服务
文章信息
- 牛浩, 吴文果
- Hao Niu, Wenguo Wu
- 纳米材料修饰微生物燃料电池阳极的研究进展
- Progress in nanomaterials modified anodes of microbial fuel cell
- 生物工程学报, 2016, 32(3): 271-283
- Chin J Biotech, 2016, 32(3): 271-283
- 10.13345/j.cjb.150250
-
文章历史
- Received: June 1, 2015
- Accepted: September 15, 2015
2 华侨大学生物材料与组织工程研究所,福建 厦门 361021
2 Institute of Biomaterials and Tissue Engineering, Huaqiao University, Xiamen 361021, Fujian, China
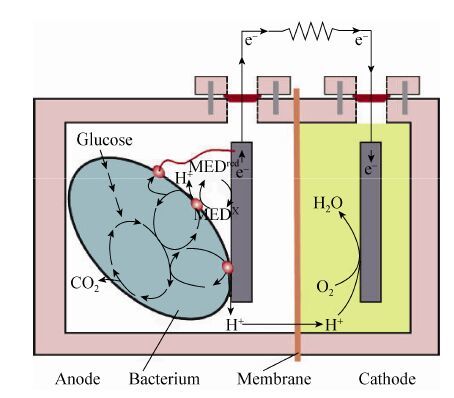
微生物燃料电池 (Microbial fuel cell,MFC)是利用微生物作催化剂将化学能转化为电能的装置[1- 2, 3]。如图 1所示,双室MFC 的结构主要由阳极、阴极和质子交换膜3个关键部件组成。目前,电量输出较低仍是限制MFC应用的主要因素。
|
| 图1 微生物燃料电池的示意图 Figure1 Concept schematic of a typical microbial fuel cell |
| |
MFC的产电效果主要取决于电子的传递速率,影响电子传递速率的主要因素有[4]:微生物对底物的氧化;电子从微生物到电极的传递;外电路的负载电阻;向阴极提供质子的过程;氧气的供给和阴极的反应[5- 6, 7]。作为微生物燃料电池中重要的结构,高性能的阳极不仅能为菌体提供附着点、电化学催化活性位点,而且还具备减小电子传递阻力、生物相容性良好的特点。基于纳米科学和技术的发展,发现纳米结构具有一些独特的性质如:表面效应、量子尺寸效应及宏观量子隧道效应等。这些性质被用于实现生物膜的附着和直接快速的电子传递,使其在性能上表现出与微米材料间的显著差异[8]。因此,将金属、半导体纳米颗粒、纳米丝和碳纳米管等[9- 10]具有纳米结构的电极材料应用于微生物燃料电池领域是目前研究的热点[11]。
2 阳极纳米材料的种类在微生物燃料电池的研究中,阳极材料的必要条件是高导电率、无腐蚀性、高比表面积、高孔隙率、无污垢、廉价、容易制造并且可放大[12]。根据这些基本特点,结合纳米材料的独特性质,研究者们对于有可能被用作阳极的纳米材料进行了广泛的研究,按照阳极纳米材料的形状分类,可将阳极分为:纳米颗粒[13]、纳米草[14]、纳米丝[15]及纳米网[16]等。按照阳极材料成分对电极进行分类,大致可分为3大类:纳米碳材料阳极、纳米金属修饰阳极、纳米导电聚合物复合阳极。
2.1 碳纳米材料阳极传统的碳材料阳极有碳棒、碳布、碳纸、碳毡、石墨片、石墨颗粒等。这些材料具有高导电性且适合细菌生长,但主要缺点是内阻较大、产电功率非常低[17]。而纳米碳材料作为传统碳材料的一种发展和衍生,不仅继承了碳材料化学稳定性、导电性、生物相容性较好的普遍优点,同时具有更大的表面积有利于产电菌的附着,为胞外电子直接传递过程提供了更多的接触位点[18]。
2.1.1 碳纳米管碳纳米管 (Carbon nanotubes,CNTs) 具有特定的孔隙结构、极高的机械强度和韧性、很大的比表面积、很高的热稳定性和化学惰性、极强的导电性等性质以及独特的一维纳米尺度,是一种十分理想的电极材料[19]。因此,被广泛应用到MFC的电极材料研究中。梁鹏等[20]考察了分别以碳纳米管、活性碳和柔性石墨为阳极材料的3种微生物燃料电池的产电性能,其中以碳纳米管作为阳极的MFC的功率密度最高,内阻最低,分别为402 mW/m2和263 Ω。研究结果表明,相比于其他两种阳极材料,碳纳米管能够有效地降低MFC的阳极内阻和欧姆内阻,因此在外电阻相同的情况下,碳纳米管阳极MFC的库伦效率也高于其他两种材料为阳极的MFC。基于以上结论推测,添加多壁碳纳米管可能更有利于电极材料电子的传递,提高电极材料的导电性。因此,Sun等[21]通过层层自组装的方法将多壁碳纳米管修饰到碳纸电极上,实验结果表明在以废水淤泥为底物的微生物燃料电池中,经碳纳米管修饰后的电极使界面电荷转移电阻从1 163 Ω降低至258 Ω,最大功率密度从241 mW/m2增加至290 mW/m2。Zhao等[22]的研究表明CNTs末端能够刺入菌体细胞膜中,促进细胞色素C与电极间的直接电子传递,使产电功率增强,更进一步地揭示了碳纳米管提高产电效果的机制。Mohanakrishna等[23]选用纯度为99%,表面积大于100 m2/g,颗粒尺寸小于50 nm的碳纳米粉将其浸泡在导电材料环氧树脂中,随后涂在石墨板上作为阳极。同时,对比了用同样的方法以多壁碳纳米管为修饰材料制备的阳极和未经修饰的阳极的产电情况。研究结果表明,在以污水为底物的MFC中,采用多壁碳纳米管MFC的功率密度最高,达到267.77 mW/m2比石墨平板电极提升了148%。表面积的增大,促使了高效的电荷迁移。为了进一步考察阳极几何特性对MFC产电性能的影响,Erbay等[24]制备了3种碳纳米管长度分别为8 μm、13 μm、19 μm,修饰密度高、低两类的5种阳极。研究发现长度为19 μm、修饰密度低的碳纳米管修饰阳极,获得的最大功率密度为3 360 mW/m2,是碳布阳极的7.4倍。其原因是碳纳米管不仅减小了内阻,同时能够如同触手一样捕捉菌体,使电子在碳环与纳米导线间产生π-π跃迁。碳纳米管作为微生物载体的阳极,也被广泛应用于新型MFC的构建中。2015年,He等[25]以碳纳米管为阳极,构建了上流式固定床微生物燃料电池反应器,并用于污水处理实验。结果表明这种新型的反应器,在有机负荷率为3.94 g COD/(L·d) 时,能够产生的最大体积功率密度为590 mW/m3。Liu等[26]采用碳纳米管固定床MFC反应器,结合水热液化法用于玉米秸秆生物质的处理。在有机负荷率为 2.41 g/(L·d) 时,获得最大体积功率密度为 680 mW/m3。这种高效反应器能够反复使用,延长了MFC的使用寿命,并增加了产电的稳定性。促进了电极与产电菌间的协同作用,更有利于电子的传递。尽管碳纳米管具有良好的电化学性质,有利于提高MFC的产电功率。然而,据文献报道[27],碳纳米管具有细胞毒性能抑制细胞增殖甚至导致细胞死亡,经修饰后对细胞的毒性减小。因此,纳米颗粒、导电聚合物材料修饰碳纳米管阳极又成为研究的热点[28, 29- 30]。
2.1.2 石墨烯石墨烯 (Graphene) 是一种二维碳材料,是单层石墨烯、双层石墨烯和多层石墨烯的统称。氧化石墨烯纳米带,是经过对多壁碳纳米管的纵向切割和拆解,制得的具有高长径比结构。Huang等[31]在以奥奈达希瓦氏菌Shewanella oneidensis MR-1为产电菌的MFC中,将氧化石墨烯纳米带用电泳沉积的方法修饰在了以碳纸为基础的阳极上,使电流密度达到0.30 A/m2,功率密度为34.2 mW/m2,相比于未修饰的碳纸阳极提高了4倍,氧化石墨烯纳米带在胞外电子传递 (Extracellular electron transfer,EET) 过程中发挥了类似纳米导线的作用,增长电子传递的距离。然而,长径比较大的情况下形成的网络结构为化学反应提供的表面积和空间是有限的,因此阳极结构由一维向更高维度进行了延伸。Wang等[32]用柔性三维石墨烯与泡沫镍通过高压热沉积制备的阳极,不仅为产电菌提供了大面积的附着位点,同时均匀的微孔结构又有利于传质。以阳极材料体积为基准,MFC最大的体积功率密度为661 W/m3,在流加培养的条件下,至少可以有效地工作一周以上。为了更进一步提升三维电极的产电性能,Yong等[33]以S. oneidensis MR-1为产电菌,利用化学气相沉积法将石墨烯修饰在泡沫镍上并加入了聚苯胺 (Polyaniline,PANI),制备出的三维复合阳极提升了胞外电子传递效率,并且多孔的结构特点有助于物质传递和菌体附着,功率密度为 768 mW/m2比采用碳纸电极MFC的功率密度高出约4倍。
2.1.3 金刚石金刚石是一种由碳元素组成的矿物,是碳元素的同素异形体。金刚石电极是一种新型的电极,在电化学传感器,电催化中具有良好的应用前景。人们利用掺硼金刚石 (Boron-doped diamond,BDD) 薄膜作为电极材料,而掺硼金刚石薄膜特殊的sp3键结构及其具有的导电性,赋予了金刚石薄膜电极优异的电化学特性,如宽的电化学势窗、较低的背景电流、较好的物理化学稳定性以及低吸附特性等[34]。
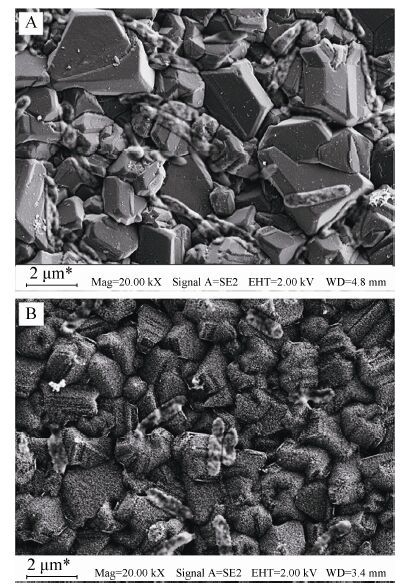
Masuda等[35]采用Al2O3模板法,通过微波等离子体化学气相沉积制备了金刚石纳米柱和类金刚石纳米管结构。香港城市大学李述汤研究组[36- 37]利用偏压辅助的微波等离子技术,通过调控刻蚀条件,在纳米金刚石表面分别刻蚀成纳米须、纳米锥、纳米柱等不同形貌的纳米结构。Yang等[38]采用纳米金刚石颗粒作为掩膜,利用反应离子刻蚀方法制备了金刚石纳米线结构。本课题组Wu等[14]利用简单的反应离子刻蚀法在高浓度掺杂金刚石薄膜上进行氧等离子处理,制备了金刚石纳米草结构电极,如图 2所示。以光伏希瓦氏菌Shewanella loihica PV-4为产电菌,得到的稳定电流密度为1.2 μA/cm2,明显高于对照组BDD薄膜0.4 μA/cm2的电流密度,证明了BDD纳米草结构电极能有效地提高电子直接传递的能力。
纳米碳材料阳极不仅继承了碳材料良好的导电性、稳定的化学性质、低廉的价格等特点,同时,较高的空隙率有助于底物在微生物间的传质、提升了电子的迁移速率、减小浓差极化造成的影响较大的表体比也提升了微生物的附着率,提高了产电效率[39]。然而,试图通过增大微生物的附着面积、提高产电微生物的附着量仅是提升微生物燃料电池产电性能研究的一个方面。研究者们还对提高质子和电子传递的措施进行了研究,制备出了纳米金属修饰的电极。
2.2 纳米金属修饰阳极纳米金属材料具有多种优异的特性,如小尺寸效应、高催化活性、良好的稳定性及生物相容性等,因而被广泛应用于电分析、电催化和电池领域[37]。纳米金属材料修饰的阳极,种类广泛。其所用到的金属元素除了常见的导电金属Fe、Au、Pt等外,还有过渡金属Mo、Ru、Mn、Ti、Cd等。
2.2.1 纳米铁材料Nakamura等[40]发现将纳米Fe2O3胶体加入MFC中能够促进S. loihica PV-4菌体相互交联形成有助于电子远距离传递的网状结构,相比于没有加入Fe2O3的情况下,MFC的电流增大了300倍。Fe2O3的加入充当了介体的作用,减小电子在菌体间传递阻力。Pandit等[41]研究表明经Fe2O3修饰后的电极还能促进腐败希瓦氏菌Shewanella putrefaciens形成生物膜,同时增加电容。在Fe2O3装载量为0.8 mg/cm2时,MFC的功率密度和库仑效率分别提高了40%和33%。Bose等[42]的研究表明,Fe2O3纳米粒子的大小和形状的不同会影响S. oneidensis MR-1菌体对纳米粒子的吸附,从而导致电子从菌体传递至纳米粒子的机理不同。Ji等[43]通过层层自组装方法将α-Fe2O3纳米杆和壳聚糖修饰至ITO电极上,研究发现修饰后的电极产生的电流比未修饰的电极提高了320倍。Peng等[44]将质量百分比为5% α-FeOOH掺入活性炭并压制入不锈钢网中制得阳极,MFC最大功率密度达到了 693 mW/m2左右,比对照组活性炭电极功率密度高出了36%。然而,高含量的α-FeOOH,也会阻碍电极的导电性。另一项工作中,Peng等[45]将聚四氟乙烯为交联剂与Fe3O4纳米颗粒混合制备的薄膜辊压在不锈钢网上作为电极,最大电容可达574.6 C/m2。
Fe3O4有益于促进短暂的阳极电荷存储并伴随着MFC功率性能的提升。综上所述,含铁元素纳米颗粒修饰的阳极,会提高阳极的电容量、增加电子传递距离、促进生物膜形成、有效减少MFC的启动时间。但是,铁元素纳米颗粒的导电性、抗腐蚀性相比与金、铂等略差。
2.2.2 纳米金及其他金属材料在纳米金属材料修饰的阳极研究中,常见的还有纳米金。2010年,Sun等[46]通过在碳纸上喷涂一层金作为微生物燃料电池的阳极,这种导电性好,表面积巨大,与S. oneidensis MR-1相容性优良的电极,使得微生物易于附着并快速形成菌膜。产电电量比直接碳纸作为阳极的微生物电池产生的电量高47%。2011年,Fan等[47]的研究发现,在以S. oneidensis MR-1为产电微生物的微生物燃料电池中,经纳米金修饰的石墨阳极产生的电流密度比未修饰的石墨阳极高20倍,同时也证明了电极形态学对产电有一定的影响。例如,纳米金颗粒越圆,费里特直径和表面积越大,增强了细菌与电极间的相互作用,使产电量得到了提升。2014年,Alatraktchi等[48]用喷涂的方法将纳米金覆盖在碳布上作为阳极,发现MFC的启动时间不仅比以前缩短了1.22倍,最大功率密度达到3 749 mW/m2,而且纳米金密度越高,阳极上产电菌群越多样化,说明纳米金具有良好的生物相容性和电化学催化特性。Wu等[49]利用层层自组装的方法将纳米金/聚电解质多层膜修饰在硼掺金刚石薄膜电极上,制备了不同层数的纳米金/聚电解质修饰硼掺金刚石薄膜电极。结果表明,随着纳米金/聚电解质复合物层数从0增加至6,S. loihica PV-4在电极上产生的峰电流从1.25 μA/cm2增加至2.93 μA/cm2。纳米金颗粒和绝缘特性的聚电解质以合适的比例层层自组装成三维网络结构,促进了电子从复合物层向电极表面的传递,使得S. loihica PV-4的电化学性能在纳米金/聚电解质修饰硼掺金刚石薄膜电极上得到了增强。
此外,利用其他金属元素修饰阳极对MFC产电效果的改善也有显著的帮助。如Lü等[50]用RuO2纳米颗粒修饰的碳毡阳极,使MFC最大功率密度为3.08 W/m2,比修饰前提高了17倍,同时也促进了电子的胞外传递。这是由于RuO2的修饰不仅增大了电极表面积,更重要的是在一个广泛的电势范围里RuO2都能够快速并可逆地进行四价与二价间的氧化还原转化,充当中介体的作用。同时,RuO2也是一种超电容材料,能够在未找到合适的电子受体前,短暂地储存电子。一旦有新的电子受体可用,就快速地将电子释放。同样,在电子传递过程中起媒介作用的还有MnO2,Park和Zeikus[51]在以乙酸盐为底物,S. putrefacians为产电菌的MFC中,用MnO2涂层的阳极,功率可达10.2 mW/m2,而对照组石墨电极的功率密度仅为0.02 mW/m2。TiO2由于介电常数较高和半导体的性能,因此具有优良的电学性能,被广泛应用于MFC阳极修饰中。Wen等[52]将TiO2修饰在碳纳米管上作为阳极,产生的最大功率密度为1.12 W/m2是单独用碳纳米管MFC的1.5倍。这种纳米复合物具有独特的一维结构,表面积大、内阻小、输出电流和功率密度高等优点,而且合成方法简单,为制备纳米复合物材料阳极提供了新的合成路线参考。Tang等[53]给丝瓜络载上蛋清蛋白包裹TiO2纳米颗粒后经碳化,形成以TiO2为核心的碳基核壳结构,性能优良且廉价的新型电极。与石墨阳极和未经TiO2纳米颗粒修饰的丝瓜络电极相比,这种新型三维阳极产生的最大功率密度为 (2.59±0.12) W/m2,分别比前两者高出201%和63%。Garcia等[54]将TiO2修饰在了碳静电纺丝纳米纤维上,展现了良好的生物相容性和电阻小的特点。Fan等[13]发现纳米钯修饰后的石墨阳极产生的电流密度范围达到 5.7-8.8 μA/cm2比未修饰的石墨阳极高50-150倍。说明经纳米钯修饰后的阳极,能够为中介体与酶的相互作用提供更大的空间,促使电极与细胞膜表面酶更直接、有效的接触,达到增强电子传递的目的。Wang等[55]用微波辅助法将Mo2C修饰在碳纳米管上作为阳极,以大肠杆菌为产电菌,发现这种含16.7 wt% Mo的阳极展现出了与含20 wt% Pt作为阳极同等能力的电催化活性。不仅如此,这种新型的阳极还具有促进生物膜形成和对氢的氧化能力。如图 3展示了该实验中两种不同的电子传递机制。Singh 等[56]通过化学蒸汽沉积的方法,将镍纳米颗粒修饰在了活性炭纤维/碳纳米纤维多尺度网络上。获得了 (710±5) mV的开路电压和 (1 145±20) mW/m2的功率密度,是采用镀有过渡金属的活性碳纤维纳米电极的9倍。虽然纳米金属修饰电极是微生物燃料电池领域的研究热点,然而金属自身特性也限制了金属修饰方法的应用。Kargi和Eker[57]利用铜及其氧化物作为阳极材料或修饰阳极,研究发现Cu在电解液中的溶解性和对产电菌的毒性被证明不适合作为MFC的电极使用。因此,寻找内阻低、电催化活性好、成本低廉、对细胞无毒性、可用于MFC产电中的纳米金属材料或纳米合金材料将会成为新的研究方向。

|
| 图3 在以Mo2C/CNTs 为阳极的MFC 中电子传递机制示意图 (Ⅰ生物膜机制通过外膜上的细胞色素 (途径A)和导电性的纳米导线 (途径B);Ⅱ代谢产物氧化机制 (途径C))[55] Figure3 Schematic electron-transfer mechanisms at Mo2C/CNTs anode of MFC. Ⅰ biofilm mechanism via outer-membrane cytochromes (PathA) and electrically conductive nanowires (PathB); Ⅱ metabolites oxidation mechanism (PathC)[55] |
| |
导电聚合物是一种新型的电极材料。相比于碳纳米材料阳极和纳米金属修饰阳极,具有易加工成各种复杂的形状和尺寸、重量轻、稳定性、电阻率可调等特点[58]。将导电聚合物修饰阳极应用于MFC引起了研究者的兴趣,纳米导电聚合修饰阳极在MFC的研究日渐增多。2007年,Qiao等[59]将碳纳米管和聚苯胺的复合物修饰到泡沫镍电极上作为MFC的阳极,实验结果发现以大肠杆菌为产电微生物的微生物燃料电池产生的最大功率密度为42 mW/m2,远高于其他阳极材料的微生物燃料电池产生的电能。
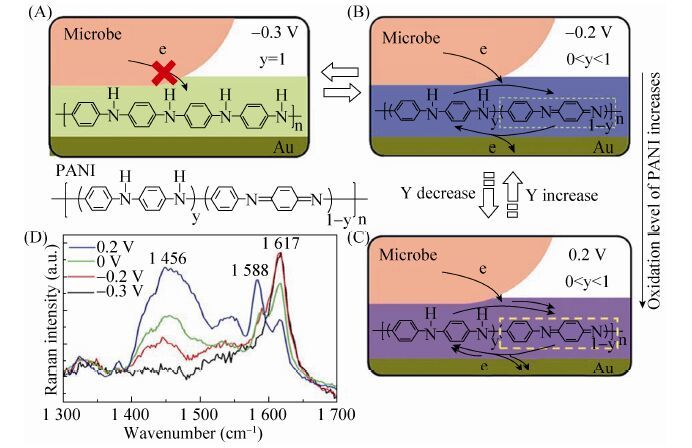
2008年Qiao等[60]的另一项研究中,用纳米结构聚苯胺/多孔TiO2复合物修饰泡沫镍阳极,使大肠杆菌产生的功率密度增加至1 495 mW/m2。2010年,Zhao等[16]对比考察了S. loihica PV-4分别在以ITO电极、聚苯胺薄膜修饰的ITO电极以及聚苯胺纳米纤维修饰的ITO电极为阳极MFC中的产电性能,研究结果发现导电聚合物修饰后的ITO电极产生的电流和功率密度高于ITO裸电极,且纳米结构聚苯胺修饰的ITO电极产生的电流和功率密度显著高于聚苯胺薄膜修饰的ITO电极。2012年,Ding等[61]将聚苯胺沉积在金电极表面形成聚苯胺纳米导线 (Polyaniline nanowire array),在与光滑的聚苯胺—金和裸露的金电极作对比后,发现聚苯胺纳
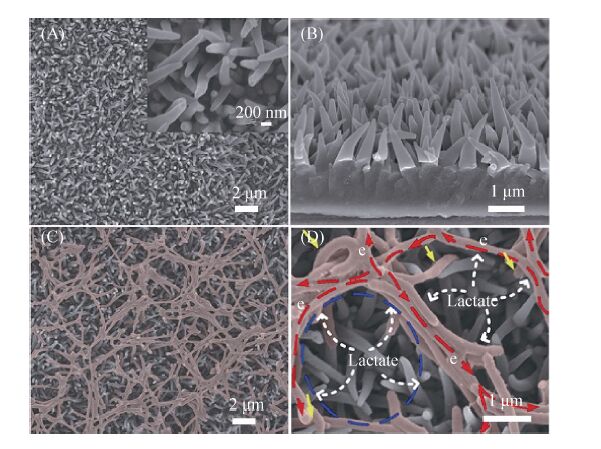
米导线电极的稳定电流输出分别是后两者的10和25倍。图 4中,阐明了聚苯胺纳米导线电极由于氧化/还原比的不同,导致了传递电子能力的差别。2014年,Ding等[62]采用的聚吡咯纳米导线 (Polypyrrole nanowire array PPy-NA) 阳极,以S. loihica PV-4为产电菌进行产电性能研究,电极形貌如图 5所示。结果表明,聚吡咯纳米线阵阳极具有长期的稳定性,能够极大地促进胞外电子传递,展现出了聚吡咯巨大的应用潜力。Liu等[63]用聚3,4-乙烯二氧噻吩修饰碳布和碳纸阳极。相比较未经修饰的阳极,这种新型阳极将功率密度提升了43%,达到140 mW/m2。循环伏安和交流阻抗分析表明采用聚3,4-乙烯二氧噻吩修饰的碳基电极增加了氧化还原位点,有效降低了电子的界面传递阻力。
|
| 图4 在PANI 修饰后的Au 电极上菌体胞外电子的传递特点。 (A-C) 示意图从分子水平揭示了在经PANI 修饰后的Au 电极上菌体的胞外电子传递. PANI 层能够通过调节电势来介导由细菌到电极间的电子传递,而这种调节能力是由于PANI 有多种不同的氧化水平。y 和1–y 分别代表在PANI 聚合物中被还原的 (苯环) 和被氧化的 (醌环) 比例。(A) 当外电势为–0.3 V 时,PANI 处于完全还原态,不能介导电子从菌体到达下层的Au 电极。(B) 当电势为–0.2 V 时,PANI 开始被氧化,并且可以传递电子。(C) 随着电势向正向移动,PANI 的氧化水平增加,导致介导胞外电子传递的能力增强。(D) 用波长为516 nm 的激发光,分别得到了电极电势为–0.3 V (黑线),–0.2V (红线),0 V (绿线),0.2 V (蓝线) 四种PANI-NA 的拉曼光谱[61] Figure4 Characteristics of bacterial extracellular electron transfer for Au anobe modified by PANI. (a-c) Schematic illustration of bacterial EET on a PANI modified Au electrode at a molecular level. The ability of PANI layers to mediate electron transfer from cells to the Au electrode can be tuned by altering the applied potential,which can be ascribed to the multiple oxidation level of PANI at various applied potentials. y and 1-y represent the ratio of reduced (benzene ring) and oxidized (quinone ring) units in the PANI polymer,respectively. (a) At an applied potential of -0.3 V,PANI is in its fully reduced state and cannot mediate anodic electron transfer from cells to the underlayer of the Au electrode. (b) At an applied potential of -0.2 V,the PANI layer starts to be oxidized and can serve as a conduit to mediate electron transfer from microbes to the Au electrode. (c) With the positive shifting of applied potential,the oxidation level of PANI increased,leading to an enhanced ability in mediating EET. (d) In situ Raman spectra of PANI-NA obtained at electrode potentials of -0.3 V (dark line),-0.2 V (red line),0 V (green line),and 0.2 V (blue line) using a laser excitation wavelength of 516 nm[61] |
| |
|
| 图5 聚吡咯纳米阳极的扫描电镜图。(a) 在大比例尺下扫描电镜图像显示聚吡咯纳米导线分布均匀。插图:高倍扫描电镜图像下PPy-NA的锥形结构清晰可见。(b) PPy-NA电极截面的扫描电镜图像,表明其高度有序排列的性质。(c) 电极电势为0.2 V时,细菌在聚吡咯纳米阳极培养60 h后,细菌在整个PPy-NA电极呈现出典型的多孔网络结构,微生物被标记为红色。(d) 高倍扫描电镜图像的微生物和聚吡咯纳电极,其中几个细菌紧密捆绑在一起,并连接相邻菌束,形成有利于电子转移的管道[62] Figure5 SEM image of the PPy nanowires as anobe. (a) SEM image of the as-prepared PPy-NA fi lm showing the homogeneous distribution of PPy nanowires at a large scale. Inset: high magni fi cation SEM image of the PPy nanowires where the conical structure is clearly seen. (b) Aside-view SEM image of the as-prepared PPy-NA,suggesting its highly ordered and aligned nature. (c) Bacterial cellsona PPy-NA electrode after 60 hours of electrochemical culture at 0.2 V,exhibiting a typical porous structured bacterial network on the whole PPy-NA electrode. The microbes were labeled in red. (d) Enlarged SEM image of microbes on the PPy-NA electrode,where several microbes are bundled tightly together and are connected with neighboring microbe bundles,forming a condui to facilitate the electron transfer[62] |
| |
纳米导电聚合物修饰后的电极具有易聚合、成本低、易掺杂等优点。有望成为MFC中贵重金属电极有效的替代品。与此同时,新型导电聚合物的发现及其在微生物燃料电池领域中的应用,也将使导电聚合物修饰的电极更具
研究价值,为揭示电子传递机理提供帮助。
综上可见,用碳纳米材料、纳米金属或纳米导电聚合物修饰阳极,可对微生物燃料电池的产电性能产生不同影响。究其原因,这主要是由3类纳米材料各自不同的理化性质和电化学性能所致。表 1对微生物燃料电池中修饰阳极的这3类纳米材料的理化性质、电化学性能、电子传递机制进行了总结。
| Nano-materials for anode modification | Physico-chemical and electrochemical properties | The mechanism of electron transfer | References |
| Carbon materials: ⅠCarbon nanotubes ⅡGraphene ⅢDiamond | High surface area. Good biocompatibility. High electrical conductivity and stability of CNTs. | Reduce the electrode resistance. Increase the amount of microbial adhesion. | [20-25] [30-34] |
| N.ano-metallic materials: ⅠNano-iron ⅡNano-glod ⅢOther metallic materials (Mn,Ti,Pd,Mo) | Providing high anode capacitance. Nano-metallic materials as efficient mediator for electron transfer from bacteria to anode. Positive effect of nano-metallic materials on the charge transfer rate compared to the anode support alone. | Reduce the electrode resistance. The large specific surface area of anode. Synergetic effects between nanometal and anode support. | [47-49] [51-54] |
| Nano-polymer conductor materials: ⅠPolyaniline ⅡPolypyrrole | High surface area. Suitable porosity. High electrical conductivity and biocompatibility. | Large surface area of PANI for the attachment of bacterial cells. High conductivity to facilitate extracellular electron transfer. Provide a suitable macroporous anode. | [59-61] |
纳米材料修饰微生物燃料电池阳极,有效减小电极内阻、增大阳极菌体的粘附量,从而显著提高微生物燃料电池的产电性能。尽管目前纳米材料修饰阳极研究取得了一些进展,但当前该领域尚处于研究阶段,存在一些问题亟待解决:1)关于纳米材料修饰的稳定性和耐用性问题的研究缺乏。2) 纳米材料如何促进胞外电子远距离传递,提高电池的产电性能的具体机制还有待进一步深入研究。
| [1] | Logan BE, Hamelers B, Rozendal R, et al. Microbial fuel cells: methodology and technology. Environ Sci Technol, 2006, 40 (17) : 5181–5192 (in Chinese). |
| [2] | Du ZW, Li HR, Gu TY. A state of the art review on microbial fuel cells: a promising technology for wastewater treatment and bioenergy. Biotechnol Adv, 2007, 25 (5) : 464–482 (in Chinese). |
| [3] | Lovely DR. Bug juice: harvesting electricity with microorganisms. Nat Rev Microbiol, 2006, 4 (7) : 497–508 (in Chinese). |
| [4] | Gil GC, Chang IS, Kim BH, et al. Operational parameters affecting the performance of a mediator-less microbial fuel cell. Biosens Bioelectron, 2003, 18 (4) : 327–334 (in Chinese). |
| [5] | Logan BE. Exoelectrogenic bacteria that power microbial fuel cells. Nat Rev Microbiol, 2009, 7 (5) : 375–381 (in Chinese). |
| [6] | Kim BH, Chang IS, Gadd GM. Challenges in microbial fuel cell development and operation. Appl Microbiol Biotechol, 2007, 76 (3) : 485–494 (in Chinese). |
| [7] | Lovley DR. Electromicrobiology. Ann Rev Microbiol, 2012 : 391–409 (in Chinese). |
| [8] |
Xu GD, Li YF, Zhang YJ.
Principle and Application of Microbial Fuel Cell. Harbin: Harbin Institute of Technology Press, 2012 : 45 -49(in Chinese).
徐功娣, 李永峰, 张永娟. 微生物燃料电池原理与应用. 哈尔滨: 哈尔滨工业大学出版社, 2012 : 45 -49. |
| [9] | Duan XJ, Fu TM, Liu J, et al. Nanoelectronics-biology frontier: from nanoscopic probes for action potential recording in live cells to three-dimensional cyborg tissues. Nano Today, 2013, 8 (4) : 351–373 (in Chinese). |
| [10] | Tian BZ, Cohen-Karni T, Qing Q, et al. Three-dimensional flexible nanoscale field-effect transistors as localized bioprobes. Science, 2010, 329 (5993) : 830–834 (in Chinese). |
| [11] |
Wu WG. The discharge characteristics of microbial fuel cell and its applications in sensing [D]. Nanjing: Southeast University, 2012 (in Chinese). 吴文果. 微生物燃料电池产电特性及其在传感器中的应用研究[D]. 南京: 东南大学, 2012. |
| [12] |
Ye YL. The study of anode configuration and performance in microbial fuel cell [D]. Hangzhou: Zhejiang University, 2014 (in Chinese). 叶遥立. 微生物燃料电池生物阳极构造和产电性能研究[D]. 杭州:浙江大学, 2014. |
| [13] | Fan YZ, Xu ST, Schaller R, et al. Nanoparticle decorated anodes for enhanced current generation in microbial electrochemical cells. Biosens Bioelectron, 2011, 26 (5) : 1908–1912 (in Chinese). |
| [14] | Wu WG, Bai LL, Liu X, et al. Nanograss array boron-doped diamond electrode for enhanced electron transfer from Shewanella loihica PV-4. Electrochem Commun, 2011, 13 (8) : 872–874 (in Chinese). |
| [15] | Zou YS, Ma KL, Zhang WJ, et al. Fabrication of diamond nanocones and nanowhiskers by bias-assisted plasma etching. Diam Relat Mater, 2007, 16 (4/7) : 1208–1212 (in Chinese). |
| [16] | Zhao Y, Watanabe K, Nakanmura R, et al. Three-dimensional conductive nanowire networks for maximing anode performance in microbial fuel cells. Chemistry, 2010, 16 (17) : 4982–4985 (in Chinese). |
| [17] | Zhou MH, Chi ML, Luo JM, et al. An overview of electrode materials in microbial fuel cells. J Power Sources, 2011, 196 (10) : 4427–4435 (in Chinese). |
| [18] | Pham TH, Aelterman P, Verstraete W. Bioanode performance in bioelectrochemical systems: recent improvements and prospects. Trends Biotechnol, 2009, 27 (3) : 168–179 (in Chinese). |
| [19] | Kauffman DR, Star A. Carbon nanotube gas and vapor sensors. Angew Chem Int Ed, 2008, 47 (35) : 6550–6570 (in Chinese). |
| [20] |
Liang P, Fan ZM, Cao XX, et al. Electricity generation by the microbial fuel cells using carbon nanotube as the anode.
Chin J Environ Sci, 2008, 29
(8)
: 2356–2360
(in Chinese).
梁鹏, 范志明, 曹效鑫, 等. 碳纳米管阳极微生物燃料电池产电特性的研究. 环境科学,2008,29 (8) :2356–2360. |
| [21] | Sun JJ, Zhao HZ, Yang QZ, et al. A novel layer-by-layer self-assembled carbon nanotubebased anode: preparation, characterization, and application in microbial fuel cell. Electrochim Acta, 2010, 55 (9) : 3041–3047 (in Chinese). |
| [22] | Zhao GC, Yin ZZ, Zhang L, et al. Direct electrochemistry of cytochrome c on a multi-walled carbon nanotubes modified electrode and its electrocatalytic activity for the reduction of H2O2. Electrochem Commun, 2005, 7 (3) : 256–260 (in Chinese). |
| [23] | Mohanakrishna G, Mohan SK, Mohan SV. Carbon based nanotubes and nanopowder as impregnated electrode structures for enhanced power generation: evaluation with real field wastewater. Appl Energy, 2012 : 31–37 (in Chinese). |
| [24] | Erbay C, Pu X, Choi W, et al. Control of geometrical properties of carbon nanotube electrodes towards high-performance microbial fuel cells. J Power Sources, 2015 : 347–354 (in Chinese). |
| [25] | He YH, Liu ZD, Xing XH, et al. Carbon nanotubes simultaneously as the anode and microbial carrier for up-flow fixed-bed microbial fuel cell. BiochemEng J, 2015 : 39–44 (in Chinese). |
| [26] | Liu ZD, He YH, Shen RX, et al. Performance and microbial community of carbon nanotube fixed-bed microbial fuel cell continuously fed with hydrothermal liquefied cornstalk biomass. Bioresour Technol, 2015 : 294–301 (in Chinese). |
| [27] | Kang S, Pinault M, Pfefferle LD, et al. Single-walled carbon nanotubes exhibit strong antimicrobial activity. Langmuir, 2007, 23 (17) : 8670–8673 (in Chinese). |
| [28] | Mottaghitalab V, Spinks GM, Wallace GG. The influence of carbon nanotubes on mechanical and electrical properties of polyaniline fibers. Synth Met, 2005, 152 (1/3) : 77–80 (in Chinese). |
| [29] | Qiao Y, Li CM, Bao SJ, et al. Carbon nanotube/polyaniline composite as anode material for microbial fuel cells. J Power Sources, 2007, 170 (1) : 79–84 (in Chinese). |
| [30] | Sharma T, Reddy ALM, Chandra TS, et al. Development of carbon nanotubes and nanofluids based microbial fuel cell. Int J Hydrogen Energy, 2008, 33 (22) : 6749–6754 (in Chinese). |
| [31] | Huang YX, Liu XW, Xie JF, et al. Graphene oxide nanoribbons greatly enhance extracellular electron transfer in bio-electrochemical systems. Chem Commun, 2011, 47 (20) : 5795–5797 (in Chinese). |
| [32] | Wang HY, Wang GM, Ling YC, et al. High power density microbial fuel cell with flexible 3D graphene-nickel foam as anode. Nanoscale, 2013, 5 (21) : 10283–10290 (in Chinese). |
| [33] | Yong YC, Dong XC, Chan-Park MB, et al. Macroporous and monolithic anode based on polyaniline hybridized three-dimensional graphene for high-performance microbial fuel cells. ACS Nano, 2012, 6 (3) : 2394–2400 (in Chinese). |
| [34] | Nelson GW, Foord JS. Nanoparticle-Based Diamond Electrodes//Yang NJ, ed. Novel Aspects of Diamond. Switzerland: Springer-Verlag, 2015: 165-204. |
| [35] | Masuda H, Yanagishita T, Yasui K, et al. Synthesis of well-aligned diamond nanocylinders. Adv Mater, 2001, 13 (4) : 247–249 (in Chinese). |
| [36] | Zhang WJ, Wu Y, Wong WK, et al. Structuring nanodiamond cone arrays for improved field emission. Appl Phys Lett, 2003, 83 (16) : 3365–3367 (in Chinese). |
| [37] | Zou YS, Yang Y, Zhang WJ, et al. Fabrication of diamond nanopillars and their arrays. Appl Phys Lett, 2008, 92 (5) : 053105 (in Chinese). |
| [38] | Yang NJ, Uetsuka H, Osawa E, et al. Vertically aligned nanowires from boron-doped diamond. Nano Lett, 2008, 8 (11) : 3572–3576 (in Chinese). |
| [39] | Wei JC, Liang P, Huang X. Recent progress in electrodes for microbial fuel cells. Bioresour Technol, 2011, 102 (20) : 9335–9344 (in Chinese). |
| [40] | Nakamura R, Kai F, Okamoto A, et al. Self-constracted electrically conductive bacterial networks. Angew Chem Int Ed, 2009, 48 (3) : 508–511 (in Chinese). |
| [41] | Pandit S, Khilari S, Roy S, et al. Improvement of power generation using Shewanella putrefaciens mediated bioanode in a single chambered microbial fuel cell: effect of different anodic operating conditions. Bioresour Technol, 2014 : 451–457 (in Chinese). |
| [42] | Bose S, Hochella Jr MF, Gorby YA, et al. Bioreduction of hematite nanoparticles by the dissmiltory iron reducing bacterium Shewanella oneidensis MR-1. Geochim Cosmochim Acta, 2009, 73 (4) : 962–976 (in Chinese). |
| [43] | Ji JY, Jia YJ, Wu WG, et al. Layer-by-layer self-assembled Fe2O3 nanorod-based composite multilayer film on ITO anode in microbial fuel cell. Colloids Surf A: Physicochem Eng Aspects, 2011, 390 (1/3) : 56–61 (in Chinese). |
| [44] | Peng XH, Yu HB, Wang X, et al. Enhanced anode performance of microbial fuel cells by adding nanosemiconductor goethite. J Power Sources, 2013, 223 (1) : 94–99 (in Chinese). |
| [45] | Peng XH, Yu HB, Ai LN, et al. Time behavior and capacitance analysis of nano-Fe3O4 added microbial fuel cells. Bioresour Technol, 2013 : 689–692 (in Chinese). |
| [46] | Sun M, Zhang F, Tong ZH, et al. A gold-sputtered carbon paper as an anode for improved electricitygeneration from a microbial fuel cell inoculated with Shewanella oneidensis MR-1. Biosens Bioelectron, 2010, 26 (2) : 338–343 (in Chinese). |
| [47] | Fan YZ, Xu ST, Schaller R, et al. Nanoparticle decorated anodes for enhanced current generation in microbial electrochemical cells. Biosens Bioelectron, 2011, 26 (5) : 1908–1912 (in Chinese). |
| [48] | Alatraktchi FA, Zhang YF, Angelidaki I. Nano-modification of the electrodes in microbial fuel cell: impact of nanoparticle density on electricity production and microbial community. Appl Energy, 2014 : 216–222 (in Chinese). |
| [49] | Wu WG, Xie RG, Bai LL, et al. Direct electrochemistry of Shewanella loihica PV-4 on gold nanoparticles-modified boron-doped diamond electrodes fabricated by layer-by-layer technique. J Nanosci Nanotechnol, 2012, 12 (5) : 3903–3908 (in Chinese). |
| [50] | Lü ZS, Xie DH, Yue XJ, et al. Ruthenium oxide-coated carbon felt electrode: a highly active anode for microbial fuel cell applications. J Power Sources, 2012 : 26–31 (in Chinese). |
| [51] | Park DH, Zeikus JG. Impact of electrode composition on electricity generation in a single-compartment fuel cell using Shewanella putrefaciens. Appl Microbiol Biotechnol, 2002, 59 (1) : 58–61 (in Chinese). |
| [52] | Wen ZH, Ci SQ, Mao S, et al. TiO2 nanoparticles-decorated carbon nanotubes for significantly improved bioelectricity generation in microbial fuel cells. J Power Sources, 2013 : 100–106 (in Chinese). |
| [53] | Tang JH, Yuan Y, Liu T, et al. High-capacity carbon-coated titanium dioxide core-shell nanoparticles modified three dimensional anodes for improved energy output in microbial fuel cells. J Power Sources, 2015 : 170–176 (in Chinese). |
| [54] | Garcia-Gomez NA, Balderas-Renteria I, Garcia-Gutierrez DI, et al. Development of mats composed by TiO2 and carbon dual electrospun nanofibers: a possible anode material in microbial fuel cells. Mater Sci Eng B, 2015 : 130–136 (in Chinese). |
| [55] | Wang YQ, Li B, Cui D, et al. Nano-molybdenum carbide/carbon nanotubes composite as bifunctional anode catalyst for high-performance Escherichia coli-based microbial fuel cell. Biosens Bioelectron, 2014 : 349–355 (in Chinese). |
| [56] | Singh S, Verma N. Fabrication of Ni nanoparticles-dispersed carbon micro-nanofibers as the electrodes of a microbial fuel cell for bio-energy production. Int J Hydrogen Energy, 2015, 40 (2) : 1145–1153 (in Chinese). |
| [57] | Kargi F, Eker S. Electricity generation with simultaneous wastewater treatment by a microbial fuel cell (MFC) with Cu and Cu-Au electrodes. J Chem Technol Biotechnol, 2007, 82 (7) : 658 (in Chinese). |
| [58] | Kumar GG, Sarathi VG, Nahm KS. Recent advances and challenges in the anode architecture and their modifications for the applications of microbial fuel cells. Biosens Bioelectron, 2013 : 461–475 (in Chinese). |
| [59] | Qiao Y, Li CM, Bao SJ, et al. Carbon nanotube/polyaniline compsite as anode material for microbial fuel cell. J Power Sources, 2007, 170 (1) : 79–84 (in Chinese). |
| [60] | Qiao Y, Bao SJ, Li CM, et al. Nanostructured polyaniline/titanium dioxide composite anode for microbial fuel cells. ACS Nano, 2008, 2 (1) : 113–119 (in Chinese). |
| [61] | Ding CM, Liu H, Zhu Y, et al. Control of bacterial extracellular electron transfer by a solid-state mediator of polyaniline nanowire arrays. Energy Environ Sci, 2012, 5 (9) : 8517–8522 (in Chinese). |
| [62] | Ding CM, Liu H, Lü ML, et al. Hybrid bio-organic interfaces with matchable nanoscale topography for durable high extracellular electron transfer activity. Nanoscale, 2014, 6 (14) : 7866–7871 (in Chinese). |
| [63] | Liu X, Wu WG, Gu ZZ. Poly(3,4-ethylene dioxythiophene) promotes direct electron transfer at the interface between Shewanella loihica and the anode in a microbial fuel cell. J Power Sources, 2015 : 110–115 (in Chinese). |
 2016, Vol. 32
2016, Vol. 32