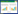服务
文章信息
- 李宁, 王祥, 柏雪源, 李志合, 张英
- Li Ning, Wang Xiang, Bai Xueyuan, Li Zhihe, Zhang Ying
- 热烟气气氛下生物质在流化床反应器中快速热解制取生物油
- Biomass fast pyrolysis for bio-oil production in a fluidized bed reactor under hot flue atmosphere
- 生物工程学报, 2015, 31(10): 1501-1511
- Chin J Biotech, 2015, 31(10): 1501-1511
- 10.13345/j.cjb.140554
-
文章历史
- Received: November 17, 2014
- Accepted: March 12, 2015
The diminishing reserves and apparent negative effects such as greenhouse gases and acid rain of fossil fuel have led the world community to recognize the importance of renewable and cleaner energy in recent years[1]. The recovery of energy from a renewable source like biomass involves chemical,biochemical and thermochemical processes,depending on the nature of the source. The advantages of using biomass are its negligible sulphur,nitrogen and metal content[2]. Fast pyrolysis of biomass is a thermochemical process for transforming solid biomass mainly into liquid production,which is referred to bio-oil,together with producing solid bio-char and non-condensable gases as valuable by-products. Bio-oil has high energy density compared to “as received” biomass and can be readily and economically transported by trucks or pipeline[3]. It is also regarded as a substitute for fuel oil or diesel in many static applications that use boilers,furnaces,engines,and turbines for heating or electricity generation[4]. The other two by-products of pyrolysis,gas can be used as an additive-fuel for the process,charcoal (bio-char) can be directly used as fuel or pre-material for activated carbon production[5]. Therefore,the technology of fast biomass pyrolysis has gained extensive attentions in recent years[6].
Fluidized beds have been considered as one of the most popular reactors of fast biomass pyrolysis due to its characteristics of high heat and mass transfer,easy scaling up and simple in operation. Previous studies have focused on the investigations of the influence of operation parameters,such as reaction temperature,the fluidising gas flow rate,vapour residence time,particle size,the feeding rate and species of the raw feedstocks,on the product yields in fluidized bed reactors[7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21]. However,the effects of bed materials on yields of bio-oil were not involved in these studies. The pyrolysis studies of cotton stalk[7],cassava plants[8],jatropha oil cake[9],rice straw[10, 11],bamboo sawdust[11],rice husk[12, 13, 14, 15],wood[16, 17],switch grass[18],sewage sludge[19],palm shells[20, 21],etc.,have been documented elsewhere. Whereas the pyrolysis studies of corn stalk (CS),furfural residue (FR),xylose residue (XR) and kelp seaweed (KS) are relatively few.
China is the biggest country in straw resources and its total output of straw resources amounts to more than 700 million tons a year. Especially,CS is one of the most abundant agricultural residues,which accounts for nearly 24% of the total output of straw resources[22]. In the countryside,the main treatments of CS were burning it and return to field or cooking food as a fuel. However,the utilization efficiency is low. So many research institutions are focused on the study of it. KS is also an abundant resource due to the vast marine areas of China. FR and XR are waste solids that contain amounts of acid. Traditional methods such as composting and incineration are not suitable to dispose these organic solid wastes as they contain low concentration of nitrogen for composting and a considerable amount of solid grains and smoke,which would be released to pollute the environment during incineration. Therefore,pyrolysis can convert these agricultural wastes to be more valuable energy forms. The technology is widely expected to offer a considerable contribution in terms of versatility,improved efficiency and environmental acceptability.
In order to maximize the energy of biomass,the technically and economically viable process should be selected. In this paper,a fluidized bed was developed for bio-oil production via fast biomass pyrolysis. The reactor uses hot flue gas generated by a coal furnace as fluidizing medium. Compared with conventional reactors,it is more economical because of flue gas being used as fluidizing gas instead of the consumption of expensive inert gases such as argon or nitrogen. In this study,fast pyrolysis experiments of four selected biomass feedstocks (CS,FR,XR and KS) were carried out using this developed fluidized bed reactor under flue gas atmosphere. Particularly,the main aim of the study was to investigate the optimal pyrolysis temperature and bed material for obtaining the highest bio-oil yield.
1 Materials and methods 1.1 MaterialsThe samples of CS,FR,XR and KS used in this study were milled,seized out to an average particle size between 0.2 mm and 0.83 mm. Prior to use,the samples were dried at 100−105 °C for 24 h in an automatic drying oven. Proximate analysis was determined by ASTM standard (E1755-01) and ultimate analysis was carried out by an EA-3000 (EuRoVector,Italy) elemental analyzer. Properties of four feedstocks,including proximate and elemental analyses were listed in Table1. The bed materials,viz.,quartz sand,aluminous soil and dolomite were also sieved to obtain a uniform size of 0.5−0.7 mm。
| Biomass feedstock | CS | FR | XR | KS |
| Proximate analysis (wt%) | ||||
| Moisture | 9.24 | 9.56 | 9.92 | 9.73 |
| Volatiles | 67.58 | 61.32 | 63.06 | 46.75 |
| Fixed carbon | 14.54 | 21.55 | 20.65 | 22.35 |
| Ash | 8.64 | 7.57 | 6.37 | 19.09 |
| HHV (kJ/kg) | 13 254 | 15 298 | 14 968 | 8 680 |
| Elemental analysis (wt%) | ||||
| C | 39.15 | 46.95 | 45.14 | 28.93 |
| H | 5.03 | 5.42 | 6.17 | 6.22 |
| O | 42.61 | 25.67 | 29.29 | 27.00 |
| N | 1.24 | 1.08 | 3.09 | 4.00 |
The experimental system used in the study is schematically shown in Fig.1. It mainly consists of a reactor,a coal furnace,a feeding system,a quenching unit,two series connected char removing cyclones,products collection and data acquisition system.
|
| Fig.1 Schematic diagram of the fluidized bed experimental system. |
The reactor was constructed of a heat resistant stainless steel pipe (Grades: 0Cr18Ni9Ti) with an internal diameter of 100 mm and a height of 900 mm. The internal diameter of the upper part of the reactor is larger than that of the bed section,which has an internal diameter of 200 mm and a height of 100 mm so as to decrease the gas turbulence,thus fluidized bed materials can be easily back to the fluidized bed section. Air was sent by a compressor into a coal furnace and in which oxygen was depleted by the combustion of coal,thus hot flue gas stream was obtained at the vent of the coal furnace. The adjustment and measurement of the gas flow rate was carried out with the helps of a pressure-relief valve and a rotameter. A gas distributor of bell type was placed at the bottom of the bed,which was served to equalize gas flow. The feeding system consisted two screw feeders. The first screw was used to control the feeding rate through rpm adjusting of the electric motor. The second one operated at a relatively high speed to feed biomass into the reactor and prevented jamming the feeding system due to the carbonization of biomass. One stream of non-condensable gas was introduced into the hopper keeping pressure balance between the fluidized bed and hopper to ensure the biomass into the hopper easily. The bio-oil collection unit consisted a shell-tube condenser and a glass ball condensing tube. The char was removed rapidly from the pyrolysis gas and solid multiphase by two series connected cyclones and collected with a stainless steel vessel. The reactor was heated externally by an electric wire heater,with the temperature being controlled by a K-type thermocouple (Tb) placed inside the reactor. The data of temperature can be obtained from the computer. The detective range of thermocouple was 0−1 100 ℃ with measurement accuracy ±1.
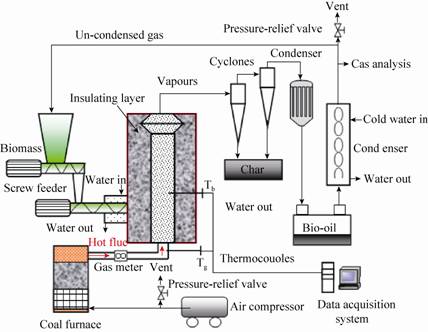
1.3 Methods 1.3.1 Determination of operating fluidization velocityA cold simulation test-bed made of plexiglas pipe,as shown in Fig.2,was built with a scale of 1:1 to determine the operating fluidization velocity. 70 mm height bed and nitrogen as fluidizing gas were chosen because it has a similar density and physical property with flue gas at ambient temperature. Experiments have been carried out by taking four different bed materials,viz.,quartz sand,aluminous soil and dolomite. In general,the flow characteristics of the materials in the bed were different with the gas flow rates. During experiments,increased the gas flow rate gradually and observed the fluidization appearance and the bed height through the meter ruler until expected fluidization phenomena occurred. The value under the best fluidization condition was chosen as the operating gas flow rate. According to the gas flow rate and diameter of the bed,the operating fluidization velocity can be determined. The ideal gas law of constant pressure was used to predict the values of gas flow rate and velocity of operating temperature.
|
| Fig.2 Schematic process of the cold simulation test-bed. |
Fast pyrolysis experiments were carried out in two series. Firstly,experiments of CS were performed to discover the optimal pyrolysis temperature and bed material for maximum bio-oil yield. The pyrolysis temperatures were selected as 450 °C,475 °C,500 °C and 525 °C as well as the bed materials were selected as quartz sand,aluminous soil and dolomite,respectively. Secondly,based on the results of the first series experiments,the optimized temperature and bed material were used to the experiments of FR,XS and KS.
All metal and glassware items used in pyrolysis experiments were weighed before and after each run in order to calculate the yields of pyrolysis products. By weighing the biomass feedstocks before and after the run,the amount of biomass fed can be calculated. The yield of solid was a combination of the char collected in the char vessels from both cyclones and reactor and transfer line. The liquid products were collected from the vessels connected with the shell-tube condenser and the glass ball condensing tube. After stratification occurred in the collected liquid,the upper phase was separated from the liquid and weight both of the remained (Heavy oil) and separated oil (Light oil),respectively. The yield of gas was calculated by the material balance. The yields of products were calculated as follows:
\[{\text{Yileds (wt% )}} = \frac{{{\text{Product (kg)}}}}{{{\text{Fed biomass (kg)}}}} \times 100\% \]
(1)
The bio-oils obtained under the experimental conditions that gave maximum oil yield (pyrolysis temperature of 500 °C,dolomite as bed material) were used for further characterization. Physical analysis of the bio-oils such as pH value,viscosity and calorific value were determined in the studies. The pH measurements were performed with a PHS-2F pH meter (Shanghai Precision and Science Instrument Co.,LTD). Dynamic viscosity data were determined with a NDJ-1 rotational viscometer (Shanghai Changyi Gealogical Instruments Co.,Ltd). Calorific values of the bio-oils were detected with a WZR-1T oxygen combustion bomb (Changsha Bente Apparatus).
1.3.4 Gas analysis The compositions of pyrolysis gas were detected by an Austrian gas analyzer (1904,Shanghai bank equipment limited company). The calorific value of the gas was calculated by the following formula (GB-T11062-1998,China).
\[\begin{gathered}
LHV/(kJ/{m^3}) = (30 \times CO\% + 25.7 \times {H_2} \hfill \\
\;\;\; + 85.4 \times C{H_4}\% + 151.3 \times {C_n}{H_m}\% ) \times 4.2 \hfill \\
\end{gathered} \]
(2)
The gas fraction was a volume ratio which can be expressed as follows:
\[{\text{Gas fraction (% )}} = \frac{{{\text{Certain gas volume (}}{{\text{m}}^{\text{3}}}{\text{)}}}}{{{\text{Totol gas volume (}}{{\text{m}}^{\text{3}}}{\text{)}}}} \times 100\% \]
(3)
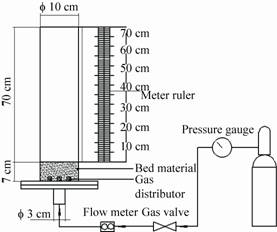
Fig.3 presents the bed height as a function of the gas flow rate. During experiments,four flow regimes were observed as the gas flow rate increasing,viz.,fixed bed (0−4 m3/h),bubbling fluidized bed (4−7 m3/h),partially fluidized bed (7−18 m3/h) and fluidized bed (18−20 m3/h). As can be seen from Fig.3,the bed height reached 640 mm as the gas flow rate was elevated to 20 m3/h. Experiments showed that a stable fluidization state occurred at these conditions and similar results were obtained when aluminous soil and dolomite were used as bed materials,respectively. Therefore,20 m3/h was the best operating fluidising flow rate. According to the diameter of the bed,operating velocity was calculated which was 0.18 m/s. It is worth noting that the pyrolysis gas should be involved in the determination of operating velocity in pyroysis experiments. Previous study has verified that 1.0 kg biomass feedstock could produce about 1.0 m3 gaseous products of ambient temperature[23]. In addition,the volume of the gas changes sharply with temperature. Therefore,biomass feeding rate and temperature changes should be taken into account in the calculation of the gas flow. In the pyrolysis experiments,the feeding rate of feedstock was controlled at 3 kg/h,thus it will produce 3 m3/h pyrolysis gas of ambient temperature. Assuming ambient temperature was 20 °C,the gas flow rate of pyrolysis temperature could be determined according to the equation of the ideal gas law. The temperature of the flue gas at the entrance of the bed was 450 °C (detected by thermocouple of Tg),so the flow rate of pyrolysis temperature also could be calculated. Based on the above analysis,the operating flow rates of the flue gas at selected pyrolysis temperature were determined (Table2) and the values could be controlled precisely by the combined action of the rotameter and the pressure-relief valve during pyrolysis experiments.

|
| Fig.3 Relationship between the bed height and gas flow rate. |
| Pyrolysis temperature (°C) | Pyrolysis gas product (m3/h) | Flue gas (m3/h) |
| 450 | 7.3 | 12.7 |
| 475 | 7.6 | 12.4 |
| 500 | 7.8 | 12.2 |
| 525 | 8.1 | 11.9 |
The temperature of the flue gas at the entrance of the fluidized bed was about 450 °C,while the selected pyrolysis temperature ranged from 450 °C to 525 °C. Consequently,the temperature of the reaction bed was actually regulated by the electric wire heater twined outside surface of the bed pipe.
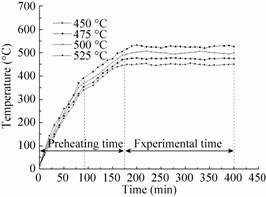
Fig.4 shows the temperature variations of the reaction bed in relation to heating time during preheating and experimental stages. Fig.4 reported that the preheating time needed was more than 175 minutes and a marked inflexion occurred at about 90 minutes in the heating curve. This may be caused by the heat loss between the bed and the environment,which would enhance as the temperature of the reactor climbed to an extent. Also,when the temperatures reached the selected values,the errors in the average reaction temperature were within ±5 °C under the controlling of the automatic temperature control unit,which proved the reactor was competent for the experiments of fast biomass pyrolysis.
|
| Fig.4 Temperature variation with heating time. |
The effect of the pyrolysis temperature on the CS products yields is shown in the Fig.5 at 450 °C,475 °C,500 °C and 525 °C,respectively,using quartz sand as a bed material. Bio-oil yield increased from 33.85 wt% to 37.6 wt% with increasing temperature at 450−500 °C and then decreased to 30.3 wt% at 525 °C. Similar behavior was also obtained in the yield of heavy oil that the yield was maximized at 11.4 wt% at 500 °C. The solid char yield decreased as the temperature increase at 450−525 °C. The gas yield was nearly constant at 450 °C and 475 °C,after a little increase at 500 °C,then increased to 42.2 wt% at 525 °C. As can be seen in Fig.5,the pyrolysis temperature affected the yields of the product significantly. The bio-oil yield increases to an optimum value with increasing temperature and then decreases. When temperature over optimum temperature 500 °C,liquid and char yields reduced and gas yield increase. It may be concluded from the results that secondary reactions of the liquid fraction of the volatiles and further decomposition of the char particles proceeded in the reactor with increasing pyrolysis temperatures. If these results were investigated with respect to maximum oil yield,it was clear that the optimal pyrolysis temperature for bio-oil production was 500 °C under the experimental conditions used. Therefore,the pyrolysis temperature of 500 °C was determined in the later experiments.
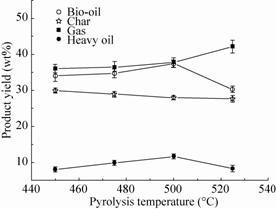
|
| Fig.5 Effect of temperature on pyrolysis product yields. |
Table3 listed the gas fractions and calorific values at four selected temperatures. As can be seen from Table3,carbon oxides (mainly CO2) were dominant about 53.77%−62.75% in the pyrolysis gas products. The amount of methane was 4.55%−6.99%,whereas hydrogen was 1.36%−2.03%. In previous study,nitrogen was used as fluidizing medium,whereas hydrogen did not be detected due to the strong dilution to fluidizing gas[24]. Carbon dioxide is a product of the primary pyroysis of cellulose and hemicelluloses[25]. Methane and carbon monoxide are mainly formed for secondary cracking of volatiles,followed by a reduction of CO2 (C+CO2=2CO)[26]. It was observed that high temperature (>500 °C) led to a high proportion of CO and CH4 while a lower temperature led to a high proportion of CO2. The oxygen was little in the gas mixture,which was beneficial to pyrolysis reaction. This result also demonstrated that most of the oxygen was depleted in the coal furnace. The gas product had a relatively high calorific value,which ranged from 6.5 to 8.5 MJ/m3,so the pyrolysis gas could be used as a gaseous fuel.
| Temperature (°C) | CO2 (%) | CnHm (%) | O2 (%) | CO (%) | CH4 (%) | H2 (%) | Calorific value (MJ/m3) |
| 450 | 62.35 | 1.86 | 1.03 | 28.85 | 4.55 | 1.36 | 6.5 |
| 475 | 61.38 | 2.09 | 1.10 | 29.62 | 4.19 | 1.60 | 6.7 |
| 500 | 60.30 | 2.23 | 1.86 | 28.47 | 5.77 | 1.36 | 7.2 |
| 525 | 53.77 | 2.50 | 1.26 | 33.45 | 6.99 | 2.03 | 8.5 |
Fig.6 presents the bio-oil yield from CS in relation to bed materials of quartz sand,aluminous soil and dolomite at different pyroysis temperatures,respectively. In Fig.6,as expected that the variation of bio-oil yields at diffident temperatures was similar with the above discussions regardless of any bed materials used. At specific temperature,the highest bio-oil yield was achieved when dolomite was used as bed material and the lowest bio-oil yield was obtained using aluminous soil as bed material. The highest yield of 43.3 wt%,bio-oil,was obtained at the pyrolysis temperature of 500 °C and dolomite as bed material. The increase of bio-oil production may come from the catalysis of dolomite on the cracking of the tar. In biomass pyrolsis,the formation of tar has adverse effects on the production of bio-oil and the reactor system. It usually adheres to the internal walls of the pipes and reactors and is difficult to be cleaned.

|
| Fig.6 Effects of bed materials on the yields of bio-oil. |
Previous studies showed that a great deal of CaO-MgO compounds were formed in dolomite after calcinations and which had a specific surface area. The big specific surface area could achieve better catalyst efficiency for tar[27]. It is worth noting that the most important factor of dolomite is the specific surface area after calcinations. In order to obtain high catalyst effect,dolomite with bigger specific surface area should be applied to fast biomass pyrolysis for bio-oil production. From the results,we can see using calcinated dolomite as bed material for pyrolysis could enhance the bio-oil production. Therefore,dolomite was used as bed material in the later experiments.
2.3.3 Product yields from different feedstocksFig.7 shows the yields of bio-oil,heavy oil and char products in relation to different biomass feedstocks at the optimal pyroysis temperature of 500 °C as well as dolomite as bed material. The distributions of the product yields were different significantly among the four kinds of biomass. The CS produced higher bio-oil than other biomass materials,but smaller difference existed between FR and XR in bio-oil yields. There were not striking differences among FR,XR and CS in heavy oil yields either. The highest amount of char was obtained from KS which reached 45.4 wt%,while the bio-oil only arrived at 22.1 wt%. These behaviors may be relative with different contents of the chemical components in the biomass feedstocks[28]. Char formation is related with the ash and fixed carbon contents. KS contained more ash and carbon and less volatiles. At the same time,hot particle char entrainment causes the secondary reaction of volatiles which led to less amount of bio-oil.

|
| Fig.7 Product yield distributions from different feedstocks. |
Table4 shows mainly physical properties of light bio-oil obtained from CS in relation to pyrolysis temperatures and bed materials. The physical properties of light oils and heavy oils obtained from different feedstocks,as shown in Table5. As can be seen from Table4,there were not striking regularities among the pyrolysis temperatures,the bed materials and the physical properties of the light oil. The pH value of light oils ranged from 3.18 to 3.99. The heat value was very low which only ranged from 7.63 MJ/kg to 8.25 MJ/kg. The kinetic viscosity ranged from 52.0 MPa·s to 59.0 MPa·s which was far higher than that of diesel fuel.
| Bed materials | Quartz sand | Aluminous soil | Dolomite | |||||||||||
| Temperature (°C) | 450 | 475 | 500 | 525 | 450 | 475 | 500 | 525 | 450 | 475 | 500 | 525 | ||
| pH value | 3.40 | 3.94 | 3.71 | 3.54 | 3.18 | 3.68 | 3.61 | 3.71 | 3.29 | 3.99 | 3.74 | 3.56 | ||
| Kinetic viscosity (MPa·s) | 53.00 | 55.00 | 55.00 | 59.00 | 55.00 | 55.00 | 54.00 | 55.00 | 52.00 | 55.00 | 55.00 | 50.00 | ||
| Calorific value(MJ/kg) | 7.68 | 7.89 | 7.73 | 7.79 | 8.21 | 8.09 | 7.79 | 7.82 | 8.15 | 7.76 | 8.25 | 7.63 | ||
| Heavy oil | Kinetic viscosity (MPa·s) | pH value | Calorific value (MJ/kg) |
| CS | 51.0 | 2.61 | 24.4 |
| FR | 49.0 | 2.45 | 23.5 |
| XS | 50.0 | 2.57 | 24.1 |
| KS | 51.0 | 2.80 | 23.9 |
| Light oil | |||
| CS | 55.0 | 3.59 | 8.25 |
| FR | 53.0 | 3.21 | 8.11 |
| XS | 54.0 | 3.14 | 7.98 |
| KS | 53.0 | 5.20 | 7.88 |
The pH values of light oils and heavy oils obtained from CS,FR,XR,KS at pyrolysis temperature of 500 °C and using dolomite as bed material are presented in Fig.8. As can be seen from Fig.8,the pH value of light oil from KS reached 5.20 which was far higher than that of from the other three feedstcoks,due to a high alkaline content in KS. The pH value of heavy oils did not vary a lot with the kind of biomass. For each feedstock,pH value of light oil was higher than that of heavy oil. This is because that more water contained in the light oils than in heavy oils,the water diluted acid.

|
| Fig.8 pH values of bio-oils from different biomass feedstocks. |
Bio-oils are very strong corrosive to aluminum,mild steel and nickel-based materials due to the low pH values. Previous researches indicated that the ph value of bio-oil reduced with temperature increasing,then the corrosiveness of bio-oil enhanced for above materials[29]. These characteristics imposed more requirements on construction materials of the vessels and upgrading process before using bio-oil in transport fuels[28].
2.4.2 ViscosityViscosity is very important in many fuel applications[30]. It plays an important role in the design and operation of the fuel injection system,as well as in the atomization quality and subsequent combustion properties of the fuel[31]. As shown in Table5,it can be observed that the kinetic viscosity varied little with the kind of feedstock. The viscosity of heavy oils ranged 48.0−51.0 MPa·s which was lower than that of light oil of 53.0−55.0 MPa·s,but they are far higher than that of diesel fuel. Extensive studies have verified that the pyrolysis oil was highly oxygenated. The high oxygen content was indicative of the presence of many polar groups leading to a high viscosity.
2.4.3 Calorific valueAs can be seen from Table5,the calorific values of light oils were only 7.63−8.25 MJ/kg and the values of the heavy oils were arrived at 23.5−24.4 MJ/kg. Because the light oils contained much more water than that of heavy oil,which led the light oils to low calorific values. The caloric value of diesel is 42.0−45.0 MJ/kg,the caloric value of coal is about 29.3 MJ/kg,and the caloric value of gasoline is 44.0 MJ/kg,which revealed that the calorific value of the pyrolysis oil was much lower than that of fossil fuels because the oxygen content of the pyrolysis oils was high.
3 ConclusionThe result of fluidization is well by the way of cold simulation in the plexiglas pipe. According to the experimental results and calculation,we can get the optimally operating fluidization velocity of the flue gas that is 12.7,12.4,12.2,11.9 m³/h in the selected temperature of 450 °C,475 °C,500 °C,525 °C,respectively. The optimal reaction conditions of fast biomass pyroysis for maximum bio-oil production were investigated using a fluidized bed under flue gas atmosphere. The optimal pyrolysis temperature was 500 °C and the bed material of dolomite could enhance the bio-oil yield. The distribution of product yields obtained from CS,FR,XR and KS were significantly different. The highest bio-oil yield of 43.3% was achieved from CS at the pyrolysis temperature of 500 °C and dolomite as bed material. Two main fractions were recovered from the stratified bio-oils: light oils and heavy oils. The physical properties of heavy oils obtained from all the feedstocks varied little. The calorific values of heavy oils were much higher than that of light oils. The pyrolysis gas product contained some combustible substances can be used as a gaseous fuel.
| [1] | Onay Ö, Beis SH, Kockar ÖM. Fast pyrolysis of rapeseed in a well-swept fixed-bed reactor. J Anal Appl Pyrolysis, 2001, 58-59: 995-1007. |
| [2] | Raja SA, Kennedy ZR, Pillai BC, et al. Flash pyrolysis of jatropha oil cake in electrically heated fluidized bed reactor. Energy, 2010, 35(7): 2819-2823. |
| [3] | Pootakham T, Kumar A. Bio-oil transport by pipeline: a techno-economic assessment. Bioresour Technol, 2010, 101(18): 7137-7143. |
| [4] | Bridgwater AV, Peacocke GVC. Fast pyrolysis processes for biomass. Renew Sust Energy Rev, 2000, 4(1): 1-73. |
| [5] | Lédé J, Broust F, Ndiaye FT, et al. Properties of bio-oils produced by biomass fast pyrolysis in a cyclone reactor. Fuel, 2007, 86(12/13): 1800-1810. |
| [6] | Özbay N, Pütün AE, Uzun BB, et al. Biocrude from biomass: pyrolysis of cottonseed cake. Renew Energy, 2001, 24(3/4): 615-625. |
| [7] | Zheng JL, Yi WM, Wang NN. Bio-oil production from cotton stalk. Ener Convers Manage, 2008, 49(6): 1724-1730. |
| [8] | Pattiya A. Bio-oil production via fast pyrolysis of biomass residues from cassava plants in a fluidised-bed reactor. Bioresour Technol, 2011, 102(2): 1959-1967. |
| [9] | Kim SW, Koo BS, Lee DH. A comparative study of bio-oils from pyrolysis of microalgae and oil seed waste in a fluidized bed. Bioresour Technol, 2014, 162: 96-102. |
| [10] | Wang SR, Fang MX, Yu CJ, et al. Flash pyrolysis of biomass particles in fluidized bed for bio-oil production. China Particuol, 2005, 3(1/2): 136-140. |
| [11] | Jung SH, Kang BS, Kim JS. Production of bio-oil from rice straw and bamboo sawdust under various reaction conditions in a fast pyrolysis plant equipped with a fluidized bed and a char separation system. J Anal Appl Pyrolysis, 2008, 82(2): 240-247. |
| [12] | Heo HS, Park HJ, Dong JI, et al. Fast pyrolysis of rice husk under different reaction conditions. J Ind Eng Chem, 2010, 16(1): 27-31. |
| [13] | Zheng JL. Bio-oil from fast pyrolysis of rice husk: yields and related properties and improvement of the pyrolysis system. J Anal Appl Pyrolysis, 2007, 80(1): 30-35. |
| [14] | Tsai WT, Lee MK, Chang YM. Fast pyrolysis of rice husk: product yields and compositions. Bioresour Technol, 2007, 98(1): 22-28. |
| [15] | Lu Q, Yang XL, Zhu XF. Analysis on chemical and physical properties of bio-oil pyrolyzed from rice husk. J Anal Appl Pyrolysis, 2008, 82(2): 191-198. |
| [16] | Luo ZY, Wang SR, Liao YF, et al. Research on biomass fast pyrolysis for liquid fuel. Biomass Bioenerg, 2004, 26(5): 455-462. |
| [17] | Luo ZY, Wang SR, Cen KF. A model of wood flash pyrolysis in fluidized bed reactor. Renew Energy, 2005, 30(3): 377-392. |
| [18] | He RH, Ye XP, English BC, et al. Influence of pyrolysis condition on switchgrass bio-oil yield and physicochemical properties. Bioresour Technol, 2009, 100(21): 5305-5311. |
| [19] | Fonts I, Azuara M, Gea G, et al. Study of the pyrolysis liquids obtained from different sewage sludge. J Anal Appl Pyrol, 2009, 85(12): 184-191. |
| [20] | Kim SJ, Jung SH, Kim JS. Fast pyrolysis of palm kernel shells: influence of operation parameters on the bio-oil yield and the yield of phenol and phenolic compounds. Bioresour Technol, 2009, 101(23): 9294-9300. |
| [21] | Abnisa F, Daud WMAW, Husin WNW, et al. Utilization possibilities of palm shell as a source of biomass energy in Malaysia by producing bio-oil in pyrolysis process. Biomass Bioenergy, 2011, 35(5): 1863-1872. |
| [22] |
Bi YY, Gao CY, Wang YJ, et al. Estimation of straw resources in China. Trans CSAE, 2009, 25(12): 211-217 (in Chinese). 毕于运, 高春雨, 王亚静, 等. 中国秸秆资源数量估算. 农业工程学校, 2009, 25(12): 211-217. |
| [23] |
Ma LL, Wu CZ, Sun L. Biomass Gasification Technology and its Application. Beijing: Chemical Industry Press, 2003 (in Chinese). 马隆龙, 吴创之, 孙立. 生物质气化技术及其应用. 北京: 化学工业出版社, 2003. |
| [24] | Yanik J, Kornmayer C, Saglam M, et al. Fast pyrolysis of agricultural wastes: characterization of pyrolysis products. Fuel Process Technol, 2007, 88(10): 942-947. |
| [25] | Blasi CD, Branca C, D'Errico G. Degradation characteristics of straw and washed straw. Thermochim Acta, 2000, 364(1/2): 133-142. |
| [26] | Bridgwater AV, Czernik S, Piskorz J. The status of biomass fast pyrolysis//Bridgwater AV, Ed. Fast Pyrolysis of Biomass A Handbook. Newbury, UK: CPLPress, 2002, 2: 1-22. |
| [27] |
Sun YJ, Jiang JC. Study on catalytic cracking of tar derived from biomass pyrolysis and gasification. Chem Ind For Prod, 2007, 27(5): 15-20 (in Chinese). 孙云娟, 蒋剑春. 生物质热解气化产物中焦油的催化裂解研究. 林产化学与工业, 2007, 27(5): 15-20. |
| [28] | Zhang Q, Chang J, Wang TJ. Review of biomass pyrolysis oil properties and upgrading research. Ener Convers Manage, 2007, 48(1): 87-92. |
| [29] | Diebold JP, Milne TA, Czernik S, et al. Proposed specifications for various grades of pyrolysis oils//Bridgwater AV, Boocock DGB, Eds. Developments in Thermochemical Biomass Conversion. London: Blackie Academic & Professional, 1997: 433-447. |
| [30] | Lu Q, Li WZ, Zhu XF. Overview of fuel properties of biomass fast pyrolysis oils. Ener Convers Manage, 2009, 50(5): 1376-1383. |
| [31] | Zheng JL. Pyrolysis oil from fast pyrolysis of maize stalk. J Anal Appl Pyrol, 2008, 83(2): 205-212. |
 2015, Vol. 31
2015, Vol. 31