中国科学院微生物研究所、中国微生物学会主办
文章信息
- 殷如, 洪葵
- Yin Ru, Hong Kui
- 丝状真菌二倍半萜化合物及其合成酶
- Filamentous fungal sesterterpenoids and their synthases
- 生物工程学报, 2016, 32(12): 1631-1641
- Chin J Biotech, 2016, 32(12): 1631-1641
- 10.13345/j.cjb.160129
-
文章历史
- Received: March 30, 2016
- Accepted: May 4, 2016
萜类化合物是所有异戊二烯聚合物及其衍生物的总称,是小分子天然产物中最大的一类化合物,到目前为止已发现超过60 000多种[1],根据所含异戊二烯单位数目不同可分为单萜、倍半萜、二萜、二倍半萜和三萜等。萜类化合物广泛存在于自然界,是构成某些植物的香精、树脂、色素等的主要成分。如玫瑰油、桉叶油、松脂等都含有多种萜类化合物。另外,某些动物的激素、维生素等也属于萜类化合物。萜类化合物是小分子天然产物中结构最多样的化合物,其在药物中也扮演着重要的角色。如单萜类的冰片、倍半萜类的过氧化物青蒿素、环状二萜类的紫杉醇、单碳环形二倍半萜类的曼诺力得等[2]。
二倍半萜是萜类化合物中的稀有类型,只占萜类总数不到2%,主要分布在植物、真菌、海洋生物海绵、地衣及昆虫分泌物中,绝大多数二倍半萜类化合物都是从海绵中提取分离得到的[3]。二倍半萜类化合物按照其结构中碳环的数量可以分为以下几类[4]:直线型二倍半萜、单碳环型二倍半萜、双碳环型二倍半萜、三碳环型二倍半萜、四碳环型二倍半萜和混杂型二倍半萜,这些化合物大多具有广泛的生理活性,如消炎[5]、抗菌[6]、抗结核[7]、抗癌[8]和细胞毒活性[9]等,在药物开发中具有良好前景[10]。相比其他萜类合成酶,二倍半萜合成酶的研究主要集中在丝状真菌二倍半萜合成酶的研究。自2013年从棒曲霉Aspergillus clavatus中克隆得到了第一个二倍半萜合成酶基因,通过大肠杆菌表达系统表达出蛇孢假壳素ophiobolin F合成酶AcOS[11],到目前为止报道了3个丝状真菌来源的二倍半萜合成酶和1个改造后的二倍半萜合成酶。随着对这些已知的二倍半萜合成酶蛋白序列、结构与功能特征的了解,越来越多的二倍半萜合成酶将被挖掘、研究和利用。本文从丝状真菌来源的二倍半萜化合物,丝状真菌二倍半萜合成酶结构与功能特征进行简要综述。
1 丝状真菌来源的二倍半萜化合物从1965年至今发现的70种丝状真菌来源的二倍半萜化合物,其化学结构如图 1所示。palinurin A (1)和B (2)[12]分离自海绵来源的小克银汉霉菌Cunninghamella sp. NRRL5695,丝状真菌来源的直线型二倍半萜至今只有这两例报道。到目前为止还没有发现丝状真菌来源的单碳环型和双碳环型二倍半萜化合物。来源于丝状真菌的三碳环型二倍半萜化合物有37种,包括29种蛇孢假壳素ophiobolins类化合物(3-31)和8种terretonins类化合物(32-39),其骨架结构不同,ophiobolins类二倍半萜化合物具有5-8-5三环骨架,terretonins类二倍半萜化合物为6-6-6三环骨架。

|
| 图 1 丝状真菌来源的二倍半萜化合物 Figure 1 Sesterterpenoids from filamentous fungi. |
| |
从胡麻斑病菌Bipolaris oryzae中分离得到的ophiobolin A (3)[13]作为首个被发现的ophiobolins类化合物,除具有细胞毒和抗疟疾活性外,还是一种重要的钙调蛋白抑制剂[14-15],最新的研究表明化合物3具有显著的抗脑胶质瘤活性[16],而其衍生物3-anhydro-6-hydroxy-ophiobolin A (5)能够促进PC12细胞α突触蛋白的降解,在帕金森病的治疗中有潜在应用[17]。6-epi-3-anhydro ophiobolin B (7)[18]首次从异旋孢腔菌Cochliobolus heterostrophus分离得到的,同样分离于该菌株中的还有ophiobolin C (8)、ophiobolin M (20-21),通过对秀丽隐杆线虫Caenorhabditis elegans运动性实验发现20和21化合物有潜在的抗线虫活性[19]。从内脐蠕孢属Drechslera gigantea中首次分离得到的ophiobolin E (9)和8-epi-ophiobolin J (17),是一种潜在的真菌除草剂,其中分离得到的ophiobolin B (6)对芽孢杆菌有很高的细胞毒活性,而分离得到的ophiobolin J (16)对芽孢杆菌只有很低或者无抗菌活性[20]。从焦曲霉Aspergillusustus分离得到的ophiobolin G (11)和H (14)都表现出对枯草芽孢杆菌的抑制作用[21]。6-epi-ophiobolin G (12)和6-epi-ophiobolin N (22)从海洋翘孢霉属Emericella variecolorGF10真菌中分离获得[22]。从双极霉属Bipolaris分离的ophiobolin I (15)具有细胞毒活性[23]。从海洋来源的曲霉属Aspergillus calidoustus真菌分离得到的ophiobolin K (18)和6-epi-ophiobolin K (19)具有抗真菌、线虫和细胞毒活性[24]。从Aspergillus属中分离得到ophiobolin O (23)和6-epi-ophiobolin O (24)[25],其中23不仅能抑制乳腺癌细胞株MCF-7的增殖,还能逆转乳腺癌耐药细胞株MCF-7/ADR对阿霉素的耐药性[26],ophiobolin O可以增加阿霉素耐药的乳腺癌细胞的敏感性,0.1 μmol/L ophiobolin O与阿霉素联合用药,可以使耐药细胞的阿霉素IC50从(74.00±0.18) μmol/L降低到(6.67±0.98) μmol/L,约11倍[27]。从地衣来源的单格孢属Ulocladium中分离到6-epi-ophiobolin G (12)、6-epi-21, 21-O-dihydroophiobolin G (13)和ophiobolin P-T (25-29),其中化合物12和29对Hep G2细胞表现出很强的细胞毒活性,其IC50分别为0.37和0.24 μmol/L,25和29化合物对芽孢杆菌表现出温和的抗菌活性[28]。从海洋来源的土曲霉Aspergillus terreus分离得到terretonins (32)和terretonins A-D (33-36)类化合物[29],后续又从曲霉属Aspergillus insuetus分离了terretonins E (37)和F (38),37和38具有抑制线粒体呼吸链的功能[30],最近又从Aspergillus sp. OPMF00272中分离到terretonins G (39)对革兰氏阳性菌有抑制作用,对革兰氏阴性菌和真菌没有抑制作用[31]。
已知来源于真菌的四碳环型二倍半萜化合物有13种,其骨架结构各异。从海洋来源的异孢镰刀菌Fusarium heterosporum中分离得到mangicols A-G (40-46),其中化合物40和41有显著的消炎活性[32],又从该属菌株中分离得到neomangicols A-C (47-49),化合物47和48对HCT-116人结肠癌细胞有细胞毒活性,其中化合物47还具有抗芽孢杆菌活性[33]。从红树林真菌Aspergillus sp. 085242分离得到的asperterpenols A (50)和B (51)抑制乙酰胆碱酯酶的IC50值分别为2.3和3.0 μmol/L[34]。从变色曲霉Aspergillus variecolorMF138中分离得到的变色曲霉素variecolin (52)也是一种新骨架结构的四碳环型二倍半萜,是一种血管紧张肽Ⅱ受体结合抑制剂[35]。
混杂型二倍半萜化合物已发现有18种,结构复杂多样,具有多种生物活性。从植物内生菌翘孢霉属Emericellasp. AST0036中分离得到了emericellenes A-E (53-57)[36],从拟茎点霉属Phomopsissp.XZ-26分离得到的terpestacin (58)能抑制肿瘤血管生成和具有温和的抗菌活性[37]。镰刀菌Fusarium subglutinans中分离得到的霉菌毒素fusaproliferin (59),对人结肠腺癌Caco-2细胞有细胞毒活性[38]。从Emericellapurpurea分离得到的variecolol (60)、variecolactone (61)、variecoacetals A (62)和B (63)是该真菌免疫调节物质成分[39]。从AspergillusCNM-713分离得到的aspergilloxide (64)[40]和从变色翘霉菌Emericella variecolor分离得到的emervaridione (65)[41]都属于混杂型二倍半萜化合物。从海绵来源的青霉属Penicillium citrinum真菌分离得到的cyclocitrinol (66)[42]、isocyclocitrinol A (67)、22-acetylisocyclocitrinol (68)是3个骨架相同的混杂型二倍半萜化合物,其中67和68化合物对表皮葡球菌和肠球菌有抗菌活性[43]。将Emericella variecolor海洋真菌二倍半萜合成酶基因在米曲霉中异源表达分离得到stellata-2, 6, 19-triene (69),在细胞色素氧化酶作用下69进一步被氧化成stellatic acid (70)[44]。
2 丝状真菌二倍半萜合成酶结构与功能特征萜类的生物合成可以分为4步:1)五碳单体二甲基丙烯焦磷酸(Dimethylallyl diphosphate,DMAPP)和异戊烯焦磷酸(Isopentenyl diphosphate,IPP)的合成;2)聚合反应形成线性多聚异戊烯焦磷酸前体;3)环化或重排形成萜类骨架;4)骨架的修饰。丝状真菌中DMAPP和IPP通过甲羟戊酸(MVA)途径合成。IPP在异构酶作用下可以转化为DMAPP。以DMAPP起始,在异戊烯基转移酶(Prenyltransferases,PT)作用下与IPP首尾连接形成线性聚异戊烯基焦磷酸前体如牻牛儿基焦磷酸(Geranyl diphosphate,GPP)、法尼基焦磷酸(Farnesyl diphosphate,FPP)、牻牛儿基牻牛儿基焦磷酸(Geranylgeranyl diphosphate,GGPP)和牻牛儿基法尼基焦磷酸(Geranylfarnesyl diphosphate,GFPP)等。然后萜类环化酶(Terpene cyclase,TC)对这些焦磷酸前体进行环化或重排形成萜类骨架,进一步后修饰(如氧化、脱水等)生成各种萜类化合物[45]。
已经发现的来源于丝状真菌的二倍半萜合成酶都是具有N端TC结构域和C端PT结构域的嵌合酶,能催化聚异戊烯基焦磷酸链延伸与环化两步反应形成二倍半萜化合物骨架。这种嵌合型的萜类合成酶最早在桃拟茎点霉菌Phomopsis amygdali中发现,是催化二萜化合物壳梭孢(菌)素合成的酶PaFS,其PT结构域与TC结构域能独立发挥功能。单独异源表达嵌合型二萜合成酶PaFS的N端和C端片段,可分别检测到萜类环化酶活性与异戊烯基转移酶活性[46]。2013年Chiba等根据PaFS的序列特征在棒曲霉菌Aspergillusclavatus基因组中同源检索这类二萜合成酶时发现了acla_76850基因[11],将该基因在米曲霉表达系统表达检测到了ophiobolin F化合物,又通过对该基因编码蛋白的体外反应证实了该蛋白是ophiobolin F合成酶AcOS。近几年来根据AcOS氨基酸序列信息在不同菌株基因组中进行同源比对分析,又挖掘出了费希新萨托菌Neosartorya fischeri中的sesterfisherol合成酶NfSS[47]和异冠裸胞壳菌Emericella variecolor中的stellatic acid合成酶EvSS[44]。根据嵌合酶的这种特性,Qin等通过来源于Emericella variecolor二倍半萜合成酶EvSS的PT结构域与variediene二萜合成酶EvVS的TC结构域重构了一种新的二倍半萜合成酶,能合成新骨架的二倍半萜化合物[48]。本课题组在对一株合成ophiobolins类化合物红树林来源的焦曲霉研究中发现了具有二倍半萜合成酶功能的蛋白Au8003。
图 2为上述4例已经发现的丝状真菌二倍半萜合成酶序列,显示这些酶的共同氨基酸序列特征,其N端为TC结构域,含有DDXXD和NSE/DTE两个保守区域,具有ClassⅠTC特征[49-51]。其中DDXXD保守区域与Mg2+结合负责脱去底物末端的焦磷酸基团,是严格保守的。而NSE/DTE是非严格保守区域,其中天冬酰胺/天冬氨酸(N/D)可以被组氨酸(H)替换[44]。其C端为PT结构域,含有两个富含天冬氨酸的保守区[52-53] DDXXD (The first aspartate-rich motif,FARM)和DDXXN (The second aspartate-rich motif,SARM)。这两个保守区是催化烯丙基底物反应的活性位点,烯丙基底物通过Mg2+与其结合,催化聚异戊烯基链的延长[54-55]。FARM及其空间结构周围关键位置的氨基酸侧链基团的大小决定了疏水口袋的深度,从而控制产物的链长[56]。与异戊烯基转移酶相比SAFM保守区最后一个氨基酸由天冬氨酸变成了天冬酰胺,这种变化对于催化机制的影响有待进一步研究。在C端的G (Q/E)比较保守,有研究表明该区域影响聚异戊烯基焦磷酸的链长[57]。

|
| 图 2 几种二倍半萜合成酶氨基酸序列比对 Figure 2 Amino acid sequence alignment of several sesterterpene synthases. Highly conserved residues in the four sequences are black, shaded in gray are amino acid positions with 76%−95% of similarity, highly divergent amino acid positions are unshaded. |
| |
丝状真菌二半萜合成酶除结构上的嵌合性特征外,在催化功能上表现出非特异性,可以分别以DMAPP、GPP、FPP及GGPP为底物,添加IPP生成二倍半萜化合物骨架[11, 46-48]。一些二倍半萜合成酶非特异性还进一步体现在以下两个方面:一方面在催化同一底物时得到多种的产物,如来源于Aspergillus clavatus的AcOS二倍半萜合成酶在催化GFPP形成ophiobolin F的同时也合成了另外3种二倍半萜化合物,这4种二倍半萜化合物的环化方式不同[11];另一方面一种二倍半萜合成酶能催化不同的反应,比如Emericella variecolor的EvVS萜类合成酶不仅催化二倍半萜(2E)-α-cericerene的合成,还可以催化二萜variediene的合成[48]。
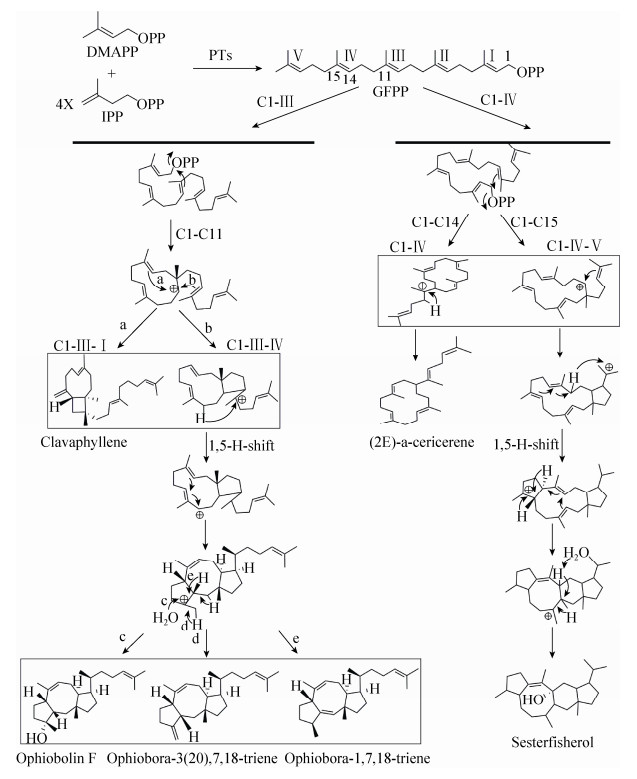
根据目前报道的真菌二半萜合成酶合成的几种二倍半萜化合物骨架结构[11, 47-48],推测这几种结构的环化机制如图 3所示,包括3步反应。第一步,脱去焦磷酸的碳正离子与双键发生亲电加成反应,主要有两种形式即C1与GFPP中的第3个双键加成(C1-Ⅲ)或与第4个双键加成(C1-Ⅳ),C1可以与双键上的任意一个碳连接成环;第二步,在第一次成环的基础上碳正离子直接进攻双键形成新环或者通过氢离子迁移进行重排后进攻双键成环。最后,碳正离子通过脱去相邻碳上的氢离子或者从H2O中获取OH-平衡电荷,完成环化反应。通过对二倍半萜化合物骨架环化机制的分析,虽然只有C1-Ⅲ和C1-Ⅳ环化方式的报道,但C1与Ⅱ、Ⅴ也有直接亲电加成的可能性,与烯键加成也有两种连接方式,后续进一步环化也具有多种选择,平衡电荷的方式也有多种可能,形成不同结构的二倍半萜化合物骨架。目前已经分离得到了具有多种骨架结构的二倍半萜化合物,其环化方式各不相同,所以二倍半萜合成酶催化底物的环化方式具有多样性。
本课题组从文昌头苑红树林木揽根系土壤中分离得到Aspergillus ustus 094102,该菌株可产生ophiobolins二倍半萜化合物[26],通过对该菌株基因组的测序与生物信息学分析,以及基因敲除发现了与ophiobolin合成相关的基因au8003,体外蛋白功能分析表明Au8003是ophiobolin F合成酶,该酶具备上述丝状真菌萜类合成酶特点,具有异戊烯基转移酶和萜类环化酶两种功能,能够以DMAPP、GPP、FPP及GGPP为底物,添加IPP生成GFPP并环化形成ophiobolin F (相关结果另文发表),其环化机制及详细的酶学特征还在进一步研究中。
3 小结与展望红树林真菌Aspergillus ustus 094102可产生一系列ophiobolins二倍半萜化合物,其中ophiobolin O对耐药乳腺癌细胞有抑制作用[26-27],有良好的成药前景,对这类化合物合成酶的认识,有利于解析ophiobolins二倍半萜化合物的生物合成途径,为后续利用生物工程手段获取大量化合物提供基础。虽然二倍半萜化合物是萜类化合物的稀有类群,丝状真菌来源的二倍半萜化合物已表现出多种潜在药用价值。目前只有少数二倍半萜化合物如ophiobolin F、sesterfisherol等的合成酶被研究,其他二倍半萜骨架结构如mangicols、terretonins、emericellenes和asperterpenol类的合成酶还有待认识。对真菌二倍半萜合成酶进一步挖掘与研究可以用来指导二倍半萜天然产物的发现。二倍半萜合成酶的晶体结构研究对揭示其底物多样性和环化机制多样性具有重要意义,可为后续二倍半萜化合物生物合成提供理论基础,有利于指导利用代谢工程和合成生物学手段进行二倍半萜化合物的人工合成。
| [1] | Guan Z, Xue D, Abdallah II, et al. Metabolic engineering of Bacillus subtilis for terpenoid production. Appl Microbiol Biotechnol, 2015, 99 (22) : 9395–9406. DOI: 10.1007/s00253-015-6950-1 |
| [2] | Ajikumar PK, Tyo K, Carlsen S, et al. Terpenoids: opportunities for biosynthesis of natural product drugs using engineered microorganisms. Mol Pharm, 2008, 5 (2) : 167–190. DOI: 10.1021/mp700151b |
| [3] | Wang L, Yang B, Lin XP, et al. Sesterterpenoids. Nat Prod Rep, 2013, 30 (3) : 455–473. DOI: 10.1039/c3np20089b |
| [4] | Liu Y, Wang L, Jung JH, et al. Sesterterpenoids. Nat Prod Rep, 2007, 24 (6) : 1401–1429. DOI: 10.1039/b617259h |
| [5] | Schumacher M, Juncker T, Schnekenburger M, et al. Natural compounds as inflammation inhibitors. Genes Nutr, 2011, 6 (2) : 89–92. DOI: 10.1007/s12263-011-0231-0 |
| [6] | Baquero F, Coque TM, de la Cruz F. Ecology and evolution as targets: the need for novel eco-evo drugs and strategies to fight antibiotic resistance. Antimicrob Agents Chemother, 2011, 55 (8) : 3649–3660. DOI: 10.1128/AAC.00013-11 |
| [7] | García A, Bocanegra-García V, Palma-Nicolás JP, et al. Recent advances in antitubercular natural products. Eur J Med Chem, 2012, 49 : 1–23. DOI: 10.1016/j.ejmech.2011.12.029 |
| [8] | Sithranga Boopathy N, Kathiresan K. Anticancer drugs from marine flora: an overview. J Oncol, 2010, 2010 : 214186. DOI: 10.1155/2010/214186 |
| [9] | Villa FA, Gerwick L. Marine natural product drug discovery: leads for treatment of inflammation, cancer, infections, and neurological disorders. Immunopharmacol Immunotoxicol, 2010, 32 (2) : 228–237. DOI: 10.3109/08923970903296136 |
| [10] | Zhang C, Liu Y. Targeting cancer with sesterterpenoids: the new potential antitumor drugs. J Nat Med, 2015, 69 (3) : 255–266. DOI: 10.1007/s11418-015-0911-y |
| [11] | Chiba R, Minami A, Gomi K, et al. Identification of ophiobolin F synthase by a genome mining approach: a sesterterpene synthase from Aspergillus clavatus. Org Lett, 2013, 15 (3) : 594–597. DOI: 10.1021/ol303408a |
| [12] | El Sayed KA, Mayer AM, Kelly M, et al. The biocatalytic transformation of furan to amide in the bioactive marine natural product palinurin. J Org Chem, 1999, 64 (25) : 9258–9260. DOI: 10.1021/jo990539j |
| [13] | Nozoe S, Morisaki M, Tsuda K, et al. The structure of ophiobolin, a C25 terpenoid having a novel skeleton. J Am Chem Soc, 1965, 87 (21) : 4968–4970. |
| [14] | Leung PC, Taylor WA, Wang JH, et al. Role of calmodulin inhibition in the mode of action of ophiobolin a. Plant Physiol, 1985, 77 (2) : 303–308. DOI: 10.1104/pp.77.2.303 |
| [15] | Peters C, Mayer A. Ca2+/calmodulin signals the completion of docking and triggers a late step of vacuole fusion. Nature, 1998, 396 (6711) : 575–580. DOI: 10.1038/25133 |
| [16] | Dasari R, Masi M, Lisy R, et al. Fungal metabolite ophiobolin A as a promising anti-glioma agent: in vivo evaluation, structure-activity relationship and unique pyrrolylation of primary amines. Bioorg Med Chem Lett, 2015, 25 (20) : 4544–4548. DOI: 10.1016/j.bmcl.2015.08.066 |
| [17] | Xue D, Wang Q, Chen Z, et al. 3-anhydro-6-hydroxy-ophiobolin A, a fungal sesterterpene from Bipolaris oryzae induced autophagy and promoted the degradation of α-synuclein in PC12 cells. Bioorg Med Chem Lett, 2015, 25 (7) : 1464–1470. DOI: 10.1016/j.bmcl.2015.02.030 |
| [18] | Shen X, Krasnoff SB, Lu SW, et al. Characterization of 6-epi-3-anhydroophiobolin B from Cochliobolus heterostrophus. J Nat Prod, 1999, 62 (6) : 895–897. DOI: 10.1021/np980462e |
| [19] | Tsipouras A, Adefarati AA, Tkacz JS, et al. Ophiobolin M and analogues, noncompetitive inhibitors of ivermectin binding with nematocidal activity. Bioorg Med Chem, 1996, 4 (4) : 531–536. DOI: 10.1016/0968-0896(96)00036-3 |
| [20] | Evidente A, Andolfi A, Cimmino A, et al. Ophiobolin E and 8-epi-ophiobolin J produced by Drechslera gigantea, a potential mycoherbicide of weedy grasses. Phytochemistry, 2006, 67 (20) : 2281–2287. DOI: 10.1016/j.phytochem.2006.07.016 |
| [21] | Cutler HG, Crumley FG, Cox RH, et al. Ophiobolins G and H: new fungal metabolites from a novel source, Aspergillus ustus. J Agric Food Chem, 1984, 32 (4) : 778–782. DOI: 10.1021/jf00124a019 |
| [22] | Hong W, Takuya Itoh, Masahiro Kinoshita, et al. Cytotoxic sesterterpenes, 6-epi-ophiobolin G and 6-epi-ophiobolin N, from marine derived fungus Emericella variecolorGF10. Tetrahedron, 2004, 60 (28) : 6015–6019. DOI: 10.1016/j.tet.2004.05.021 |
| [23] | Ahn JW, Lee MK, Choi SU, et al. Cytotoxic ophiobolins produced byBipolaris sp. J Microbiol Biotechnol, 1998, 8 : 406–408. |
| [24] | Rodrigues de Carvalho C, Vieira Mde L, Cantrell CL, et al. Biological activities of ophiobolin K and 6-epi-ophiobolin K produced by the endophytic fungus Aspergillus calidoustus. Nat Prod Res, 2016, 30 (4) : 478–481. DOI: 10.1080/14786419.2015.1022777 |
| [25] | Zhang D, Fukuzawa S, Satake M, et al. Ophiobolin O and 6-epi-ophiobolin O, two new cytotoxic sesterterpenes from the marine derived fungus Aspergillussp. Nat Prod Commun, 2012, 7 (11) : 1411–1414. |
| [26] | Yang T, Lu Z, Meng L, et al. The novel agent ophiobolin O induces apoptosis and cell cycle arrest of MCF-7 cells through activation of MAPK signaling pathways. Bioorg Med Chem Lett, 2012, 22 (1) : 579–585. DOI: 10.1016/j.bmcl.2011.10.079 |
| [27] | Sun W, Lv C, Zhu T, et al. Ophiobolin-O reverses adriamycin resistance via cell cycle arrest and apoptosis sensitization in adriamycin-resistant human breast carcinoma (MCF-7/ADR) cells. Marine Drugs, 2013, 11 (11) : 4570–4584. DOI: 10.3390/md11114570 |
| [28] | Wang QX, Bao L, Yang XL, et al. Ophiobolins P-T, five new cytotoxic and antibacterial sesterterpenes from the endolichenic fungus Ulocladiumsp. Fitoterapia, 2013, 90 : 220–227. DOI: 10.1016/j.fitote.2013.08.002 |
| [29] | Li GY, Li BG, Yang T, et al. Sesterterpenoids, terretonins A-D, and an alkaloid, asterrelenin, from Aspergillus terreus. J Nat Prod, 2005, 68 (8) : 1243–1246. DOI: 10.1021/np0501738 |
| [30] | López-Gresa MP, Cabedo N, González-Mas MC, et al. Terretonins E and F, inhibitors of the mitochondrial respiratory chain from the marine-derived fungus Aspergillus insuetus. J Nat Prod, 2009, 72 (7) : 1348–1351. DOI: 10.1021/np900085n |
| [31] | Fukuda T, Kurihara Y, Kanamoto A, et al. Terretonin G, a new sesterterpenoid antibiotic from marine-derived Aspergillussp.OPMF00272. J. Antibiot, 2014, 67 (8) : 593–595. DOI: 10.1038/ja.2014.46 |
| [32] | Renner MK, Jensen PR, Fenical W. Mangicols: structures and biosynthesis of a new class of sesterterpene polyols from a marine fungus of the genus Fusarium. J Org Chem, 2000, 65 (16) : 4843–4852. DOI: 10.1021/jo000081h |
| [33] | Renner MK., Jensen PR, Fenica W. Neomangicols: structures and absolute stereochemistries of unprecedented halogenated sesterterpenes from a marine fungus of the genus Fusarium. J Org Chem, 1998, 63 (23) : 8346–8354. DOI: 10.1021/jo981226b |
| [34] | Xiao Z, Huang H, Shao C, et al. Asperterpenols A and B, new sesterterpenoids isolated from a mangrove endophytic fungus Aspergillus sp. 085242. Org Lett, 2013, 15 (10) : 2522–2525. DOI: 10.1021/ol401005j |
| [35] | Hensens OD, Zink D, Williamson JM, et al. Variecolin, a sesterterpenoid of novel skeleton from Aspergillus variecolor MF138. J Org Chem, 1991, 56 (10) : 3399–3403. DOI: 10.1021/jo00010a040 |
| [36] | Xu YM, Espinosa-Artiles P, Liu MX, et al. Secoemestrin D, a cytotoxic epitetrathiodio xopiperizine and emericellenes A-E, five sesterterpenoids from Emericella sp. AST0036, a fungal endophyte of Astragalus lentiginosus. J Nat Prod, 2013, 27, 76 (12) : 2330–2336. |
| [37] | Xiang L, Lu C, Hu Z, et al. Secondary metabolites of Phomopsis sp. XZ-26, an endophytic fungus from Camptotheca acuminate. Eur J Org Chem, 2009, 2009 (18) : 2975–2982. DOI: 10.1002/ejoc.v2009:18 |
| [38] | Sewram V, Nieuwoudt TW, Marasas WF, et al. Determination of the fusarium mycotoxins, fusaproliferin and beauvericin by high-performance liquid chromatography-electrospray ionization mass spectrometry. J Chromatogr A, 1999, 858 (2) : 175–185. DOI: 10.1016/S0021-9673(99)00814-6 |
| [39] | Fujimoto H, Nakamura E, Okuyama E, et al. Immunomodulatory constituents from an ascomycete, Emericella aurantio-brunnea. Chem Pharm Bull, 2000, 48 (10) : 1436–1441. DOI: 10.1248/cpb.48.1436 |
| [40] | Cueto M, Jensen PR, Fenical W. Aspergilloxide, a novel sesterterpene epoxide from a marine-derived fungus of the genus Aspergillus. Org Lett, 2002, 4 (9) : 1583–1585. DOI: 10.1021/ol0258076 |
| [41] | Liangsakul J, Pornpakakul S, Sangvichien E, et al. Emervaridione and varioxiranediol, two new metabolites from the endophytic fungus, Emericella variecolor. Tetrahedron Lett, 2011, 52 (48) : 6427–6430. DOI: 10.1016/j.tetlet.2011.09.079 |
| [42] | Kozlovsky AG, Zhelifonova VP, Ozerskaya SM, et al. Cyclocitrinol, a new fungal metabolite from Penicillium citrinum. Pharmazie, 2000, 55 (6) : 470–471. |
| [43] | Amagata T, Amagata A, Tenney K, et al. Unusual C25 steroids produced by a sponge-derivedpenicillium citrinum. Org Lett, 2003, 5 (23) : 4393–4396. DOI: 10.1021/ol0356800 |
| [44] | Matsuda Y, Mitsuhashi T, Quan Z, et al. Molecular basis for stellatic acid biosynthesis: a genome mining approach for discovery of sesterterpene synthases. Org Lett, 2015, 17 (18) : 4644–4647. DOI: 10.1021/acs.orglett.5b02404 |
| [45] | Chang MC, Keasling JD. Production of isoprenoid pharmaceuticals by engineered microbes. Nat Chem Biol, 2006, 2 (12) : 674–681. DOI: 10.1038/nchembio836 |
| [46] | Toyomasu T, Tsukahara M, Kaneko A, et al. Fusicoccins are biosynthesized by an unusual chimera diterpene synthase in fungi. Proc Natl Acad Sci USA, 2007, 104 (9) : 3084–3088. DOI: 10.1073/pnas.0608426104 |
| [47] | Ye Y, Minami A, Mandi A, et al. Genome mining for sesterterpenes using bifunctional terpene synthases reveals a unified intermediate of di/sesterterpenes. J Am Chem Soc, 2015, 137 (36) : 11846–11853. DOI: 10.1021/jacs.5b08319 |
| [48] | Qin B, Matsuda Y, Mori T, et al. An unusual chimeric diterpene synthase from emericella variecolor and its functional conversion into a sesterterpene synthase by domain swapping. Angew Chem Int Ed Engl, 2016, 55 (5) : 1658–1661. DOI: 10.1002/anie.201509263 |
| [49] | Gao Y, Honzatko RB, Peters RJ. Terpenoid synthase structures: a so far incomplete view of complex catalysis. Nat Prod Rep, 2012, 29 (10) : 1153–1175. DOI: 10.1039/c2np20059g |
| [50] | Wendt KU, Schulz GE. Isoprenoid biosynthesis: manifold chemistry catalyzed by similar enzymes. Structure, 1998, 6 (2) : 127–133. DOI: 10.1016/S0969-2126(98)00015-X |
| [51] | Christianson DW. Structural biology and chemistry of the terpenoid cyclases. Chem Rev, 2006, 106 (8) : 3412–3442. DOI: 10.1021/cr050286w |
| [52] | Chen A, Kroon PA, Poulter CD. lsoprenyl diphosphate synthases: protein sequence comparisons, a phylogenetic tree and predictions of secondary structure. Protein Sci, 1994, 3 (4) : 600–607. |
| [53] | Wang K, Ohnuma S. Chain-length determination mechanism of isoprenyl diphosphate synthases and implications for molecular evolution. Trends Biochem Sci, 1999, 24 (11) : 445–451. DOI: 10.1016/S0968-0004(99)01464-4 |
| [54] | Song L, Poulter CD. Yeast farnesyl-diphosphate synthase site-directed mutagenesis of residues in highly conserved prenyltransferase domain-I and domain-II. Proc Natl Acad Sci USA, 1994, 91 (8) : 3044–3048. DOI: 10.1073/pnas.91.8.3044 |
| [55] | Tarshis LC, Proteau PJ, Kellogg BA, et al. Regulation of product chain length by isoprenyl diphosphate synthases. Proc Natl Acad Sci USA, 1996, 93 (26) : 15018–15023. DOI: 10.1073/pnas.93.26.15018 |
| [56] | Wang C, Chen Q, Fan D, et al. Structural analyses of short-chain prenyltransferases identify an evolutionarily conserved GFPPS clade in brassicaceae plants. Mol Plant, 2016, 9 (2) : 195–204. DOI: 10.1016/j.molp.2015.10.010 |
| [57] | Hemmi H, Noike M, Nakayama T, et al. An alternative mechanism of product chain-length determination in type III geranylgeranyl diphosphate synthase. Eur J Biochem, 2003, 270 (10) : 2186–2194. DOI: 10.1046/j.1432-1033.2003.03583.x |
 2016, Vol. 32
2016, Vol. 32