中国科学院微生物研究所、中国微生物学会主办
文章信息
- 叶双双, 周丽, 周哲敏
- Ye Shuangshuang, Zhou Li, Zhou Zhemin
- 定点突变提高苯丙氨酸羟化酶的热稳定性
- Thermal stability improvement for phenylalanine hydroxylase by site-directed mutagenesis
- 生物工程学报, 2016, 32(9): 1243-1254
- Chin J Biotech, 2016, 32(9): 1243-1254
- 10.13345/j.cjb.160022
-
文章历史
- Received: January 12, 2016
- Accepted: May 30, 2016
苯丙氨酸在人体中主要代谢途径是通过苯丙氨酸羟化酶(Phenylalanine hydroxylase,PAH,EC 1.14.16.1)的催化生成酪氨酸。若人体苯丙氨酸羟化酶存在缺陷,将导致血液中苯丙氨酸含量升高,最终形成苯丙酮尿症[1](Phenylketonuria,PKU)。目前,国际上苯丙酮尿症的临床治疗方法都是摄入低苯丙氨酸食品或人工合成营养食品[2]。这种治疗方式虽然能在一定程度上控制苯丙酮尿症,但是也会导致患者其他营养素缺乏[3-6]。如果能将苯丙氨酸羟化酶添加到食物中,直接催化苯丙氨酸生成酪氨酸,可有效控制血液中苯丙氨酸含量,同时不影响其他营养物质的摄入。
苯丙氨酸羟化酶是一种非血红素铁单加氧酶,广泛存在于动物、植物和微生物中,是苯丙氨酸代谢过程中的关键酶和限速酶。科研工作者对多种来源的苯丙氨酸羟化酶进行研究,发现与其他来源的苯丙氨酸羟化酶相比,紫色色杆菌Chromobacterium violaceum来源的苯丙氨酸羟化酶(CvPAH)具有结构简单[7]、与人类来源苯丙氨酸羟化酶催化区域几乎完全一致[8]、能够在人体温度和pH条件下催化苯丙氨酸羟化反应[7, 9]、催化活性及稳定性高[7]等特点。至2013年,Yew等[10]已通过红细胞封装的手段将CvPAH导入正常小鼠中,并降低小鼠血液中苯丙氨酸的水平,为其在功能食品领域的运用提供了参考。
本研究室在前期研究中,从紫色色杆菌中提取CvPAH基因,实现了在大肠杆菌中高效表达,并对其酶学性质进行了系统地研究[9]。结果表明该酶在37 ℃、pH 7.5时活性高于其他来源的苯丙氨酸羟化酶。然而该酶在高温条件下迅速失活,增加了其医药应用的成本。因此,提高CvPAH的热稳定性对其应用至关重要。
定向进化能够通过引入随机突变提高酶的热稳定性,但是该方法需要构建足够大的突变体库,因此往往消耗大量的人力、物力及财力[11-12];计算机模拟设计也能提高蛋白热稳定性,然而影响蛋白质稳定性的因素复杂,大大降低计算机预测的准确性[13];基于B-factor的进化策略能够降低酶局部的柔性达到降低酶的热稳定性的目的,但是该方法需要建立在获得晶体结构的基础上[12],限制了该方法的应用。影响蛋白质热稳定性的因素众多[14-20],蛋白质的氨基酸组成与其热稳定性关系密切。如果能够以蛋白质的一级结构为基础,建立提高酶热稳定性的方法,不仅避免了高级结构的限制,还能快速准确地获得突变体。
1979年,Argos等[21]对嗜热蛋白和嗜中温蛋白氨基酸组成进行了统计分析,发现相对于嗜中温蛋白,嗜热蛋白中氨基酸序列中有如下替换趋势:即甘氨酸(Glycine,Gly)替换为丙氨酸(Alanine,Ala),赖氨酸(Lysine,Lys)替换为精氨酸(Arginine,Arg)。Zhang等[22]在T4溶菌酶中介入了单个或多个Ala突变,有效提高该酶的热稳定性。此外,Mrabet等[23]利用将Lys突变成Arg的策略有效提高木聚糖异构酶的热稳定性。然而,尚未见将Ala和Arg突变策略组合的研究,以及应用上述策略提高PAH热稳定性的研究。
本实验选取CvPAH上所有的Gly及Lys,分别突变成Ala和Arg,构建突变体,筛选热稳定性提高的突变酶,进一步进行组合突变,并研究突变体的酶学性质。
1 材料与方法 1.1 材料 1.1.1 菌种与培养基菌种:大肠杆菌Escherichia coli JM109、E. coli BL21 (DE3)购自Novangen公司,E. coli BL21 (DE3)/pET24a-pah由本实验室构建并保存[9]。
LB培养基(g/L):胰蛋白胨10,酵母膏5,NaCl 10,琼脂粉20 (配制固体培养基)。
2YT培养基(g/L):胰蛋白胨16,酵母膏10,NaCl 10,琼脂粉20 (配制固体培养基)。
1.1.2 工具酶和生化试剂工具酶、DNA Marker和Protein Marker购自宝生物工程(大连)有限公司;质粒提取试剂盒购自天根生化科技有限公司;胶回收试剂盒和PCR产物纯化试剂盒购自东洋纺(上海)生物科技有限公司;引物由上海生物工程公司合成;酵母提取物、蛋白胨购自Oxford公司;IPTG、卡那霉素购自上海生物工程公司;其余试剂均为国产分析纯。
1.2 方法 1.2.1 突变位点选择及引物设计根据嗜热蛋白对Ala的偏好性明显高于Gly、对Arg的偏好性高于Lys的特点,选取CvPAH上所有Gly和Lys分别突变成Ala和Arg。根据GenBank提供的CvPAH基因序列(Gene ID:AF146711),以重组质粒pET24a-pah为模板,用软件Primer 5.0设计引物(表 1)。
| Primer name | Primer sequence (5′-3′) | Size (bp) |
| G19A up | CACCCGCAAGAATGTCGCCCTGAGCCACGAC | 31 |
| G19A down | TGGCGTCGTGGCTCAGGGCGACATTCTTGCG | 31 |
| G57A up | ATGCAAGCTGCTGCCCGCCCGCGCCTGCGAC | 31 |
| G57A down | ACTCGTCGCAGGCGCGGGCGGGCAGCAGCTT | 31 |
| G66A up | CGACGAGTTTCTGGAAGCCCTGGAGCGCCTG | 31 |
| G66A down | CTTCCAGGCGCTCCAGGGCTTCCAGAAACTC | 31 |
| G92A up | GCTGATGGCCGCCACCGCCTGGAAGATCGTC | 31 |
| G92A down | CCGCGACGATCTTCCAGGCGGTGGCGGCCATC | 32 |
| G100A up | GATCGTCGCGGTGCCGGCCCTGATTCCCGAC | 31 |
| G100A down | CGTCGTCGGGAATCAGGGCCGGCACCGCGAC | 31 |
| G142A up | GTTCCACGACCTGTTCGCCCACGTGCCGCTG | 31 |
| G142A down | TCAGCAGCGGCACGTGGGCGAACAGGTCGTG | 31 |
| G160A up | TTACCTGGAGGCCTACGCCAAGGGCGGGGTG | 31 |
| G160A down | CCTTCACCCCGCCCTTGGCGTAGGCCTCCAG | 31 |
| G162A up | GGAGGCCTACGGCAAGGCCGGGGTGAAGGCG | 31 |
| G162A down | CCTTCGCCTTCACCCCGGCCTTGCCGTAGGC | 31 |
| G163A up | AGGCCTACGGCAAGGGCGCCGTGAAGGCGAAG | 32 |
| G163A down | GCGCCTTCGCCTTCACGGCGCCCTTGCCGTAG | 32 |
| G170A up | GAAGGCGAAGGCGCTGGCCGCGCTGCCGATG | 31 |
| G170A down | CCAGCATCGGCAGCGCGGCCAGCGCCTTCGC | 31 |
| G186A up | GTACACGGTGGAATTCGCCCTGATCAATACTC | 32 |
| G186A down | CCGGAGTATTGATCAGGGCGAATTCCACCGTG | 32 |
| G193A up | GATCAATACTCCGGCCGCCATGCGCATCTAC | 31 |
| G193A down | CGCCGTAGATGCGCATGGCGGCCGGAGTATTG | 32 |
| G198A up | CGGCATGCGCATCTACGCCGCCGGCATCTTG | 31 |
| G198A down | TGGACAAGATGCCGGCGGCGTAGATGCGCATG | 32 |
| G200A up | GCGCATCTACGGCGCCGCCATCTTGTCCAGC | 31 |
| G200A down | ACTTGCTGGACAAGATGGCGGCGCCGTAGATG | 32 |
| G221A up | CAGCCCCAACCGCGTCGCCTTCGACCTGAT | 30 |
| G221A down | TGCGCATCAGGTCGAAGGCGACGCGGTTGGG | 31 |
| G272A up | CGACGCGCAACCGTGGGCCGCCGGCGACATC | 31 |
| G272A down | GCGCGATGTCGCCGGCGGCCCACGGTTGCGC | 31 |
| G274A up | GCAACCGTGGGGCGCCGCCGACATCGCGCCG | 31 |
| G274A down | CGTCCGGCGCGATGTCGGCGGCGCCCCACGG | 31 |
| G286A up | CCTGGTGCTGAATGCCGCCGACCGCCAAGG | 31 |
| G286A down | CCCATCCTTGGCGGTCGGCGGCATTCAGCAC | 31 |
| G290A up | TGCCGGCGACCGCCAAGCCTGGGCGGATACC | 31 |
| G290A down | CTTCGGTATCCGCCCAGGCTTGGCGGTCGC C | 31 |
| K16R up | CCGACATCACCACCCGCCGCAATGTCGGACTG | 32 |
| K16R down | GGCTCAGTCCGACATTGCGGCGGGTGGTGATG | 32 |
| K53R up | GTACCAGCGCCAATGCCGCCTGCTGCCCGGC | 31 |
| K53R down | CGCGGCCGGGCAGCAGGCGGCATTGGCGCTG | 31 |
| K82R up | GGTGCCGGACTTCAATCGCCTCAACGAGAAG | 31 |
| K82R down | TCAGCTTCTCGTTGAGGCGATTGAAGTCCGG | 31 |
| K86R up | CAATAAGCTCAACGAGCGCCTGATGGCCGCC | 31 |
| K86R down | CGGTGGCGGCCATCAGGCGCTCGTTGAGCTT | 31 |
| K94R up | GGCCGCCACCGGCTGGAGGATCGTCGCGGTG | 31 |
| K94R down | CCGGCACCGCGACGATCCTCCAGCCGGTGGC | 31 |
| K161R up | CCTGGAGGCCTACGGCCGCGGCGGGGTGAAG | 31 |
| K161R down | TCGCCTTCACCCCGCCGCGGCCGTAGGCCTC | 31 |
| K165R up | CGGCAAGGGCGGGGTGCGCGCGAAGGCGCTG | 31 |
| K165R down | CGCCCAGCGCCTTCGCGCGCACCCCGCCCTTG | 32 |
| K167R up | GGGCGGGGTGAAGGCGCGCGCGCTGGGCGCG | 31 |
| K205R up | CGGCATCTTGTCCAGCCGCTCGGAATCCATC | 31 |
| K205R down | AGTAGATGGATTCCGAGCGGCTGGACAAGATG | 32 |
| K239R up | GATCGACACCTTCCAGCGCACCTACTTCGTC | 31 |
| K239R down | CGATGACGAAGTAGGTGCGCTGGAAGGTGTC | 31 |
| K248R up | CGTCATCGACAGCTTCCGGCAGCTGTTCGAC | 31 |
| K248R down | TGGCGTCGAACAGCTGCCGGAAGCTGTCGATG | 32 |
| The mutation sites are underlined. | ||
PCR反应体系:10 μL 5×PrimeSATAR Buffer (5 mmol/L Mg2+ plus),4 μL浓度的dNTPs (2.5 mmol/L each),1 μL上游引物(10 mmol/L),1 μL下游引物(10 mmol/L),1 μL模板pET24a-pah,0.5 μL PrimeSTAR HS DNA Polymerase,超纯水补至50 μL。
PCR扩增条件:95 ℃预变性5 min;98 ℃变性1 min,58 ℃退火30 s,72 ℃延伸6.5 min,25个循环;72 ℃再延伸10 min。
用Dpn Ⅰ消化PCR模板(在37 ℃下反应1 h),再进行纯化,获得带有突变基因的质粒。将构建的质粒转化感受态细胞E. coli JM109,涂布含有卡那霉素(50 mg/L)的抗性平板,筛选获得阳性克隆,提取质粒转化感受态细胞E. coli BL21 (DE3)。
1.2.3 突变酶的表达与纯化从平板上挑取突变菌种的单菌落,分别接种至5 mL含有卡那霉素(50 mg/L)的LB培养基,37 ℃、200 r/min过夜培养。取1 mL培养液接种至100 mL含有卡那霉素(50 mg/L)的2YT培养基中,37 ℃、200 r/min培养至菌浓度达到OD600=0.6,加入终浓度0.6 mmol/L的IPTG,诱导表达24 h。
离心收集菌体,用适当体积20 mmol/L Tris-HCl (pH 7.5)重悬后用超声破碎仪破碎(工作3 s,停7 s),离心(12 000 ×g,4 ℃,30 min)取上清液,0.45 μm微孔滤膜过滤,使用HiTrap TM 1 mL DEAE FF于AKATA pure层析系统中纯化蛋白,纯化方法参见文献[9]。采用Bradford法测定蛋白质浓度[10]。
1.2.4 CvPAH酶活测定CvPAH酶液稀释至0.5 mg/mL,取50 μL加到250 μL 100 mmol/L HEPES (pH 7.5)缓冲液中,然后在该体系中加入50 μL浓度为10 mmol/L的L-苯丙氨酸(L-phe)、50 μL浓度为50 mmol/L的DTT、50 μL浓度为50 μmol/L FeSO4、50 μL浓度为2 mmol/L的DMPH4,37 ℃反应10 min,加入500 μL甲醇终止反应。15 000 ×g离心30 min,取上清,用0.22 μm微孔有机滤膜过滤,利用HPLC检测产物生成量,计算酶活[9]。CvPAH活性测定反应体系见表 2。
| Composition | Volume (μL) | Final concentration |
| HEPES | 250.0 | 50.0 mmol/L |
| FeSO4 | 50.0 | 5.0 μmol/L |
| DTT | 50.0 | 5.0 mmol/L |
| L-phe | 50.0 | 1.0 mmol/L |
| CvPAH | 50.0 | 0.05mg/mL |
| DMPH4 | 50.0 | 0.2 mmol/L |
最适反应温度:根据表 2的反应体系,将突变酶分别置于20 ℃、30 ℃、35 ℃、40 ℃、45 ℃、50 ℃、60 ℃条件反应10 min,测定残余酶活。
温度稳定性:突变酶在50 ℃水浴中热处理15 min、30 min、45 min、60 min,立刻冰浴30 min。根据表 2配制反应体系,迅速振荡混匀,置于37 ℃水浴锅中反应10 min,测定残余酶活。
1.2.6 突变酶Tm值测定用圆二色谱仪测定原始酶及突变酶Tm。温度范围为35-70 ℃,0.1 mg/L蛋白样溶于20 mmol/L Tris-HCl (pH 7.5),在222 nm的波长条件下测定。用软件Origin 8.0进行平滑处理。
1.2.7 突变酶动力学参数测定以不同浓度(0.2、0.4、0.6、0.8、1.0 mmol/L) L-phe为底物,在37 ℃、pH 7.5的条件下反应3 min,测定突变酶的初始反应速率。利用软件GraphPad Prism 5进行非线性拟合,计算动力学参数(Vmax、Km、kcat和kcat/Km)。
1.2.8 突变酶的结构分析取0.2 mg/mL蛋白溶解于20 mmol/L Tris-HCl缓冲液(pH 7.5)中,使用圆二色谱分析仪,扫描波长从190-250 nm,检测蛋白质的二级结构。
以CvPAH (PDB ID:1LTZ)为模板,利用SWISS-MODEL对突变酶进行同源建模。利用Pymol软件对蛋白质的三级结构进行分析。
2 结果与分析 2.1 突变位点保守性分析为了比较CvPAH中Gly和Lys的保守性,从动物和植物中分别选取人苯丙氨酸羟化酶(hPAH)和绿藻Chlamydomonas reinhardtii苯丙氨酸羟化酶(CrPAH),与CvPAH进行序列比对(图 1)。结果表明,CvPAH氨基酸序列中有19个Gly,其中有7个保守位点,分别是92、100、142、160、186、198和200位;有11个Lys,并且都不保守。

|
| 图 1 紫色色杆菌PAH与人和绿藻PAH氨基酸序列比对 Figure 1 Alignment of amino acid sequences for the PAH from C. violaceum, human and Chlamydomonas reinhardtii. Red boxs indicated conserved amino acids. Triangles indicated amino acids around the active center. |
| |
用粗酶液初步测定突变酶相对酶活和热稳定性。相对酶活(图 2中A、B)表明,Lys突变为Arg对酶活影响较小,除了K86R酶活降至原始酶的45.5%,其余酶的相对酶活均保持在50%以上。Gly突变成Ala对CvPAH活性影响较大,其中G100A、G198A和G200A的酶活分别降至原始酶的10.4%、24.4%和28.1%。根据晶体结构(数据未显示)分析可知G198和G200均处于活性中心周围,对酶的活性部位的微环境有较大的影响,而G100直接参与辅酶(6, 7-dimethyltetrahydropterin,DMPH4)的结合,突变后直接影响辅酶结合,降低酶活。
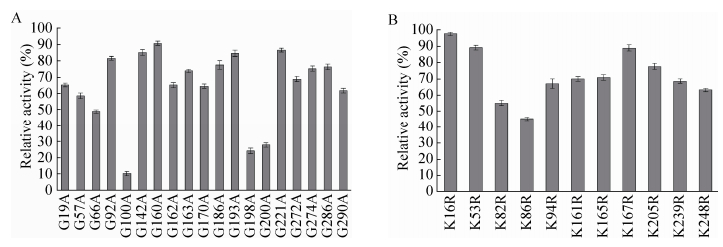
|
| 图 2 突变酶相对酶活 Figure 2 Relative activity of mutant enzymes. Relativity activity of mutant enzymes that mutated from Gly to Ala is show in figure A. Relativity activity of mutant enzymes that mutated from Lys to Arg is shown in figure B. The error bars show standard deviations calculated for three replicate experiments. |
| |
选取Gly突变成Ala后相对酶活保持在50%以上的突变酶和所有由Lys突变成Arg的突变酶初步测定热稳定性(在50 ℃保温,测定15 min、30 min时的酶活保留率)。图 3表明,大部分突变酶的热稳定性下降或影响较小,只有G221A、K94R热稳定性明显提高。因此,后续对G221A、K94R和组合突变酶K94R/G221A展开研究。
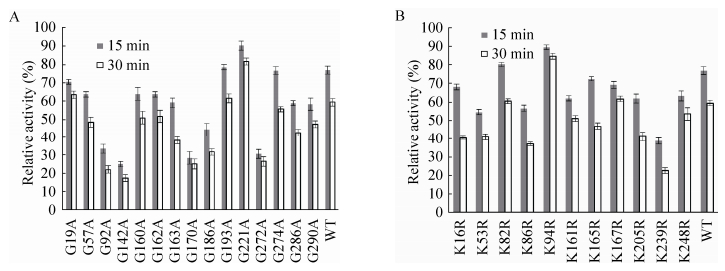
|
| 图 3 突变酶热稳定性 Figure 3 Thermal stability of mutant enzymes. Thermal stability of mutant enzymes that mutated from Gly to Ala is show in figure A. Thermal stability of mutant enzymes that mutated from Lys to Arg is shown in figure B. The error bars show standard deviations calculated for three replicate experiments. |
| |
对各突变位点在晶体结构中所处的位置进行比较分析,Gly分布为:19、57、92、100、170、186、193、198、221、272、274、286、290均位于该酶的柔性区,66、142、160、162、163、200均位于α-螺旋中,且19、92、170、193、221、272、274、286、290均位于酶分子表面。Lys分布为:所有点均分布在酶分子表面,且94、167、239位于柔性区,其余位点位于α-螺旋中。可见,蛋白表面氨基酸性质对酶的热稳定性影响较大,对分子表面的柔性区进行改造能有效提高突变位点选择的效率,大大降低工作量。
2.3 突变酶的酶学性质 2.3.1 突变酶纯化突变酶经过DEAE阴离子柱纯化,进行SDS-PAGE电泳检测(图 4),突变酶的分子量大小与原始酶一致,均在35 kDa左右。
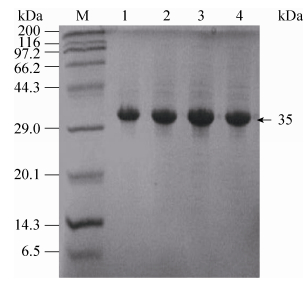
|
| 图 4 突变酶纯化结果 Figure 4 SDS-PAGE analysis of purified mutant enzymes. M: protein marker; 1: parent enzyme; 2: K94R; 3: G221A; 4: K94R/G221A. |
| |
突变酶的活性研究结果表明,K94R、G221A和K94R/G221A的比酶活分别为473.8、413.6和294.3 U/mg,与原始酶(562.7 U/mg)相比,分别下降了15.8%、26.5%和47.7%。最适反应温度结果(图 5)表明,突变酶和原始酶均在40 ℃左右达到最高酶活,随着温度升高或降低,酶活均会降低,其中K94R/G221A在温度降低过程中酶活降低速度最慢。
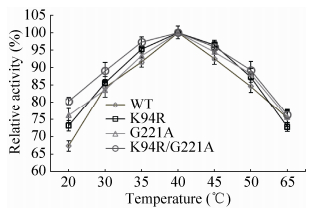
|
| 图 5 突变酶最适反应温度 Figure 5 Optimum temperature of the mutant enzymes. Enzymes reacted at different temperatures (20 ℃, 30 ℃, 35 ℃, 40 ℃, 45 ℃, 50 ℃, 60 ℃), and residual activities were determined. The error bars show standard deviations calculated for three replicate experiments. |
| |
热稳定性对比分析(图 6)显示,在50 ℃处理时,原始酶的酶活下降速度明显比突变酶快,尤其在前15 min,原始酶活性迅速丧失,最终只剩25.1%,而突变酶K94R、G221A和K94R/G221A酶活保留率分别达到65.5%、53.4%和80.2%。K94R和G221A在50 ℃的半衰期分别为26.2 min、16.8 min,比原始酶(9.0 min)分别提高了1.9倍、0.9倍,同时K94R/G221A在50 ℃处理1 h后仍保留65.6%的酶活,比原始酶(8.6%)高出6.6倍。

|
| 图 6 突变酶热稳定性比较 Figure 6 Comparison of thermostability of the mutant enzymes. Enzymes were heated at 50 ℃ for increasing times. Then residual activities were determined taking L-phe as substrate. The error bars show standard deviations calculated for three replicate experiments. |
| |
通过圆二色谱仪对突变酶K94R、G221A、K94R/G221A及原始酶进行Tm测定,结果如图 7所示,原始酶的Tm值约51.5 ℃,而突变酶K94R、G221A及K94R/G221A分别达到53.8 ℃、53.1 ℃、54.8 ℃。因此,相比较原始酶,突变酶具有更高的耐热性。
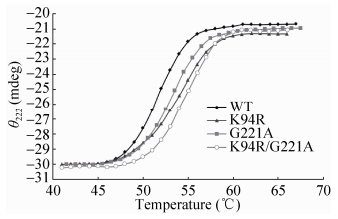
|
| 图 7 圆二色谱测定突变酶Tm值 Figure 7 The melting temperature of the mutant enzymes measured by CD spectroscopy. Temperature-induced unfolding profile of parent enzyme and mutant enzymes derived from data monitored by changes in ellipticity at 222 nm. |
| |
以不同浓度的L-phe为底物,测定突变酶的动力学参数。如表 3所示,与原始酶相比,突变后最大反应速率Vmax有所降低,其中K94R/G221A的Vmax最低,降到5.8 μmol/(L·s)。突变酶对底物的米氏常数Km均有不同程度下降,其中K94R对L-phe的Km最低,为119.3 μmol/L,可见突变酶对底物的结合能力均有所提高,同时kcat也有不同程度的下降。最终,催化效率(kcat/Km)仅略低于原始酶,即该突变对酶活的影响较小。结合稳定性研究结果可知,相比原酶,突变酶更具有应用价值。
| Enzymes | Vmax(μmol/(L·s)) | Km(μmol/L) | kcat(s-1) | kcat/Km(L/(s·mmol)) |
| WT | 10.2±0.2 | 163.1±1.5 | 5.2±0.1 | 319.6±3.2 |
| K94R | 6.4±0.1 | 119.3±1.2 | 2.8±0.1 | 230.5±2.7 |
| G221A | 7.6±0.3 | 130.4±1.3 | 3.2±0.2 | 247.7±4.3 |
| K94R/G221A | 5.8±0.1 | 128.9±1.3 | 2.7±0.1 | 211.8±3.5 |
二级结构分析结果如图 8。突变酶与原始酶α-螺旋特征波长208 nm和222 nm以及β-折叠特征波长195 nm和216 nm处的椭圆率没有显著差别。可见K94R、G221A两个位点突变对酶的二级结构几乎没有影响。
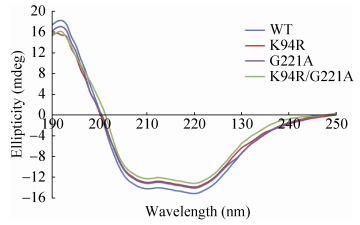
|
| 图 8 突变酶二级结构 Figure 8 Secondary structure of the mutant enzymes. The circular dichroism spectrum of the mutant enzymes was not dramatically different from that of parent enzyme. |
| |
同源建模发现,突变酶与原始酶的三级结构没有显著差别,因此对氨基酸之间成键情况进行分析。如图 9所示,94位氨基酸位于α-螺旋与β-折叠之间的柔性环上,由Lys突变成带有更大侧链基团的Arg,与Ile95形成额外的氢键,有助于稳定该环的结构,从而提高酶的稳定性;此外,该位点位于蛋白质表面区域,突变成Arg后胍基能够与水分子之间形成偶极-电荷作用,使蛋白表面形成水化膜,从而提高蛋白质热稳定性[24]。

|
| 图 9 位点94突变前(A)后(B)三维结构模拟 Figure 9 Simulated three-dimensional structure prior (A) and after (B) residue 94 mutation. The red dotted line is hydrogen bond. In the parent enzyme, Lys94 (A) forms hydrogen bonds with Arg114 and Phe116, respectively. Arg94 (B) forms an extract hydrogen bond with Ile95 in K94R. |
| |
如图 10所示,Gly221处于柔性区并且与酶分子的C-端柔性区接近,Gly没有侧链基团,构象熵大。突变成Ala后,增加了一个疏水性基团(甲基),拉近了与酶C-端Leu281的距离,与Leu281疏水侧链出现重叠区域(图 10),推测形成疏水作用[25-26],不仅增加了柔性环的稳定性,同时起到锚定C-端柔性区的作用,提高了突变酶的热稳定性。
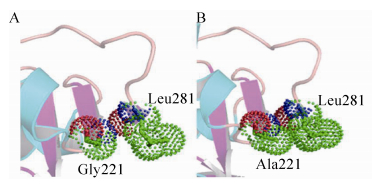
|
| 图 10 位点22突变前(A)后(B)三维结构模拟 Figure 10 Simulated three-dimensional structure prior (A) and after (B) residue 221 mutation. The green dashed ball is hydrophobic group. The Ala221 (B) has a bigger hydrophobic group, forming hydrophobic interaction with Leu281. |
| |
通过Gly替换成Ala以及Lys替换成Arg的策略得到2个突变酶K94R和G221A,其热稳定性较原始酶分别提高了0.9倍和1.9倍。进一步对两个位点进行组合突变,获得K94R/ G221A,其热稳定性较原始酶提高了6.6倍。圆二色谱数据进一步证明突变酶的具有更高的耐热性。动力学分析结果表明,突变酶对底物的亲和力提高了,同时催化效率仅略低于原始酶。最后,通过蛋白三级结构模拟发现,K94R和G221A分别通过形成氢键和增强局部疏水作用提高了酶的热稳定性。
| [1] | Flydal MI, Martinez A. Phenylalanine hydroxylase: function, structure, and regulation. IUBMB Life,2013, 65 (4) : 341 –349. DOI: 10.1002/iub.v65.4 |
| [2] | Williams RA, Mamotte CDS, Burnett JR. Phenylketonuria: an inborn error of phenylalanine metabolism. Clin Biochem Rev,2008, 29 (1) : 31 –41. |
| [3] | Przyrembel H, Bremer HJ. Nutrition, physical growth, and bone density in treated phenylketonuria. Eur J Pediatr,2000, 159 (2 Supl) : S129 –S135. |
| [4] | Chanoine JP. Selenium and thyroid function in infants, children and adolescents. Biofactors,2003, 19 (3/4) : 137 –143. |
| [5] | Beblo S, Reinhardt H, Demmelmair H, et al. Effect of fish oil supplementation on fatty acid status, coordination, and fine motor skills in children with phenylketonuria. J Pediatr,2007, 150 (5) : 479 –484. DOI: 10.1016/j.jpeds.2006.12.011 |
| [6] | Colomé C, Artuch R, Vilaseca MA, et al. Ubiquinone-10 content in lymphocytes of phenylketonuric patients. Clin Biochem,2002, 35 (1) : 81 –84. DOI: 10.1016/S0009-9120(02)00278-3 |
| [7] | Nakata H, Yamauchi T, Fujisawa H. Phenylalanine hydroxylase from Chromobacterium violaceum purification and characterization. J Biol Chem,1979, 254 (6) : 1829 –1833. |
| [8] | Onishi A, Liotta LJ, Benkovic SJ. Cloning and expression of Chromobacterium violaceum phenylalanine hydroxylase in Escherichia coli and comparison of amino acid sequence with mammalian aromatic amino acid hydroxylases. J Biol Chem,1991, 266 (28) : 18454 –18459. |
| [9] |
Cao SH, Zhou L, Cui WJ, et al. Heterologous expression and characterization of phenylalanine hydroxylase from Chromobacterium violaceum.
Biotechnol Bull,2014 (8) : 152 –158.
(in Chinese). 曹淑慧, 周丽, 崔文璟, 等. 紫色色杆菌苯丙氨酸羟化酶的异源表达及重组酶学性质研究. 生物技术通报, 2014 (8) : 152-158. |
| [10] | Yew NS, Dufour E, Przybylska M, et al. Erythrocytes encapsulated with phenylalanine hydroxylase exhibit improved pharmacokinetics and lowered plasma phenylalanine levels in normal mice. Mol Genet Metab,2013, 109 (4) : 339 –344. DOI: 10.1016/j.ymgme.2013.05.011 |
| [11] | Turner NJ. Directed evolution drives the next generation of biocatalysts. Nat Chem Biol,2009, 5 (8) : 56 –573. |
| [12] | Reetz MT, Carballeira JD, Vogel A. Iterative saturation mutagenesis on the basis of B factors as a strategy for increasing protein thermostability. Angew Chem Int Ed Engl,2006, 45 (46) : 7745 –7751. DOI: 10.1002/(ISSN)1521-3773 |
| [13] | Polizzi KM, Bommarius AS, Broering JM, et al. Stability of biocatalysts. Curr Opin Chem Biol,2007, 11 (2) : 220 –225. DOI: 10.1016/j.cbpa.2007.01.685 |
| [14] | Martin A, Kather I, Schmid FX. Origins of the high stability of an in vitro-selected cold-shock protein. J Mol Biol,2002, 318 (5) : 1341 –1349. DOI: 10.1016/S0022-2836(02)00243-7 |
| [15] | Bagautdinov B, Yutani K. Structure of indole-3-glycerol phosphate synthase from Thermus thermophilus HB8: implication for thermal stability. Acta Crystallogr D,2011, 67 (12) : 1054 –1064. DOI: 10.1107/S0907444911045264 |
| [16] | Chakravarty S, Varadarajan R. Elucidation of factors responsible for enhanced thermal stability of proteins: a structural genomics based study. Biochemistry,2002, 41 (25) : 8152 –8161. DOI: 10.1021/bi025523t |
| [17] | Delboni LF, Mande SC, Rentier-Delrue F, et al. Crystal structure of recombinant triosephosphate isomerase from Bacillus stearothermophilus: an analysis of potential thermostability factors in 6 isomerases with known 3-dimensional structures points to the importance of hydrophobic interactions. Protein Sci,1995, 4 (12) : 2594 –2604. DOI: 10.1002/pro.v4:12 |
| [18] | Vogt G, Woell S, Argos P. Protein thermal stability, hydrogen bonds, and ion pairs. J Mol Biol,1997, 269 (4) : 631 –643. DOI: 10.1006/jmbi.1997.1042 |
| [19] | Ma YF, Eglinton JK, Evans DE, et al. Removal of the four C-terminal glycine-rich repeats enhances the thermostability and substrate binding affinity of barley β-amylase. Biochemistry,2000, 39 (44) : 13350 –13355. DOI: 10.1021/bi000688s |
| [20] | Mahanta P, Bhardwaj A, Kumar K, et al. Structural insights into N-terminal to C-terminal interactions and implications for thermostability of a (β/α)8-triosephosphate isomerase barrel enzyme. FEBS J,2015, 282 (18) : 3543 –3555. DOI: 10.1111/febs.2015.282.issue-18 |
| [21] | Argos P, Rossman MG, Grau UM, et al. Thermal stability and protein structure. Biochemistry,1979, 18 (25) : 5698 –5703. DOI: 10.1021/bi00592a028 |
| [22] | Zhang XJ, Baase WA, Matthews BW. Multiple alanine replacements within α-helix 126-134 of T4 lysozyme have independent, additive effects on both structure and stability. Protein Sci,1992, 1 (6) : 761 –776. DOI: 10.1002/pro.v1:6 |
| [23] | Mrabet NT, Van den Broeck A, Van den Brande I, et al. Arginine residues as stabilizing elements in proteins. Biochemistry,1992, 31 (8) : 2239 –2253. DOI: 10.1021/bi00123a005 |
| [24] | Rahman RNZA, Fujiwara S, Nakamura H, et al. Ion pairs involved in maintaining a thermostable structure of glutamate dehydrogenase from a hyperthermophilic archaeon. Biochem Bioph Res Co,1998, 248 (3) : 920 –926. DOI: 10.1006/bbrc.1998.8933 |
| [25] | Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem,1976, 72 (1/2) : 248 –254. |
| [26] | Martin A, Sieber V, Schmid FX. In-vitro selection of highly stabilized protein variants with optimized surface. J Mol Biol,2001, 309 (3) : 717 –726. DOI: 10.1006/jmbi.2001.4698 |
 2016, Vol. 32
2016, Vol. 32




