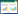中国科学院微生物研究所、中国微生物学会主办
文章信息
- 王雨辰, 文孟良, 李铭刚, 赵江源, 韩秀林
- Wang Yuchen, Wen Mengliang, Li Minggang, Zhao Jiangyuan, Han Xiulin
- 檀香烯与檀香醇生物合成研究进展
- Progress in biosynthesis of santalene and santalol
- 生物工程学报, 2018, 34(6): 862-875
- Chinese Journal of Biotechnology, 2018, 34(6): 862-875
- 10.13345/j.cjb.170465
-
文章历史
- Received: November 24, 2017
- Accepted: January 19, 2018
2 云南省保山市中医药高等专科学校, 云南 保山 678000
2 Baoshan College of Traditional Chinese Medicine, Baoshan 678000, Yunnan, China
萜类化合物是天然产物中种类最多、结构多样性最丰富的家族之一,目前已在动物、植物和微生物体内发现80 000余种[1],萜类在生物个体或细胞间的交流、防御和适应性演化过程中起重要作用,具有广泛的抗菌、抗肿瘤和抗氧化等药理活性,其中有些还具有特殊的香气,因而在医药、食品、化妆品以及生物能源等领域具有广泛的应用价值[2-3]。萜类主要从植物中提取获得,普遍存在产率与纯度低、成本高及易污染环境等许多问题;而萜类的立体选择性合成难度大,导致萜类尚未大规模工业生产及应用。近年来代谢工程与合成生物学的发展让酿酒酵母Saccharomyces cerevisiae和大肠杆菌Escherichia coli等微生物细胞工厂异源合成稀缺萜类成为可能[4-11],这对于资源稀缺、成本昂贵的萜类提供了新的可持续的绿色生产途径(表 1)。
| Terpenes | Structure | Molecular formula | Host | Substrate | Yield | Reference |
| Amorphadiene |  |
C15H24 | Saccharomyces cerevisiae | Glucose +Ethanol | 40 g/L | [4] |
| Artemisinic acid |  |
C15H22O2 | Saccharomyces cerevisiae | IPM+Glucose +Ethanol | 25 g/L | [5] |
| Taxadiene |  |
C20H32 | Saccharomyces cerevisiae | Glucose | 8.7 mg/L | [6] |
| Oleanolic acid |  |
C30H48O3 | Saccharomyces cerevisiae | Glucose | 71 mg/L | [7] |
| β-amyrin | 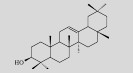 |
C30H50O | Saccharomyces
cerevisiae | Glucose | 107 mg/L | [7] |
| Dammarenediol-Ⅱ | 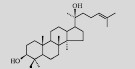 |
C30H52O2 | Saccharomyces cerevisiae | Glucose | 1 548 mg/L | [8] |
| Protopanaxatriol | 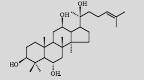 |
C30H52O4 | Saccharomyces cerevisiae |
Glucose | 15.9 mg/L | [7] |
| Protopanaxadiol | 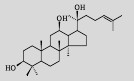 |
C30H52O3 | Saccharomyces cerevisiae |
Glucose | 1 189 mg/L | [8] |
| Ginsenoside CK |  |
C30H52O3 | Saccharomyces cerevisiae |
Galactose | 1.4 mg/L | [9] |
| β-carotene |  |
C40H56 | Saccharomyces cerevisiae |
Glucose | 18 mg/g DCW | [10] |
| Astaxanthin |  |
C40H52O4 | Xanthophyllomyces dendrorhous | Glucose | 9 mg/g DCW | [11] |
| IPM: isopropyl myristate oil; DCW: dry cell weight. | ||||||
来源于檀香树Santalum album的倍半萜檀香烯和檀香醇是檀香精油的主要成分,具有重要的应用价值。但由于檀香树生长条件苛刻、生长期长、精油含量低且市场需求旺盛,导致檀香树被过度采伐,檀香精油产量下滑,价格上涨[12]。如果采用微生物细胞工厂异源生物合成檀香烯和檀香醇,则能提高效率、降低成本、打破自然条件限制而有效缓解檀香精油的供需矛盾。文中对檀香烯合酶(Santalene synthase,SaSS)和来源于檀香树的细胞色素P450单加氧酶(Cytochrome P450 monooxygenase,CYP450)进行总结,同时对生物合成檀香烯的甲羟戊酸途径(Mevalonate pathway,MVA)改造的研究现状进行综述,分析蛋白质工程在萜类合酶的成功案例,提出综合利用蛋白质工程技术结合代谢路径改造的方法对檀香烯生物合成进行定向改造的思路,为檀香烯和檀香醇的优产研究提供参考。
1 檀香精油主要成分及功用市售檀香精油(CAS No. 8006-87-9)主要通过水蒸汽蒸馏印度檀香Santalum album、新喀里多尼亚檀香Santalum austrocaledonicum和澳洲檀香Santalum spicatum的心材和根来获得[13]。迄今已从S. album精油中鉴定出以倍半萜类为主的230多种香气成分,其中(Z)-(+)-α-檀香醇(1)和(Z)-(-)-β-檀香醇(2)是两个最主要香气成分;1的气味类似雪松木和α-雪松烯,2的丰度较低,但香气更强烈,是S. album精油独特香味的主要成分[14];市售S. album精油中1的含量在41%−55%,2的含量在16%−24%,主要成分结构式及相对含量分别见图 1和表 2[15-16]。
| Component | Molecular formula |
CAS Accession No. |
S. album (%) |
S. austrocaledonicum (%) |
S. spicatum (%) |
| 1, (Z)-(+)-β-santalol | C15H24O | 115-71-9 | 48.0 | 38.0 | 20 |
| 2, (Z)-(-)-α-santalol | 77-42-9 | 20.0 | 19.0 | 9 | |
| 3, (Z)-β-trans-bergamotol | 88034-74-6 | 6.0 | 9.0 | 5 | |
| 4, (E)-α-curzerene-12-ol | 942226-77-9 | 2.0 | 9.0 | 2 | |
| 5, (Z)-γ-bisabolene-12-ol | 1006030-74-5 | 2.0 | 2.0 | 11 | |
| 6, (6R, 7S)-iso-α-bisabolol | C15H26O | 496868-45-2 | 1.0 | 1.0 | 3 |
| 7, (6R, 7R)-iso-α-bisabolol | 496868-45-3 | ||||
| 8, (6S, 7S)-iso-α-bisabolol | 496868-45-5 | ||||
| 9, (6S, 7R)-iso-α-bisabolol | 496868-45-4 | ||||
| 10, (6S, 7S)-α-bisabolol | 15352-77-9 | 0.2 | 0.6 | 5 | |
| 11, (6R, 7S)-α-bisabolol | 106035-75-0 | ||||
| 12, (6S, 7R)-α-bisabolol | 106035-76-1 | ||||
| 13, (6R, 7R)-α-bisabolol | 700358-60-7 | ||||
| 14, (E, E)-farnesol | 106-28-5 | - | 1.0 | 10 | |
| 15, epi-β-bisabolol | 72059-10-0 | - | - | 7 | |
| Total | 79.2 | 79.6 | 65 |
檀香精油在动物体内毒性较低,无致突变性,因此欧美多国均认为是公认安全的食品添加剂,虽然对人偶有刺激或致敏反应,但未见其他副作用,推荐安全剂量0.007 4 mg/(kg·d)[13]。除用于香料和化妆品外,檀香精油及其主要成分还有镇静安神[17]、抗炎镇痛[18]、抗菌[19]、抗病毒[20]、抗氧化[21]、抗肿瘤[22-23]作用,对皮肤疾病、支气管炎、黏膜炎、抑郁失眠等具有一定的疗效。但目前只有德之馨(Symrise)、国际香精香料(IFF)、奇华顿(Givaudan)、芬美意(Firmenich)等几个香料寡头公司以α-龙脑烯醛为原料化学合成8个(16-23)香气接近檀香醇的替代品上市(图 2),而且生产工艺复杂,品质还不能取代天然的檀香精油[24]。
植物中的萜类是通过位于细胞质中的MVA途径和质体中的2-C-甲基赤藓醇-4-磷酸(2-C- methylerythritol-4-phosphate,MEP)途径合成异戊二烯(Isoprene),进一步得到异戊烯基二磷酸(Isopentenyl diphosphate,IPP)和二甲基烯丙基二磷酸(Dimethylallyl diphosphate,DMAPP),然后以IPP和DMAPP为单位生成香叶基二磷酸(Geranyl diphosphate,GPP)、法尼烯二磷酸(Farnesyl diphosphate,FPP)和香叶基香叶烯二磷酸(Geranylgeranyl diphosphate,GGPP)等,根据异戊二烯(C5)的数量不同分为单萜(C10)、倍半萜(C15)、二萜(C20)、三萜(C30)和四萜(C40)等[25-26]。其中倍半萜檀香烯和檀香醇的生物合成途径及参与合成的关键酶已基本清楚,首先S. album中的α-/β-檀香烯通过MVA途径得到FPP,再经SaSS酶环化及多重反应合成α-、β-及epi-β-檀香烯,最后在CYP450和NADPH依赖型的细胞色素P450还原酶(Cytochrome P450 reductase,CPR)协作下对C12羟基化形成檀香醇[27-28] (图 3)。

|
| 图 3 檀香烯与檀香醇的生物合成途径 Figure 3 The bio-synthetic pathway of santalene and santalenol. |
| |
因此,FPP作为合成檀香烯等倍半萜类及其他萜类(C≥15)的通用底物,其产率直接影响目标萜类产量,由于目前对宿主MVA途径的各种酶缺乏充足的酶动力学信息,以及影响宿主代谢物质通量的因素复杂多样,所以很难建立一个高效、通用的系统来作为公共底盘细胞平台生产FPP。尽管如此,朱发银等利用大肠杆菌E. coli表达、纯化来源于苹果Malus x domestica的法尼烯合酶(α-farnesene synthase,AFS)及E. coli、酿酒酵母S. cerevisiae MVA途径中的8个关键酶,以乙酰-CoA为底物进行体外催化实验,使法尼烯的产量达1.1 g/L,并得知各种酶的最佳比例[29];应用该策略生产番茄红素产量达1.44 g/L[30]。虽然通过该策略能鉴定出代谢路径中各种酶的比例及重要性,并简化关键路径筛选,但体外实验无法真正模拟宿主体内生理条件,只能作为代谢路径优化设计的参考;毕竟宿主的各条代谢路径是错综复杂、有机联系的,目前技术上也不具备精确表达多种蛋白质的能力,因此在宿主体内建立一个能精确表达代谢路径各种酶组成的系统尚待完成。
2.1 檀香烯合酶基因克隆及功能验证Misra等最早报道S. album各组织(愈伤组织、胚芽、种子、老树、幼树)中均含有SaSS酶,提取的蛋白在体外实验中能催化FPP合成檀香烯等产物,这与精油中的主要成分相吻合[31-32]。此后从S. album、S. austrocaledonicum和S. spicatum中分别克隆到SaSS基因并进行了序列测定、异源表达和功能验证[33-34]。除此之外,从野生多毛番茄Solanum habrochaites[35]、香根草Vetiver zizanioides[36]和黄皮Clausena lansium[37]中克隆到SaSS同源基因,并进行了相关验证(表 3)。
| Species | Genes/NCBI Accession No. |
Enzyme | Substrate | Product | Reference |
| Santalum album | None | Tissue extraction mixture |
FPP | α-/β-santalene, α-/β-santalol, Farnesol, α-bergamotene, Z-β-trans-bergamotol, β-bisabolene, Z-lanceol, Z-nuciferol |
[31] |
| Solanum habrochaites | Zfps/FJ194969 | Z, Z-farnesyl diphosphate synthase | IPP+DMAPP | Z, Z- FPP | [35] |
| SBS/FJ194970 | Santalene/bergamotene synthase | Z, Z-FPP | (+)-β-santalene, (+)-endo-α-bergamotene, (-)-endo-β-bergamotene |
||
| Santalum album | SaFDS/JQ023564 | Farnesyl diphosphate synthase | IPP+DMAPP | GPP | [34] |
| GPP+IPP | FPP | ||||
| SaSS/JX826486 | Santalene synthase |
(2E, 6E) FPP | α/β-santalene, epi-α-santalene α-bergamotene diphosphate |
||
| Santalum album | SaSSy/HQ343276 | Sa santalene synthase |
E, E-FPP | α/β-santalene, epi-α-santalene α-exo-bergamotene |
[33] |
| Z, Z-FPP | α-endo-bergamotene,
α-/β-/epi-α-santalene, (Z)-α-farnesene |
||||
| Santalum austrocaledonicum | SauSSy/ HQ343277 | Sau santalene synthase |
E, E-FPP | α/β-santalene, epi-α-santalene α-exo-bergamotene |
|
| Santalum spicatum | SspiSSy/HQ343278 | Sspi santalene synthase |
E, E-FPP | α/β-santalene, epi-α-santalene α-exo-bergamotene |
|
| Vetiver zizanioides | Unpublished | Santalene synthase |
E, E-FPP | epi-α-santalene, α-bergamotene, β-bisabolene | [36] |
| Clausena lansium | ClTps2-1/HQ452480 | α-santalene synthase |
E, E-FPP | α-santalene | [37] |
| IPP: isopentenyl diphosphate; DMAPP: dimethyl allyl diphosphate; GPP: geranyl diphosphate; FPP: farnesyl diphosphate. | |||||
微生物以其生长迅速、营养简单以及基因工程操作方便和遗传背景清晰的一系列优点,适合作为生物合成的宿主,并且E. coli和S. cerevisiae等宿主天然含有萜类生物合成的途径,因此多位学者利用S. cerevisiae和小立碗藓Physcomitrella patens等作底盘细胞,构建表达质粒来合成檀香烯[38-41],但宿主本身的MVA或MEP途径代谢效率并不高,因此需要对宿主代谢途径的关键酶进行优化,提高檀香烯的产量。例如Chen等过表达宿主的乙醇脱氢酶基因ADH2和NADPH依赖型醛脱氢酶基因ALD6,使用葡萄糖调节的HXT7启动子,导入密码子优化的乙酰-CoA合成酶基因ACS,过表达乙酰-CoA酰基转移酶ERG10等步骤,使乙酰-CoA朝着乙酰乙酰-CoA转化,最终S. cerevisiae工程菌的α-檀香烯产率从2.08 mg/L增加到8.29 mg/L[38];Scalcinati等将S. cerevisiae鲨烯合酶基因ERG9自带的启动子替换为葡萄糖敏感型启动子HXT1,减少FPP合成鲨烯的物质流量,同时敲除编码FPP脱磷酸化的2个基因LPP1和DPP1,减少FPP向法尼醇(Farnesol)转化;此外过表达ERG20基因以增加IPP产量,让葡萄糖富集而固醇的合成减少,增加碳代谢流偏向α-檀香烯合成,最终使S. cerevisiae工程菌的α-檀香烯产量达到92 mg/L,比出发菌株提高3.4倍[39-40]。此外,Zhan等通过35S启动子调控HMGR基因表达后,使α/β-檀香烯在宿主P. patens中的产量达到0.039 mg/g干重,这是檀香烯在自养型宿主中异源表达的首次报道,开辟了光驱动宿主生产高价值香料的道路[41],优化方法与结果见表 4。
| Target gene | Optimization methods | Host | Final yield | Reference | |
| ADH2 | Using the glucose-regulatable HXT7 promoter | S. cerevisiae | 8.29 mg/L | [38] | |
| ACS | Codon optimization (L641P) | ||||
| ALD6, ERG10 | Over-expression | ||||
| CIT2, MLS1 | Inhibition | ||||
| ERG9 | Replaced by the HXT1 promoter to down-regulate | S. cerevisiae | 92 mg/L | [39] | |
| ERG9 | Using HXT2 promoter to express an ERG9 antisense construct |
||||
| HMGR | Over-expression | ||||
| LPP1, DPP1 | Deletion | ||||
| ERG9 | The promoter is replaced with PHXT1 | S. cerevisiae | 39.4 mg/L | [40] | |
| tHMG1, ERG20, GDH2, SanSynopt | Over-expression | ||||
| GDH1, LPP1, DPP1 | Deletion | ||||
| HMGR | Over-expression under the control of the 35S promoter | P. patens | 0.039 mg/g DCW | [41] | |
| ADH2: alcohol dehydrogenase gene; ALD6: NADP-dependent aldehyde dehydrogenase gene; ACS: acetyl-CoA synthetase gene; ERG10: acetyl-CoA C-acetyltransferase gene; CIT2: peroxisomal citrate synthase gene; MLS1: cytosolic malate synthase gene; HMGR: 3-hydroxyl-3-methyl-glutaryl-CoA reductase gene; LPP1: lipid phosphate phosphatase gene; DPP1: diacylglycerol pyrophosphate phosphatase gene; tHMG1: truncated HMG-CoA reductase gene; ERG9: squalene synthase gene; ERG20: FPP synthase gene; GDH1: NADP-dependent glutamate dehydrogenase gene; GDH2: NAD-dependent glutamate dehydrogenase gene; SanSynopt: codon optimization α-santalene synthase gene sequence; DCW: dry cell weight. | |||||
总之,α/β-檀香烯合酶表达优化方法主要是:1)替换启动子,使被调控基因具备更好的可调节性,通过改变培养条件调节表达量;2)增加基因拷贝数过表达目标酶,利用强启动子等过表达底物合成基因,增加底物量;3)敲除或抑制代谢路径分支点的基因,减少不必要的底物消耗;4)对目的基因进行密码子优化,促进转录,提高目的基因与宿主的协调适应性;5)改变宿主类型,利用自养型宿主作为底盘细胞工厂,降低生产成本。
2.3 檀香树CY P450基因克隆及功能验证CYP450能以各种萜类为底物对不同位置的C羟基化,因此能催化檀香烯的C12位羟基化,形成檀香醇[42]。多位学者通过分析挖掘S. album转录组数据库信息,从S. album克隆得到多个CYP450基因,大部分都能催化檀香烯得到檀香醇,其中Sa-CYP76F39v1的催化率接近100%[28, 43-44]。从S. album中得到的CYP450及其催化产物见表 5。
| CYP450 | NCBI Accession No. | Substrate | Product | Reference |
| Sa-CYP76F37v1 | KC533717 | α/β-santalene epi-β-santalene α-exo-bergamotene |
(E)-α-santalol,
*(E)-α-exo-bergamotol, (E)-β-santalol |
[43] |
| Sa-CYP76F37v2 | KC698966 | Products same as Sa-CYP76F37v1 | ||
| Sa-CYP76F38v1 | KC533715 | Products same as Sa-CYP76F37v1 | ||
| Sa-CYP76F38v2 | KC533718 | Products same as Sa-CYP76F37v1 | ||
| Sa-CYP76F39v1 | KC533716 | (Z)-α-santalol,
(Z)-α-exo-bergamotol *(E)-α-santalol, (E)-α-exo-bergamotol, (Z)-epi-β-santalol, (Z)-β-santalol, (E)-epi-β-santalol, *(E)-β-santalol |
||
| Sa-CYP76F39v2 | KC698967 | (Z)-α-santalol (Z)-α-exo-bergamotol, *(E)-α-santalol, (E)-α-exo-bergamotol, (Z)-β-santalol, (E)-epi-β-santalol, *(E)-β-santalol |
||
| Sa-CYP76F40 | KC698968 | (E)-α-exo-bergamotol,
(Z)-β-santalol *(E)-β-santalol |
||
| Sa-CYP76F41 | KC698969 | (Z)-α-santalol,
(Z)-α-exo-bergamotol, *(E)-α-santalol, (E)-α-exo-bergamotol, (Z)-epi-β-santalol, (Z)-β-santalol, (E)-epi-β-santalol, (E)-β-santalol |
||
| Sa-CYP76F42 | KC698965 | (Z)-α-santalol,
(Z)-α-exo-bergamotol (E)-α-santalol, *(E)-α-exo-bergamotol, (Z)-epi-β-santalol, (Z)-β-santalol, (E)-epi-β-santalol, (E)-β-santalol |
||
| Sa-CYP76F43 | KC533719 | α/β-santalene epi-β-santalene α-exo-bergamotene |
Not detected | |
| Sa-CYP736A167 | KU169302 | α-santalene | (Z)-α/β-santalol,
(Z)-epi-β-santalol, (Z)-α-exo-bergamotol |
[28] |
| *: star marked product has the highest ratio. | ||||
SaSS酶属于植物萜类环化酶中的一种,根据目前报道的植物来源萜类合酶结构,这类酶都包含相似的保守结构,能把无环的GPP、FPP或GGPP分别环化为单萜、倍半萜或二萜类化合物。Bohlmann等综合28种植物的33个萜类合酶家族序列和其他萜类合酶蛋白三维结构分析发现这类酶的活性部位是1个反平行α-螺旋构成的大中央空腔,空腔内壁相对位置有2个富含Asp的绝对保守结构域DDxxD (“x”表示任意氨基酸),Asp的侧链基团通过与Mg2+桥接后介导与底物上的磷酸结合,从而诱导酶构象发生改变,在活性空腔其他侧链基团作用下,使底物(GPP、FPP或GGPP)上的二磷酸断裂并从空腔逸出,脱下二磷酸的底物产生1个烯丙基碳正离子亲电攻击C=C双键,进一步降低烃链对第1个环闭合的影响,促进第1个环及更多的环产生,而酶蛋白N-端区域在空腔入口处形成的盖子状结构能维持空腔疏水性作用,防止烃链逃逸[45-47]。基于此,多位学者根据萜类合酶的结构进行了定向突变研究,方法集中在以下几个方面。
3.1 基于X-ray衍射单晶结构的定向诱变此方法利用X-ray衍射技术,对比酶、酶结合底物类似物复合体的晶体结构,发现起关键作用的基团从而进行定向突变,比如Gennadios等以树棉Gossypium arboreum的(+)-δ-荜澄茄烯合酶((+)-δ-Cadinene synthase,DCS)为研究对象,对DDxxD结构域进行定向突变(D308A),使该酶的Km值增加了13倍[48]。同理把烟草Nicotiana tabacum的5-epi-马兜铃烯合酶(5-epi-aristolochene synthase,TEAS)和天仙子Hyoscyamus muticus的premnaspirodiene合酶(HPS)的2个活性残基进行对调突变,结果使这2个酶的产物也发生了对调[49]。
3.2 基于分子进化理论的定向突变在缺乏相关蛋白的晶体结构数据情况下,可根据分子进化分析数据及同源蛋白中氨基酸突变概率,对大量同源基因序列及中心代谢途径相关酶氨基酸序列进行分析,找到最保守的氨基酸残基并对其进行定向突变。如对宿主S. cerevisiae的羟甲基戊二酸单酰-CoA还原酶(tHMGR)和巨冷杉Abies grandis的γ-蛇麻烯合酶(γ-humulene synthase,HUM)分别进行9个和6个位点定向突变,最终使宿主生长量翻了3-4倍,γ-蛇麻烯产量提高近1 000倍[50],而以PDB公布的5-epi-马兜铃烯合酶的晶体结构作为依据,对HUM 19个候选残基定向突变,其中S484经饱和诱变后与野生型比较产物提高100-1 000倍[51]。另外,易错PCR (Error-prone PCR)技术也能进行定向突变,但文库筛选工作量大,并且融合蛋白标签的活性并不一定能真实反映诱变蛋白活性[52]。
3.3 SaSS蛋白模拟建模及其定向突变思考根据NCBI中保守结构域数据库(Conserved Domain Database,CDD https://www.ncbi.nlm.nih.gov/cdd/)公布的信息,SaSS酶(Protein ID in NCBI:ADO87000,569aa)属于植物萜类环化酶1类,预测SaSS酶三维结构是由α-螺旋通过短小的环和转折连接组成,包含两个独特的结构域:N-末端结构与糖苷水解酶的一个未知功能结构类似;C-末端结构域是催化檀香烯合成的区域,该区域的D-螺旋和H-螺旋分别有D321DGYD325基序和N463DIGT467SPDE471基序,这两个基序位于活性空腔入口的相对位置,靠近入口位置还有2个无序的A-C环和J-K环。因此认为D321DGYD325和N463DIGT467SPDE471这两个基序在结合Mg2+后能固定底物FPP上的二磷酸基团,在此基础上,J-K环形成一个盖子在活性空腔入口处,维持空腔疏水性防止烃链逃逸。而参与结合Mg2+的基序在植物倍半萜合酶中很保守,一旦突变则活性丧失,因此对于定向突变则应关注疏水性空腔的残基[46, 53-54]。所以推测其构成盖子部位活性残基可能是32-39,470-471,473-476,478-481,542-545;底物结合口袋的残基可能是284,293,314,316-318,321,325,396,460-461,463-464,467,471,539,543,545;底物-Mg2+结合部位残基可能是321、325、463、467、471;富Asp残基是321-325和463-471,这些残基可作为定向进化参考位点[45-47] (图 4)。
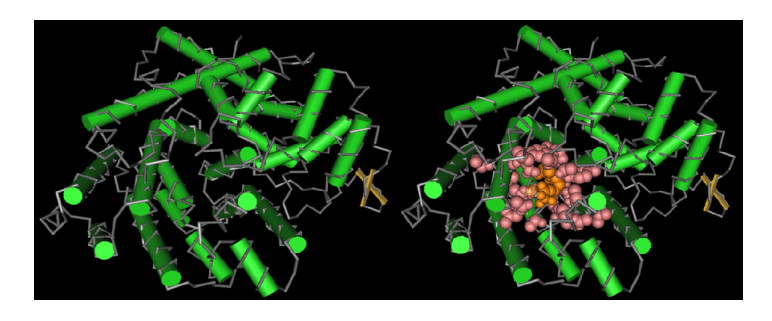
|
| 图 4 CDD模拟SaSS未结合底物与结合底物的模型 Figure 4 The simulation model of SaSS unbound substrate and substrate binding from the CDD. |
| |
此外,利用SWISS-MODEL在线工具(https://swissmodel.expasy.org/)对SaSS酶三级结构建模,共搜索到已公布X-ray衍射晶体结构的相似酶95个,相似度10.42%−45.49%;利用Phyre2 (http://www.sbg.bio.ic.ac.uk/phyre2/html/page.cgi?id=index)进行模拟建模,共搜索到同源蛋白99个,有3D模型的20个;根据CSA (Catalytic Site Atlas http://www.ebi.ac.uk/thornton-srv/databases/CSA/)对已知萜类合酶的催化活性位点的标注(表 6),得到与SaSS酶相似度最高的12个酶蛋白结构数据,可考虑用相似度最高的(+)-limonene synthase蛋白分子三维结构数据作为SaSS酶定向突变的标准模板,并结合CDD公布的保守残基综合考虑,决定定向突变位点。综合比较数据得知SaSS酶序列中的293W、545F位点是潜在的保守催化活性位点,可尝试对这两个位点进行定点突变来检测活性变化。
| Protein | PDB Id | Seq identity (%) |
X-ray | Range | Coverage | Catalytic residue from the CSA |
Corresponding sites in SaSS | Reference |
| (+)-limonene synthase |
5uv0.1.A | 45.49 | 2.30 | 31-569 | 0.93 | Unknown | Unknown | [55] |
| Isoprene synthase |
3n0f.1.A | 40.07 | 2.70 | 34-569 | 0.94 | Unknown | Unknown | [56] |
| 4S-limonene synthase |
2ong.1.A | 38.46 | 2.70 | 31-564 | 0.94 | 324 W/579H | 293W/545F | [57] |
| (+)-bornyl diphosphate synthase |
1n24.1.A | 38.88 | 2.30 | 31-563 | 0.94 | 323W/578F | 293W/545F | [47] |
| 5-epi- aristolochene synthase | 5eat.1.A | 33.90 | 2.80 | 34-566 | 0.92 | 264R/273S/401T/ 402T/403T/441R/ 444D/520Y/ 525D/527Y |
284R/293W/ 421S/422I/ 423G/460R/ 463N/539Y/ 544D/546F | [46] |
| Putative γ-terpinene synthase | 5c05.1.A | 36.16 | 1.65 | 41-563 | 0.93 | Unknown | Unknown | [58] |
| Amorpha-4, 11- diene synthase |
4gax.1.A | 33.14 | 1.99 | 35-566 | 0.92 | Unknown | Unknown | [59] |
| 1, 8-cineole synthase |
2j5c.1.A | 37.45 | 1.95 | 61-565 | 0.94 | 317W/571F | 293W/545F | [60] |
| α-bisabolene synthase |
3sae.1.A | 37.40 | 1.96 | 72-566 | 0.86 | Unknown | Unknown | [61] |
| (+)-δ-cadinene synthase isozyme xc1 | 3g4d.1.A | 32.83 | 2.40 | 35-566 | 0.93 | 279W | 293W | [48] |
| Taxadiene synthase |
3p5r.1.A | 31.05 | 2.25 | 66-566 | 0.87 | Unknown | Unknown | [62] |
| Abietadiene synthase |
3s9v.1.A | 31.85 | 2.30 | 66-565 | 0.87 | Unknown | Unknown | [63] |
目前各数据库公布了很多植物来源的萜类合酶三维结构(表 6),能利用这些数据为定向突变提供指导,可能采取的方法及思路主要是:基于X-ray得到酶与底物类似物结合后的复合体三维结构,分析得知活性中心中的柔性残基(与底物结合紧密,并对催化反应起关键作用,具有定向突变潜力的氨基酸残基),然后对这些氨基酸进行定点突变;或者基于已知的同源蛋白三维结构来模拟构建目的蛋白三维结构,并进一步筛选活性位点的柔性残基进行突变;也可以搜索同源蛋白的一级结构建立数据库,来与目的蛋白比对得到保守残基位点,以此筛选柔性残基并对其进行突变。在以上方法中,如何确定柔性残基位点以及突变成何种氨基酸是实验成功与否的关键。
4 总结与展望综上所述,目前檀香烯的生物合成方法主要是通过对底盘细胞MVA途径中关键基因的上调或者下调、替换或增加高效启动子元件、利用自养型宿主来替换S. cerevisiae降低资源消耗等方法,最终让微生物细胞工厂合成檀香烯。但由于底物FPP产率低及分支代谢太多使檀香烯产量低,改造MVA代谢途径也有其局限性,无论使用多高效率的调控元件来启动目的基因表达,宿主也不可能消耗过多能量及资源来合成它自身并不需要的异源产物;甚至某些目标产物对宿主有毒性,况且宿主的平衡调节机制也不允许自身过量表达异源产物。若要从根本上打破这个局限性而达到工业化生产要求,可从以下几方面考虑:1)在工程学思想的指导下构建更为合理的表达模块,结合酶动力学、转录组学、代谢组学和蛋白质组学的辅助分析,了解宿主MVA代谢途径各酶表达水平和代谢中间物质流规律,定向改造代谢途径,尽可能提高通用底物表达水平,最终使构建的表达模块更为协调、高效而合理。2)利用基因编辑技术如CRISPR-Cas9系统来对宿主S. cerevisiae的特定位点进行切割,增加宿主中表达模块的拷贝数,以利于产物的累积。3)利用冷冻电镜解析关键酶蛋白分子结构,结合人工智能和大数据来分析指导酶蛋白定向改造策略,以及从量子水平探索酶结构与功能的关系,从而对目标酶进行理性设计和定向突变,这样能从根本上打破单纯改造代谢途径的顶板效应,大大提高酶活性甚至能得到一个全新的酶。4)对S. cerevisiae的代谢组进行立体研究,构建高效、精准表达各种酶的宿主,为其他萜类合酶基因导入提供通用底盘细胞平台。5)选择更广泛的宿主,比如自养型微生物或者人造S. cerevisiae (人工设计合成基因组,遗传背景清楚,敲除了冗余无效基因的S. cerevisiae)作宿主,利于精确的整体表达调控及节省成本。
目前我们已经优化合成SaSS基因及体外功能验证,克隆到MVA途径关键的ERG20和MVA基因,构建了δ位点切割的CRISPR-Cas9系统及表达元件,为后续研究奠定了一定基础。相信随着量子生物学、功能基因组学、转录组学、代谢工程技术、计算机辅助设计、蛋白质工程技术和组合合成生物学技术等多学科的发展和交融,越来越多重要萜类化合物会实现在微生物宿主中的高产并达到工业化生产的要求,开创微生物细胞工厂生产萜类化合物的绿色可持续发展的新型工业。
| [1] | Christianson DW. Structural and chemical biology of terpenoid cyclases. Chem Rev, 2017, 117(17): 11570–11648. DOI: 10.1021/acs.chemrev.7b00287 |
| [2] | Abbas F, Ke Y, Yu R, et al. Volatile terpenoids:multiple functions, biosynthesis, modulation and manipulation by genetic engineering. Planta, 2017, 246(5): 803–816. DOI: 10.1007/s00425-017-2749-x |
| [3] | Lu X, Tang K, Li P. Plant metabolic engineering strategies for the production of pharmaceutical terpenoids. Front Plant Sci, 2016, 7(1647): 1–11. |
| [4] | Westfall PJ, Pitera DJ, Lenihan JR, et al. Production of amorphadiene in yeast, and its conversion to dihydroartemisinic acid, precursor to the antimalarial agent artemisinin. Proc Natl Acad Sci USA, 2012, 109(3): E111–E118. |
| [5] | Paddon CJ, Westfall PJ, Pitera DJ, et al. High-level semi-synthetic production of the potent antimalarial artemisinin. Nature, 2013, 496(7446): 528–532. DOI: 10.1038/nature12051 |
| [6] | Engels B, Dahm P, Jennewein S. Metabolic engineering of taxadiene biosynthesis in yeast as a first step towards Taxol (Paclitaxel) production. Metab Eng, 2008, 10(3-4): 201–206. DOI: 10.1016/j.ymben.2008.03.001 |
| [7] | Dai Z, Wang B, Liu Y, et al. Producing aglycons of ginsenosides in bakers' yeast. Sci Rep, 2014, 4(3698): 1–6. |
| [8] | Dai Z, Liu Y, Zhang X, et al. Metabolic engineering of Saccharomyces cerevisiae for production of ginsenosides. Metab Eng, 2013, 20(5): 146–156. |
| [9] | Yan X, Fan Y, Wei W, et al. Production of bioactive ginsenoside compound K in metabolically engineered yeast. Cell Res, 2014, 24(6): 770–773. DOI: 10.1038/cr.2014.28 |
| [10] | Reyes LH, Gomez JM, Kao KC. Improving carotenoids production in yeast via adaptive laboratory evolution. Metab Eng, 2014, 21(1): 26–33. |
| [11] | Gassel S, Breitenbach J, Sandmann G. Genetic engineering of the complete carotenoid pathway towards enhanced astaxanthin formation in Xanthophyllomyces dendrorhous starting from a high-yield mutant. Appl Microbiol Biotechnol, 2014, 98(1): 345–350. DOI: 10.1007/s00253-013-5358-z |
| [12] | Teixeira da Silva JA, Kher MM, Soner D, et al. Sandalwood:basic biology, tissue culture, and genetic transformation. Planta, 2016, 243(4): 847–887. DOI: 10.1007/s00425-015-2452-8 |
| [13] | Burdock GA, Carabin IG. Safety assessment of sandalwood oil (Santalum album L.). Food Chem Toxicol, 2008, 46(2): 421–432. DOI: 10.1016/j.fct.2007.09.092 |
| [14] | Krotz A, Helmchen G. Total syntheses of sandalwood fragrances:(Z)- and (E)-β-santalol and their enantiomers, ent-β-santalene. Tetrahedr Asymmetry, 1990, 1(8): 537–540. DOI: 10.1016/S0957-4166(00)80544-3 |
| [15] | Baldovini N, Delasalle C, Joulain D. Phytochemistry of the heartwood from fragrant Santalum species:a review. Flavour Fragr J, 2011, 26(1): 7–26. DOI: 10.1002/ffj.v26.1 |
| [16] | Hammerschmidt FJ, Krammer GE, Meier L, et al. Authentification of essential oils. ACS Symposium Series, 2006, 952(6): 87–108. |
| [17] | Okugawa H, Ueda R, Matsumoto K, et al. Effect of α-santalol and β-santalol from sandalwood on the central nervous system in mice. Phytomedicine, 1995, 2(2): 119–126. DOI: 10.1016/S0944-7113(11)80056-5 |
| [18] | Saneja A, Kaushik P, Kaushik D, et al. Antioxidant, analgesic and anti-inflammatory activities of Santalum album. Planta Medica, 2009, 75(4): 452–453. |
| [19] | Jirovetz L, Buchbauer G, Denkova Z, et al. Comparative study on the antimicrobial activities of different sandalwood essential oils of various origin. Flavour Fragr J, 2006, 21(3): 465–468. DOI: 10.1002/(ISSN)1099-1026 |
| [20] | Paulpandi M, Kannan S, Thangam R, et al. In vitro anti-viral effect of β-santalol against influenza viral replication. Phytomedicine, 2012, 19(3/4): 231–235. |
| [21] | Misra BB, Dey S. Evaluation of in vivo anti-hyperglycemic and antioxidant potentials of α-santalol and sandalwood oil. Phytomedicine, 2013, 20(5): 409–416. DOI: 10.1016/j.phymed.2012.12.017 |
| [22] | Santha S, Dwivedi C. α-Santalol, a skin cancer chemopreventive agent with potential to target various pathways involved in photocarcinogenesis. Photochem Photobiol, 2013, 89(4): 919–926. DOI: 10.1111/php.12070 |
| [23] | Bommareddy A, Rule B, Vanwert AL, et al. α-Santalol, a derivative of sandalwood oil, induces apoptosis in human prostate cancer cells by causing caspase-3 activation. Phytomedicine, 2012, 19(8/9): 804–811. |
| [24] | Brocke C, Eh M, Finke A. Recent developments in the chemistry of sandalwood odorants. Chem Biodivers, 2008, 5(6): 1000–1010. DOI: 10.1002/cbdv.v5:6 |
| [25] | Rohmer M, Knani M, Simonin P, et al. Isoprenoid biosynthesis in bacteria:a novel pathway for the early steps leading to isopentenyl diphosphate. Biochem J, 1993, 295(Pt2): 517–524. |
| [26] | Newman JD, Chappell J. Isoprenoid biosynthesis in plants:carbon partitioning within the cytoplasmic pathway. Crit Rev Biochem Mol Bio, 1999, 34(2): 95–106. DOI: 10.1080/10409239991209228 |
| [27] | Bick JA, Lange BM. Metabolic cross talk between cytosolic and plastidial pathways of isoprenoid biosynthesis:unidirectional transport of intermediates across the chloroplast envelope membrane. Arch Biochem Biophys, 2003, 415(2): 146–154. DOI: 10.1016/S0003-9861(03)00233-9 |
| [28] | Celedon JM, Chiang A, Yuen MM, et al. Heartwood-specific transcriptome and metabolite signatures of tropical sandalwood (Santalum album) reveal the final step of (Z)-santalol fragrance biosynthesis. Plant J, 2016, 86(4): 289–299. DOI: 10.1111/tpj.13162 |
| [29] | Zhu FY, Lu L, Fu S, et al. Targeted engineering and scale up of lycopene overproduction in Escherichia coli. Process Biochem, 2015, 50(3): 341–346. DOI: 10.1016/j.procbio.2014.12.008 |
| [30] | Zhu FY, Zhong XF, Hu MZ, et al. In vitro reconstitution of mevalonate pathway and targeted engineering of farnesene overproduction in Escherichia coli. Biotechnol Bioeng, 2014, 111(7): 1396–1405. DOI: 10.1002/bit.25198 |
| [31] | Misra BB, Dey S. Developmental variations in sesquiterpenoid biosynthesis in East Indian sandalwood tree (Santalum album L.). Trees, 2013, 27(4): 1071–1086. DOI: 10.1007/s00468-013-0858-0 |
| [32] | Jindal G, Sunoj RB. Revisiting sesquiterpene biosynthetic pathways leading to santalene and its analogues:a comprehensive mechanistic study. Org Biomol Chem, 2012, 10(39): 7996–8006. DOI: 10.1039/c2ob26027a |
| [33] | Jones CG, Moniodis J, Zulak KG, et al. Sandalwood fragrance biosynthesis involves sesquiterpene synthases of both the terpene synthase (TPS)-a and TPS-b subfamilies, including santalene synthases. J Biol Chem, 2011, 286(20): 17445–17454. DOI: 10.1074/jbc.M111.231787 |
| [34] | Rani A, Ravikumar P, Reddy MD, et al. Molecular regulation of santalol biosynthesis in Santalum album L. Gene, 2013, 527(2): 642–648. DOI: 10.1016/j.gene.2013.06.080 |
| [35] | Sallaud C, Rontein D, Onillon S, et al. A novel pathway for sesquiterpene biosynthesis from Z, Z-farnesyl pyrophosphate in the wild tomato Solanum habrochaites. Plant Cell, 2009, 21(1): 301–317. DOI: 10.1105/tpc.107.057885 |
| [36] | Schalk M. Novel sesquiterpene synthases and methods of their use. International Patent, 2006: WO 2006/134523. |
| [37] | Schalk M. Method for producing α-santalene. US Patent, 2011: US2011/0008836. |
| [38] | Chen Y, Daviet L, Schalk M, et al. Establishing a platform cell factory through engineering of yeast acetyl-CoA metabolism. Metab Eng, 2013, 15(1): 48–54. |
| [39] | Scalcinati G, Knuf C, Partow S, et al. Dynamic control of gene expression in Saccharomyces cerevisiae engineered for the production of plant sesquitepene α-santalene in a fed-batch mode. Metab Eng, 2012, 14(2): 91–103. DOI: 10.1016/j.ymben.2012.01.007 |
| [40] | Scalcinati G, Partow S, Siewers V, et al. Combined metabolic engineering of precursor and co-factor supply to increase α-santalene production by Saccharomyces cerevisiae. Microb Cell Fact, 2012, 11(1): 117–133. DOI: 10.1186/1475-2859-11-117 |
| [41] | Zhan X, Zhang YH, Chen DF, et al. Metabolic engineering of the moss Physcomitrella patens to produce the sesquiterpenoids patchoulol and α/β-santalene. Front Plant Sci, 2014, 5: 636. |
| [42] | Weitzel C, Simonsen HT. Cytochrome P450-enzymes involved in the biosynthesis of mono- and sesquiterpenes. Phytochem Rev, 2015, 14(1): 7–24. DOI: 10.1007/s11101-013-9280-x |
| [43] | Diazchavez ML, Moniodis J, Madilao LL, et al. Biosynthesis of sandalwood oil:Santalum album CYP76F cytochromes P450 produce santalols and bergamotol. PLoS ONE, 2013, 8(9): e75053. DOI: 10.1371/journal.pone.0075053 |
| [44] | Zerbe P, Hamberger B, Yuen MM, et al. Gene discovery of modular diterpene metabolism in nonmodel systems. Plant Physiol, 2013, 162(2): 1073–1091. DOI: 10.1104/pp.113.218347 |
| [45] | Bohlmann J, Meyer-Gauen G, Croteau R. Plant terpenoid synthases:molecular biology and phylogenetic analysis. Proc Natl Acad Sci USA, 1998, 95(8): 4126–4133. DOI: 10.1073/pnas.95.8.4126 |
| [46] | Starks CM, Back K, Chappell J, et al. Structural basis for cyclic terpene biosynthesis by tobacco 5-epi-aristolochene synthase. Science, 1997, 277(5333): 1815–1820. DOI: 10.1126/science.277.5333.1815 |
| [47] | Whittington DA, Wise ML, Urbansky M, et al. Bornyl diphosphate synthase:structure and strategy for carbocation manipulation by a terpenoid cyclase. Proc Natl Acad Sci USA, 2002, 99(24): 15375–15380. DOI: 10.1073/pnas.232591099 |
| [48] | Gennadios HA, Gonzalez V, Costanzo LD, et al. Crystal structure of (+)-δ-cadinene synthase from Gossypium arboreum and evolutionary divergence of metal binding motifs for catalysis. Biochemistry, 2009, 48(26): 6175–6183. DOI: 10.1021/bi900483b |
| [49] | Greenhagen BT, O'Maille PE, Noel JP, et al. Identifying and manipulating structural determinates linking catalytic specificities in terpene synthases. Proc Natl Acad Sci USA, 2006, 103(26): 9826–9831. DOI: 10.1073/pnas.0601605103 |
| [50] | Yoshikuni Y, Dietrich JA, Nowroozi FF, et al. Redesigning enzymes based on adaptive evolution for optimal function in synthetic metabolic pathways. Chem Biol, 2008, 15(6): 607–618. DOI: 10.1016/j.chembiol.2008.05.006 |
| [51] | Yoshikuni Y, Ferrin TE, Keasling JD. Designed divergent evolution of enzyme function. Nature, 2006, 440(7087): 1078–1082. DOI: 10.1038/nature04607 |
| [52] | Yoshikuni Y, Martin VJ, Ferrin TE, et al. Engineering cotton (+)-δ-cadinene synthase to an altered function:germacrene D-4-ol synthase. Chem Biol, 2006, 13(1): 91–98. DOI: 10.1016/j.chembiol.2005.10.016 |
| [53] | Christianson DW. Structural biology and chemistry of the terpenoid cyclases. Chem Rev, 2006, 106(8): 3412–3442. DOI: 10.1021/cr050286w |
| [54] | Degenhardt J, K llner TG, Gershenzon J. Monoterpene and sesquiterpene synthases and the origin of terpene skeletal diversity in plants. Phytochemistry, 2009, 70(15-16): 1621–1637. DOI: 10.1016/j.phytochem.2009.07.030 |
| [55] | Morehouse BR, Kumar RP, Matos JO, et al. Functional and structural characterization of a (+)-limonene synthase from Citrus sinensis. Biochemistry, 2017, 56(12): 1706–1715. DOI: 10.1021/acs.biochem.7b00143 |
| [56] | Kksal M, Zimmer I, Schnitzler JP, et al. Structure of isoprene synthase illuminates the chemical mechanism of teragram atmospheric carbon emission. J Mol Biol, 2010, 402(2): 363–373. DOI: 10.1016/j.jmb.2010.07.009 |
| [57] | Hyatt DC, Youn B, Zhao Y, et al. Structure of limonene synthase, a simple model for terpenoid cyclase catalysis. Proc Natl Acad Sci USA, 2007, 104(13): 5360–5365. DOI: 10.1073/pnas.0700915104 |
| [58] | Rudolph K, Parthier C, Egerersieber C, et al. Expression, crystallization and structure elucidation of γ-terpinene synthase from Thymus vulgaris. Acta Crystallogr A, 2016, 72(1): 16–23. |
| [59] | Li JX, Fang X, Zhao Q, et al. Rational engineering of plasticity residues of sesquiterpene synthases from Artemisia annua:product specificity and catalytic efficiency. Biochem J, 2013, 451(3): 417–426. DOI: 10.1042/BJ20130041 |
| [60] | Kampranis SC, Ioannidis D, Purvis A, et al. Rational conversion of substrate and product specificity in a salvia monoterpene synthase:structural insights into the evolution of terpene synthase function. Plant Cell, 2007, 19(6): 1994–2005. DOI: 10.1105/tpc.106.047779 |
| [61] | Mcandrew RP, Peraltayahya PP, Degiovanni A, et al. Structure of a three-domain sesquiterpene synthase:a prospective target for advanced biofuels production. Structure, 2011, 19(12): 1876–1884. DOI: 10.1016/j.str.2011.09.013 |
| [62] | K ksal M, Jin Y, Coates RM, et al. Taxadiene synthase structure and evolution of modular architecture in terpene biosynthesis. Nature, 2011, 469(7328): 116–120. DOI: 10.1038/nature09628 |
| [63] | Zhou K, Gao Y, Hoy JA, et al. Insights into diterpene cyclization from structure of bifunctional abietadiene synthase from Abies grandis. J Biol Chem, 2012, 287(9): 6840–6850. DOI: 10.1074/jbc.M111.337592 |
 2018, Vol. 34
2018, Vol. 34