中国科学院微生物研究所,中国微生物学会
文章信息
- 王婉妮, 李滢, 曾嘉辉, 谢新强, 吴清平. 2024
- WANG Wanni, LI Ying, ZENG Jiahui, XIE Xinqiang, WU Qingping.
- 益生菌对特应性皮炎的防治及机制研究进展
- Research progress in probiotics for prevention and treatment of atopic dermatitis
- 微生物学报, 64(5): 1378-1391
- Acta Microbiologica Sinica, 64(5): 1378-1391
-
文章历史
- 收稿日期:2023-11-06
- 网络出版日期:2024-03-18
2. 广东省科学院微生物研究所 华南应用微生物国家重点实验室 广东省微生物安全与健康重点实验室 国家卫健委微生物食品营养与安全科技创新平台, 广东 广州 510070
2. State Key Laboratory of Applied Microbiology Southern China, Guangdong Provincial Key Laboratory of Microbial Safety and Health, National Health Commission Science and Technology Innovation Platform for Nutrition and Safety of Microbial Food, Institute of Microbiology, Guangdong Academy of Sciences, Guangzhou 510070, Guangdong, China
皮肤(skin)被覆于体表,作为人体抵御外界因素侵扰的第一道防线,具有屏障保护、结构支撑、调节体温以及维持人体内环境稳定等功能[1]。特应性皮炎(atopic dermatitis, AD)是一种临床上最常见的慢性炎症性皮肤病,以反复发作和严重瘙痒为主要特征,常与食物过敏、哮喘和过敏性鼻炎等其他过敏反应有关[2]。据统计,全球约20%的儿童和10%的成年人受此影响[3]。研究指出,社会心理压力、空气温湿度变化、过敏原刺激、皮肤与肠道菌群失调、皮肤屏障减弱和免疫细胞活性增强为诱发AD的主要因素;AD通常影响身体的特定部位,如脸部、颈部、四肢及皮肤皱褶处,以皮肤干燥、红斑、丘疹、苔藓样硬化、渗出和结痂为典型病理特征;尽管AD是以过敏的形式影响皮肤系统,但近年研究发现其与心血管疾病、神经系统疾病、自身免疫性疾病以及肥胖等非过敏性疾病的高发病率也存在联系[4-7]。除了对患者躯体造成伤害外,AD也因为其持续的瘙痒症状严重影响患者的生活质量以及工作效率,危害患者心理健康[3]。
为缓解AD患者的临床症状、减少疾病复发及并发症的发生,临床医生根据成人和儿童皮炎的严重程度,制定了包括回避疗法、漂白浴、润肤剂、外用皮质类固醇(topical corticosteroids, TCS)、钙调神经磷酸酶抑制剂(topical calcineurin inhibitor, TCI)和Janus激酶(Janus kinase, JAK)抑制剂等系列方法,但这些治疗具有诸如皮肤萎缩、多毛、色素沉着、应用部位产生灼烧感和瘙痒、生长迟缓、骨质疏松、鼻咽炎、头痛、恶心和腹泻等较严重的副作用[8]。抗组胺药是治疗AD皮肤瘙痒的主要疗法,但该药具有嗜睡的副作用,且对儿童认知能力存在影响[9]。因此,亟需研发出一种新的安全、有效、经济的生物制剂用于AD的预防与治疗。
1930年,皮肤病学家John H. Stokes提出关于情绪、肠道菌群及皮肤炎症之间相互关系的开创性理论,揭示了肠道-皮肤轴的存在[10]。随着新一代测序技术的出现,研究发现AD的发生不仅与皮肤微生物组有关,且与肠道微生物组有关[11]。肠道菌群失调通过促炎细胞因子增加肠道通透性,进而促进免疫失调,导致包括AD、痤疮以及银屑病等皮肤疾病的发生[12]。益生菌(probiotics)是一种活的微生物,当给予足够数量时将对宿主的健康产生有益作用[13]。益生菌具有增强上皮屏障的完整性、抑制病原体增殖以及重新恢复机体免疫平衡的作用,是预防和治疗AD在内的各种过敏性疾病的新手段[14]。作者所在研究团队累积了超过3万株的健康功能微生物,建成了相应的菌种资源及基因组数据库,并对其进行功能挖掘,目前已证明益生微生物能通过调节肠道菌群在降血压[15]、降糖降脂[16]、促睡眠[17]、抗幽门螺杆菌[18]、抗病毒[19]、修复皮肤光老化[20]以及缓解溃疡性结肠炎[21]等方面有显著的益生功能。肠道-皮肤轴的存在也为益生菌防治AD提供了理论支撑。因此,本文将从AD的皮肤和肠道微生态特征、病理机制及益生菌在AD领域的研究现状,系统综述益生菌在防治AD的研究中取得的最新进展。
1 AD特征性人体微生态 1.1 AD的皮肤微生态皮肤和肠道菌群是人体的2个主要微生态系统[22]。皮肤表面定殖有数百万微生物,主要为丙酸杆菌(Propionibacteria)、链球菌(Streptococcus)、葡萄球菌(Staphylococcus)和棒状杆菌(Corynebacterium)[23]。皮肤免疫系统的动态平衡很大程度依赖于皮肤菌群的平衡[24],皮肤微生态一旦失衡,不仅会增加病原微生物的感染风险,且极易诱发系列慢性皮肤疾病,如AD[25]。在过去十年里,高通量测序技术的进步为我们提供了关于皮肤健康与微生态组成的新见解:健康人的皮肤微生物群通常是稳定的,但AD患者皮肤表现出显著的失调,表现为微生物多样性减少,且皮损部位被大量金黄色葡萄球菌(Staphylococcus aureus)定殖[26];除了S. aureus外,表皮葡萄球菌、溶血葡萄球菌在AD发病部位也有所增加[27];此外,真菌及感染在AD患者中也较为常见,马拉色菌及白色念珠菌在AD患者的皮肤中比健康人更多[28],而单纯疱疹病毒感染也常在AD儿童中被发现[29]。
1.2 AD的肠道微生态既往研究表明,肠道菌群的定殖与免疫系统的发育密切相关,肠道菌群失调与过敏性疾病的发生相关,且常先于疾病发生[30]。Watanabe等[31]发现,AD患者肠道中乳杆菌、双歧杆菌的相对丰度显著降低,而葡萄球菌的比例显著高于健康人群;Fieten等[32]的研究表明,伴有食物过敏的AD患儿粪便微生物中假链双歧杆菌和大肠杆菌相对较多,而青春双歧杆菌、短双歧杆菌、普拉梭菌及嗜黏蛋白阿克曼氏菌较少;除了双歧杆菌数量减少外,AD患儿肠道拟杆菌数量降低,而肠杆菌数量增加[33];Fujimura等[34]的研究表明,在患上AD及哮喘等高过敏性疾病风险的儿童中,阿克曼氏菌、双歧杆菌及粪杆菌的水平较低,而假丝酵母及红酵母含量较高;在其他研究中还发现,艰难梭菌在生命早期的定殖也导致了随后AD的发生[35];进一步研究指出,AD儿童肠道共生细菌对肠黏膜的黏附性较差,导致肠道屏障功能出现障碍,肠道中抗炎因子白介素10 (interleukin, IL-10)的产生减少,而促炎细胞因子水平升高[36]。综上,肠道微生物多样性及组成变化与AD等过敏性疾病的发生发展密切相关,调节肠道菌群或可改善过敏症状的发展,成为预防和治疗AD的靶点。
2 特应性皮炎的发病机制研究 2.1 AD的病理机制医学研究指出,AD属于由IgE抗体介导的Ⅰ型超敏反应,涉及到多个过程的免疫反应是AD致病机制的核心。当过敏原入侵皮肤屏障后,被皮肤抗原递呈细胞(antigen-presenting cells, APC)如朗格汉斯细胞和树突细胞摄取,随后APC将过敏原递呈至组织与血液中的初始辅助性T细胞(naive helper T cell, Th0),并诱导Th0细胞向Th2细胞分化增殖;分化后Th2细胞分泌大量过敏性细胞因子如IL-4、IL-5、IL-13,形成过度的Th2型免疫反应(图 1)。一方面,Th2分泌的细胞因子可募集更多Th0细胞并向Th2分化,另一方面,Th2细胞作用于B细胞,诱导其转化为分泌特异性IgE的浆细胞;这些浆细胞来源的IgE将激活皮肤肥大细胞(mast cell, MC)表面的Fc受体,使MC发生脱颗粒,释放大量致敏效应物质组胺(histamine)进入皮肤组织与血液,引起皮肤红肿、瘙痒等系列症状[37]。组胺是目前研究较为关注的过敏介质,而人体细胞表面已被证实具有4种组胺受体(histamine receptor, HR),其中H1R和H4R的异常激活是导致AD患者产生瘙痒症状的主要原因[38]。皮肤肥大细胞释放出的组胺作用于皮肤感觉神经末梢表达的组胺受体,继而激活神经末梢的信号传导,并通过脊髓丘脑束将瘙痒的感觉传递到大脑,导致患者进入瘙痒-抓挠-严重瘙痒的恶性循环[39]。
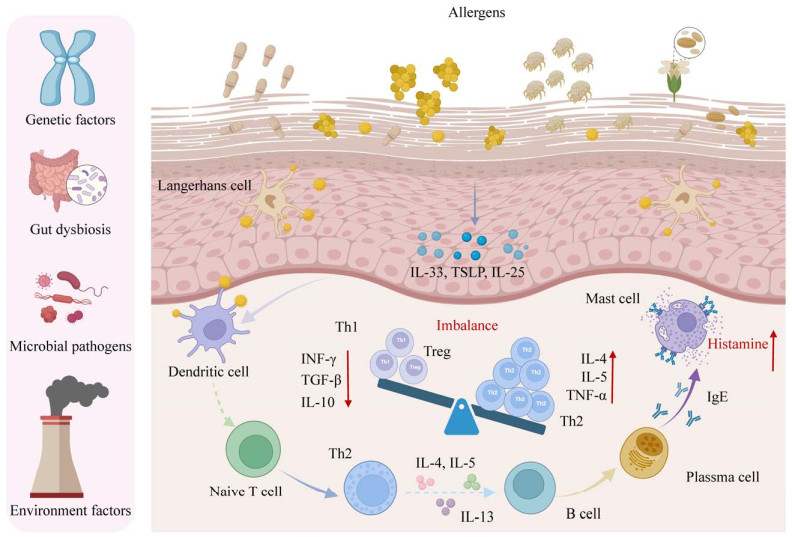
|
| 图 1 特应性皮炎的病理机制 Figure 1 The pathogenesis of atopic dermatitis. |
2.2 AD的影响因素
AD的发病机制是复杂和多因素的,涉及到遗传易感性、肠道和皮肤微生态失调、局部和全身免疫失衡以及环境因素之间的相互作用[40]。
2.2.1 遗传易感性AD具有很强的遗传性[41],目前已确定的最大遗传风险与由flg基因编码的皮肤屏障蛋白——微丝蛋白的突变有关[42]。flg基因突变将导致微丝蛋白表达下降甚至完全丧失[43],从而使患AD的风险比未突变人群高3−5倍,同时也增加了哮喘和花生过敏的风险[44]。此外,位于染色体5q31.1上的基因座包含2型免疫细胞因子IL-4、IL-13的基因以及调控细胞因子表达的rad50基因突变也会引起AD患病风险增高。位于染色体11q13.5上的2个候选基因emsy和lrrc32之间的第3个广泛复制的基因与多种特应性表型相关[45]。在AD中,迄今已识别有34个基因座突变与疾病发病相关,另外还可能存在其他未识别的基因座或可遗传的表观效应导致AD发病。
2.2.2 微生物组失调如前文所述,AD患者的皮肤存在表皮屏障功能障碍,表现为经皮水分损失(transepidermal water loss, TEWL)增加、pH升高、皮肤通透性增加、水分保留率降低以及脂质成分改变[46],因此也更容易受到细菌感染。S. aureus和Malassezia spp.是主要的定殖菌和病原体,可引发或加剧AD的皮肤炎症[47-48]。另外,与健康人群相比,患者肠道微生物多样性降低,乳杆菌、双歧杆菌等有益微生物的相对丰度显著降低,而大肠杆菌、艰难梭菌和S. aureus的比例增加。特别在生命早期,肠道微生物的定殖与改变早于临床表现,表明肠道菌群失调也是AD发生的一大影响因素。
2.2.3 免疫反应失衡过敏反应是一种免疫失调性疾病,Th1/Th2平衡破坏导致Th2细胞亚群及其分泌的细胞因子增多也是AD发生的原因[49]。在始发阶段,皮肤屏障功能出现障碍,增加了外源性微生物和过敏原的经皮渗透,降低了对抗原和刺激物的炎症阈值;受损表皮中的角质形成细胞通过胸腺基质淋巴生成素(thymic stromal lymphopoietin, TSLP)及IL-33等细胞因子发出促炎和瘙痒信号,激活Th2介导的免疫反应,进一步导致组织损伤[50]。TSLP通过其受体激活未成熟的树突细胞,促进APC的成熟,同时促进嗜酸性粒细胞的活性,增强IL-4、IL-5和IL-13的表达[51],而IL-4及IL-13的上调降低了表皮屏障蛋白的产生,从而加重皮肤屏障缺陷;IL-25可诱导多种趋化因子促进Th2细胞募集[52];IL-33通过受体激活核因子κB (nuclear factor kappa-B, NF-κB)及丝裂原激活蛋白激酶,从而刺激与Th2反应相关的细胞因子的产生[53]。另外,先天淋巴样细胞(innate lymphoid cells, ILCs)能够刺激细胞因子的产生,影响局部组织中的免疫和非免疫细胞。ILC2具有分泌IL4、IL-5和IL-13等促过敏细胞因子的能力,通过启动Th2反应而参与AD等各种过敏性疾病[54]。
2.2.4 环境因素宿主所处的生活环境以及生活方式也会影响AD的发生发展,其中气候变化[55]、空气污染物[56]、心理压力[57]、日晒不足[58]及高硬度水[59]等被认为是AD发病的驱动因素。研究指出,空气中的屋尘螨、花粉,以及牛奶、蛋清、大豆和花生等常见食物过敏源都是诱发儿童AD及加重疾病最常见的环境刺激物,直接引发免疫异常[37]。另外,长期暴露在低湿度的环境中会加速AD患者皮肤的水分损失,加重皮肤屏障缺陷[60]。同时,压力引起的糖皮质激素变化会抑制皮肤神经酰胺、胆固醇和游离脂肪酸的合成,进而破坏了皮肤的疏水屏障导致水分丢失,进一步加重AD并诱发其他皮肤炎症[61]。
3 益生菌防治AD的机制益生菌是一类能够附着在胃肠道黏膜且具有多样化临床及免疫能力的活性微生物[62]。据报道,目前能减轻Ⅰ型过敏反应的益生菌主要来源于乳杆菌属和双歧杆菌属[63]。一项使用动物双歧杆菌的随机、双盲安慰剂对照研究发现,8周后益生菌组的受试者瘙痒症状显著改善[64]。Enomoto等[65]的研究发现,使用短双歧杆菌后,益生菌组特应性皮炎评分(scoring of atopic dermatitis, SCORAD)指数下降。成人AD患者服用唾液乳杆菌LS01后,益生菌治疗组的SCORD评分显著降低,粪便微生物中葡萄球菌载量减少[66]。使用副干酪乳杆菌K71后,益生菌组的皮肤严重程度评分降低[67]。联合使用唾液乳杆菌LS01 DSM 2275和短双歧杆菌BR03 DSM 16604的随机对照试验发现,受试者在临床分数上有显著改善且血浆脂多糖(lipopolysaccharides, LPS)水平有所下降[68]。此外,孕期及婴儿早期补充鼠李糖乳杆菌HN001似乎可以降低患AD的风险[69]。本文列举了近年来益生菌防治AD的研究进展(表 1),并对益生菌缓解AD的相关机制进行概述。
| Strain | Form and dosage | Test sample and quantity | Mechanism | Effect |
| Lactobacillus rhamnosus HN001 | Capsulated probiotic 6×109 CFU/d |
157 infants | Shape the immune system and increase IFN-γ level | The prevalence of eczema were significantly reduced[69] |
| Lactobacillus plantarum CCFM8610 | Lyophilized powder 1×109 CFU/d |
109 adult patients | Regulate the gut microbiota; maintain the immune responses and barrier integrity; change in functional genes of the gut microbiota | Improve SCORAD index; increase the expression levels of IL-10; decrease the F/B ratio; increase species evenness and diversity[70] |
| Lactobacillus plantarum IS-10506 |
Microencapsulated probiotic 2×1010 CFU/d |
30 adult patients: 15 controls vs. 15 interventions |
Suppression of the Th2 adaptive immune response and increase in the Th1 adaptive immune response; increase the Treg immune response and decrease Th17 response | The SCORAD score, IL-4, and IL-17 levels were significantly lower; the IFN-γ and Foxp3+ levels were significantly higher[71] |
| Lactobacillus rhamnosus IDCC 3201 |
Tyndallized probiotics 1×1010 CFU/d |
66 children: 33 controls vs. 33 tests |
Decreased production or activity of IL-31 and eosinophils | The SCORAD score, eosinophil count, and the IL-31 levels were decreased[72] |
| Lactobacillus paracasei KBL382 |
Live bacteria 1×109 CFU/d |
NC/Nga mice: 7 controls vs. 7 models vs. 7 tests |
Increase the immunosuppressive response and change the metabolic functions of gut microbiota | Reduced AD-associated skin lesions, serum levels of IgE, and immune cell infiltration[73] |
| Lactobacillus plantarum CJLP55 |
Lyophilized powder 1×1010 CFU/d |
NC/Nga mice: 8 controls vs. 8 models vs. 8 tests |
Alter the balance of Th1/Th2 ratio or induce IL-10 production | Suppressed AD-like skin lesions, high serum IgE levels; diminished the accumulation of eosinophils and mast cells; decreased production of IL-4 and IL-5[74] |
| Kazachstania turicensis CAU Y1706 |
Freeze-dried 1×1010 CFU/d |
BALB/c mice: 10 controls vs. 10 models vs. 10 tests |
Interactions between gut microbiota, SCFA, and immune modulation between Th1 and Th2 | Reduced IgE levels and the number of mast cells and eosinophil; the serum concentrations of Th2 cytokines were significantly lower[75] |
| Limosilactobacillus reuteri FN041 | Live bacteria 1×109 CFU/d |
BALB/c mice: 12 controls vs. 12 models vs. 12 tests |
Regulate the ileum microbiota, strengthen the ileal mucosal barrier |
The plasma IgE were significantly decreased; the intestinal mucosal barrier was enhanced[76] |
| Lactobacillus plantarum LM1004 |
Lyophilized powder 2×1012 cells/g 0.01 g/kg-bw |
SD rats: 10 controls vs. 10 models vs. 10 tests |
Inhibited Th2 cell responses; activated the responses of Treg cell; modulated gut microbiota | Decreased the scratching behaviors; reduced vasodilation and serum histamine; decreased serum IgE contents[77] |
| Bifidobacterium adolescentis | Live bacteria 1×109 CFU/d |
C57bl/6 mice: 5 controls vs. 5 models vs. 5 tests |
Promote Tregs population in the spleen; restore the balance between Th1- and Th2-type immune responses; regulate the gut microbiota composition | Reduced ear and skin thickness and suppressed eosinophils and mast cells infiltration[78] |
| Bifidobacterium longum | Cell-free culture supernatant 1×107 CFU/d |
Hairless mice: 5 controls vs. 5 models vs. 5 tests |
Attenuate skin inflammation and enhance skin barrier function | Attenuated DNCB-induced skin inflammation, abnormal TEWL, AD-like skin, and deficiency of epidermal barrier proteins[79] |
| Lactococcus chungangensis CAU 28T | Live bacteria 1×109 CFU/mL |
RAW 264.7 cells; human mast cell |
Inhibit the release of allergy-associated proteins | Inhibited the production of the proinflammatory mediators; reduced the release of histamine[80] |
3.1 增强屏障功能
AD患者肠道菌群失衡,多样性降低,肠道屏障也出现了类似皮肤的屏障功能受损[81]。研究指出,益生菌通过上调黏蛋白类糖蛋白(mucin-type glycoprotein, MUC)-1、MUC-2和MUC-3促进杯状细胞的黏蛋白分泌,从而限制病原菌在黏膜中的移动;另外,益生菌分泌的α-防御素、β-防御素等抗菌肽通过上调跨膜紧密连接蛋白和细胞间紧密连接蛋白,增强了紧密连接的稳定性并防止细菌增殖,从而降低上皮对病原体及其产物的通透性[82]。丁酸等益生菌源短链脂肪酸(short chain fatty acid, SCFA)也有助于调节紧密连接蛋白的表达,增强上皮屏障完整性[83]。Ma等[84]发现婴儿双歧杆菌YLGB-1496也表现出良好的抗氧化性能,并能够上调皮肤屏障基因增强皮肤屏障功能。作者所在研究团队也发现发酵乳杆菌XJC60可通过分泌烟酰胺等抗氧化物质,维持皮肤角质层的完整性,具有维系皮肤屏障功能的作用[85]。
3.2 抑制病原体增殖多项研究发现,益生菌能够与病原体竞争黏蛋白或上皮细胞的结合位点,防止致病微生物过度生长,纠正AD患者微生态的失调;而益生菌产生的抗菌肽、细菌素及SCFA也具有抑制或杀死病原体的作用(图 2)。Fang等[70]发现植物乳杆菌CCFM8610治疗下调了涉及肠道金黄色葡萄球菌感染的功能基因,通过调节肠道微生物组成以及免疫反应,从而改善AD患者的临床症状。另外,本研究团队也证实人源性长双歧杆菌能分泌新型溶菌酶样蛋白,具有调节机体肠道菌群稳定的生物活性[86]。此外,益生菌还能够增加胃肠道中IgA的含量,分泌型IgA通过连接病原体的抗原从而保护肠上皮细胞免受定殖或侵袭,诱导抗原向DC的逆向运输,下调促炎反应[87]。
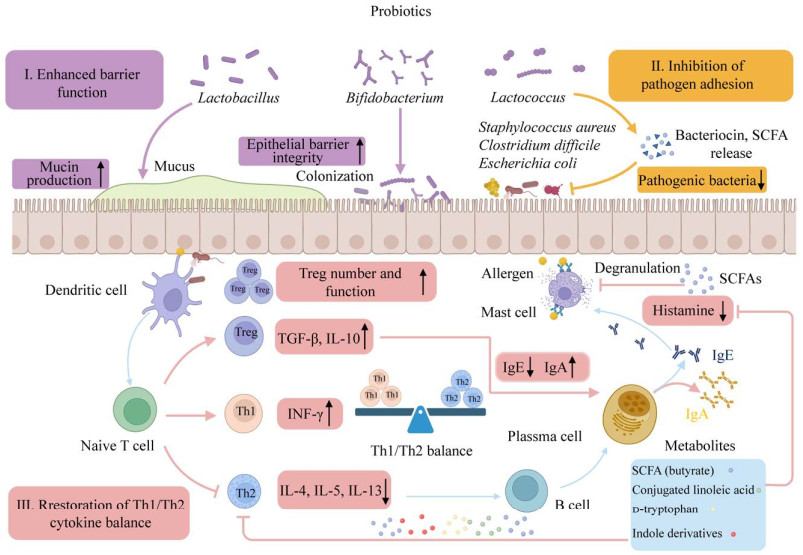
|
| 图 2 益生菌调控AD发病的作用机制 Figure 2 Mechanisms of probiotics in regulating AD pathogenesis. |
3.3 调节Th1/Th2免疫应答
益生菌在AD中发挥免疫调节作用,一方面通过分泌不同的细胞因子刺激Th1应答,降低Th2应答来平衡Th1/Th2免疫反应;另一方面在IL-2和TGF-β的作用下能够诱导调节性T细胞分化增殖,下调IgE合成,减少过敏反应,维持免疫稳态[88]。Won等[74]发现从泡菜中分离的植物乳杆菌CJLP55能够降低IL-4、IL-5水平,增加IL-10产生,通过平衡Th1/Th2免疫反应缓解小鼠AD样皮损。另外,益生菌还可通过产生SCFA、共轭亚油酸、d-色氨酸和吲哚衍生物发挥免疫调节作用;SCFA诱导肠道树突细胞表达视黄醛脱氢酶,促使维生素A转化为视黄酸从而促进Treg细胞的扩增和分化[89]。SCFA特别是丁酸能够抑制MC上高亲和力Fc受体与IgE结合所引发的脱颗粒,从而降低组胺等炎症介质的释放,减少过敏反应的发生[90]。Kim等[77]的研究发现,植物乳杆菌LM1004能显著降低血清中IgE与组胺水平,增加Th1与Treg细胞转录因子水平以及与丁酸产生相关的菌群丰度,减轻AD模型小鼠的皮肤瘙痒。以上发现均表明,益生菌通过调节宿主免疫系统和肠道菌群对防治AD及相关皮肤过敏性疾病具有很大潜力。
4 结语与展望综上所述,flg基因功能缺失突变导致的遗传因素,瘙痒-抓挠-严重瘙痒引起的表皮功能障碍,皮肤及肠道菌群失调,Th1/Th2免疫调节失衡,气候变化、空气污染、社会心理压力等环境影响,这些因素共同作用促进了AD的发生。益生菌通过与病原菌竞争黏附位置、产生细菌素等抗菌物质、阻止细菌生物膜的形成和促进Th1型细胞因子分泌,抑制Th2型细胞因子水平,从而抑制病原菌生长,修复受损皮肤及肠道屏障,促进失衡菌群平衡重建,恢复Th1/Th2免疫失调,最终减轻及改善患者皮肤过敏及临床症状。随着研究的深入,益生菌对AD的作用已在许多临床试验中进行了研究,但益生菌缓解AD的有效物质基础仍不明确。为了阐明益生菌、肠道菌群及皮肤之间具体的互作机制,精准补充益生菌来达到防治AD的作用,需要结合基因组学、转录组学和代谢组学以揭示益生菌对功能基因、肠道菌群、代谢途径以及具体代谢物的影响,全面解析益生菌对AD的缓解机制。为使治疗完全有效,在未来的研究中,也可考虑将益生菌补充剂与局部应用含有益生菌的乳膏相结合,以期开发出新型微生态方法来改善与治疗AD及相关皮肤过敏性疾病。
| [1] | McLOUGHLIN IJ, WRIGHT EM, TAGG JR, JAIN R, HALE JDF. Skin microbiome-the next frontier for probiotic intervention[J]. Probiotics and Antimicrobial Proteins, 2022, 14(4): 630-647 DOI:10.1007/s12602-021-09824-1. |
| [2] | SCHULER CF 4th, BILLI AC, MAVERAKIS E, TSOI LC, GUDJONSSON JE. Novel insights into atopic dermatitis[J]. The Journal of Allergy and Clinical Immunology, 2023, 151(5): 1145-1154 DOI:10.1016/j.jaci.2022.10.023. |
| [3] | CHOVATIYA R. Atopic dermatitis (eczema)[J]. JAMA, 2023, 329(3): 268 DOI:10.1001/jama.2022.21457. |
| [4] | SILVERBERG JI. Association between adult atopic dermatitis, cardiovascular disease, and increased heart attacks in three population-based studies[J]. Allergy, 2015, 70(10): 1300-1308 DOI:10.1111/all.12685. |
| [5] | FABRAZZO M, CIPOLLA S, SIGNORIELLO S, CAMERLENGO A, CALABRESE G, GIORDANO GM, ARGENZIANO G, GALDERISI S. A systematic review on shared biological mechanisms of depression and anxiety in comorbidity with psoriasis, atopic dermatitis, and hidradenitis suppurativa[J]. European Psychiatry, 2021, 64(1): e71 DOI:10.1192/j.eurpsy.2021.2249. |
| [6] | MOHAN GC, SILVERBERG JI. Association of vitiligo and alopecia areata with atopic dermatitis: a systematic review and meta-analysis[J]. JAMA Dermatology, 2015, 151(5): 522-528 DOI:10.1001/jamadermatol.2014.3324. |
| [7] | ZHANG A, SILVERBERG JI. Association of atopic dermatitis with being overweight and obese: a systematic review and metaanalysis[J]. Journal of the American Academy of Dermatology, 2015, 72(4): 606-616.e4 DOI:10.1016/j.jaad.2014.12.013. |
| [8] | LI HG, ZHANG Z, ZHANG H, GUO YF, YAO ZR. Update on the pathogenesis and therapy of atopic dermatitis[J]. Clinical Reviews in Allergy & Immunology, 2021, 61(3): 324-338. |
| [9] | HE A, FELDMAN SR, FLEISCHER AB. An assessment of the use of antihistamines in the management of atopic dermatitis[J]. Journal of the American Academy of Dermatology, 2018, 79(1): 92-96 DOI:10.1016/j.jaad.2017.12.077. |
| [10] | STOKES JH. The effect on the skin of emotional and nervous states[J]. Archives of Dermatology and Syphilology, 1930, 22(6): 962 DOI:10.1001/archderm.1930.01440180008002. |
| [11] | FANG ZF, LI LZ, ZHANG H, ZHAO JX, LU WW, CHEN W. Gut microbiota, probiotics, and their interactions in prevention and treatment of atopic dermatitis: a review[J]. Frontiers in Immunology, 2021, 12: 720393 DOI:10.3389/fimmu.2021.720393. |
| [12] | SALEM I, RAMSER A, ISHAM N, GHANNOUM MA. The gut microbiome as a major regulator of the gut-skin axis[J]. Frontiers in Microbiology, 2018, 9: 1459 DOI:10.3389/fmicb.2018.01459. |
| [13] | HILL C, GUARNER F, REID G, GIBSON GR, MERENSTEIN DJ, POT B, MORELLI L, CANANI RB, FLINT HJ, SALMINEN S, CALDER PC, SANDERS ME. The International Scientific Association for probiotics and prebiotics consensus statement on the scope and appropriate use of the term probiotic[J]. Nature Reviews Gastroenterology & Hepatology, 2014, 11: 506-514. |
| [14] | SUEZ J, ZMORA N, SEGAL E, ELINAV E. The pros, cons, and many unknowns of probiotics[J]. Nature Medicine, 2019, 25: 716-729 DOI:10.1038/s41591-019-0439-x. |
| [15] |
WU QP, YUAN L, DAI JS, XIE XQ, LI Y, DING Y, WANG J, CHEN MT, XUE L, WU S, PANG R, ZHANG JM, CHEN HY. Two strains of Lactobacillus plantarum SR37-3 and SR61-2 with remarkable blood pressure reducing function and application of Lactobacillus plantarum SR37-3 and SR61-2: CN115747092A[P]. 2023-03-07 (in Chinese). 吴清平, 袁林, 代京莎, 谢新强, 李滢, 丁郁, 王涓, 陈谋通, 薛亮, 吴诗, 庞锐, 张菊梅, 陈惠元. 两株具有显著降血压功能的植物乳杆菌SR37-3和SR61-2及应用: CN115747092A[P]. 2023-03-07. |
| [16] |
WU QP, JIANG T, DAI JS, XIE XQ, LI Y, DING Y, WANG J, CHEN MT, XUE L, YE QH, WU S, GU QH, ZHANG JM, PANG R, ZHANG YX. Bifidobacterium longum 070103 with effects of remarkably reducing blood sugar and blood fat by targeting glucokinase and application of Bifidobacterium longum 070103: CN114774335B[P]. 2022-09-02 (in Chinese). 吴清平, 蒋同, 代京莎, 谢新强, 李滢, 丁郁, 王涓, 陈谋通, 薛亮, 叶青华, 吴诗, 古其会, 张菊梅, 庞锐, 张友雄. 具有靶向葡萄糖激酶显著降血糖和降血脂功效的长双歧杆菌070103及其应用: CN114774335B[P]. 2022-09-02. |
| [17] |
WU QP, LI LY, ZHONG HJ, XIE XQ, LI Y, DING Y, WANG J, XUE L, CHEN MT, ZHAO H, ZHANG JM, YE QH, WU S, CHEN HY, WU J. Lactobacillus plantarum L5 with function of relieving idiopathic tremor and sleep disorder and application of Lactobacillus plantarum L5: CN115873753A[P]. 2023-03-31 (in Chinese). 吴清平, 李龙岩, 钟豪杰, 谢新强, 李滢, 丁郁, 王涓, 薛亮, 陈谋通, 赵辉, 张菊梅, 叶青华, 吴诗, 陈惠元, 吴军林. 具有缓解特发性震颤和睡眠障碍功能的植物乳杆菌L5及其应用: CN115873753A[P]. 2023-03-31. |
| [18] |
WU QP, SHANG YY, LI Y, XIE XQ, DING Y, WANG J, XUE L, CHEN MT, ZHANG JM, YE QH, WU S, CHEN HY, WU JL. Lactobacillus plantarum LP1Z capable of producing lysozyme and efficiently antagonizing multidrug-resistant helicobacter pylori and application of Lactobacillus plantarum LP1Z: CN114350578B[P]. 2022-05-27 (in Chinese). 吴清平, 商燕燕, 李滢, 谢新强, 丁郁, 王涓, 薛亮, 陈谋通, 张菊梅, 叶青华, 吴诗, 陈惠元, 吴军林. 一株产溶菌酶并高效拮抗多药耐药幽门螺杆菌的植物乳杆菌LP1Z及其应用: CN114350578B[P]. 2022-05-27. |
| [19] |
LI Y, XUE L, WU QP, GAO JS, SHANG YY, XIE XQ, JIANG T, CHEN HZ, LI LY, CAI WC, CHENG T, ZHANG JM. Lactobacillus fermentum PV22 with anti-virus capacity, and application of Lactobacillus fermentum PV22: CN112522160B[P]. 2022-05-10 (in Chinese). 李滢, 薛亮, 吴清平, 高珺珊, 商燕燕, 谢新强, 蒋同, 陈慧贞, 李龙岩, 蔡伟程, 程彤, 张菊梅. 一株具有抗病毒能力的发酵乳杆菌PV22及其应用: CN112522160B[P]. 2022-05-10. |
| [20] |
WU QP, CHEN HZ, LI Y, XIE XQ, ZHANG JM, YANG N, CHEN HY, DAI JS, CHEN L, LIU ZJ. Lactobacillus fermentum capable of efficiently synthesizing nicotinamide and resisting light aging and application of Lactobacillus fermentum: CN113832050B[P]. 2022-10-21 (in Chinese). 吴清平, 陈慧贞, 李滢, 谢新强, 张菊梅, 杨宁, 陈惠元, 代京莎, 陈玲, 刘振杰. 一株高效合成烟酰胺、抗光老化的发酵乳杆菌XJC60及其应用: CN113832050B[P]. 2022-10-21. |
| [21] |
XIE XQ, DU MZ, CAI SZ, BAI JL, ZHANG JM, WU QP, YANG SH, LI Y, ZHAO H, LI FF, LIANG TT, JIANG T, YANG J, LI LY, WU L. Bifidobacterium longum 050101 with effect of relieving ulcerative colitis and application thereof: CN115820458A[P]. 2023-03-21 (in Chinese). 谢新强, 杜明珠, 蔡淑珍, 柏建玲, 张菊梅, 吴清平, 杨双红, 李滢, 赵辉, 李芬发, 梁婷婷, 蒋同, 杨娟, 李龙岩, 吴磊. 具有缓解溃疡性结肠炎功效的长双歧杆菌050101及其应用: CN115820458A[P]. 2023-03-21. |
| [22] | HUTTENHOWER C, GEVERS D, KNIGHT R, ABUBUCKER S, BADGER JH, CHINWALLA AT, CREASY HH, EARL AM, FITZGERALD MG, FULTON RS, GIGLIO MG, HALLSWORTH-PEPIN K, LOBOS EA, MADUPU R, MAGRINI V, MARTIN JC, MITREVA M, MUZNY DM, SODERGREN EJ, VERSALOVIC J, et al. Structure, function and diversity of the healthy human microbiome[J]. Nature, 2012, 486: 207-214 DOI:10.1038/nature11234. |
| [23] | THOMAS CL, FERNÁNDEZ-PEÑAS P. The microbiome and atopic eczema: more than skin deep[J]. The Australasian Journal of Dermatology, 2017, 58(1): 18-24 DOI:10.1111/ajd.12435. |
| [24] | FRIEDRICH AD, PAZ ML, LEONI J, GONZÁLEZ MAGLIO DH. Message in a bottle: dialog between intestine and skin modulated by probiotics[J]. International Journal of Molecular Sciences, 2017, 18(6): 1067 DOI:10.3390/ijms18061067. |
| [25] | SEITE S, BIEBER T. Barrier function and microbiotic dysbiosis in atopic dermatitis[J]. Clinical, Cosmetic and Investigational Dermatology, 2015, 8: 479-483. |
| [26] | SIMPSON EL, VILLARREAL M, JEPSON B, RAFAELS N, DAVID G, HANIFIN J, TAYLOR P, BOGUNIEWICZ M, YOSHIDA T, de BENEDETTO A, BARNES KC, LEUNG DYM, BECK LA. Patients with atopic dermatitis colonized with Staphylococcus aureus have a distinct phenotype and endotype[J]. Journal of Investigative Dermatology, 2018, 138(10): 2224-2233 DOI:10.1016/j.jid.2018.03.1517. |
| [27] | OH J, FREEMAN AF, COMPARATIVE SEQUENCING PROGRAM NISC, PARK M, SOKOLIC R, CANDOTTI F, HOLLAND SM, SEGRE JA, KONG HH. The altered landscape of the human skin microbiome in patients with primary immunodeficiencies[J]. Genome Research, 2013, 23(12): 2103-2114 DOI:10.1101/gr.159467.113. |
| [28] | NOWICKA D, NAWROT U. Contribution of Malassezia spp. to the development of atopic dermatitis[J]. Mycoses, 2019, 62(7): 588-596 DOI:10.1111/myc.12913. |
| [29] | TRAIDL S, ROESNER L, ZEITVOGEL J, WERFEL T. Eczema herpeticum in atopic dermatitis[J]. Allergy, 2021, 76(10): 3017-3027 DOI:10.1111/all.14853. |
| [30] | LEE SY, LEE E, PARK YM, HONG SJ. Microbiome in the gut-skin axis in atopic dermatitis[J]. Allergy, Asthma & Immunology Research, 2018, 10(4): 354-362. |
| [31] | WATANABE S, NARISAWA Y, ARASE S, OKAMATSU H, IKENAGA T, TAJIRI Y, KUMEMURA M. Differences in fecal microflora between patients with atopic dermatitis and healthy control subjects[J]. Journal of Allergy and Clinical Immunology, 2003, 111(3): 587-591 DOI:10.1067/mai.2003.105. |
| [32] | FIETEN KB, TOTTÉ JEE, LEVIN E, REYMAN M, MEIJER Y, KNULST A, SCHUREN F, PASMANS SGMA. Fecal microbiome and food allergy in pediatric atopic dermatitis: a cross-sectional pilot study[J]. International Archives of Allergy and Immunology, 2018, 175(1/2): 77-84. |
| [33] | ABRAHAMSSON TR, JAKOBSSON HE, ANDERSSON AF, BJÖRKSTÉN B, ENGSTRAND L, JENMALM MC. Low diversity of the gut microbiota in infants with atopic eczema[J]. Journal of Allergy and Clinical Immunology, 2012, 129(2): 434-440.e2 DOI:10.1016/j.jaci.2011.10.025. |
| [34] | FUJIMURA KE, SITARIK AR, HAVSTAD S, LIN DL, LEVAN S, FADROSH D, PANZER AR, LaMERE B, RACKAITYTE E, LUKACS NW, WEGIENKA G, BOUSHEY HA, OWNBY DR, ZORATTI EM, LEVIN AM, JOHNSON CC, LYNCH SV. Neonatal gut microbiota associates with childhood multisensitized atopy and T cell differentiation[J]. Nature Medicine, 2016, 22: 1187-1191 DOI:10.1038/nm.4176. |
| [35] | PENDERS J, THIJS C, van den BRANDT PA, KUMMELING I, SNIJDERS B, STELMA F, ADAMS H, van REE R, STOBBERINGH EE. Gut microbiota composition and development of atopic manifestations in infancy: the KOALA Birth Cohort Study[J]. Gut, 2007, 56(5): 661-667 DOI:10.1136/gut.2006.100164. |
| [36] | ENGEVIK MA, VERSALOVIC J. Biochemical features of beneficial microbes: foundations for therapeutic microbiology[J]. Microbiology Spectrum, 2017, 5(5): 10.1128. |
| [37] | WERFEL T, ALLAM JP, BIEDERMANN T, EYERICH K, GILLES S, GUTTMAN-YASSKY E, HOETZENECKER W, KNOL E, SIMON HU, WOLLENBERG A, BIEBER T, LAUENER R, SCHMID-GRENDELMEIER P, TRAIDL-HOFFMANN C, AKDIS CA. Cellular and molecular immunologic mechanisms in patients with atopic dermatitis[J]. Journal of Allergy and Clinical Immunology, 2016, 138(2): 336-349 DOI:10.1016/j.jaci.2016.06.010. |
| [38] | SIMONS FER, SIMONS KJ. Histamine and H1-antihistamines: celebrating a century of progress[J]. Journal of Allergy and Clinical Immunology, 2011, 128(6): 1139-1150.e4 DOI:10.1016/j.jaci.2011.09.005. |
| [39] | ANDREW D, CRAIG AD. Spinothalamic lamina I neurons selectively sensitive to histamine: a central neural pathway for itch[J]. Nature Neuroscience, 2001, 4(1): 72-77 DOI:10.1038/82924. |
| [40] | KIM J, KIM BE, LEUNG DYM. Pathophysiology of atopic dermatitis: clinical implications[J]. Allergy and Asthma Proceedings, 2019, 40(2): 84-92 DOI:10.2500/aap.2019.40.4202. |
| [41] | THOMSEN SF, ULRIK CS, KYVIK KO, HJELMBORG JVB, SKADHAUGE LR, STEFFENSEN I, BACKER V. Importance of genetic factors in the etiology of atopic dermatitis: a twin study[J]. Allergy and Asthma Proceedings, 2007, 28(5): 535-539 DOI:10.2500/aap2007.28.3041. |
| [42] | PALMER CNA, IRVINE AD, TERRON- KWIATKOWSKI A, ZHAO YW, LIAO HH, LEE SP, GOUDIE DR, SANDILANDS A, CAMPBELL LE, SMITH FJD, O'REGAN GM, WATSON RM, CECIL JE, BALE SJ, COMPTON JG, DiGIOVANNA JJ, FLECKMAN P, LEWIS-JONES S, ARSECULERATNE G, SERGEANT A, et al. Common loss-of-function variants of the epidermal barrier protein filaggrin are a major predisposing factor for atopic dermatitis[J]. Nature Genetics, 2006, 38: 441-446 DOI:10.1038/ng1767. |
| [43] | McALEER MA, IRVINE AD. The multifunctional role of filaggrin in allergic skin disease[J]. Journal of Allergy and Clinical Immunology, 2013, 131(2): 280-291 DOI:10.1016/j.jaci.2012.12.668. |
| [44] | BROWN SJ, ASAI YK, CORDELL HJ, CAMPBELL LE, ZHAO YW, LIAO HH, NORTHSTONE K, HENDERSON J, ALIZADEHFAR R, BEN-SHOSHAN M, MORGAN K, ROBERTS G, MASTHOFF LJN, PASMANS SGMA, van den AKKER PC, WIJMENGA C, HOURIHANE JO, PALMER CNA, LACK G, CLARKE A, et al. Loss-of-function variants in the filaggrin gene are a significant risk factor for peanut allergy[J]. The Journal of Allergy and Clinical Immunology, 2011, 127(3): 661-667 DOI:10.1016/j.jaci.2011.01.031. |
| [45] | MARENHOLZ I, ESPARZA-GORDILLO J, RÜSCHENDORF F, BAUERFEIND A, STRACHAN DP, SPYCHER BD, BAURECHT H, MARGARITTE- JEANNIN P, SÄÄF A, KERKHOF M, EGE M, BALTIC S, MATHESON MC, LI J, MICHEL S, ANG WQ, McARDLE W, ARNOLD A, HOMUTH G, DEMENAIS F, et al. Meta-analysis identifies seven susceptibility loci involved in the atopic March[J]. Nature Communications, 2015, 6: 8804 DOI:10.1038/ncomms9804. |
| [46] | TSAKOK T, WOOLF R, SMITH CH, WEIDINGER S, FLOHR C. Atopic dermatitis: the skin barrier and beyond[J]. British Journal of Dermatology, 2019, 180(3): 464-474 DOI:10.1111/bjd.16934. |
| [47] | GEOGHEGAN JA, IRVINE AD, FOSTER TJ. Staphylococcus aureus and atopic dermatitis: a complex and evolving relationship[J]. Trends in Microbiology, 2018, 26(6): 484-497 DOI:10.1016/j.tim.2017.11.008. |
| [48] | BRODSKÁ P, PANZNER P, PIZINGER K, SCHMID-GRENDELMEIER P. IgE-mediated sensitization to malassezia in atopic dermatitis: more common in male patients and in head and neck type[J]. Dermatitis: Contact, Atopic, Occupational, Drug, 2014, 25(3): 120-126. |
| [49] | UMETSU DT, DeKRUYFF RH. The regulation of allergy and asthma[J]. Immunological Reviews, 2006, 212: 238-255 DOI:10.1111/j.0105-2896.2006.00413.x. |
| [50] | KLONOWSKA J, GLEŃ J, NOWICKI RJ, TRZECIAK M. New cytokines in the pathogenesis of atopic dermatitis-new therapeutic targets[J]. International Journal of Molecular Sciences, 2018, 19(10): 3086 DOI:10.3390/ijms19103086. |
| [51] | SANO Y, MASUDA K, TAMAGAWA-MINEOKA R, MATSUNAKA H, MURAKAMI Y, YAMASHITA R, MORITA E, KATOH N. Thymic stromal lymphopoietin expression is increased in the horny layer of patients with atopic dermatitis[J]. Clinical and Experimental Immunology, 2013, 171(3): 330-337 DOI:10.1111/cei.12021. |
| [52] | XU M, DONG C. IL-25 in allergic inflammation[J]. Immunological Reviews, 2017, 278(1): 185-191 DOI:10.1111/imr.12558. |
| [53] | IMAI Y, YASUDA K, SAKAGUCHI Y, HANEDA T, MIZUTANI H, YOSHIMOTO T, NAKANISHI K, YAMANISHI K. Skin-specific expression of IL-33 activates group 2 innate lymphoid cells and elicits atopic dermatitis-like inflammation in mice[J]. Proceedings of the National Academy of Sciences of the United States of America, 2013, 110(34): 13921-13926. |
| [54] | PASHA MA, PATEL G, HOPP R, YANG Q. Role of innate lymphoid cells in allergic diseases[J]. Allergy and Asthma Proceedings, 2019, 40(3): 138-145 DOI:10.2500/aap.2019.40.4217. |
| [55] | SILVERBERG JI, HANIFIN J, SIMPSON EL. Climatic factors are associated with childhood eczema prevalence in the United States[J]. Journal of Investigative Dermatology, 2013, 133(7): 1752-1759 DOI:10.1038/jid.2013.19. |
| [56] | KWON JH, KIM E, CHANG MH, PARK EA, HONG YC, HA MN, PARK H, KIM Y, PARK C, HA EH. Indoor total volatile organic compounds exposure at 6 months followed by atopic dermatitis at 3 years in children[J]. Pediatric Allergy and Immunology: Official Publication of the European Society of Pediatric Allergy and Immunology, 2015, 26(4): 352-358 DOI:10.1111/pai.12393. |
| [57] | SCHMID-OTT G, JAEGER B, MEYERA B, STEPHANA B, KAPP A, WERFEL T. Different expression of cytokine and membrane molecules by circulating lymphocytes on acute mental stress in patients with atopic dermatitis in comparison with healthy controls[J]. Journal of Allergy and Clinical Immunology, 2001, 108(3): 455-462 DOI:10.1067/mai.2001.117800. |
| [58] | THYSSEN JP, ZIRWAS MJ, ELIAS PM. Potential role of reduced environmental UV exposure as a driver of the current epidemic of atopic dermatitis[J]. Journal of Allergy and Clinical Immunology, 2015, 136(5): 1163-1169 DOI:10.1016/j.jaci.2015.06.042. |
| [59] | PERKIN MR, CRAVEN J, LOGAN K, STRACHAN D, MARRS T, RADULOVIC S, CAMPBELL LE, MacCALLUM SF, IRWIN MCLEAN WH, LACK G, FLOHR C. Association between domestic water hardness, chlorine, and atopic dermatitis risk in early life: a population-based cross-sectional study[J]. Journal of Allergy and Clinical Immunology, 2016, 138(2): 509-516 DOI:10.1016/j.jaci.2016.03.031. |
| [60] | KANTOR R, SILVERBERG JI. Environmental risk factors and their role in the management of atopic dermatitis[J]. Expert Review of Clinical Immunology, 2017, 13(1): 15-26 DOI:10.1080/1744666X.2016.1212660. |
| [61] | SUÁREZ AL, FERAMISCO JD, KOO J, STEINHOFF M. Psychoneuroimmunology of psychological stress and atopic dermatitis: pathophysiologic and therapeutic updates[J]. Acta Dermato-Venereologica, 2012, 92(1): 7-15 DOI:10.2340/00015555-1188. |
| [62] | de ANDRADE PDSMA, MARIA E SILVA J, CARREGARO V, SACRAMENTO LA, ROBERTI LR, ARAGON DC, CARMONA F, ROXO-JUNIOR P. Efficacy of probiotics in children and adolescents with atopic dermatitis: a randomized, double-blind, placebo-controlled study[J]. Frontiers in Nutrition, 2022, 8: 833666 DOI:10.3389/fnut.2021.833666. |
| [63] | WIEËRS G, BELKHIR L, ENAUD R, LECLERCQ S, PHILIPPART de FOY JM, DEQUENNE I, de TIMARY P, CANI PD. How probiotics affect the microbiota[J]. Frontiers in Cellular and Infection Microbiology, 2020, 9: 454 DOI:10.3389/fcimb.2019.00454. |
| [64] | MATSUMOTO M, EBATA T, HIROOKA J, HOSOYA R, INOUE N, ITAMI S, TSUJI K, YAGINUMA T, MURAMATSU K, NAKAMURA A, FUJITA A, NAGAKURA T. Antipruritic effects of the probiotic strain LKM512 in adults with atopic dermatitis[J]. Annals of Allergy, Asthma & Immunology, 2014, 113(2): 209-216.e7. |
| [65] | ENOMOTO T, SOWA M, NISHIMORI K, SHIMAZU S, YOSHIDA A, YAMADA K, FURUKAWA F, NAKAGAWA T, YANAGISAWA N, IWABUCHI N, ODAMAKI T, ABE F, NAKAYAMA J, XIAO JZ. Effects of bifidobacterial supplementation to pregnant women and infants in the prevention of allergy development in infants and on fecal microbiota[J]. Allergology International, 2014, 63(4): 575-585 DOI:10.2332/allergolint.13-OA-0683. |
| [66] | DRAGO L, IEMOLI E, RODIGHIERO V, NICOLA L, de VECCHI E, PICONI S. Effects of Lactobacillus salivarius LS01 (DSM 22775) treatment on adult atopic dermatitis: a randomized placebo-controlled study[J]. International Journal of Immunopathology and Pharmacology, 2011, 24(4): 1037-1048 DOI:10.1177/039463201102400421. |
| [67] | MOROI M, UCHI S, NAKAMURA K, SATO S, SHIMIZU N, FUJII M, KUMAGAI T, SAITO M, UCHIYAMA K, WATANABE T, YAMAGUCHI H, YAMAMOTO T, TAKEUCHI S, FURUE M. Beneficial effect of a diet containing heat-killed Lactobacillus paracasei K71 on adult type atopic dermatitis[J]. The Journal of Dermatology, 2011, 38(2): 131-139 DOI:10.1111/j.1346-8138.2010.00939.x. |
| [68] | IEMOLI E, TRABATTONI D, PARISOTTO S, BORGONOVO L, TOSCANO M, RIZZARDINI G, CLERICI M, RICCI E, FUSI A, de VECCHI E, PICONI S, DRAGO L. Probiotics reduce gut microbial translocation and improve adult atopic dermatitis[J]. Journal of Clinical Gastroenterology, 2012, 46(suppl): S33-S40. |
| [69] | TAN-LIM CSC, ESTEBAN-IPAC NAR, RECTO MST, CASTOR MAR, CASIS-HAO RJ, NANO ALM. Comparative effectiveness of probiotic strains on the prevention of pediatric atopic dermatitis: a systematic review and network meta-analysis[J]. Pediatric Allergy and Immunology: Official Publication of the European Society of Pediatric Allergy and Immunology, 2021, 32(6): 1255-1270 DOI:10.1111/pai.13514. |
| [70] | FANG ZF, LU WW, ZHAO JX, ZHANG H, QIAN L, WANG Q, CHEN W. Probiotics modulate the gut microbiota composition and immune responses in patients with atopic dermatitis: a pilot study[J]. European Journal of Nutrition, 2020, 59(5): 2119-2130 DOI:10.1007/s00394-019-02061-x. |
| [71] | PRAKOESWA CRS, BONITA L, KARIM A, HERWANTO N, UMBOROWATI MA, SETYANINGRUM T, HIDAYATI AN, SURONO IS. Beneficial effect of Lactobacillus plantarum IS-10506 supplementation in adults with atopic dermatitis: a randomized controlled trial[J]. The Journal of Dermatological Treatment, 2022, 33(3): 1491-1498 DOI:10.1080/09546634.2020.1836310. |
| [72] | JEONG K, KIM M, JEON SA, KIM YH, LEE S. A randomized trial of Lactobacillus rhamnosus IDCC 3201 tyndallizate (RHT3201) for treating atopic dermatitis[J]. Pediatric Allergy and Immunology: Official Publication of the European Society of Pediatric Allergy and Immunology, 2020, 31(7): 783-792 DOI:10.1111/pai.13269. |
| [73] | KIM WK, JANG YJ, HAN DH, JEON K, LEE C, HAN HS, KO G. Lactobacillus paracasei KBL382 administration attenuates atopic dermatitis by modulating immune response and gut microbiota[J]. Gut Microbes, 2020, 12(1): 1-14. |
| [74] | WON TJ, KIM B, LIM YT, SONG DS, PARK S, PARK ES, LEE DI, HWANG KW. Oral administration of Lactobacillus strains from Kimchi inhibits atopic dermatitis in NC / Nga mice[J]. Journal of Applied Microbiology, 2011, 110(5): 1195-1202 DOI:10.1111/j.1365-2672.2011.04981.x. |
| [75] | KIM JH, KIM K, KANJANASUNTREE R, KIM W. Kazachstania turicensis CAU Y1706 ameliorates atopic dermatitis by regulation of the gut-skin axis[J]. Journal of Dairy Science, 2019, 102(4): 2854-2862 DOI:10.3168/jds.2018-15849. |
| [76] | ZHOU JB, XU GS, LI XY, TU HY, LI HY, CHANG H, CHEN J, YU RQ, QI C, SUN J. Limosilactobacillus reuteri FN041 prevents atopic dermatitis in pup mice by remodeling the ileal microbiota and regulating gene expression in Peyer's patches after vertical transmission[J]. Frontiers in Nutrition, 2022, 9: 987400 DOI:10.3389/fnut.2022.987400. |
| [77] | KIM IS, LEE SH, KWON YM, ADHIKARI B, KIM JA, YU DY, KIM GI, LIM JM, KIM SH, LEE SS, MOON YS, CHOI IS, CHO KK. Oral administration of β-glucan and Lactobacillus plantarum alleviates atopic dermatitis-like symptoms[J]. Journal of Microbiology and Biotechnology, 2019, 29(11): 1693-1706 DOI:10.4014/jmb.1907.07011. |
| [78] | FANG ZF, LI LZ, ZHAO JX, ZHANG H, LEE YK, LU WW, CHEN W. Bifidobacteria adolescentis regulated immune responses and gut microbial composition to alleviate DNFB-induced atopic dermatitis in mice[J]. European Journal of Nutrition, 2020, 59(7): 3069-3081 DOI:10.1007/s00394-019-02145-8. |
| [79] | KIM S, HAN SY, LEE J, KIM NR, LEE BR, KIM H, KWON M, AHN K, NOH Y, KIM SJ, LEE P, KIM D, KIM BE, KIM J. Bifidobacterium longum and galactooligosaccharide improve skin barrier dysfunction and atopic dermatitis-like skin[J]. Allergy, Asthma & Immunology Research, 2022, 14(5): 549-564. |
| [80] | CHOI WJ, KONKIT M, KIM Y, KIM MK, KIM W. Oral administration of Lactococcus chungangensis inhibits 2,4-dinitrochlorobenzene-induced atopic-like dermatitis in NC/Nga mice[J]. Journal of Dairy Science, 2016, 99(9): 6889-6901 DOI:10.3168/jds.2016-11301. |
| [81] | ROSENFELDT V, BENFELDT E, VALERIUS NH, PÆRREGAARD A, MICHAELSEN KF. Effect of probiotics on gastrointestinal symptoms and small intestinal permeability in children with atopic dermatitis[J]. The Journal of Pediatrics, 2004, 145(5): 612-616 DOI:10.1016/j.jpeds.2004.06.068. |
| [82] | OHLAND CL, MACNAUGHTON WK. Probiotic bacteria and intestinal epithelial barrier function[J]. American Journal of Physiology Gastrointestinal and Liver Physiology, 2010, 298(6): G807-G819 DOI:10.1152/ajpgi.00243.2009. |
| [83] | ESLAMI M, BAHAR A, KEIKHA M, KARBALAEI M, KOBYLIAK NM, YOUSEFI B. Probiotics function and modulation of the immune system in allergic diseases[J]. Allergologia et Immunopathologia, 2020, 48(6): 771-788 DOI:10.1016/j.aller.2020.04.005. |
| [84] | MA X, PAN Y, ZHAO W, SUN PW, ZHAO JF, YAN SY, WANG R, HAN YQ, LIU WH, TAN SJ, HUNG WL. Bifidobacterium infantis strain YLGB-1496 possesses excellent antioxidant and skin barrier-enhancing efficacy in vitro[J]. Experimental Dermatology, 2022, 31(7): 1089-1094 DOI:10.1111/exd.14583. |
| [85] | CHEN HZ, LI Y, XIE XQ, CHEN MT, XUE L, WANG J, YE QH, WU S, YANG RS, ZHAO H, ZHANG JM, DING Y, WU QP. Exploration of the molecular mechanisms underlying the anti-photoaging effect of Limosilactobacillus fermentum XJC60[J]. Frontiers in Cellular and Infection Microbiology, 2022, 12: 838060 DOI:10.3389/fcimb.2022.838060. |
| [86] | DU MZ, XIE XQ, YANG SH, LI Y, JIANG T, YANG J, LI LY, HUANG YX, WU QP, CHEN W, ZHANG JM. Lysozyme-like protein produced by Bifidobacterium longum regulates human gut microbiota using in vitro models[J]. Molecules, 2021, 26(21): 6480 DOI:10.3390/molecules26216480. |
| [87] | MANTIS NJ, ROL N, CORTHÉSY B. Secretory IgA's complex roles in immunity and mucosal homeostasis in the gut[J]. Mucosal Immunology, 2011, 4(6): 603-611 DOI:10.1038/mi.2011.41. |
| [88] | YAO Y, CHEN CL, YU D, LIU Z. Roles of follicular helper and regulatory T cells in allergic diseases and allergen immunotherapy[J]. Allergy, 2021, 76(2): 456-470 DOI:10.1111/all.14639. |
| [89] | TAN J, McKENZIE C, VUILLERMIN PJ, GOVERSE G, VINUESA CG, MEBIUS RE, MACIA L, MACKAY CR. Dietary fiber and bacterial SCFA enhance oral tolerance and protect against food allergy through diverse cellular pathways[J]. Cell Reports, 2016, 15(12): 2809-2824 DOI:10.1016/j.celrep.2016.05.047. |
| [90] | LUU M, MONNING H, VISEKRUNA A. Exploring the molecular mechanisms underlying the protective effects of microbial SCFAs on intestinal tolerance and food allergy[J]. Frontiers in Immunology, 2020, 11: 1225 DOI:10.3389/fimmu.2020.01225. |
 2024, Vol. 64
2024, Vol. 64