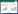中国科学院微生物研究所、中国微生物学会主办
文章信息
- 王辉, 杨波, 赵可胜, 李家吉, 李新, 孔萌萌, 宫春杰, 王毅, 陶冶, 张秋, 胡征
- Wang Hui, Yang Bo, Zhao Kesheng, Li Jiaji, Li Xin, Kong Mengmeng, Gong Chunjie, Wang Yi, Tao Ye, Zhang Qiu, Hu Zheng
- 卡他莫拉菌UspA1蛋白多克隆抗体的制备及鉴定
- Preparation and identification of polyclonal antibodies against Moraxella catarrhalis UspA1
- 生物工程学报, 2018, 34(1): 102-109
- Chinese Journal of Biotechnology, 2018, 34(1): 102-109
- 10.13345/j.cjb.170095
-
文章历史
- Received: March 8, 2017
- Accepted: April 19, 2017
卡他莫拉菌(Moraxella catarrhalis, Mc)由Kirchner首次分离于1890年,当时称之为卡他微球菌Micrococcus catarrhalis[1],之后又称为卡他奈瑟菌Neisseria catarrhalis和卡他布兰汉菌Branhamella catarhalis。最初被认为是对人体无致病性的上呼吸道正常寄居菌,后来越来越多的研究发现,该菌不仅可以引起儿童和老年人的上呼吸道感染,而且还是引起儿童上颌窦炎、中耳炎、肺炎以及成人的慢性下呼吸道感染的最常见致病菌[2-6]。因多数分离株具有β-内酰胺类抗生素抗性[7-16],所以需要确诊后再选择合适的治疗方案。但临床上该菌与其他呼吸道病原体引起的感染症状类似,因此常难以根据临床表现、X-射线检查等得出结论,确诊往往依赖于实验室诊断。由于儿童疾病起病急、转归快的特点,要求建立基于抗原的敏感、快速、实用的病原体检测方法,因此寻找制备方法简便、特异性高的针对性抗体具有重要意义。
普遍存在的表面蛋白(Ubiquitous surface protein A,UspA)又称为高分子量外膜蛋白,是Mc外膜蛋白中研究较成熟的蛋白。1994年,Helminen等[17]发现M. catarrhalis O35E菌株UspA蛋白表面暴露区存在单克隆抗体(MAb 17C7)识别位点。此后,该蛋白被广泛报道。1997年,Aebi等[18]发现UspA蛋白包括2种蛋白分子,uspa1编码蛋白的UspA1 (88 kDa)和uspa2编码的UspA2 (62 kDa)。两者均含MAb 17C7识别位点,分别存在相似性达93%的氨基酸序列片段(由140个氨基酸组成)。1998年,Aebi等[19]证明UspA1蛋白在Mc黏附到上皮细胞过程中起作用,而UspA2蛋白存在时宿主血清杀菌能力受到限制。Mcmichael等[20]报道,UspA1和UspA2为不同的毒力功能服务,但都有很好的免疫原性和反应原性。Chen等[21]报道抗UspA1抗体和抗UspA2抗体有交叉反应,两者都与免疫血清的杀菌活性有关。Lafontaine等[22]发现UspA1蛋白和由基因uspA2H编码的UspA2H蛋白共同调节Mc对人上皮细胞的黏附。Meier等[23]报道外膜蛋白UspA1和UspA2在幼儿咽炎分离株中高度保守。Hays等[24]认为UspA1和UspA2可作为疫苗候选抗原。Murphy等[25]认为UspA1和UspA2可作为抗体的靶标。由此可见,UspA1和UspA2具有良好的免疫原性和反应原性,可用于抗体的制备。
然而,该蛋白在卡他莫拉菌内表达量较少,提取天然蛋白后制备抗体存在困难。目前,国内外未见用UspA1重组蛋白制备多克隆抗体的报道。本研究通过对UspA1蛋白进行生物信息学分析,获取了胞外结构域中抗原表位最为丰富的肽段,并对其密码子进行原核优化后获得了经修饰的uspa1基因。通过全基因合成和分子克隆技术构建了重组质粒pET-28a-uspa1,经过诱导表达及纯化获得重组蛋白UspA1-His,进一步免疫新西兰大白兔制备了抗UspA1蛋白PcAb,为进一步建立卡他莫拉菌快速检测技术奠定了基础。
1 材料与方法 1.1 菌种、质粒及实验动物卡他莫拉菌ATCC 25238购自ATCC。克隆宿主菌E. coli Top 10、表达宿主菌E. coli Rosetta (DE3)、表达载体pET-28a(+)均为本实验室保存。新西兰大白兔由湖北省疾病预防控制中心提供。
1.2 主要试剂核酸分子量标准、蛋白质分子量标准、彩色预染蛋白分子量标准、限制性内切酶XhoⅠ和NdeⅠ、T4 DNA连接酶、质粒DNA小量试剂盒、胶回收试剂盒均购自TaKaRa公司。96孔板、羊抗兔IgG-HRP、TMB、硝酸纤维素膜、聚偏二氟乙烯膜(PVDF)购自武汉博士德生物工程有限公司。弗氏完全佐剂、弗氏不完全佐剂购自Sigma公司。羧基水溶性量子点(12-18 nm)购自武汉珈源量子点技术开发有限责任公司。组氨酸亲和预装柱、Protein A亲和预装柱为GE healthcare公司产品。
1.3 uspa1基因克隆与重组表达载体pET-28a-uspa1的构建对天然Mc表面蛋白UspA1 (GenBank accession number为AAF36416)进行生物信息学分析,获取其胞外保守结构域中抗原表位最为丰富的肽段(522-710 aa),找到其对应的DNA编码序列,引入大肠杆菌偏好性密码子,优化后在其5′引入酶切位点NdeⅠ、3′端引入终止信号TAA和酶切位点XhoⅠ后化学合成全基因序列(图 1),记为uspa1 (由南京金斯瑞生物科技有限公司合成后连于载体pUC57-Sample上)。将质粒pUC57-Sample-uspa1和载体pET-28a(+)双酶切回收后进行连接。将连接产物转入E. coli Top 10感受态细胞中,用卡那霉素抗性平板和酶切鉴定筛选阳性单克隆进行测序。选择测序未突变且读码框正确的重组原核表达载体pET-28a-uspa1。

|
| 图 1 uspa1核苷酸基因全序列 Figure 1 The whole genome sequence of uspa1. The underlined sequences are recognition sites of restriction endonuclease. |
| |
将重组载体pET-28a-uspa1和空载体pET-28a(+)分别转入到E. coli Rosetta (DE3) 表达菌中,挑取单菌落加入到100 mL含有硫酸卡那霉素的LB培养基中,220 r/min、37 ℃振荡培养12 h,取1 mL接入1 L含有硫酸卡那霉素的LB培养基中,220 r/min、37 ℃培养至OD600为0.6,加入IPTG至终浓度为1 mmol/L,37 ℃诱导表达4 h,离心收集菌体。菌体经超声裂解后,12 000 r/min离心15 min,收集上清。上清进行亲和层析纯化,得到重组蛋白。
1.5 抗UspA1-His融合蛋白PcAb的制备及纯化将上述纯化的重组UspA1-His融合蛋白稀释后,按照200 μg (1 mL)与1 mL弗氏完全佐剂混匀乳化后免疫2只雄性新西兰大白兔,于背部皮下多点注射。间隔7 d后再免疫1次,再过14 d后用上述纯化的重组UspA1-His融合蛋白按照200 μg (1 mL)与1 mL弗氏不完全佐剂混匀乳化后进行加强免疫,加强免疫7 d后,再按上述同样方法再加强免疫一次。7 d后取血以UspA1-His包被酶标板,间接ELISA法测定效价。若效价大于1×105,则心脏采血,分离血清,否则需再进行加强免疫。以A蛋白亲和层析预装柱纯化PcAb IgG,用A280测定抗体浓度,并用磷酸盐缓冲液调整为1 mg/mL,-20 ℃保藏备用。
1.6 抗UspA1-His融合蛋白PcAb的效价测定用间接ELISA方法测定抗UspA1-His融合蛋白PcAb效价。以4 μg/mL的UspA1-His包被酶标板(100 μL/孔),37 ℃恒温孵育1 h,经洗涤、封闭后,加入100 μL倍比稀释的抗UspA1-His融合蛋白PcA,初始浓度为10 μg/mL。以辣根过氧化物酶标记的羊抗兔IgG (HRP-IgG)为二抗进行测定。阴性参照以免疫前血清代替一抗。根据450 nm处吸光值(OD450)判断结果。判定方法,S/N=(待检OD450) / (阴性对照OD450)。S/N>2.1为阳性。以S/N>2.1所对应的抗体最高稀释倍数作为该抗体的效价。
1.7 抗UspA1-His融合蛋白PcAb鉴定 1.7.1 Western blotting方法鉴定利用Western blotting鉴定抗UspA1-His融合蛋白PcAb对天然Mc的识别能力。将Mc超声破胞上清及UspA1-His融合蛋白处理后进行SDS-PAGE分析。经转膜、干燥、封闭后,加入待检多抗,以HRP-IgG为二抗进行反应。清洗后,以超敏ECL化学发光即用型底物混合物完全覆盖印迹膜2-3 min,超灵敏多功能成像仪下获取胶片。
1.7.2 免疫荧光方法鉴定利用直接免疫荧光法,鉴定UspA1-His融合蛋白PcAb对Mc抗原的识别能力。首先用可溶性羧基量子点标记抗UspA1-His融合蛋白PcAb (按说明书操作),经1%琼脂糖凝胶电泳后紫外光下观察标记效果。再以该标记抗体作为特异性荧光抗体,将其滴加于固定有Mc菌液的载玻片上,用水洗去多余未参加反应的荧光抗体。干燥后用超分辨荧光显微镜观察Mc菌体与量子点标记抗体的结合情况。
1.7.3 间接ELISA方法鉴定利用间接ELISA法,测定UspA1-His融合蛋白PcAb对Mc抗原的识别能力。以Mc菌液(OD460=0.504)包被酶标板,封闭后加入100 ng/mL的待检抗体,以辣根过氧化物酶标记的羊抗兔IgG (HRP-IgG)为二抗进行测定。阴性参照以待检抗体缓冲液为一抗,阳性参照包被UspA1-His融合蛋白。根据450 nm处吸光值(OD450)判断结果。判定方法:S/N= (待检OD450) / (阴性对照OD450)。S/N>2.1为阳性。
2 结果与分析 2.1 uspa1基因克隆与重组表达载体pET-28a-uspa1的构建如图 2A中显示,通过双酶切方法,从质粒pUC57-Sample-uspa1中获得大小为570 bp的目的片段,重组质粒pET-28a-uspa1经NdeⅠ和XhoⅠ双酶切鉴定,出现大小与目的片段一致的条带(图 2B)。基因测序结果正确。
|
| 图 2 uspa1基因克隆与重组表达载体pET-28a-uspa1的构建 Figure 2 Cloning of uspa1 gene and construction of its recombinant expression vector pET-28a-uspa1. (A) Digestion of pET-28a and pUC57-Sample-uspa1 plasmid with restriction enzyme NdeⅠ and XhoⅠ. (B) Digestion of pET-28a-uspa1 plasmid with restriction enzyme NdeⅠand XhoⅠ. |
| |
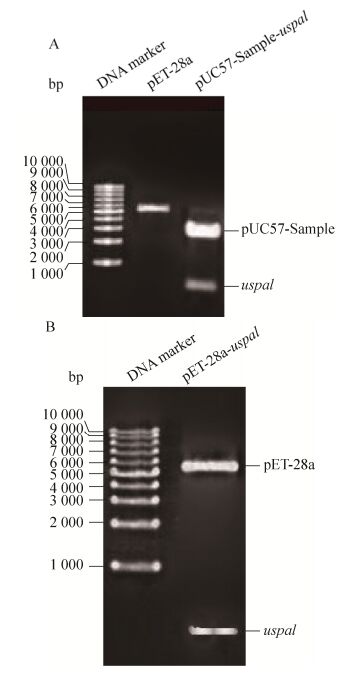
对含质粒pET-28a-uspa1的重组菌进行诱导表达,在24 kDa处诱导表达出目的蛋白(图 3A)。超声裂解后在上清和沉淀中均含有目的蛋白。取诱导表达上清用镍柱进行纯化,得到纯化的UspA1-His。以A280测定蛋白浓度为0.4 mg/mL (图 3B)。
|
| 图 3 UspA1-His蛋白的表达与纯化 Figure 3 Expression and purification of recombinant protein UspA1. (A) Expression of recombinant protein UspA1. M: protein marker; lane 1: UspA1-His before expression; lane 2: UspA1-His after expression; lane 3: supernatant after ultrasonication; lane 4: precipitation after ultrasonication. (B) Purification of recombinant protein UspA1-His. M: protein marker; lane 1: flow-through solution; lane 2: elution buffer with 10 mmol/L glyoxaline; lane 3: elution buffer with 20 mmol/L glyoxaline; lane 4: elution buffer with 40 mmol/L glyoxaline; 5: elution buffer with 100 mmol/L glyoxaline; lane 6: elution buffer with 150 mmol/L glyoxaline. |
| |
兔子经4次免疫后,以间接ELISA测定血清中抗体的效价可达到1:512 000以上,经A蛋白亲和层析纯化后最终可获得450 mg的特异性抗体。
2.4 间接ELISA测定抗UspA1-His融合蛋白PcAb的效价抗UspA1-His融合蛋白PcAb的效价测定结果(图 4)显示,当用倍比稀释法将待检抗体稀释512 000倍时,S/N=2.69>2.1,为阳性结果。若继续稀释,S/N将小于2.1,因此所得抗体的效价为1:512 000。

|
| 图 4 间接ELISA方法检测抗UspA1-His融合蛋白PcAb效价 Figure 4 The titer assay of the PcAb against recombinant protein UspA1-His. |
| |
Western blotting鉴定抗体结果见图 5,泳道1中出现条带,表明抗UspA1-His PcAb能够识别UspA1-His融合蛋白。泳道2中72-95 kDa之间出现的条带与UspA1 (88 kDa)分子量大小相符,表明抗UspA1-His融合蛋白PcAb能够识别Mc表面蛋白UspA1。

|
| 图 5 Western blotting鉴定抗UspA1-His融合蛋白PcAb Figure 5 Identification of the PcAb against recombinant protein UspA1-His by Western blotting. M: protein marker; lane 1: recombinant protein UspA1-His; lane 2: supernatant of Mc after ultrasonication. |
| |
用可溶性羧基量子点标记抗UspA1-His融合蛋白PcAb,1%琼脂糖凝胶电泳后紫外光下鉴定结果见图 6A。结果显示,标记了抗体的量子点比未标记抗体的量子点分子量大,说明抗体标记成功。量子点标记抗体与Mc菌体反应后,超分辨荧光显微镜下观察结果见图 6B。结果显示,视野中可观察到红色亮点,说明量子点标记PcAb能够识别Mc表面蛋白UspA1的胞外暴露区。

|
| 图 6 免疫荧光法鉴定 Figure 6 Identification by direct immunofluorescence. (A) Detection of the labelled PcAb. Lane 1: the unlabelled PcAb; lane 2: the labelled PcAb. (B) The labelled PcAb's recognition to Mc. |
| |
间接ELISA法鉴定抗体结果显示,S/N=46.701>2.1,结果为阳性,说明待检抗UspA1-His融合蛋白PcAb能有效识别Mc表面蛋白UspA1的胞外暴露区。该结果与2.5.2中免疫荧光鉴定结果一致。
3 讨论本研究对卡他莫拉菌UspA1蛋白进行生物信息学分析,获取了其胞外保守结构域中抗原表位最为丰富的肽段(522-710 aa),避免了多余肽段的无效表达,提高了重组抗原的有效性。密码子优化协调是进行蛋白质异源系统表达常用的手段。本文对uspa1基因进行原核密码子优化后,获得了可溶性表达的目的蛋白。重组表达的小分子蛋白免疫原性强,免疫后所得多克隆抗体量大、亲和力高。本文用800 μg表达的重组蛋白UspA1-His免疫新西兰兔,经4次免疫后得到总量450 mg的多克隆抗体,效价可达1:512 000。
Ackermann等[26]研究发现UspA1作为Oca家族成员,其保守功能域(742-832 aa)与YadA、EibA、Hia等多个蛋白的相关结构域在三维结构上高度相似,但氨基酸序列一致性很低(最高为10.99%),因此本文依据该保守结构域及其附近区域(522-710 aa)制备的多克隆抗体可保证其特异性。
本研究所制备的抗卡他莫拉菌表面蛋白UspA1胞外结构域的多克隆抗体不仅能识别UspA1-His重组蛋白,而且可识别Mc表面蛋白UspA1的胞外暴露区。该研究为进一步建立基于抗原的卡他莫拉菌快速检测方法奠定了基础。
目前,正在利用该表面暴露区制备相应的单克隆抗体。单克隆抗体制备完成后将与该多克隆抗体进行配对,制备基于量子点免疫层析的检测试剂盒。
| [1] | Arkwright JA. On the occurrence of the Micrococcus catarrhalis in normal and catarrhal noses and its differentiation from other gram-negative cocci. J Hyg, 1907, 7(1): 145–154. DOI: 10.1017/S0022172400033180 |
| [2] | Leinonen M, Luotonen J, Herva E, et al. Preliminary serologic evidence for a pathogenic role of Branhamella catarrhalis. J Infect Dis, 1981, 144(6): 570–574. DOI: 10.1093/infdis/144.6.570 |
| [3] | Wald ER, Milmoe GJ, Bowen A, et al. Acute maxillary sinusitis in children. N Engl J Med, 1981, 304(13): 749–754. DOI: 10.1056/NEJM198103263041302 |
| [4] | Nicotra B, Rivera M, Luman JI, et al. Branhamella catarrhalis as a lower respiratory tract pathogen in patients with chronic lung disease. Arch Intern Med, 1986, 146(5): 890–893. DOI: 10.1001/archinte.1986.00360170090015 |
| [5] | van Hare GF, Shurin PA, Marchant CD, et al. Acute otitis media caused by Branhamella catarrhalis: biology and therapy. Clin Infect Dis, 1987, 9(1): 16–27. DOI: 10.1093/clinids/9.1.16 |
| [6] | Wright PW, Wallace RJ Jr, Shepherd R. A descriptive study of 42 cases of Branhamella catarrhalis pneumonia. Am J Med, 1990, 88(5Suppl.1): S2–S8. |
| [7] | Shurin PA, Marchant CD, Kim CH, et al. Emergence of beta-lactamase-producing strains of Branhamella catarrhalis as important agents of acute otitis media. Pediatr Infect Dis, 1983, 2(1): 34–38. DOI: 10.1097/00006454-198301000-00009 |
| [8] | Wallace JR, Nash DR, Steingrube VA. Antibiotic susceptibilities and drug resistance in Moraxella(Branhamella) catarrhalis. Am J Med, 1990, 88(5 Suppl.1): S46–S50. |
| [9] | Kovatch AL, Wald ER, Michaels RH. β-lactamase-producing Branhamella catarrhalis causingotitis media in children. J Pediat, 1983, 102(2): 261–264. DOI: 10.1016/S0022-3476(83)80537-X |
| [10] | Zhanel GG, Palatnick L, Nichol KA, et al. Antimicrobial resistance in Haemophilus influenzae andMoraxella catarrhalis respiratory tract isolates: results of the Canadian Respiratory Organism Susceptibility Study, 1997 to 2002. Antimicrob Agents Chemother, 2003, 47(6): 1875–1881. DOI: 10.1128/AAC.47.6.1875-1881.2003 |
| [11] |
Luo R, Huang Y, Liu L, et al. Clinical features and drug resistance of Moraxella catarrhalis in children with lower respiratory infections in Chongqing.
Acta Acad Med Mil Tert, 2007, 29(18): 1818–1820.
(in Chinese). 罗蓉, 黄英, 刘岚, 等. 小儿下呼吸道感染中卡他莫拉菌的分离特点与耐药性分析. 第三军医大学学报, 2007, 29(18): 1818-1820. DOI:10.3321/j.issn:1000-5404.2007.18.023 |
| [12] | Abdullah FE, Ahuja KR, Kumar H. Prevalence and emerging resistance of Moraxella catarrhalis in lower respiratory tract infections in Karachi. J Pak Med Assoc, 2013, 63(11): 1342–1344. |
| [13] | Elrhman EMA, Ibrahim AH, Abdelhalim KA. Frequency of Moraxella catarrhalis from patients with lower respiratory tract infection in Khartoum State, Sudan. World Journal of Pharmaceutical Research, 2015, 4(5): 2286–2293. |
| [14] |
Liu J, Le CY, Wang XD, et al. Drug resistance of Moraxella catarrhalis causing lower respiratory tract infection in patients of traditional Chinese medicine hospital.
Chin J Nosocomiol, 2014, 24(22): 5506–5507.
(in Chinese). 刘军, 乐朝阳, 王炫德, 等. 中医院患者下呼吸道感染卡他莫拉菌耐药性分析. 中华医院感染学杂志, 2014, 24(22): 5506-5507. |
| [15] |
Huang LY, Li DM, Hu XH, et al. Distribution and drug resistance of fastidious bacteria of children with respiratory tract infection in Nanning Area.
J Pediat Pharm, 2016, 22(1): 46–48.
(in Chinese). 黄丽英, 李东明, 胡雪桦, 等. 南宁地区儿童呼吸道感染苛养菌分布及耐药性分析. 儿科药学杂志, 2016, 22(1): 46-48. |
| [16] | Doern GV, Brueggemann AB, Pierce G, et al. Prevalence of antimicrobial resistance among 723 outpatient clinical isolates of Moraxella catarrhalis in the United States in 1994 and 1995: results of a 30-center national surveillance study. Antimicrob Agents Chemother, 1996, 40(12): 2884–2886. |
| [17] | Helminen ME, Maciver I, Latimer JL, et al. A large, antigenically conserved protein on the surface of Moraxella catarrhalis is a target for protective antibodies. J Infect Dis, 1994, 170(4): 867–872. DOI: 10.1093/infdis/170.4.867 |
| [18] | Aebi C, Maciver I, Latimer J L, et al. A protective epitope of Moraxella catarrhalis is encoded by two different genes. Infect Immun, 1997, 65(11): 4367–4377. |
| [19] | Aebi C, Lafontaine ER, Cope LD, et al. Phenotypic effect of isogenic uspA1 and uspA2 mutations on Moraxella catarrhalis 035E. Infect Immun, 1998, 66(7): 3113–3119. |
| [20] | Mcmichael JC, Fiske MJ, Fredenburg RA, et al. Isolation and characterization of two proteins from Moraxella catarrhalis that bear a common epitope. Infect Immun, 1998, 66(9): 4374–4381. |
| [21] | Chen DX, Barniak V, van der Meid KR, et al. The levels and bactericidal capacity of antibodies directed against the UspA1 and UspA2 outer membrane proteins of Moraxella (Branhamella) catarrhalis in adults and children. Infect Immun, 1999, 67(3): 1310–1316. |
| [22] | Lafontaine ER, Cope LD, Aebi C, et al. The UspA1 protein and a second type of UspA2 protein mediate adherence of Moraxella catarrhalis to human epithelial cells in vitro. J Bacteriol, 2000, 182(5): 1364–1373. DOI: 10.1128/JB.182.5.1364-1373.2000 |
| [23] | Meier PS, Troller R, Grivea IN, et al. The outer membrane proteins UspA1 and UspA2 of Moraxella catarrhalis are highly conserved in nasopharyngeal isolates from young children. Vaccine, 2002, 20(13/14): 1754–1760. |
| [24] | Hays JP, van der Schee C, Loogman A, et al. Total genome polymorphism and low frequency of intra-genomic variation in the uspA1 and uspA2 genes of Moraxella catarrhalis in otitis prone and non-prone children up to 2 years of age: Consequences for vaccine design?. Vaccine, 2003, 21(11/12): 1118–1124. |
| [25] | Murphy TF, Brauer AL, Aebi C, et al. Identification of surface antigens of Moraxella catarrhalis as targets of human serum antibody responses in chronic obstructive pulmonary disease. Infect Immun, 2005, 73(6): 3471–3478. DOI: 10.1128/IAI.73.6.3471-3478.2005 |
| [26] | Ackermann N, Tiller M, Anding G, et al. Contribution of trimeric autotransporter C-terminal domains of oligomeric coiled-coil adhesin (Oca) family members YadA, UspA1, EibA, and Hia to translocation of the YadA passenger domain and virulence of Yersinia enterocolitica. J Bacteriol, 2008, 190(14): 5031–5043. DOI: 10.1128/JB.00161-08 |
 2018, Vol. 34
2018, Vol. 34