693 |
| [17] | Eidi A, Eidi M, Sokhteh M. Effect of fenugreek (Trigonella foenum-graecum L) seeds on serum parameters in normal and streptozotocin-induced diabetic rats. Nutr Res, 2007, 27(11): 728–733. DOI: 10.1016/j.nutres.2007.09.006 |
| [18] | Narender T, Puri A, Shweta, et al. 4-Hydroxyisoleucine an unusual amino acid as antidyslipidemic and antihyperglycemic agent. Bioorg Medic Chem Lett, 2006, 16(2): 293–296. DOI: 10.1016/j.bmcl.2005.10.003 |
| [19] | Kandhare AD, Bodhankar SL, Mohan V, et al. Effect of glycosides based standardized fenugreek seed extract in bleomycin-induced pulmonary fbrosis in rats:Decisive role of Bax, Nrf2, NF-κB, Muc5ac, TNF-α and IL-1β. Chem Biol Interact, 2015, 237: 151–165. DOI: 10.1016/j.cbi.2015.06.019 |
| [20] | Aboubakr HA, Nauertz A, Luong NT, et al. In vitro antiviral activity of clove and ginger aqueous extracts against feline calicivirus, a surrogate for human norovirus. J Food Protect, 2016, 79(6): 1001–1012. DOI: 10.4315/0362-028X.JFP-15-593 |
| [21] | Sakr SA, Mahran HA, Abo-El-Yazid SM. Effect of fenugreek seeds extract on cyclophosphamide-induced histomorphometrical, ultrastructural and biochemical changes in testes of albino mice. Toxicol Ind Health, 2012, 28(3): 276–288. DOI: 10.1177/0748233711412427 |
| [22] | Panda S, Tahiliani P, Kar A. Inhibition of triiodothyronine production by fenugreek seed extract in mice and rats. Pharmacol Res, 1999, 40(5): 405–409. DOI: 10.1006/phrs.1999.0510 |
| [23] | Lamfon H. Effect of fenugreek seed extract on carbendazim-inhibited spermatogenesis in albino rats. J App Pharmaceu Sci, 2012, 2(4): 9–13. |
| [24] | Becker PM, Widjaja-Greefkes HCA, Van Wikselaar PG. Inhibition of binding of the AB5-type enterotoxins LT-I and cholera toxin to ganglioside GM1 by galactose-rich dietary components. Foodborne Pathog Dis, 2010, 7(3): 225–233. DOI: 10.1089/fpd.2009.0387 |
| [25] | Bin-Hafeez B, Haque R, Parvez S, et al. Immunomodulatory efects of fenugreek (Trigonella foenum graecum L. ) extract in mice. Int Immunopharmacol, 2003, 3(2): 257-265. https://www.sciencedirect.com/science/article/pii/S1567576902002928 |
| [26] | Mukthamba P, Srinivasan K. Hypolipidemic infuence of dietary fenugreek (Trigonella foenum-graecum) seeds and garlic (Alliumsativum) in experimental myocardial infarction. Food Funct, 2015, 6(9): 3117–3125. DOI: 10.1039/C5FO00240K |
| [27] | Petit P, Sauvaire Y, Ponsin G, et al. Effects of a fenugreek seed extract on feeding behaviour in the rat:metabolic-endocrine correlates. Pharmacol Biochem Behav, 1993, 45(2): 369–374. DOI: 10.1016/0091-3057(93)90253-P |
| [28] | Fowden L, Pratt HM, Smith A. 4-Hydroxyisoleucine from seed of Trigonella foenum-graecum. Phytochemistry, 1973, 12(7): 1707–1711. DOI: 10.1016/0031-9422(73)80391-7 |
| [29] | Sauvaire Y, Petit P, Broca C, et al. 4-Hydroxyisoleucine:a novel amino acid potentiator of insulin secretion. Diabetes, 1998, 47(2): 206–210. DOI: 10.2337/diab.47.2.206 |
| [30] | Wang Q, Ouazzani J, Sasaki N A, et al. A practical synthesis of (2S, 3R, 4S)-4-hydroxyisoleucine, a potent insulinotropic α-amino acid from fenugreek. Eur J Org Chem, 2002, 2002(5): 834–839. DOI: 10.1002/(ISSN)1099-0690 |
| [31] | Rolland-Fulcrand V, Rolland M, Roumestant ML, et al. Chemoenzymatic synthesis of enantiomerically pure (2S, 3R, 4S)-4-hydroxyisoleucine, an insulinotropic amino acid isolated from Fenugreek seeds. Eur J Org Chem, 2004, 2004(4): 873–877. DOI: 10.1002/(ISSN)1099-0690 |
| [32] | Ogawa J, Yamanaka H, Mano J, et al. Synthesis of 4-hydroxyisoleucine by the aldolase-transaminase coupling reaction and basic characterization of the aldolase from Arthrobacter simplex AKU 626. Biosci Biotechnol Biochem, 2007, 71(7): 1607–1615. DOI: 10.1271/bbb.60655 |
| [33] | Haefelé C, Bonfils C, Sauvaire Y. Characterization of a dioxygenase from Trigonella foenum-graecum involved in 4-hydroxyisoleucine biosynthesis. Phytochemistry, 1997, 44(4): 563–566. DOI: 10.1016/S0031-9422(96)00620-6 |
| [34] | Smirnov SV, Kodera T, Samsonova NN, et al. Metabolic engineering of Escherichia coli to produce (2S, 3R, 4S)-4-hydroxyisoleucine. Appl Microbiol Biotechnol, 2010, 88(3): 719–726. DOI: 10.1007/s00253-010-2772-3 |
| [35] | Shi X, Miyakawa T, Nakamura A, et al. Engineering a short-chain dehydrogenase/reductase for the stereoselective production of (2S, 3R, 4S)-4-hydroxyisoleucine with three asymmetric centers. Sci Rep, 2017, 7(1): 13703. DOI: 10.1038/s41598-017-13978-w |
| [36] | Berkeš D, Kolarovič A, Považanec F. Stereoselective sodium borohydride reduction, catalyzed by manganese(Ⅱ) chloride, of γ-oxo-α-amino acids. A practical approach to syn-γ-hydroxy-α-amino acids. Tetrahedron Lett, 2010, 41(27): 5257–5260. |
| [37] | Remuzon P. Trans-4-hydroxy-L-proline, a useful and versatilechiral starting block. Tetrahedron, 1996, 52(44): 13803–13835. DOI: 10.1016/0040-4020(96)00822-8 |
| [38] | Mueller C, Emmrich J, Jaster R, et al. cis-Hydroxyproline-induced inhibition of pancreatic cancer cell growth is mediated by endoplasmic reticulum stress. World J Gastroenterol, 2006, 12(10): 1569–1576. DOI: 10.3748/wjg.v12.i10.1569 |
| [39] | Shibasaki T, Mori H, Ozaki A. Cloning of an isozyme of proline 3-hydroxylase and its purification from recombinant Escherichia coli. Biotechnol Lett, 2000, 22(24): 1967–1973. DOI: 10.1023/A:1026792430742 |
| [40] | Mori H, Shibasaki T, Yano K, et al. Purification and cloning of a proline 3-hydroxylase, a novel enzyme which hydroxylate free L-proline to cis-3-hydroxy-L-proline. J Bacteriol, 1997, 179(18): 5677–5683. DOI: 10.1128/jb.179.18.5677-5683.1997 |
| [41] | Johnston RM, Chu LN, Liu M, et al. Hydroxylation of L-proline to cis-3-hydroxy-L-proline by recombinant Escherichia coli expressing a synthetic L-proline-3-hydroxylase gene. Enzym Micro Technol, 2009, 45(6/7): 484–490. |
| [42] | Wieland T. Poisonous principles of mushrooms of the genus Amanita. Four-carbon amines acting on the central nervous system and cell-destroying cyclic peptides are produced. Science, 1968, 159(3818): 946–952. DOI: 10.1126/science.159.3818.946 |
| [43] | Takagi H, Yamada S. Roles of enzymes in anti-oxidative response system on three species of chenopodiaceous halophytes under NaCl-stress condition. Soil Sci Plant Nutrn, 2013, 59(4): 603–611. DOI: 10.1080/00380768.2013.809600 |
| [44] | Tan EML, Ryhänen LU. Proline analogues inhibit human skin fibroblast growth and collagen production in culture. J Invest Dermatol, 1983, 80(4): 261–267. DOI: 10.1111/1523-1747.ep12534593 |
| [45] | Eldridge CF, Bunge RP, Bunge MB. Effect of cis-4-hydroxy-Lproline, an inhibitor of Schwann cell differentiation, on the secretion of collagens and noncollagenous protein by Schwann cells. Exp Cell Res, 1988, 174(2): 491–501. DOI: 10.1016/0014-4827(88)90318-7 |
| [46] | Shibasaki T, Hashimoto S, Mori H, et al. Construction of novel hydroxyproline-producing recombinant Escherichia coli by introducing a proline 4-hydroxylase gene. J Biosci Bioeng, 2000, 90(5): 522–525. DOI: 10.1016/S1389-1723(01)80033-5 |
| [47] | Shibasaki T, Mori H, Chiba S, et al. Microbial proline 4-hydroxylase screening and gene cloning. Appl Environ Microbiol, 1999, 65(9): 4028–4031. |
| [48] | Matsuoka T, Serizawa N, Hosoya T, et al. Isolated cultures of microorganisms of Clonostachys cylindrospora, Gliocladium and Nectria gliocladioides. US, 5407826. 1995-04-18. |
| [49] | Hosoya T, Matsuoka T, Serizawa N, et al. Two morphological groups derived from Clonostachys cylindrospora and their relationship to trans-4-L-proline productivity. Mycoscience, 1995, 36(2): 193–197. DOI: 10.1007/BF02268557 |
| [50] | Hara R, Kino K. Characterization of novel 2-oxoglutarate dependent dioxygenases converting L-proline to cis-4-hydroxy-Lproline. Biochem Biophys Res Commun, 2009, 379(4): 882–886. DOI: 10.1016/j.bbrc.2008.12.158 |
| [51] | Bach TMH, Hara R, Kino K, et al. Microbial production of N-acetyl cis-4-hydroxy-L-proline by coexpression of the Rhizobium L-proline cis-4-hydroxylase and the yeast N-acetyltransferase Mpr1. Appl Microbiol Biotechnol, 2013, 97(1): 247–257. DOI: 10.1007/s00253-012-4204-z |
| [52] | Hoa BTM, Hibi T, Nasuno R, et al. Production of N-acetyl cis-4-hydroxy-L-proline by the yeast N-acetyltransferase Mpr1. J Biosci Bioeng, 2012, 114(2): 160–165. DOI: 10.1016/j.jbiosc.2012.03.014 |
| [53] | Nicolodi M, Sicuteri F. Fibromyalgia and migraine, two faces of the same mechanism. Serotonin as the common clue for pathogenesis and therapy//Filippini GA, Costa CVL, Bertazzo A, eds. Recent Advances in Tryptophan Research. Advances in Experimental Medicine and Biology. Boston, MA: Springer, 1996, 398: 373-379. |
| [54] | Cangiano C, Ceci F, Cascino A, et al. Eating behavior and adherence to dietary prescriptions in obese adult subjects treated with 5-hydroxytryptophan. Am J Clin Nutr, 1992, 56(5): 863–867. DOI: 10.1093/ajcn/56.5.863 |
| [55] | Guilleminault C, Cathala JP, Castaigne P. Effects of 5-hydroxytryptophan on sleep of a patient with a brain-stem lesion. Electroencephalogr Clin Neurophys, 1973, 34(2): 177–184. DOI: 10.1016/0013-4694(73)90045-X |
| [56] | Bono G, Criscuoli M, Martignoni E, et al. Serotonin precursors in migraine prophylaxis. Adv Neurol, 1982, 33: 357–363. |
| [57] | Birdsall TC. Therapeutic applications of taurine. Altern Med Rev, 1998, 3(2): 128–136. |
| [58] | Turner EH, Loftis JM, Blackwell AD. Serotonin a la carte:Supplementation with the serotonin precursor 5-hydroxytryptophan. Pharmacol Therapeut, 2006, 109(3): 325–338. DOI: 10.1016/j.pharmthera.2005.06.004 |
| [59] | Winnicka E, Kańska M. Synthesis of[3-14C]-L-tryptophan and 5'-hydroxy-[3-14C]-L-tryptophan. J Radioanal Nucl Chem, 2009, 280(1): 79–84. DOI: 10.1007/s10967-008-7415-0 |
| [60] | Boroda E, Kański R, Kańska M. Synthesis of[14C]-l-tryptophan and[14C]-5'-hydroxy-l-tryptophan labeled in the carboxyl group. J Labelled Compd Rad, 2003, 46(5): 441–447. DOI: 10.1002/(ISSN)1099-1344 |
| [61] | Hibi M, Kasahara T, Kawashima T, et al. Multi-enzymatic synthesis of optically pure β-hydroxy α-amino acids. Adv Synth Catal, 2015, 357(4): 767–774. DOI: 10.1002/adsc.v357.4 |
| [62] | Hibi M, Kawashima T, Sokolov PM, et al. L-leucine 5-hydroxylase of Nostoc punctiforme is a novel type of Fe(Ⅱ)/α-ketoglutarate-dependent dioxygenase that is useful as a biocatalyst. Appl Microbiol Biotechnol, 2013, 97(6): 2467–2472. DOI: 10.1007/s00253-012-4136-7 |
| [63] | Cheng AX, Han XJ, Wu YF, et al. The function and catalysis of 2-oxoglutarate-dependent oxygenases involved in plant flavonoid biosynthesis. Int. J Mol Sci, 2014, 15(1): 1080–1095. DOI: 10.3390/ijms15011080 |
| [64] | Hegg EL, Que L Jr. The 2-His-1-carboxylate facial triad:an emerging structural motif in mononuclear non-heme iron(Ⅱ) enzymes. Eur J Biochem, 1997, 250(3): 625–629. DOI: 10.1111/ejb.1997.250.issue-3 |
| [65] | Aik W, McDonough MA, Thalhammer A, et al. Role of the jelly-roll fold in substrate binding by 2-oxoglutarate oxygenases. Curr Opin Struct Biol, 2012, 22(6): 691–700. DOI: 10.1016/j.sbi.2012.10.001 |
| [66] | Clifton IJ, McDonough MA, Ehrismann D, et al. Structural studies on 2-oxoglutarate oxygenases and related double-stranded β-helix fold proteins. J Inorg Biochem, 2006, 100(4): 644–669. DOI: 10.1016/j.jinorgbio.2006.01.024 |
| [67] | Henshaw TF, Feig M, Hausinger RP. Aberrant activity of the DNA repair enzyme AlkB. J Inorg Biochem, 2004, 98(5): 856–861. DOI: 10.1016/j.jinorgbio.2003.10.021 |
| [68] | McDonough MA, Loenarz C, Chowdhury R, et al. Structural studies on human 2-oxoglutarate dependent oxygenases. Curr Opin Struct Biol, 2010, 20(6): 659–672. DOI: 10.1016/j.sbi.2010.08.006 |
| [69] | Chowdhury R, McDonough MA, Mecinović J, et al. Structural basis for binding of hypoxia-inducible factor to the oxygensensing prolyl hydroxylases. Structure, 2009, 17(7): 981–989. DOI: 10.1016/j.str.2009.06.002 |
| [70] | Elkins JM, Ryle MJ, Clifton IJ, et al. Xray crystal structure of Escherichia coli taurine/a-ketoglutarate dioxygenase complexed to ferrous iron and substrates. Biochemistry, 2002, 41(16): 5185–5192. DOI: 10.1021/bi016014e |
| [71] | Hanauske-Abel HM, Günzler V. A stereochemical concept for the catalytic mechanism of prolylhydroxylase:applicability to classification and design of inhibitors. J Theor Biol, 1982, 94(2): 421–455. DOI: 10.1016/0022-5193(82)90320-4 |
| [72] | Zhou J, Kelly WL, Bachmann BO, et al. Spectroscopic studies of substrate interactions with clavaminate synthase 2, a multifunctional alpha-KG-dependent non-heme iron enzyme:correlation with mechanisms and reactivities. J Am Chem Soc, 2001, 123(30): 7388–7398. DOI: 10.1021/ja004025+ |
| [73] | Borowski T, Bassan A, Siegbahn PEM. Mechanism of dioxygen activation in 2-oxoglutarate-dependent enzymes:a hybrid DFT study. Chem Eur J, 2004, 10(4): 1031–1041. DOI: 10.1002/(ISSN)1521-3765 |
| [74] | Dambrova M, Liepinsh E, Kalvinsh I. Mildronate:cardioprotective action through carnitine-lowering effect. Trends Cardiovas Med, 2002, 12(6): 275–279. DOI: 10.1016/S1050-1738(02)00175-5 |
| [75] | Hopkinson RJ, Hamed RB, Rose NR, et al. Monitoring the activity of 2-oxoglutarate dependent histone demethylases by NMR spectroscopy:direct observation of formaldehyde. ChemBioChem, 2010, 11(4): 506–510. DOI: 10.1002/cbic.v11:4 |
| [76] | Tuderman L, Myllylä R, Kivirikko KI. Nutrition classics. European Journal of Biochemistry, Volume 80, 1977:Mechanism of the prolyl hydroxylase reaction. 1. Role of co-substrates. Nutr Rev, 1982, 40(10): 306–309. |
 2018, Vol. 34
2018, Vol. 34