服务
文章信息
- 梁康, 赵新新, 易洁, 刘琼, 刘青, 孔庆科
- Kang Liang, Xinxin Zhao, Jie Yi, Qiong Liu, Qing Liu, Qingke Kong
- 应用遗传改造的沙门菌介导肿瘤治疗的研究进展
- Advances in tumor-therapy using genetically modified Salmonella
- 生物工程学报, 2016, 32(5): 565-576
- Chin J Biotech, 2016, 32(5): 565-576
- 10.13345/j.cjb.150393
-
文章历史
- Received: September 12, 2015
- Accepted: November 24, 2015
2 四川农业大学 动物医学院 生物工程系,四川 成都 611130
2 Department of Bioengineering, College of Veterinary Medicine, Sichuan Agricultural University, Chengdu 611130,Sichuan, China
常规的肿瘤治疗方法具有明显的局限性,如对正常细胞有毒性、肿瘤靶向性低、无法渗透到肿瘤组织内部发挥作用等,而只能对肿瘤造成不完全的破坏。因此有必要寻找更有效的肿瘤治疗手段。早期研究发现某些厌氧菌或兼性厌氧菌也可以介导抗肿瘤作用,例如梭状芽胞杆菌、双歧杆菌、大肠杆菌和沙门菌中的某些菌种。尤其鼠伤寒沙门氏菌,该菌是革兰氏阴性、胞内侵袭、兼性厌氧菌,应用于肿瘤治疗具有许多潜在的优势:1) 能选择性富集于肿瘤组织及抑制肿瘤生长,定殖肿瘤和正常组织的比值超过1 000∶1[1],且该菌具有运动能力,可以渗透到肿瘤组织深部;2) 可以侵袭多种肿瘤,如黑色素瘤、乳腺肿瘤、胰腺肿瘤、结肠肿瘤和前列腺肿瘤等[2-8],而且由于在有氧和厌氧条件下均能生长,该菌既能定殖于大的肿瘤又能定殖于小的转移性肿瘤[9-10];3) 遗传操作方便,经适当减毒的鼠伤寒沙门氏菌可携带外源基因或递呈肿瘤治疗分子用于肿瘤治疗;4) 毒力充分减弱的沙门菌由于对机体免疫系统的躲避、抵抗能力下降,最终能被机体清除出去;5) 易于生产,亦便于储存和使用。在过去的一二十年里,对于沙门菌尤其鼠伤寒沙门氏菌介导肿瘤治疗的研究越来越深入,相关研究已经进行了大量的动物试验和少数小规模的人体临床试验[11-12]。
1 沙门菌的抗肿瘤机制沙门菌可以选择性富集于肿瘤,这很可能与肿瘤不同于正常组织的微环境以及细菌自身的特性有关。目前主要有3种机制用来解释沙门菌的肿瘤靶向性:1) 趋化作用。具有运动能力的沙门菌可以通过肿瘤微环境中分泌的某些化学物质趋化至肿瘤;且目前而言,有运动能力的细菌是用来治疗肿瘤所有区域唯一可能的手段。研究发现,天冬氨酸受体控制鼠伤寒沙门氏菌向肿瘤的迁移,丝氨酸受体起始细菌向肿瘤内部的渗透作用,核糖/半乳糖受体介导细菌进入肿瘤坏死区域富集[13]。野生型鼠伤寒沙门氏菌更偏爱肿瘤坏死区域的死亡肿瘤细胞;而敲除核糖/半乳糖受体的编码基因会导致细菌富集在肿瘤静止区并诱导肿瘤细胞死亡[14]。由此可见,通过遗传操作控制特定化学受体的表达可以使具有运动能力的沙门菌靶向肿瘤的任何区域。2) 选择性生长。研究表明,野生型沙门菌更偏爱生长在发生坏死的组织,而肿瘤细胞增殖过快导致肿瘤内部产生坏死区和低氧区,使肿瘤有别于细胞生长良好的正常组织。此外,与正常组织相比,肿瘤内营养丰富,富含嘌呤、氨基酸和各种生长因子等。因此通过遗传操作改造沙门菌构建特定的营养缺陷株,可以让沙门菌选择性地迁移至富含所需营养的肿瘤区域生长,而几乎不在正常组织中生长[15-16]。3) 肿瘤内有限的免疫清除。细菌的毒力抑制和清除是由机体多种防御机制介导的,包括粒细胞和巨噬细胞介导的吞噬作用,抗菌肽、抗体和补体系统的作用等,而在肿瘤内对于细菌的免疫清除作用是有限的。在肿瘤组织中,很少可以观察到粒细胞[17],这可能是由于肿瘤细胞或基质细胞分泌的TGF-β抑制了粒细胞的浸润;研究发现,多种肿瘤细胞的表面都存在补体激活的抑制剂[18];由于肿瘤内不规则的血管结构和血压,抗体和补体成分向肿瘤组织内的渗透存在生理屏障[19]。肿瘤微环境中有限的免疫清除条件很有利于沙门菌的生存和增殖。此外,沙门菌也可能通过其侵袭的宿主细胞 (如巨噬细胞) 被递送至肿瘤。而且研究表明,不管沙门菌是如何到达肿瘤的,只需要少量的沙门菌就能引起其在肿瘤组织内的广泛侵染。
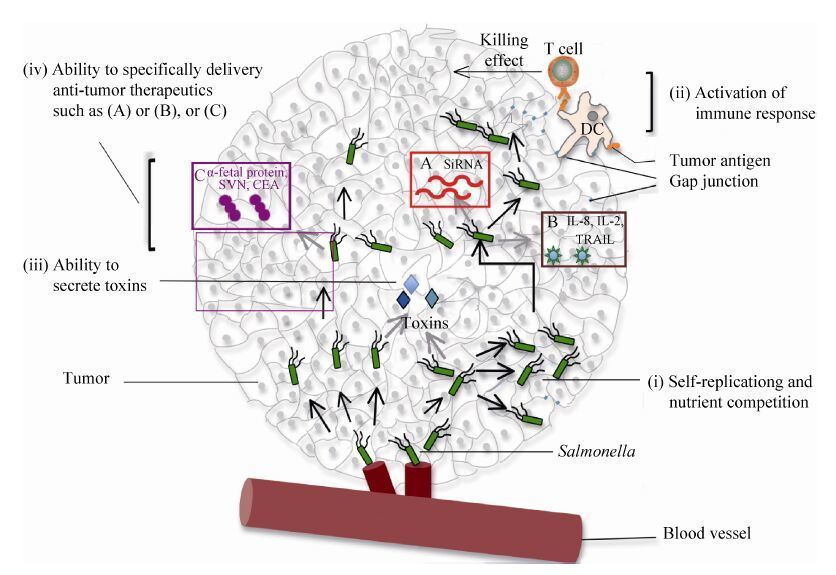
沙门菌可以通过多种途径抑制肿瘤 (图 1)。1) 定殖肿瘤的沙门菌可以与肿瘤细胞竞争营养物质。2) 沙门菌可以分泌毒素至胞外,或者通过Ⅲ型分泌系统 (type Ⅲ secretion system) 将毒力蛋白直接注入肿瘤细胞胞质[20]而对肿瘤造成杀伤。Ⅲ型分泌系统是许多革兰氏阴性致病菌都具有的、由多组分蛋白构成的蛋白分泌系统。3) 沙门菌可以从肿瘤边缘部位向中心迁移,并通过激活Caspase和下调AKT/mTOR/p70S6K信号通路等途径诱导肿瘤细胞凋亡[21]。4) 沙门菌抑制肿瘤还依赖于其对机体免疫反应的诱导[22]。研究表明,沙门菌可通过TLR4 (toll-like receptor-4) 信号通路诱导机体的先天性免疫应答[23],从而产生抗肿瘤效应。沙门菌可明显上调干扰素-γ (IFN-γ) 及其诱导的某些趋化因子 (如单核因子、干扰素诱导蛋白-10) 在肿瘤的表达,这些趋化因子可以将外围活化的效应细胞征集至肿瘤,比如NK细胞和T细胞[24]。沙门菌可以促使肿瘤细胞与树突状细胞 (DCs) 之间形成连接,然后DCs递呈从肿瘤细胞获得的多肽,进而刺激T细胞识别、杀灭肿瘤细胞[25]。由此可以看出,沙门菌可以通过征集大量效应细胞在肿瘤浸润以及诱导肿瘤抗原的交叉递呈,发挥对肿瘤细胞间接的毒性作用。5) 诸多研究表明,经遗传改造的沙门菌可向肿瘤靶向递呈某些治疗分子从而发挥相应的抗肿瘤作用。
|
| 图1 用于肿瘤治疗的沙门菌抗肿瘤的主要机制[26] Figure1 Schematic representation of the major anti-tumor mechanisms of Salmonella as a cancer therapeutic agent[26]. Salmonella can inhibit tumor through many ways like (i) self-replicating and nutrient competition; (ii) activation of immune response by inducing gap junctions and cross-presentation of tumor antigen; (iii) ability to secrete toxins that can induce the apoptosis of tumor cells; (iv) ability to be genetically engineered to specifically delivery. (a) siRNA (red box),or (b) cytokines (brown box),or (c) tumor-associated antigens,or antibodies (purple box). |
| |
与应用沙门菌防治其他疾病一样,用于肿瘤治疗的沙门菌也必须经过适当的毒力弱化。通过随机的转座子突变或者在基因组删除某个或某些特定基因等遗传操作可使沙门菌减弱毒力,同时保持甚至提高其定殖肿瘤的靶向性,从而介导更有效的抗肿瘤效应。
Low等[27]构建了鼠伤寒沙门氏菌突变株VNP20009,purⅠ基因的缺失使该菌株不能合成腺嘌呤,所需的腺嘌呤只能从外源获取,而肿瘤细胞中大量存在腺嘌呤;msbB基因的缺失则使细菌lipid A结构发生一定改变,从而降低菌株毒力,使其全身给药时诱发机体败血性休克的可能性降低。动物试验发现,VNP20009毒力减弱,半数致死量与野生株相比增加了10 000倍左右,诱导肿瘤坏死因子-α(TNF-α) 的能力大大降低;能作用于多种小鼠和人类肿瘤,定殖肿瘤和正常组织 (肝脏) 的能力相差250至25 000倍。基于动物肿瘤模型上观察到的良好抗肿瘤效果及安全性,VNP20009随后被应用于人体临床试验。24位转移性黑色素瘤患者和1位转移性肾细胞癌患者接受VNP20009静脉注射治疗,虽然VNP20009可以安全地用于肿瘤患者,但只有3例观察到菌株在肿瘤定殖,而所有患者都没有呈现理想的肿瘤治疗效果[12]。动物模型和肿瘤患者可能在细菌进入肿瘤的途径、细菌在肿瘤中的生长,以及细菌从肿瘤和外周血液循环的清除等方面存在差异,从而导致VNP20009在动物试验和人体临床试验中呈现不同的肿瘤治疗效果。例如,在一期临床试验中,VNP20009从外周血液被清除的速度非常快,比动物试验中的清除速度要快很多;而过快地从外周血液被清除阻碍了足量的VNP20009向肿瘤定殖。
沙门菌氨基酸营养缺陷株也能选择性定殖和杀伤肿瘤。Zhao等通过亚硝基胍 (NTG) 化学诱变及筛选所构建的鼠伤寒沙门氏菌突变株A1丧失了合成亮氨酸和精氨酸的能力,在动物模型可以选择性定殖于肿瘤[16];将A1感染其他荷瘤小鼠后再提取出来,菌株的肿瘤靶向性提高,命名为A1-R。A1-R经静脉注射可使裸鼠原位移植的人乳腺肿瘤[8]、人胰腺肿瘤[4]和前列腺肿瘤[7]退化并延长小鼠生存时间;A1-R对一些转移性肿瘤[9-10, 28]同样有效。
已用于抗肿瘤研究的减毒沙门菌所突变的基因及其功能见表 1。
| Mutated genes | Functions | References |
| msb B/ PurⅠ | msb B,modifying lipid-A; PurⅠ,regulating adenine synthesis | [3, 5, 11-12, 27, 30, 32, 34-35, 38-40, 51-52, 54-55] |
| phoP/phoQ | Regulating acid phosphatase synthesis | [31, 44, 49, 52] |
| relA | Encoding ppGpp synthetase | [33] |
| htrA | Encoding Heat Shock Protein | [37] |
| aroA | Key gene of aromatic amino acids synthesis | [36, 43, 45-47, 50, 55] |
| cya/crp | Encoding cAMP (cyclic adenosine monophosphate) synthetase/ cAMP receptor protein | [29] |
| asd | Key gene of DAP (diaminopimelic acid) synthesis | [2, 29] |
为获得更好的肿瘤治疗效果,沙门菌结合其他方式的联合治疗也处于不停的尝试和研究之中。过去的十多年,研究者不断尝试以减毒沙门菌作为载体向肿瘤靶向递呈各种治疗分子,即利用减毒沙门菌将细胞因子、细胞毒性剂、肿瘤相关抗原或者前药转化酶等肿瘤治疗分子的编码DNA递呈至肿瘤细胞进行表达 (真核表达系统),或者通过沙门菌直接表达肿瘤治疗分子(原核表达系统)。所期望的是,这些外源DNA表达后可以在肿瘤内产生最大的抗肿瘤作用,如细胞毒性和破坏血管的效应等,而浓度降低1 000倍时对正常的组织器官基本没有毒性;并且表达的肿瘤治疗分子或者前药转化酶激活的药物最好可以在不影响沙门菌生存增殖的基础上与沙门菌协同发挥抗肿瘤作用。相关研究已经开展了大量的动物试验,并取得不同程度的成效。
细胞因子可以通过促进免疫细胞的活化、增殖和迁移而诱导对肿瘤细胞的杀伤。由于将某些细胞因子作为化疗药物直接使用会产生较严重的系统毒性,研究者尝试利用沙门菌向肿瘤靶向递呈各种细胞因子。Sorenson等[29]应用缺失cya/crp/asd基因的减毒沙门菌向肿瘤递呈IL-2,发现重组菌株经口服可以有效引起局部和系统性NK细胞增殖并抑制小鼠骨肉瘤的肺部转移。Loeffler等[30]将可表达IL-18的减毒沙门菌静脉注射处理荷瘤小鼠,发现大量白细胞渗透入肿瘤,NK细胞、T细胞浸润以及多种细胞因子的表达增加,小鼠皮下肿瘤及转移性肺癌被明显抑制。用表达LIGHT或者CCL21的减毒沙门菌处理荷瘤小鼠,可以诱导白细胞在肿瘤浸润并抑制肿瘤生长[31-32]。
应用沙门菌递呈可以发挥细胞毒性剂对肿瘤细胞的直接杀伤作用,同时减少细胞毒性剂对机体的毒性。细菌毒素是最常见的细胞毒性剂,例如,细胞溶素A (ClyA) 可以使哺乳动物细胞的胞膜产生孔径从而诱导凋亡。研究表明用表达ClyA的沙门菌处理荷瘤小鼠可以抑制肿瘤生长[33]。FasL (Fas ligand)、TRAIL (TNF-related apoptosis-inducing ligand) 均是属于肿瘤坏死因子-α家族的细胞毒性剂,两者可以通过死亡受体途径诱导细胞凋亡并能选择性对肿瘤细胞而非正常细胞发挥细胞毒性作用,但是经全身给药,两者具有明显的肝细胞毒性和较短的循环半衰期。而通过沙门菌递呈FasL、TRAIL可以克服这些缺陷,并且使肿瘤内药物浓度维持在较高的水平[34-35]。CD40L对恶变的B细胞而非正常免疫细胞有生长抑制作用。研究发现,应用沙门菌递呈CD40L可使小鼠产生针对CD40+ B细胞淋巴癌的显著保护力[36]。
通过沙门菌递呈肿瘤相关抗原可以致敏免疫系统,从而产生抗肿瘤效应,而且针对表达相应抗原的肿瘤的保护力可以由机体免疫系统保留下来。生存素 (SVN) 是基本所有实体瘤都过量表达而正常组织几乎不表达的一种理想肿瘤相关抗原。Manuel等[37]发现,可过量表达SVN的减毒鼠伤寒沙门氏菌经口服能够抑制小鼠黑色素瘤的生长,此基础上再应用减毒沙门菌递呈STAT3-siRNA (STAT3,signal transducers and activators of transcription;siRNA,small interfering RNA) 则产生更强的抗肿瘤效果。Bereta等[38]构建了VNP20009衍生菌株,该菌株能在其表面表达癌胚抗原 (CEA) 特异性的抗体因而肿瘤靶向性更高。
如果某些抗肿瘤药物对机体系统毒性较大,可以考虑沙门菌递呈前药转化酶结合使用前药的途径。细胞毒性抗代谢药5-FU (5-fluorouracil) 能干扰静止细胞和增殖细胞的核酸合成从而可以用于抗肿瘤,但5-FU具有很大的系统毒性;其前药5-FC (5-fluorocytosine) 对人、小鼠却是无毒的,因为哺乳动物细胞不能合成可将5-FC转化为5-FU的转化酶CDase。King等[39]先用可表达CDase的减毒鼠伤寒沙门氏菌TAPET-CD静脉注射处理荷瘤小鼠,几天后开始使用前药5-FC,发现在近20 d的联合治疗期间,小鼠肿瘤体积几乎没有变大,对小鼠造成的毒性作用也较弱。随后,TAPET-CD被应用于人体临床试验,共有3位患者接受治疗,其中两例发现有菌株在肿瘤定殖并表达CDase,这两例的肿瘤-血清5-FU比值大约是3,表明菌株定殖的靶向性并不高[11]。近年来,有研究者构建了能更有效表达CDase并对5-FU耐受的菌株,从而提高其对肿瘤细胞的毒性作用[40]。
相比沙门菌直接表达肿瘤治疗分子,利用沙门菌向肿瘤细胞递呈肿瘤治疗分子编码基因的方法有优点,同时也有缺点。借助哺乳动物表达系统可以产生对递呈基因更强、更稳定的表达;但是递呈基因的表达更难以控制,表达量也会因递呈效率不高而受限,基因还可能被转移到非目的组织。早期有研究者应用减毒沙门菌递呈抗血管生成因子的编码基因,如内皮抑素 (Endostatin)[41]和血栓粘合素 (Thrombospondin)[42],两者可通过抑制血管形成和切断营养供给而杀伤肿瘤。Li等[43]将分别递呈白介素-12 (IL-12) 和粒细胞-巨噬细胞集落刺激因子 (GM-CSF) 基因的两株重组减毒沙门菌口服应用于荷瘤小鼠,发现两者均可延长小鼠生存时间;但两者合用的抗肿瘤效果不及单独递呈GM-CSF基因,IL-12和GM-CSF在抗肿瘤方面似乎并不发挥协同作用。血管生成是肿瘤生长演变的一个关键,内皮抑素作为血管生成抑制因子,被证明能够抑制肿瘤生长。Li等[44]用减毒沙门菌递呈内皮抑素和STAT3干扰RNA的共表达质粒,将其应用于前列腺肿瘤小鼠模型,发现有明显的肿瘤抑制效果。VEGFR2 (flk-1) 在肿瘤内皮细胞增殖和血管形成中扮演着重要的角色;有研究发现,通过减毒沙门菌递呈此肿瘤抗原的编码DNA可以有效抑制小鼠肺癌[45]和恶性胶质瘤[46]生长。甲胎蛋白 (α-Fetoprotein) 也是一种肿瘤相关抗原,递呈甲胎蛋白编码基因的减毒沙门菌经口服可以抑制肝细胞癌和结肠癌[47]。RBM5 (RNA-binding motif protein 5) 被认为是一种肿瘤抑制基因,Shao等[48]将携带真核表达质粒pcDNA3.1-RBM5的减毒沙门菌应用于肺腺癌裸鼠模型,发现RBM5过量表达,肿瘤细胞被诱导大量凋亡。
RNA干扰技术作为一种下调基因表达的工具已被广泛应用于基因功能的研究以及疾病治疗等,其中化学合成的siRNA (Small interfering RNA) 可介导更有效、更安全的基因沉默因此受到不少关注。而应用减毒沙门菌递呈特定siRNA介导肿瘤治疗的研究也取得一些进展。例如,STAT3[49]、Bcl 2 (B-cell lymphoma 2)[50]和IDO (Indoleamine-pyrrole 2,3-dioxygenase,a tryptophan-catabolizing enzyme)[51]等可促进肿瘤发生发展,通常在肿瘤内过度表达,于是有研究者设计了相应siRNA的表达序列然后应用沙门菌递呈,并在小鼠实验中观察到显著的肿瘤生长抑制。Cheng等[52]将VNP20009基因组phoP/phoQ编码基因删除,实验表明,以此构建的新菌株能有效递呈siRNA表达质粒。
已用于抗肿瘤研究的重组沙门菌所递呈的肿瘤治疗分子及其功能见表 2。
| Tumor therapeutic agents | Functions | References |
| Prokaryotic expression systems | ||
| Cytokines | ||
| IL-2 | Related to the activation of T cells and NK cells | [29] |
| IL-18 | Inducing production of IFN-γ; stimulating the proliferation of T cells and NK cells and their secretion of cytokines | [30] |
| LIGHT | Growth factor of DCs; inducing the expression of chemokines after binding to its receptor | [31] |
| CCL21 | As one chemokine,controlling the migration of T cells,DCs and NK cells | [32] |
| Cytotoxic agents | ||
| Cytolysin | Cytotoxic protein | [33, 55] |
| TRAIL | Inducing the apoptosis of tumor cells through death receptor pathway | [34] |
| FasL | Inducing the apoptosis of Fas-expressing cells after binding to its receptor | [35] |
| CD40L | Suppressing malignant B cells; promoting the proliferation and differentiation of B cells after binding to CD40 | [36] |
| 5-FU | Disturbing the synthesis of DNA and RNA after being converted to another form | [11, 39-40] |
| Antigens/antibodies | ||
| SVN | Inhibitor of apoptosis proteins; expressed by almost all solid tumors | [37] |
| CEA-scFv | Single-chain antibody fragment of CEA | [38] |
| Eukaryotic expression systems | ||
| Cytokines | ||
| IL-12 | Stimulating the proliferation of T cells and NK cells and their secretion of INF-γ | [43] |
| GM-CSF | Activating granulocytes and macrophages | [43] |
| Endostatin | Selectively inhibiting the proliferation and migration of vascular endothelial cells | [41, 44] |
| Thrombospondin | Inhibiting angiogenesis | [42] |
| Tumor-associated antigens | ||
| VEGFR2 (flk-1) | Only expressed in vascular endothelial cells and related to the differentiation of endothelial cells and angiogenesis | [45, 46] |
| α-Fetoprotein | The specific antigen of hepatic cells carcinoma | [47] |
| siRNA | ||
| STAT3-siRNA | Stat3,chronic activation of which is related to the abnormal cell proliferation and malignant transformation | [37, 44, 49] |
| Bcl2-siRNA | Bcl2,an anti-apoptosis protein; expressed in >90% of all malignant melanomas | [50] |
| IDO-siRNA | IDO,acts as one potent suppressor of adaptive immunity | [51] |
在过去的十几年里,对沙门菌抗肿瘤的研究已经向前跨越了一大步。相关研究已在许多动物试验中取得不少成功,证明了沙门菌在肿瘤治疗方面的潜力,但其真正应用于临床还需要解决几个基本的问题。安全性是首先需要考虑的问题。如果未充分减毒,经全身给药的沙门菌可能在血液中大量繁殖同时发挥其内毒素致病作用,从而对患者机体造成严重后果。通过遗传操作使沙门菌染色体缺失某个或某些基因,如调节LPS、氨基酸和嘌呤合成的基因,虽然可以使沙门菌不同程度地减毒,但减毒的同时可能也影响了沙门菌的侵袭、定殖能力,从而影响沙门菌对肿瘤治疗分子的递呈效率以及最终的抗肿瘤效果。在这种情况下可以考虑应用延迟减毒系统[53]。该系统的主要原理是,用可调控启动子 (如阿拉伯糖调控子) 将某些基因的启动子替换,在体外诱导条件下该基因可以正常表达,沙门菌可以如野生菌一样感染宿主,而一旦沙门菌到达缺乏诱导条件的体内环境,该基因不再表达,从而引起细菌毒力弱化。总之,对沙门菌的毒力弱化要适度,既要保证安全性,又要避免过度减毒而影响其介导的抗肿瘤效应,而在两者之间取得平衡是不易的,需要开展大量的实验来筛选这种毒力适中的菌株。沙门菌的抗肿瘤应用还存在其他隐患,比如有些免疫功能相对较弱的肿瘤患者不能将沙门菌清除体外而使其可以长期存在于机体内。通过应用与上述类似的调控技术应该可以消除这个隐患,例如在需要停止沙门菌治疗的情况下,通过向机体注射相应的诱导分子启动或者阻止特定基因的表达,从而诱导体内沙门菌的裂解、将沙门菌清除出去,我们目前也正在研究这类调控技术。此外,以定殖肿瘤和正常组织的能力相差1 000倍的标准,沙门菌选择性定殖肿瘤的同时,仍有相当数量的沙门菌会定殖于肝脏和脾脏等正常器官组织。因此,为了尽量减少沙门菌递呈的肿瘤治疗分子在正常器官中的表达以及其在沙门菌迁移至肿瘤过程中的散布,有必要通过遗传操作使沙门菌更加专一地向肿瘤递呈治疗分子。应用低氧诱导的启动子HIP-1[54]和FF+20*[55],沙门菌携带的肿瘤治疗基因在细菌载体到达肿瘤的低氧区域后才会被诱导表达,而在正常组织中几乎不表达;将放射诱导的RecA启动子系统[34]应用于减毒沙门菌递呈肿瘤治疗分子,也可以从时间和空间上控制递呈。沙门菌不充分的肿瘤定殖是人体临床试验中发现的一大肿瘤治疗障碍[11-12],而这在动物试验中并不明显;克服此障碍是沙门菌应用于肿瘤治疗的关键,通过对沙门菌进行各种遗传改造尝试并对其肿瘤定殖效率和抗肿瘤效果加以验证,相信未来会成功克服这个障碍。沙门菌抗肿瘤的人体临床试验开展得太少,有必要进一步研究阐明动物肿瘤模型和肿瘤患者之间的差异,构建更充分模拟肿瘤患者的动物肿瘤模型,从而筛选出优良的抗肿瘤沙门菌用于开展更多的人体临床试验,甚至最终的肿瘤治疗。基于以上存在的问题,我们拟以Curtiss实验室开发的与沙门菌疫苗递呈相关的多种技术,加上本实验团队发展的LPS改造技术,来开发肿瘤治疗性沙门菌疫苗,重点放在lipid A的改造上,希望筛选出肿瘤治疗沙门菌的最优lipid A结构。
虽然沙门菌介导的肿瘤治疗在人体临床试验中还没有取得预期的效果,但沙门菌用于肿瘤治疗具有诸多优点和潜力,所以仍然值得深入研究。相信随着相关研究的进一步深入以及现代生物技术的不断发展,当前遇到的问题将来都可以找到答案。
| [1] | Pawelek JM, Low KB, Bermudes D. Tumor-targeted Salmonella as a novel anticancer vector. Cancer Res, 1997, 57 (20) : 4537–4544 (in Chinese). |
| [2] | Guo ZL, Yu B, Ning BT, et al. Genetically modified "obligate" anaerobic Salmonella typhimurium as a therapeutic strategy for neuroblastoma. J Hematol Oncol, 2015 : 99 (in Chinese). |
| [3] | Jia LJ, Wei DP, Sun QM, et al. Oral delivery of tumor-targeting Salmonella for cancer therapy in murine tumor models. Cancer Sci, 2007, 98 (7) : 1107–1112 (in Chinese). |
| [4] | Nagakura C, Hayashi K, Zhao M, et al. Efficacy of a genetically-modified Salmonella typhimurium in an orthotopic human pancreatic cancer in nude mice. Anticancer Res, 2009, 29 (6) : 1873–1878 (in Chinese). |
| [5] | Rosenberg SA, Spiess PJ, Kleiner DE. Antitumor effects in mice of the intravenous injection of attenuated Salmonella typhimurium. J Immunother, 2002, 25 (3) : 218–225 (in Chinese). |
| [6] | Wang WK, Lu MF, Kuan YD, et al. The treatment of mouse colorectal cancer by oral delivery tumor-targeting Salmonella. Am J Cancer Res, 2015, 5 (7) : 2222–2228 (in Chinese). |
| [7] | Zhao M, Geller J, Ma HY, et al. Monotherapy with a tumor-targeting mutant of Salmonella typhimurium cures orthotopic metastatic mouse models of human prostate cancer. Proc Natl Acad Sci USA, 2007, 104 (24) : 10170–10174 (in Chinese). |
| [8] | Zhao M, Yang M, Ma HY, et al. Targeted therapy with a Salmonella typhimurium leucine-arginine auxotroph cures orthotopic human breast tumors in nude mice. Cancer Res, 2006, 66 (15) : 7647–7652 (in Chinese). |
| [9] | Hayashi K, Zhao M, Yamauchi K, et al. Cancer metastasis directly eradicated by targeted therapy with a modified Salmonella typhimurium. J Cell Biochem, 2009, 106 (6) : 992–998 (in Chinese). |
| [10] | Hayashi K, Zhao M, Yamauchi K, et al. Systemic targeting of primary bone tumor and lung metastasis of high-grade osteosarcoma in nude mice with a tumor-selective strain of Salmonella typhymurium. Cell Cycle, 2009, 8 (6) : 870–875 (in Chinese). |
| [11] | Nemunaitis J, Cunningham C, Senzer N, et al. Pilot trial of genetically modified, attenuated Salmonella expressing the E. Cancer Gene Ther, 2003, 10 (10) : 737–744 (in Chinese). |
| [12] | Toso JF, Gill VJ, Hwu P, et al. Phase I study of the intravenous administration of attenuated Salmonella typhimurium to patients with metastatic melanoma. J Clin Oncol, 2002, 20 (1) : 142–152 (in Chinese). |
| [13] | Kasinskas RW, Forbes NS. Salmonella typhimurium lacking ribose chemoreceptors localize in tumor quiescence and induce apoptosis. Cancer Res, 2007, 67 (7) : 3201–3209 (in Chinese). |
| [14] | Zhang MM, Forbes NS. Trg-deficient Salmonella colonize quiescent tumor regions by exclusively penetrating or proliferating. J Controlled Release, 2015 : 180–189 (in Chinese). |
| [15] | Hoffman RM. Tumor-seeking Salmonella amino acid auxotrophs. Curr Opin Biotechnol, 2011, 22 (6) : 917–923 (in Chinese). |
| [16] | Zhao M, Yang M, Li XM, et al. Tumor-targeting bacterial therapy with amino acid auxotrophs of GFP-expressing Salmonella typhimurium. Proc Natl Acad Sci USA, 2005, 102 (3) : 755–760 (in Chinese). |
| [17] | Westphal K, Leschner S, Jablonska J, et al. Containment of tumor-colonizing bacteria by host neutrophils. Cancer Res, 2008, 68 (8) : 2952–2960 (in Chinese). |
| [18] | Gorter A, Meri S. Immune evasion of tumor cells using membrane-bound complement regulatory proteins. Immunol Today, 1999, 20 (12) : 576–582 (in Chinese). |
| [19] | Jain RK, Cook AW, Steele EL. Haemodynamic and transport barriers to the treatment of solid tumours. Int J Radiat Biol, 1991, 60 (1/2) : 85–100 (in Chinese). |
| [20] | Galán JE, Collmer A. Type Ⅲ secretion machines: bacterial devices for protein delivery into host cells. Science, 1999, 284 (5418) : 1322–1328 (in Chinese). |
| [21] | Ganai S, Arenas RB, Sauer JP, et al. In tumors Salmonella migrate away from vasculature toward the transition zone and induce apoptosis. Cancer Gene Ther, 2011, 18 (7) : 457–466 (in Chinese). |
| [22] | Chang WW, Lee CH. Salmonella as an innovative therapeutic antitumor agent. Int J Mol Sci, 2014, 15 (8) : 14546–14554 (in Chinese). |
| [23] | Maeshima N, Fernandez RC. Recognition of lipid A variants by the TLR4-MD-2 receptor complex. Front Cell Infect Microbiol, 2013 : 3 (in Chinese). |
| [24] | Lee CH, Wu CL, Shiau AL. Toll-like receptor 4 mediates an antitumor host response induced by Salmonella choleraesuis. Clin Cancer Res, 2008, 14 (6) : 1905–1912 (in Chinese). |
| [25] | Saccheri F, Pozzi C, Avogadri F, et al. Bacteria-induced gap junctions in tumors favor antigen cross-presentation and antitumor immunity. Sci Transl Med, 2010, 2 (44) : 44ra57 (in Chinese). |
| [26] | Wall DM, Srikanth CV, McCormick BA. Targeting tumors with Salmonella Typhimurium- potential for therapy. Oncotarget, 2010, 1 (8) : 721–728 (in Chinese). |
| [27] | Low KB, Ittensohn M, Le T, et al. Lipid A mutant Salmonella with suppressed virulence and TNFα induction retain tumor-targeting in vivo. Nat Biotechnol, 1999, 17 (1) : 37–41 (in Chinese). |
| [28] | Miwa S, Yano S, Zhang Y, et al. Tumor-targeting Salmonella typhimurium A1-R prevents experimental human breast cancer bone metastasis in nude mice. Oncotarget, 2014, 5 (16) : 7119–7125 (in Chinese). |
| [29] | Sorenson BS, Banton KL, Frykman NL, et al. Attenuated Salmonella typhimurium with IL-2 gene reduces pulmonary metastases in murine osteosarcoma. Clin Orthop Relat Res, 2008, 466 (6) : 1285–1291 (in Chinese). |
| [30] | Loeffler M, Le'Negrate G, Krajewska M, et al. IL-18-producing Salmonella inhibit tumor growth. Cancer Gene Ther, 2008, 15 (12) : 787–794 (in Chinese). |
| [31] | Loeffler M, Le'Negrate G, Krajewska M, et al. Attenuated Salmonella engineered to produce human cytokine LIGHT inhibit tumor growth. Proc Natl Acad Sci USA, 2007, 104 (31) : 12879–12883 (in Chinese). |
| [32] | Loeffler M, Le’Negrate G, Krajewska M, et al. Salmonella typhimurium engineered to produce CCL21 inhibit tumor growth. Cancer Immunol Immunother, 2009, 58 (5) : 769–775 (in Chinese). |
| [33] | Nguyen VH, Kim HS, Ha JM, et al. Genetically engineered Salmonella typhimurium as an imageable therapeutic probe for cancer. Cancer Res, 2010, 70 (1) : 18–23 (in Chinese). |
| [34] | Ganai S, Arenas RB, Forbes NS. Tumour-targeted delivery of TRAIL using Salmonella typhimurium enhances breast cancer survival in mice. Br J Cancer, 2009, 101 (10) : 1683–1691 (in Chinese). |
| [35] | Loeffler M, Le’Negrate G, Krajewska M, et al. Inhibition of tumor growth using Salmonella expressing Fas ligand. J Nat Cancer Inst, 2008, 100 (15) : 1113–1116 (in Chinese). |
| [36] | Urashima M, Suzuki H, Yuza Y, et al. An oral CD40 ligand gene therapy against lymphoma using attenuated Salmonella typhimurium. Blood, 2000, 95 (4) : 1258–1263 (in Chinese). |
| [37] | Manuel ER, Blache CA, Paquette R, et al. Enhancement of cancer vaccine therapy by systemic delivery of a tumor-targeting Salmonella-based STAT3 shRNA suppresses the growth of established melanoma tumors. Cancer Res, 2011, 71 (12) : 4183–4191 (in Chinese). |
| [38] | Bereta M, Hayhurst A, Gajda M, et al. Improving tumor targeting and therapeutic potential of Salmonella VNP20009 by displaying cell surface CEA-specific antibodies. Vaccine, 2007, 25 (21) : 4183–4192 (in Chinese). |
| [39] | King I, Bermudes D, Lin S, et al. Tumor-targeted Salmonella expressing cytosine deaminase as an anticancer agent. Hum Gene Ther, 2002, 13 (10) : 1225–1233 (in Chinese). |
| [40] | Mesa-Pereira B, Medina C, Camacho EM, et al. Improved cytotoxic effects of Salmonella-producing cytosine deaminase in tumour cells. Microb Biotechnol, 2015, 8 (1) : 169–176 (in Chinese). |
| [41] | Lee CH, Wu CL, Shiau AL. Endostatin gene therapy delivered by Salmonella choleraesuis in murine tumor models. J Gene Med, 2004, 6 (12) : 1382–1393 (in Chinese). |
| [42] | Lee CH, Wu CL, Shiau AL. Systemic administration of attenuated Salmonella choleraesuis carrying thrombospondin-1 gene leads to tumor-specific transgene expression, delayed tumor growth and prolonged survival in the murine melanoma model. Cancer Gene Ther, 2005, 12 (2) : 175–184 (in Chinese). |
| [43] | Li YH, Guo KY, Chen H, et al. Oral cytokine gene therapy against murine tumor using attenuated Salmonella typhimurium. Int J Cancer, 2001, 94 (3) : 438–443 (in Chinese). |
| [44] | Li X, Li Y, Wang B, et al. Delivery of the co-expression plasmid pEndo-Si-Stat3 by attenuated Salmonella serovar typhimurium for prostate cancer treatment. J Cancer Res Clin Oncol, 2013, 139 (6) : 971–980 (in Chinese). |
| [45] | Zuo SG, Chen Y, Wu ZP, et al. Orally administered DNA vaccine delivery by attenuated Salmonella typhimurium targeting fetal liver kinase 1 inhibits murine Lewis lung carcinoma growth and metastasis. Biol Pharm Bull, 2010, 33 (2) : 174–182 (in Chinese). |
| [46] | Feng KK, Zhao HY, Chen J, et al. Anti-angiogenesis effect on glioma of attenuated Salmonella typhimurium vaccine strain with flk-1 gene. J Huazhong Univ Sci Technol, 2004, 24 (4) : 389–391 (in Chinese). |
| [47] | Chou CK, Hung JY, Liu JC, et al. An attenuated Salmonella oral DNA vaccine prevents the growth of hepatocellular carcinoma and colon cancer that express α-fetoprotein. Cancer Gene Ther, 2006, 13 (8) : 746–752 (in Chinese). |
| [48] | Shao C, Yang BX, Zhao LJ, et al. Tumor suppressor gene RBM5 delivered by attenuated Salmonella inhibits lung adenocarcinoma through diverse apoptotic signaling pathways. World J Surg Oncol, 2013 : 123 (in Chinese). |
| [49] | Zhang L, Gao LF, Zhao LJ, et al. Intratumoral delivery and suppression of prostate tumor growth by attenuated Salmonella enterica serovar typhimurium carrying plasmid-based small interfering RNAs. Cancer Res, 2007, 67 (12) : 5859–5864 (in Chinese). |
| [50] | Yang N, Zhu XY, Chen LS, et al. Oral administration of attenuated S. Cancer Biol Ther, 2008, 7 (1) : 145–151 (in Chinese). |
| [51] | Blache CA, Manuel ER, Kaltcheva TI, et al. Systemic delivery of Salmonella typhimurium transformed with IDO shRNA enhances intratumoral vector colonization and suppresses tumor growth. Cancer Res, 2012, 72 (24) : 6447–6456 (in Chinese). |
| [52] | Cheng X, Zhang X, Zhou Y, et al. A Salmonella typhimurium mutant strain capable of RNAi delivery: higher tumor-targeting and lower toxicity. Cancer Biol Ther, 2014, 15 (8) : 1068–1076 (in Chinese). |
| [53] | Curtiss Ⅲ R, Xin W, Li Y, et al. New technologies in using recombinant attenuated Salmonella vaccine vectors. Crit Rev Immunol, 2010, 30 (3) : 255–270 (in Chinese). |
| [54] | Mengesha A, Dubois L, Lambin P, et al. Development of a flexible and potent hypoxia-inducible promoter for tumor-targeted gene expression in attenuated Salmonella. Cancer Biol Ther, 2006, 5 (9) : 1120–1128 (in Chinese). |
| [55] | Ryan RM, Green J, Williams PJ, et al. Bacterial delivery of a novel cytolysin to hypoxic areas of solid tumors. Gene Ther, 2009, 16 (3) : 329–339 (in Chinese). |
 2016, Vol. 32
2016, Vol. 32