中国科学院微生物研究所,中国微生物学会
文章信息
- 孙丽薇, 耿倩, 郑国华. 2024
- SUN Liwei, GENG Qian, ZHENG Guohua.
- 肠道菌群-肠-脑-肌轴信号交流的研究进展
- Research progress in signaling of gut microbiota-gut-brain-muscle axis
- 微生物学报, 64(5): 1364-1377
- Acta Microbiologica Sinica, 64(5): 1364-1377
-
文章历史
- 收稿日期:2023-10-23
- 网络出版日期:2024-03-08
2. 上海健康医学院护理与健康管理学院, 上海 201318
2. School of Nursing and Health Management, Shanghai University of Medicine & Health Sciences, Shanghai 201318, China
随着对肠道菌群研究的不断深入,人们逐渐认识到肠道菌群具有独立代谢的功能,其代谢物质可参与机体的神经—内分泌—免疫功能的调节过程,在维持机体动态平衡中发挥重要作用。肠道菌群与宿主相互作用、相互影响,共生形成较为稳定的肠道微生态,被称为人类的“第二大脑”,在维持机体健康、促进疾病发生与发展过程中发挥着至关重要的作用,逐渐被认为是宿主生理学和病理生理学的关键调节因子[1]。研究表明,肠道菌群和中枢神经系统之间可通过肠-脑轴进行双向调节,也可通过肠-肌轴的交流机制调节肌肉骨骼系统的生理、病理过程,影响神经系统和肌肉骨骼系统疾病的发展进程[2]。为了深入了解和探究肠道菌群与肠、脑、肌之间的信号交流机制,本文就其研究进展进行综述。
1 人体肠道菌群与健康的关系肠道菌群和肠黏膜以及肠道免疫系统共同构成复杂的肠道微生态系统[3],而作为肠道微生态的核心,肠道菌群主要包括厚壁菌门、拟杆菌门、变形菌门、放线菌门及疣微菌门5个门类,其中厚壁菌门与拟杆菌门是人体主要的菌群,占健康个体肠道细菌的90%以上[4]。正常情况下,人体和肠道菌群间维持着一种相对稳定的“动态平衡”,共同维护人体健康;而当这种动态平衡失调时,可能诱发多种疾病[5],如代谢性疾病[6]、神经系统与心理疾病[7]、肌肉骨骼疾病[8]的发展进程。
1.1 肠道菌群对人体健康的维护肠道菌群对人体健康的作用是多维度、动态、复杂且广泛的,其对机体的健康维护作用主要是通过参与机体新陈代谢和免疫系统的调节,并维持机体的动态平衡。如一些有益的肠道菌群能将食物中不可消化的多糖分解成各种代谢产物,如短链脂肪酸(short chain fatty acids, SCFAs),不仅可为肠道上皮细胞提供能量,维持肠道上皮黏膜屏障的完整性,还是一种有效的抗炎化合物,可通过抑制核因子-κB (nuclear factor-κB, NF-κB)的活性而减少白介素-6 (interleukin-6, IL-6)、肿瘤坏死因子-α (tumor necrosis factor alpha, TNF-α)等细胞因子的产生[9],在控制慢性炎症等方面发挥重要作用。Leeuwendaal等[10]发现一些肠道菌群可促进人类维生素K、维生素B12、叶酸和必需氨基酸的合成,参与骨代谢、脂质代谢及血糖代谢的生理过程。在免疫方面,有些肠道菌群可通过与肠道上皮细胞的竞争性结合,直接或间接地触发对外源性病原体的保护性免疫反应;也可通过诱导免疫球蛋白A (immunoglobulin A, IgA)、T细胞来激活获得性免疫系统,参与全身免疫调节[11]。如有研究发现,肠道炎症可导致内分泌紊乱、影响肠道免疫功能,随着肠道菌群失衡,肠道中免疫球蛋白M (immunoglobulin M, IgM)、IgA的表达量显著降低[12]。
1.2 肠道菌群失调参与多种疾病的发展进程正常情况下,肠道菌群与肠上皮细胞和免疫细胞共同维持着肠道微生态的平衡,但当受到年龄、饮食、药物、遗传、环境等的不良影响时,肠道微生态的平衡被破坏而引起肠道菌群失调,可促进人体多种疾病的发生与发展。肠道菌群失调,包括细菌组成失衡或对共生菌群的异常免疫反应等,是炎症性肠病(inflammatory bowel disease, IBD)、代谢性疾病、阿尔茨海默病(Alzheimer’s disease, AD)及肌肉骨骼疾病发生发展的潜在机制。失调的肠道菌群可引起机体免疫、炎症反应,诱发炎症性肠病的发生。例如,当厚壁菌门丰度下降,变形菌门和放线菌门丰度上升,脆弱拟杆菌的功能及活性降低,可能会促进IBD的发生发展[13]。有文献报道,普拉梭菌在体内外均具有很强的抗炎作用,可维持肠道健康,而该菌减少可致肠黏膜保护功能降低,加速肠道疾病的发展进程[14]。另外,肠道炎症也会损伤正常的肠屏障功能,导致肠道菌群失调并发生细菌移位,从而加大炎症反应和代谢相关疾病的风险。有研究发现,长期进行高脂饮食的肠道菌群会增加肠道黏膜通透性、加剧全身炎症和脑部炎症[15]。如嗜黏蛋白阿克曼菌是一种黏蛋白分解细菌,能够有效改善高脂饮食诱导的结肠黏膜屏障功能障碍[16]。失调的肠道菌群也可影响机体淀粉样蛋白和神经递质的产生,并通过人体中枢系统影响认知以及情绪。如阿尔茨海默病是典型的中枢神经系统退行性疾病,其神经病理学特征是淀粉样蛋白β沉积,引发神经炎症,导致突触缺失和神经元死亡[17]。有研究发现,在肠道菌群失调的情况下,肠道菌群中的某些成分,如枯草芽孢杆菌和大肠杆菌,可分泌大量的脂多糖(lipopolysaccharide, LPS)和淀粉样蛋白,它们可能直接穿过因衰老或疾病而受损的肠道屏障或血脑屏障,促进AD的发展[18]。失调的肠道菌群也可影响营养的代谢与摄取、肠道炎症、胆汁酸代谢、维生素合成和肌酸降解过程从而影响肌少症的发生发展[19]。有研究发现,肌少症患者肠道菌群结构显著改变,肠杆菌科丰度增加,乳酸杆菌丰度明显减少[20]。
2 肠道菌群-肠-脑轴信号交流机制肠道菌群-肠-脑轴(microbiota-gut-brain axis, MGBA)作为机体的肠道和大脑神经系统之间的双向交流途径,可通过免疫、内分泌和神经系统等多种途径对机体的大脑行为及认知功能产生影响,在维持肠脑关系中起着重要作用。
2.1 免疫调节途径许多研究表明肠道菌群可通过影响先天免疫和适应性免疫参与肠脑轴的调节。先天免疫包括外周系统中的单核细胞和巨噬细胞、大脑中的小胶质细胞和星形胶质细胞等神经胶质细胞以及肠道中的树突状细胞和先天淋巴样细胞[21]。肠道的先天免疫反应包括激活NF-κB为特征的炎症反应[22],也可以通过识别大多数微生物表达的病原相关分子模式(pathogen/microbe-associated molecular patterns, PAMPs/MAMPs)来激活[23]。上述反应一方面会产生趋化因子、促炎细胞因子诱导炎症反应[24];另一方面也可激活免疫细胞释放抗菌素来维持和保护肠上皮黏膜屏障[25]。适应性免疫中肠道菌群及其代谢产物可通过促进T细胞释放炎症因子和抗炎细胞因子,影响大脑的功能和精神状态[21]。Powell等[26]发现肠道菌群代谢产物SCFA、LPS可以促进调节T细胞产生白介素-10 (interleukin-10, IL-10)。另有研究也发现无菌小鼠(GF小鼠)免疫系统功能较无特定性病原体小鼠(SPF小鼠)明显降低,但将正常菌群定殖GF小鼠后免疫功能会恢复正常状态[27]。因此,在维持大脑-肠道稳态中,免疫系统发挥关键的调节作用。
2.2 内分泌信号途径下丘脑-垂体-肾上腺(hypothalamic-pituitary- adrenal, HPA)轴是机体主要的神经内分泌系统,可通过调节激素的分泌,影响消化、免疫系统,以及情绪和行为,是MGB轴双向调节的重要途径。当机体处于应激状态时,HPA轴被激活,下丘脑的脑室旁核可分泌促皮质素释放因子(corticotropin releasing factor, CRF)释放到垂体前叶后增加促肾上腺皮质激素(adrenocorticotropic hormone, ACTH)的合成和释放,后者又作用于肾上腺,促进糖皮质激素(glucocorticoids, GCs)的合成[28]。GCs是HPA轴的主要效应分子,可与多种组织细胞内受体结合,调节压力应激的生理适应。有研究报道肠道菌群及其代谢产物可激活肠道内分泌细胞释放促皮质素释放因子、肾上腺皮质酮以及酪酪肽(peptide YY, PYY)、生长素释放肽、胰高血糖素样肽-1 (glucagon-like peptide-1, GLP-1)等脑肠道肽,进而激活内分泌和神经信号影响中枢神经系统的能量调节[29]。例如,有研究表明肠道代谢产物SCFAs可以通过调节肠道激素的内分泌途径来改变肠道-大脑轴的功能。SCFAs通过激活G蛋白偶联受体(G-protein-coupled receptors, GPCR)促进肠内分泌L细胞释放PYY和GLP1[30-31]。GLP-1已被证明能增强海马突触可塑性,改善学习、记忆和运动功能,这表明它对中枢神经退行性疾病具有潜在的神经保护作用。
2.3 神经信号通路神经信号通路主要依靠中枢神经系统(central nervous system, CNS)、自主神经系统(autonomic nervous system, ANS)以及肠神经系统(enteric nervous system, ENS)三者之间进行交流。ENS是ANS中最大的分支,在调节肠道菌群结构、肠道菌群相关的代谢物、神经递质和免疫信号,以及保护肠道屏障方面发挥着多方面的作用[32-33]。CNS和ENS之间的双向交流通路构成MGBA的部分网络。迷走神经是大脑和肠道交流最直接的通路,肠道菌群或其代谢产物能够直接作用于肠神经系统内初级传入神经元的功能突触而向迷走神经传入发出信号[34],一方面促进迷走神经调节代谢平衡和进食行为;另一方面促进迷走神经参与大脑、肠道和身体其他器官的炎症机制[33]。如鼠李糖乳杆菌分泌的神经递质γ-氨基丁酸(γ-aminobutyricacid, GABA)、5-羟色胺、组胺等可以通过迷走神经传递到中枢神经系统,发挥抗抑郁或抗焦虑作用[35]。在小鼠实验研究中,迷走神经切断术已被证明可以阻断乳酸杆菌和双歧杆菌属的中枢信号传导。具体而言,影响乳酸杆菌和双歧杆菌代谢产生的乙酰胆碱,进而改变体内神经递质水平,导致其情绪调节作用失效[35-36]。也有研究发现肠道代谢产物LPS和SCFA与肠内分泌细胞受体结合时可释放GLP-1和PYY等介质,作用于迷走神经内的受体,调节肠道运动和分泌功能、炎症反应和黏膜防御等各种自主神经反应[37]。
3 肠道菌群-肠-肌轴信号交流机制肠道菌群-肠-肌轴(microbiota-gut-muscle axis, MGMA)是指肠道菌群通过炎症、代谢及线粒体通路调控肠道屏障,促进蛋白质等营养物质的消化与吸收,参与骨骼肌能量代谢等途径。
3.1 炎症通路肠道菌群的调节可以影响肠道的屏障功能,从而在维持促炎和抗炎反应的平衡方面发挥重要作用[38]。健康的肠道微生态环境可以在肠黏膜内诱导多种宿主反应,从而加强肠道屏障功能,在肠道内外发挥免疫调节作用[39]。抗炎因子和炎症因子的免疫平衡不仅可以抵御外来细菌的入侵,而且可以增加身体免疫耐受能力[40]。在一定条件下,肠道菌群的改变会损害肠道屏障的完整性,使LPS等有害产物进入血液,引发全身炎症,导致代谢紊乱,肌肉功能和质量下降[41]。有研究发现LPS水平升高会激活Toll样受体4 (Toll-like receptors 4, TLR4)信号传导,促进自噬小体的形成以及肌肉萎缩相关基因1 (atrogin-1)和肌肉环指蛋白-1 (muscle ring finger protein-1, MuRF-1)的表达而诱导C2C12肌管细胞萎缩[42]。TLR4信号通路还可通过炎症级联反应释放大量炎性因子,诱导全身炎症反应,激活多条骨骼肌蛋白分解代谢通路,导致蛋白质合成与分解失衡[43]。Zhang等[44]研究也发现,将老年菌群定殖给年轻大鼠,可增加年轻小鼠肠道通透性,使血浆中LPS浓度增加。Bindels等[45]在白血病小鼠实验中明确了补充乳杆菌菌株的小鼠中肌肉萎缩标记物的表达下降,小鼠的肌肉质量和力量增加。因此,炎症反应在骨骼肌质量和功能中起着关键作用,TLR4信号通路与肠道菌群-肠-肌轴密切相关。进一步研究炎症反应和肠道菌群之间的关系可能有助于治疗以骨骼肌质量和功能降低为特征的疾病。
3.2 代谢通路肠道菌群是重要的“内分泌器官”,能够分泌多种生物活性物质,通过代谢通路影响人体骨骼肌代谢。过氧化物酶体增殖物激活受体(peroxisome proliferator-activated receptors, PPAR)是由配体激活的转录因子,参与调控骨骼肌葡萄糖和脂质代谢,是肌纤维表型的主要调节因子。研究发现,一些肠道菌群(如厚壁菌、梭杆菌、放线杆菌、粪肠球菌等)及其代谢产物是对过氧化物酶体增殖活化受体δ (peroxisome proliferator-activated receptors δ, PPARδ)强有效的激活因子[46]。PPARδ的表达可以激活腺苷单磷酸激活的蛋白激酶(AMPK)信号通路,增加过氧化物酶体增殖物激活受体γ-共激活因子1-α (peroxisome proliferators-activated receptors gamma co-activator 1α, PGC-1α)的水平,从而促进脂肪酸摄取和氧化、葡萄糖摄取和糖原合成,抑制脂肪生成和糖酵解[47]。有研究发现在PPARδ敲除小鼠肌肉和脂肪细胞中观察到能量代谢异常和肌纤维减少,以及葡萄糖耐受不良伴胰岛素抵抗[48]。PPAR激动剂则可改善强直蛋白缺乏,补偿肌纤维损失,从而改善强直性肌营养不良[49]。另有研究证实肠道菌群产生的次级胆汁酸(bile acids, BAs)可作为信号分子调节肌肉中G蛋白偶联胆汁酸受体1 (G protein-coupled bile acid receptor 1, GPBAR1),促进甲状腺激素的活化,从而改善人体骨骼肌细胞的能量消耗[50]。同时BAs也可以通过激活核受体法尼糖X受体(farnese X receptor, FXR)调节机体能量、葡萄糖和脂质代谢[51],改善骨骼肌细胞的能量代谢。
3.3 线粒体通路骨骼肌线粒体功能障碍是肌肉质量和功能下降的原因之一。线粒体通过产生三磷酸腺苷(adenosine triphos-phate, ATP)满足肌肉纤维内的代谢需求,同时也是活性氧(reactive oxygen species, ROS)的主要来源。在衰老等情况下线粒体稳态的破坏造成ROS产生过多,合成骨骼肌蛋白质所需的ATP减少,从而加速衰老所引起的肌肉质量与力量的丧失。Lahiri等[8]将GF小鼠的骨骼肌与SPF小鼠的骨骼肌进行了比较,发现GF小鼠表现为肌肉萎缩,胰岛素样生长因子-1 (insulin-like growth factor-1, IGF-1)、骨骼肌生长和线粒体功能相关琥珀酸脱氢酶表达减少;而将SPF小鼠的肠道菌群移植到GF小鼠肠道后,参与ATP生成的各种酶在线粒体中被高度乙酰化,可显著改善GF小鼠肌肉萎缩程度和增加线粒体中能量的生成。提示肠道菌群代谢产物参与骨骼肌与线粒体的功能调节。最近有研究报道肠道菌群与线粒体在不同疾病的病理生理学中的密切联系[52]。肠道菌群通过释放代谢物、蛋白质或毒素来调节ROS的产生和线粒体的活性,从而加强与线粒体的联系。因此,肠道微生物群代谢物可能是导致线粒体功能失调的关键介质,是肌少症等疾病的重要促进因素[53]。建立菌群与线粒体之间的交流模式对于理解环境、微生物组和宿主之间错综复杂且不断变化的相互作用至关重要,最终将有助于更好地理解它们对健康和疾病的影响[54]。
4 肠道菌群-肠-脑-肌轴信号交流机制肠道菌群通过肠-脑轴和肠-肌轴实现肠道菌群与大脑、肠道菌群与骨骼肌之间的双向交流,调节机体大脑和骨骼肌的功能。最近多项研究显示肠道菌群也可通过肠-脑-肌轴(gut-brain-muscle axis, GBMuA)与机体进行信号交流,参与GBMuA的潜在交流途径包括菌群代谢产物、肠道通透性、免疫-神经通路等。
4.1 肠道菌群代谢产物 4.1.1 短链脂肪酸SCFAs是由杆菌属、双歧杆菌属、丙酸杆菌属等发酵膳食纤维产生的代谢产物[55]。在胃肠道系统中的SCFAs能通过与肠上皮上的G蛋白偶联游离脂肪酸受体结合实现信号传导,也可以通过被动或主动转运进入到循环系统之中。细胞研究证实,SCFAs可以穿过血脑屏障进入大脑影响小胶质细胞的形态和功能,调节神经营养因子水平,促进血清素生物合成,改善神经元稳态和神经炎症[56-58]。目前的研究者普遍认为,SCFAs具有抗炎作用[59-60],也可通过维持肠道屏障的完整性、调节紧密连接蛋白的表达来增强肠道屏障功能以及促进胃肠道的黏液生成,保护上皮细胞免受胃酸的损伤,抑制胃肠道炎症[61]。SCFAs还可通过循环与骨骼肌上的游离脂肪酸受体2和3 (free fatty acid receptor 2/3, FFAR 2/3)结合,并通过促进葡萄糖摄取和代谢的机制释放IGF-1,激活胞内磷脂酰肌醇激酶(PI3K)-苏氨酸蛋白激酶C (Akt)-雷帕霉素靶蛋白(mTOR)信号通路,刺激骨骼肌组织中的蛋白质合成并阻断蛋白质水解[62]。
研究表明骨骼肌的运动收缩可以调节肠道菌群组成,增加体内SCFAs水平,上调PGC-1α的表达,显著提高纤连蛋白III型结构域蛋白5 (fibronectin type III domain containing 5, FNDC5)和鸢尾素的表达水平,激活肠-肌轴,改善线粒体功能和扩充肌肉线粒体体积,发挥其生物效应进而改善线粒体功能障碍,从而延缓肌少症的发生发展。而鸢尾素可经血液循环透过血脑屏障(blood brain barrier, BBB)进入脑组织,促进脑源性神经营养因子(brain-derived neurotrophic factor, BDNF)表达,产生神经保护效应。另外,SCFAs也可通过激活肠-脑轴,增加下丘脑与海马体中BDNF的释放,进一步影响学习、记忆及抗焦虑行为[63]。
4.1.2 犬尿氨酸肠道菌群可以直接分泌色氨酸-犬尿氨酸代谢途径所需的酶,如铜绿假单胞菌可产生吲哚胺-2, 3-双加氧酶(indoleamine 2, 3-dioxygenase, IDO)和色氨酸-2, 3-双加氧酶(tryptophan 2, 3-dioxygenase, TDO)[64],对色氨酸进行分解代谢,调节犬尿氨酸(kynurenine, KYN)的生成。GF小鼠因免疫系统中识别胃肠道微生物成分的Toll样受体表达减少、激活IDO的作用下降,直接导致KYN代谢下降[65]。KYN在中枢神经系统中通过不同细胞代谢产生不同的代谢产物,星形胶质细胞代谢产生具有神经保护性的犬尿喹啉酸(kynurenic acid, KYNA),小胶质细胞代谢产生具有神经毒性的喹啉酸(quinolinic acid, Quin),二者相互拮抗[66]。大量研究表明,色氨酸代谢及其下游代谢分支紊乱与阿尔茨海默病密切相关。在患有AD、帕金森病、亨廷顿病等神经退行性疾病的老年患者中观察到KYN的失调,具体表现为Quin水平升高及KYNA下降[67-69]。Kaiser等[70]发现,通过给予小鼠KYN药物治疗会降低肌肉质量和大小,同时增加肌肉脂质过氧化、蛋白质分解代谢和ROS水平。ROS水平长期升高和脂质过氧化产物增加均由高水平的KYN诱导,与肌肉减少有关[71-72]。自愿轮跑可促进骨骼肌PGC-1α和犬尿氨酸转移酶(kynurenine aminotransferase, KAT)的释放,增加野生型小鼠血浆中KYN代谢物KYNA的水平[73]。这表明运动可以通过PGC-1α/KAT途径平衡KYN代谢,减轻KYN的神经毒性作用,从而改善认知功能。
4.1.3 脂肪酸酰胺脂肪酸酰胺(fatty acid amides, FAAs)是由革兰氏阴性菌产生的肠道代谢物[74]。研究显示FAAs可通过模仿人类信号分子参与机体的神经传递和免疫调节作用[75]。动物实验[76]也发现FAAs可调节小鼠的身体活动,提高小鼠的运动表现,其机制为脂肪酸酰胺通过与内源性大麻受体CB1结合刺激肠内感觉神经,经脊椎与大脑相连,使大脑的腹侧纹状体增加,提高机体内多巴胺的含量,从而加强运动欲望来提高运动表现。
4.2 免疫-神经途径肠道菌群-免疫-神经机制是连接肠道菌群-肠-脑-肌轴的另一个重要途径,肠道菌群在黏膜屏障免疫平衡中发挥重要作用。肠道菌群生态失衡所产生的LPS等有害肠道代谢产物进入血液后,可激活TLR-4[77],导致NF-κB蛋白水平和c-Jun N端激酶(c-Jun N-terminal kinase, JNK)磷酸化显著升高,诱导促炎细胞因子IL-6和TNF-α上调,从而导致代谢紊乱,肌肉功能和质量下降,诱导全身炎症[78]。Rubio等[79]发现老年大鼠产LPS的肠道菌群明显增多,加剧肠黏膜屏障受损,使通透性增加形成“肠漏”。此外,LPS也可通过激活TLR4促进自噬小体的形成,使得促炎细胞因子分泌增加,肌肉萎缩盒F基因(muscle atrophy F-box protein, MAFbx/atrogin-1)和肌肉环指蛋白-1 (muscle ring finger protein-1, MuRF-1)释放诱导C2C12肌管细胞萎缩[80]。
作为内分泌器官,骨骼肌可分泌IL-6、IL-10,一方面改善肠道炎症反应,重塑肠道微生物系统,增加肠道菌群多样性,加强肠道屏障功能,降低细菌易位率和调节肠道通透性[81];另一方面刺激肠道L细胞分泌PYY、胰多肽和GLP-1等脑-肠肽在血液中释放,触发迷走神经传入神经作用于中枢神经系统[82]。骨骼肌还可通过独立于肠道菌群的免疫途径降低外周炎症反应水平,如通过促进肌肉PGC-1α的表达,抑制外周NF-κB活性[83];激活HPA轴,减少TNF-α释放,改善外周炎症反应[84]。
4.3 肠-脑-肌轴与疾病病理机制的潜在关联最近的研究结果表明,肠道菌群通过肠-脑-肌轴在改变或调节大脑和骨骼肌的功能方面发挥着重要作用,主要通过调节肠道通透性、SCFAs生成、炎症作用等途径影响多种疾病的发生发展进程[85]。研究表明由于增龄等因素所引起的“肠道生态失调”可导致肠漏增加,使细菌易位进入血液[86],一方面使产生SCFAs的有益菌(如厚壁菌和放线菌)的数量减少而无法抑制LPS诱导的炎症;另一方面有害菌如拟杆菌等生成过多的LPS,通过刺激TLR2、TLR4受体,引发炎症反应[87];而有些菌群如变形杆菌可产生丰富的细菌淀粉样蛋白,如卷曲纤维等可与Toll样受体-2结合激活巨噬细胞,促进TNF-α、IL-6和白介素-1β (interleukin-1 β, IL-1β)等炎症细胞因子升高[88],生成更多的ROS并促进氧化应激反应,导致神经炎症和肌肉萎缩[89];这些细胞因子还可激活NF-κB信号通路,刺激促炎微小RNA (microRNA, miRNA)的转录,激活神经炎症介质,并抑制小胶质细胞中的吞噬作用,导致神经退行性疾病的进展[90],同时还会影响骨骼肌代谢和线粒体生物合成,进而影响骨骼肌和身体功能[91]。另外,肠道菌群改变也可引起肠道屏障损伤、促进肠道菌群及其代谢产物进入血液循环,进而通过促进炎性细胞因子的表达导致全身处于慢性低度炎症状态,而慢性低度炎症可通过炎症自噬等途径影响肌肉中的蛋白质分解合成,进而影响肌肉的质量和强度,甚至造成肌肉功能的损失[92-93]。肠道慢性炎症还可以激发免疫反应,降低血脊髓屏障和血脑屏障膜完整性,引起中枢神经系统炎症反应,进而导致神经变性,并通过引起脑部炎症和破坏BBB功能来促进认知衰弱的发展(图 1)[94]。
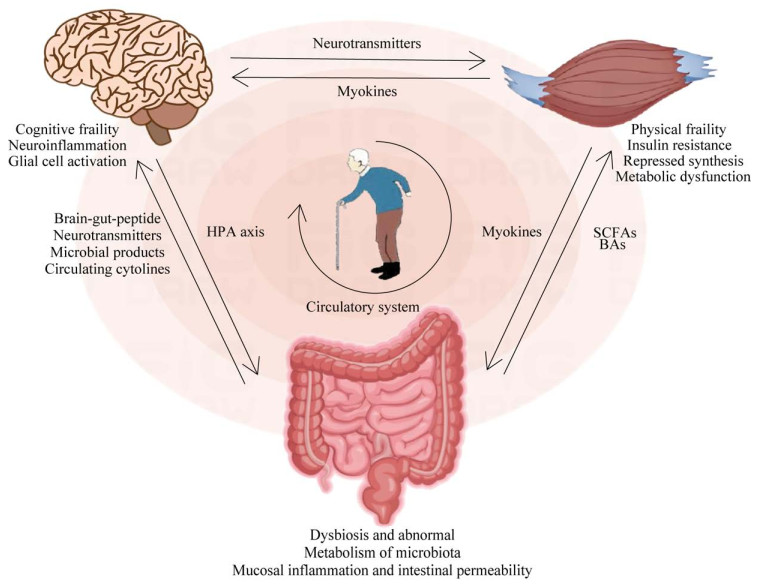
|
| 图 1 肠-脑-肌轴与认知衰弱间的潜在机制 Figure 1 Gut-brain-muscle potential mechanism between the shaft and cognitive decline. SCFAs: Short-chain fatty acids; BAs: Bile acids; HPA axis: Hypothalamic-pituitary-adrenal axis. SCFAs:短链脂肪酸;Bas:胆汁酸;HPA轴:下丘脑-垂体-肾上腺轴 |
5 小结
综上所述,肠道菌群及其代谢产物通过肠-脑、肠-肌或肌-肠-脑轴与机体进行双向动态的信号交流,并调节机体的免疫、代谢、神经系统和骨骼肌肉系统功能,对维持机体胃肠道、中枢神经、免疫和微生物系统的内环境平衡具有重要作用。肠道菌群与机体之间的双向交流影响多种疾病的发生发展,有助于深入探讨肠-脑-肌轴失调相关疾病的早期诊断等相关问题,同时也为防治这些疾病开辟了新的途径,拓宽了对认知功能、骨骼肌功能、最佳肠道菌群状态之间联系的理解。然而,虽然不断扩大的临床前研究为肠-脑-肌轴所涉及的机制途径提供了关键的见解,仍然需要强有力的临床研究来转化这些临床发现。
| [1] | PATTERSON E, RYAN PM, CRYAN JF, DINAN TG, ROSS RP, FITZGERALD GF, STANTON C. Gut microbiota, obesity and diabetes[J]. Postgraduate Medical Journal, 2016, 92(1087): 286-300 DOI:10.1136/postgradmedj-2015-133285. |
| [2] | FETISSOV SO. Role of the gut microbiota in host appetite control: bacterial growth to animal feeding behaviour[J]. Nature Reviews Endocrinology, 2017, 13: 11-25 DOI:10.1038/nrendo.2016.150. |
| [3] | LUCKEY TD. Introduction to intestinal microecology[J]. The American Journal of Clinical Nutrition, 1972, 25(12): 1292-1294 DOI:10.1093/ajcn/25.12.1292. |
| [4] | CRYAN JF, O'RIORDAN KJ, COWAN CSM, SANDHU KV, BASTIAANSSEN TFS, BOEHME M, CODAGNONE MG, CUSSOTTO S, FULLING C, GOLUBEVA AV, GUZZETTA KE, JAGGAR M, LONG-SMITH CM, LYTE JM, MARTIN JA, MOLINERO-PEREZ A, MOLONEY G, MORELLI E, MORILLAS E, O'CONNOR R, et al. The microbiota-gut-brain axis[J]. Physiological Reviews, 2019, 99(4): 1877-2013 DOI:10.1152/physrev.00018.2018. |
| [5] | HE JQ, XU SB, ZHANG BZ, XIAO CX, CHEN ZR, SI FY, FU JF, LIN XM, ZHENG GH, YU GC, CHEN J. Gut microbiota and metabolite alterations associated with reduced bone mineral density or bone metabolic indexes in postmenopausal osteoporosis[J]. Aging, 2020, 12(9): 8583-8604 DOI:10.18632/aging.103168. |
| [6] | YANG XF, LU M, YOU LJ, GEN H, YUAN L, TIAN TN, LI CY, XU KL, HOU J, LEI M. Herbal therapy for ameliorating nonalcoholic fatty liver disease via rebuilding the intestinal microecology[J]. Chinese Medicine, 2021, 16(1): 62 DOI:10.1186/s13020-021-00470-x. |
| [7] |
崔佳瞿, 陈启仪, 李宁. 肠道微生物群-肠-脑轴在神经精神系统疾病中的研究进展[J]. 上海医药, 2023, 44(1): 14-18.
DOI:10.3969/j.issn.1006-1533.2023.01.004 CUI JQ, CHEN QY, LI N. Research progress of gut microbiota-gut-brain axis in neuropsychiatric disorders[J]. Shanghai Medical & Pharmaceutical Journal, 2023, 44(1): 14-18 (in Chinese). |
| [8] | LAHIRI S, KIM H, GARCIA-PEREZ I, REZA MM, MARTIN KA, KUNDU P, COX LM, SELKRIG J, POSMA JM, ZHANG HB, PADMANABHAN P, MORET C, GULYÁS B, BLASER MJ, AUWERX J, HOLMES E, NICHOLSON J, WAHLI W, PETTERSSON S. The gut microbiota influences skeletal muscle mass and function in mice[J]. Science Translational Medicine, 2019, 11(502): eaan5662 DOI:10.1126/scitranslmed.aan5662. |
| [9] | FRANZOSA EA, SIROTA-MADI A, AVILA-PACHECO J, FORNELOS N, HAISER HJ, REINKER S, VATANEN T, HALL AB, MALLICK H, McIVER LJ, SAUK JS, WILSON RG, STEVENS BW, SCOTT JM, PIERCE K, DEIK AA, BULLOCK K, IMHANN F, PORTER JA, ZHERNAKOVA A, et al. Gut microbiome structure and metabolic activity in inflammatory bowel disease[J]. Nature Microbiology, 2019, 4: 293-305. |
| [10] | LEEUWENDAAL NK, CRYAN JF, SCHELLEKENS H. Gut peptides and the microbiome: focus on ghrelin[J]. Current Opinion in Endocrinology, Diabetes, and Obesity, 2021, 28(2): 243-252 DOI:10.1097/MED.0000000000000616. |
| [11] | FU QH, SONG TY, MA XQ, CUI J. Research progress on the relationship between intestinal microecology and intestinal bowel disease[J]. Animal Models and Experimental Medicine, 2022, 5(4): 297-310 DOI:10.1002/ame2.12262. |
| [12] |
李叔芝. 溃疡性结肠炎肠道菌群失调与免疫球蛋白水平的相关性研究[J]. 中国医学工程, 2018, 26(10): 90-92.
LI SZ. Correlation between intestinal bacilli illness and immunoglobulin levels in ulcerative colitis[J]. China Medical Engineering, 2018, 26(10): 90-92 (in Chinese). |
| [13] | GLASSNER KL, ABRAHAM BP, QUIGLEY EMM. The microbiome and inflammatory bowel disease[J]. The Journal of Allergy and Clinical Immunology, 2020, 145(1): 16-27 DOI:10.1016/j.jaci.2019.11.003. |
| [14] | WANG Z, XU CM, LIU YX, WANG XQ, ZHANG L, LI M, ZHU SW, XIE ZJ, WANG PH, DUAN LP, ZHU HQ. Characteristic dysbiosis of gut microbiota of Chinese patients with diarrhea-predominant irritable bowel syndrome by an insight into the pan-microbiome[J]. Chinese Medical Journal, 2019, 132(8): 889-904 DOI:10.1097/CM9.0000000000000192. |
| [15] | LIM SM, JEONG JJ, WOO KH, HAN MJ, KIM DH. Lactobacillus sakei OK67 ameliorates high-fat diet-induced blood glucose intolerance and obesity in mice by inhibiting gut microbiota lipopolysaccharide production and inducing colon tight junction protein expression[J]. Nutrition Research, 2016, 36(4): 337-348 DOI:10.1016/j.nutres.2015.12.001. |
| [16] | ZHU LD, LU XX, LIU L, VOGLMEIR J, ZHONG X, YU QH. Akkermansia muciniphila protects intestinal mucosa from damage caused by S. pullorum by initiating proliferation of intestinal epithelium[J]. Veterinary Research, 2020, 51(1): 34 DOI:10.1186/s13567-020-00755-3. |
| [17] | KÖHLER CA, MAES M, SLYEPCHENKO A, BERK M, SOLMI M, LANCTÔT KL, CARVALHO AF. The gut-brain axis, including the microbiome, leaky gut and bacterial translocation: mechanisms and pathophysiological role in Alzheimer's disease[J]. Current Pharmaceutical Design, 2016, 22(40): 6152-6166 DOI:10.2174/1381612822666160907093807. |
| [18] | MANCUSO C, SANTANGELO R. Alzheimer's disease and gut microbiota modifications: the long way between preclinical studies and clinical evidence[J]. Pharmacological Research, 2018, 129: 329-336 DOI:10.1016/j.phrs.2017.12.009. |
| [19] |
李姝敏, 徐哲荣. 肠道微生态改变在肌少症发病机制中的作用[J]. 中华老年病研究电子杂志, 2018, 5(1): 13-16.
DOI:10.3877/cma.j.issn.2095-8757.2018.01.004 LI SM, XU ZR. The role of intestinal microecological changes in the pathogenesis of sarcopenia[J]. Chinese Journal of Geriatrics Research (Electronic Edition), 2018, 5(1): 13-16 (in Chinese). |
| [20] |
杨云梅. 老年肌肉衰减症研究新进展[J]. 中华危重症医学杂志(电子版), 2018, 11(5): 289-294.
DOI:10.3877/cma.j.issn.1674-6880.2018.05.001 YANG YM. New progress in the study of senile muscular dystrophy[J]. Chinese Journal of Critical Care Medicine (Electronic Edition), 2018, 11(5): 289-294 (in Chinese). |
| [21] | ROUBALOVÁ R, PROCHÁZKOVÁ P, PAPEŽOVÁ H, SMITKA K, BILEJ M, TLASKALOVÁ-HOGENOVÁ H. Anorexia nervosa: gut microbiota-immune-brain interactions[J]. Clinical Nutrition, 2020, 39(3): 676-684 DOI:10.1016/j.clnu.2019.03.023. |
| [22] | MARTIN-GALLAUSIAUX C, GARCIA-WEBER D, LASHERMES A, LARRAUFIE P, MARINELLI L, TEIXEIRA V, ROLLAND A, BÉGUET-CRESPEL F, BROCHARD V, QUATREMARE T, JAMET A, DORÉ J, GRAY-OWEN SD, BLOTTIÈRE HM, ARRIEUMERLOU C, LAPAQUE N. Akkermansia muciniphila upregulates genes involved in maintaining the intestinal barrier function via ADP-heptose-dependent activation of the ALPK1/TIFA pathway[J]. Gut Microbes, 2022, 14(1): 2110639 DOI:10.1080/19490976.2022.2110639. |
| [23] | WILLIAMS LM, ERRIDGE C, GRANT C, MORRIS AC, DUNCAN SH, WALLACE RJ, WALKER AW, FLINT HJ. High-fat diet induced changes in gut microbiota and pathogen associate molecular patterns (PAMPS)[J]. Proceedings of the Nutrition Society, 2014, 73(OCE1): E30 DOI:10.1017/S0029665114000445. |
| [24] | MAYNARD CL, ELSON CO, HATTON RD, WEAVER CT. Reciprocal interactions of the intestinal microbiota and immune system[J]. Nature, 2012, 489: 231-241 DOI:10.1038/nature11551. |
| [25] | AVILA EE. Functions of antimicrobial peptides in vertebrates[J]. Current Protein & Peptide Science, 2017, 18(11): 1098-1119. |
| [26] | POWELL N, WALKER MM, TALLEY NJ. The mucosal immune system: master regulator of bidirectional gut–brain communications[J]. Nature Reviews Gastroenterology & Hepatology, 2017, 14: 143-159. |
| [27] | SUDO N, CHIDA Y, AIBA YJ, SONODA J, OYAMA N, YU XN, KUBO C, KOGA Y. Postnatal microbial colonization programs the hypothalamic-pituitary-adrenal system for stress response in mice[J]. The Journal of Physiology, 2004, 558(Pt 1): 263-275. |
| [28] | ILCHMANN-DIOUNOU H, MENARD S. Psychological stress, intestinal barrier dysfunctions, and autoimmune disorders: an overview[J]. Frontiers in Immunology, 2020, 11: 1823 DOI:10.3389/fimmu.2020.01823. |
| [29] | YIN YJ, GUO QP, ZHOU XH, DUAN YH, YANG YH, GONG SM, HAN MM, LIU YT, YANG ZK, CHEN QH, LI FN. Role of brain-gut-muscle axis in human health and energy homeostasis[J]. Frontiers in Nutrition, 2022, 9: 947033 DOI:10.3389/fnut.2022.947033. |
| [30] | PSICHAS A, SLEETH ML, MURPHY KG, BROOKS L, BEWICK GA, HANYALOGLU AC, GHATEI MA, BLOOM SR, FROST G. The short chain fatty acid propionate stimulates GLP-1 and PYY secretion via free fatty acid receptor 2 in rodents[J]. International Journal of Obesity (2005), 2015, 39(3): 424-429 DOI:10.1038/ijo.2014.153. |
| [31] | LARRAUFIE P, MARTIN-GALLAUSIAUX C, LAPAQUE N, DORE J, GRIBBLE FM, REIMANN F, BLOTTIERE HM. SCFAs strongly stimulate PYY production in human enteroendocrine cells[J]. Scientific Reports, 2018, 8: 74 DOI:10.1038/s41598-017-18259-0. |
| [32] | MARGOLIS KG, CRYAN JF, MAYER EA. The microbiota-gut-brain axis: from motility to mood[J]. Gastroenterology, 2021, 160(5): 1486-1501 DOI:10.1053/j.gastro.2020.10.066. |
| [33] | YOO BB, MAZMANIAN SK. The enteric network: interactions between the immune and nervous systems of the gut[J]. Immunity, 2017, 46(6): 910-926 DOI:10.1016/j.immuni.2017.05.011. |
| [34] | TAN AH, LIM SY, LANG AE. The microbiome-gut-brain axis in Parkinson disease: from basic research to the clinic[J]. Nature Reviews Neurology, 2022, 18: 476-495 DOI:10.1038/s41582-022-00681-2. |
| [35] | BERCIK P, PARK AJ, SINCLAIR D, KHOSHDEL A, LU J, HUANG X, DENG Y, BLENNERHASSETT PA, FAHNESTOCK M, MOINE D, BERGER B, HUIZINGA JD, KUNZE W, McLEAN PG, BERGONZELLI GE, COLLINS SM, VERDU EF. The anxiolytic effect of Bifidobacterium longum NCC3001 involves vagal pathways for gut-brain communication[J]. Neurogastroenterology and Motility, 2011, 23(12): 1132-1139 DOI:10.1111/j.1365-2982.2011.01796.x. |
| [36] | BRAVO JA, FORSYTHE P, CHEW MV, ESCARAVAGE E, SAVIGNAC HM, DINAN TG, BIENENSTOCK J, CRYAN JF. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve[J]. Proceedings of the National Academy of Sciences of the United States of America, 2011, 108(38): 16050-16055. |
| [37] | DOCKRAY GJ. Enteroendocrine cell signalling via the vagus nerve[J]. Current Opinion in Pharmacology, 2013, 13(6): 954-958 DOI:10.1016/j.coph.2013.09.007. |
| [38] | LOCHLAINN MN, BOWYER RCE, STEVES CJ. Dietary protein and muscle in aging people: the potential role of the gut microbiome[J]. Nutrients, 2018, 10(7): 929 DOI:10.3390/nu10070929. |
| [39] | LIN L, ZHANG JQ. Role of intestinal microbiota and metabolites on gut homeostasis and human diseases[J]. BMC Immunology, 2017, 18(1): 2 DOI:10.1186/s12865-016-0187-3. |
| [40] |
耿雪, 张双双, 李志慧, 覃飞, 瞿超艺, 冯亦唯, 张成岗, 赵杰修. 肠道菌群对运动系统和运动机能的影响研究进展[J]. 中国运动医学杂志, 2021, 40(1): 61-66.
GENG X, ZHANG SS, LI ZH, QIN F, QU CY, FENG YW, ZHANG CG, ZHAO JX. Research progress on the influence of intestinal flora on motor system and motor function[J]. Chinese Journal of Sports Medicine, 2021, 40(1): 61-66 (in Chinese). |
| [41] | MITTERREITER JG, OUWENDIJK WJD, van VELZEN M, van NIEROP GP, OSTERHAUS ADME, VERJANS GMGM. Satellite glial cells in human trigeminal Ganglia have a broad expression of functional Toll-like receptors[J]. European Journal of Immunology, 2017, 47(7): 1181-1187 DOI:10.1002/eji.201746989. |
| [42] | DUCHARME JB, FENNEL ZJ, McKENNA ZJ, NAVA RC, DEYHLE MR. Stimulated myotube contractions regulate membrane-bound and soluble TLR4 to prevent LPS-induced signaling and myotube atrophy in skeletal muscle cells[J]. American Journal of Physiology Cell Physiology, 2023, 325(1): C300-C313 DOI:10.1152/ajpcell.00007.2023. |
| [43] | BUDUI SL, ROSSI AP, ZAMBONI M. The pathogenetic bases of sarcopenia[J]. Clinical Cases in Mineral and Bone Metabolism, 2015, 12(1): 22-26. |
| [44] | ZHANG Y, ZHANG S, LI BL, LUO YC, GONG YT, JIN XX, ZHANG JW, ZHOU Y, ZHUO XZ, WANG ZX, ZHAO XB, HAN XJ, GAO YL, YU H, LIANG DS, ZHAO SQ, SUN DH, WANG DY, XU W, QU GJ, BO WL, LI D, WU Y, LI Y. Gut microbiota dysbiosis promotes age-related atrial fibrillation by lipopolysaccharide and glucose-induced activation of NLRP3-inflammasome[J]. Cardiovascular Research, 2022, 118(3): 785-797 DOI:10.1093/cvr/cvab114. |
| [45] | BINDELS LB, BECK R, SCHAKMAN O, MARTIN JC, de BACKER F, SOHET FM, DEWULF EM, PACHIKIAN BD, NEYRINCK AM, THISSEN JP, VERRAX J, CALDERON PB, POT B, GRANGETTE C, CANI PD, SCOTT KP, DELZENNE NM. Restoring specific lactobacilli levels decreases inflammation and muscle atrophy markers in an acute leukemia mouse model[J]. PLoS One, 2012, 7(6): e37971 DOI:10.1371/journal.pone.0037971. |
| [46] | MANICKAM R, DUSZKA K, WAHLI W. PPARs and microbiota in skeletal muscle health and wasting[J]. International Journal of Molecular Sciences, 2020, 21(21): 8056 DOI:10.3390/ijms21218056. |
| [47] | YARMOHAMMADI F, HAYES AW, KARIMI G. Targeting PPARs signaling pathways in cardiotoxicity by natural compounds[J]. Cardiovascular Toxicology, 2022, 22(4): 281-291 DOI:10.1007/s12012-021-09715-5. |
| [48] | ESPINOSA-JIMÉNEZ T, BUSQUETS O, CANO A, SÁNCHEZ-LÓPEZ E, VERDAGUER E, PARCERISAS A, OLLOQUEQUI J, AULADELL C, FOLCH J, WAHLI W, VÁZQUEZ-CARRERA M, CAMINS A, ETTCHETO M. Peroxisomal proliferator-activated receptor β/δ deficiency induces cognitive alterations[J]. Frontiers in Pharmacology, 2022, 13: 902047 DOI:10.3389/fphar.2022.902047. |
| [49] | FANG WY, LIN CL, CHANG WH, CHANG CH, HUANG YC, TSAI YH, CHANG FR, LO YC. Protective effects of the Chalcone-based derivative AN07 on inflammation-associated myotube atrophy induced by lipopolysaccharide[J]. International Journal of Molecular Sciences, 2022, 23(21): 12929 DOI:10.3390/ijms232112929. |
| [50] | SASAKI T, KUBOYAMA A, MITA M, MURATA S, SHIMIZU M, INOUE J, MORI K, SATO R. The exercise-inducible bile acid receptor Tgr5 improves skeletal muscle function in mice[J]. The Journal of Biological Chemistry, 2018, 293(26): 10322-10332 DOI:10.1074/jbc.RA118.002733. |
| [51] | SUN LL, XIE C, WANG G, WU Y, WU Q, WANG XM, LIU J, DENG YY, XIA JL, CHEN B, ZHANG SY, YUN CY, LIAN G, ZHANG XJ, ZHANG H, BISSON WH, SHI JM, GAO XX, GE PP, LIU CH, KRAUSZ KW, NICHOLS RG, CAI JW, RIMAL B, PATTERSON AD, WANG X, GONZALEZ FJ, JIANG CT. Gut microbiota and intestinal FXR mediate the clinical benefits of metformin[J]. Nature Medicine, 2018, 24: 1919-1929 DOI:10.1038/s41591-018-0222-4. |
| [52] | GRUBER J, KENNEDY BK. Microbiome and longevity: gut microbes send signals to host mitochondria[J]. Cell, 2017, 169(7): 1168-1169 DOI:10.1016/j.cell.2017.05.048. |
| [53] | LI GY, JIN BH, FAN Z. Mechanisms involved in gut microbiota regulation of skeletal muscle[J]. Oxidative Medicine and Cellular Longevity, 2022, 2022: 2151191. |
| [54] | HAN B, LIN CC J, HU G, WANG MC. "Inside Out"- a dialogue between mitochondria and bacteria[J]. The FEBS Journal, 2019, 286(4): 630-641 DOI:10.1111/febs.14692. |
| [55] | VERBEKE KA, BOOBIS AR, CHIODINI A, EDWARDS CA, FRANCK A, KLEEREBEZEM M, NAUTA A, RAES J, van TOL EAF, TUOHY KM. Towards microbial fermentation metabolites as markers for health benefits of prebiotics[J]. Nutrition Research Reviews, 2015, 28(1): 42-66 DOI:10.1017/S0954422415000037. |
| [56] | den BESTEN G, van EUNEN K, GROEN AK, VENEMA K, REIJNGOUD DJ, BAKKER BM. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism[J]. Journal of Lipid Research, 2013, 54(9): 2325-2340 DOI:10.1194/jlr.R036012. |
| [57] | MITCHELL RW, ON NH, del BIGIO MR, MILLER DW, HATCH GM. Fatty acid transport protein expression in human brain and potential role in fatty acid transport across human brain microvessel endothelial cells[J]. Journal of Neurochemistry, 2011, 117(4): 735-746. |
| [58] | VICENTINI FA, KEENAN CM, WALLACE LE, WOODS C, CAVIN JB, FLOCKTON AR, MACKLIN WB, BELKIND-GERSON J, HIROTA SA, SHARKEY KA. Intestinal microbiota shapes gut physiology and regulates enteric neurons and glia[J]. Microbiome, 2021, 9(1): 210. |
| [59] | SAAD MJA, SANTOS A, PRADA PO. Linking gut microbiota and inflammation to obesity and insulin resistance[J]. Physiology, 2016, 31(4): 283-293. |
| [60] | SOLDAVINI J, KAUNITZ JD. Pathobiology and potential therapeutic value of intestinal short-chain fatty acids in gut inflammation and obesity[J]. Digestive Diseases and Sciences, 2013, 58(10): 2756-2766. |
| [61] | ZHANG D, JIAN YP, ZHANG YN, LI Y, GU LT, SUN HH, LIU MD, ZHOU HL, WANG YS, XU ZX. Short-chain fatty acids in diseases[J]. Cell Communication and Signaling, 2023, 21(1): 212. |
| [62] | SCHIAFFINO S, MAMMUCARI C. Regulation of skeletal muscle growth by the IGF1-Akt/PKB pathway: insights from genetic models[J]. Skeletal Muscle, 2011, 1(1): 4. |
| [63] | BERMÚDEZ-HUMARÁN LG, SALINAS E, ORTIZ GG, RAMIREZ-JIRANO LJ, MORALES JA, BITZER-QUINTERO OK. From probiotics to psychobiotics: live beneficial bacteria which act on the brain-gut axis[J]. Nutrients, 2019, 11(4): 890. |
| [64] | ROAGER HM, LICHT TR. Microbial tryptophan catabolites in health and disease[J]. Nature Communications, 2018, 9: 3294. |
| [65] | ZHAO LP, WU J, QUAN W, ZHOU Y, HONG H, NIU GY, LI T, HUANG SB, QIAO CM, ZHAO WJ, CUI C, SHEN YQ. DSS-induced colitis activates the kynurenine pathway in serum and brain by affecting IDO-1 and gut microbiota[J]. Frontiers in Immunology, 2023, 13: 1089200. |
| [66] | LIM CK, FERNÁNDEZ-GOMEZ FJ, BRAIDY N, ESTRADA C, COSTA C, COSTA S, BESSEDE A, FERNANDEZ-VILLALBA E, ZINGER A, HERRERO MT, GUILLEMIN GJ. Involvement of the kynurenine pathway in the pathogenesis of Parkinson's disease[J]. Progress in Neurobiology, 2017, 155: 76-95. |
| [67] | OXENKRUG G, van der HART M, ROESER J, SUMMERGRAD P. Peripheral tryptophan-kynurenine metabolism associated with metabolic syndrome is different in Parkinson's and Alzheimer's diseases[J]. Endocrinology, Diabetes and Metabolism Journal, 2017, 1(4): 113. |
| [68] | SCHWARCZ R, STONE TW. The kynurenine pathway and the brain: challenges, controversies and promises[J]. Neuropharmacology, 2017, 112(Pt B): 247. |
| [69] | VENKATESAN D, IYER M, NARAYANASAMY A, SIVA K, VELLINGIRI B. Kynurenine pathway in Parkinson's disease-An update[J]. eNeurologicalSci, 2020, 21: 100270. |
| [70] | KAISER H, YU KL, PANDYA C, MENDHE B, ISALES CM, MCGEE-LAWRENCE ME, JOHNSON M, FULZELE S, HAMRICK MW. Kynurenine, a tryptophan metabolite that increases with age, induces muscle atrophy and lipid peroxidation[J]. Oxidative Medicine and Cellular Longevity, 2019, 2019: 9894238. |
| [71] | BELLANTI F, ROMANO AD, BUGLIO AL, CASTRIOTTA V, GUGLIELMI G, GRECO A, SERVIDDIO G, VENDEMIALE G. Oxidative stress is increased in sarcopenia and associated with cardiovascular disease risk in sarcopenic obesity[J]. Maturitas, 2018, 109: 6-12. |
| [72] | COTO MONTES A, BOGA JA, BERMEJO MILLO C, RUBIO GONZÁLEZ A, POTES OCHOA Y, VEGA NAREDO I, MARTÍNEZ REIG M, ROMERO RIZOS L, SÁNCHEZ JURADO PM, SOLANO JJ, ABIZANDA P, CABALLERO B. Potential early biomarkers of sarcopenia among independent older adults[J]. Maturitas, 2017, 104: 117-122. |
| [73] | AGUDELO LZ, FEMENÍA T, ORHAN F, PORSMYR-PALMERTZ M, GOINY M, MARTINEZ-REDONDO V, CORREIA JC, IZADI M, BHAT M, SCHUPPE-KOISTINEN I, PETTERSSON AT, FERREIRA DMS, KROOK A, BARRES R, ZIERATH JR, ERHARDT S, LINDSKOG M, RUAS JL. Skeletal muscle PGC-1α1 modulates kynurenine metabolism and mediates resilience to stress-induced depression[J]. Cell, 2014, 159(1): 33-45. |
| [74] | COHEN LJ, KANG HS, CHU J, HUANG YH, GORDON EA, REDDY BVB, TERNEI MA, CRAIG JW, BRADY SF. Functional metagenomic discovery of bacterial effectors in the human microbiome and isolation of commendamide, a GPCR G2A/132 agonist[J]. Proceedings of the National Academy of Sciences of the United States of America, 2015, 112(35): E4825-E4834. |
| [75] | CHANG FY, SIUTI P, LAURENT S, WILLIAMS T, GLASSEY E, SAILER AW, GORDON DB, HEMMERLE H, VOIGT CA. Gut-inhabiting Clostridia build human GPCR ligands by conjugating neurotransmitters with diet- and human-derived fatty acids[J]. Nature Microbiology, 2021, 6: 792-805. |
| [76] | DOHNALOVÁ L, LUNDGREN P, CARTY JRE, GOLDSTEIN N, WENSKI SL, NANUDORN P, THIENGMAG S, HUANG KP, LITICHEVSKIY L, DESCAMPS HC, CHELLAPPA K, GLASSMAN A, KESSLER S, KIM J, COX TO, DMITRIEVA-POSOCCO O, WONG AC, ALLMAN EL, GHOSH S, SHARMA N, et al. A microbiome-dependent gut–brain pathway regulates motivation for exercise[J]. Nature, 2022, 612: 739-747. |
| [77] | GHOSH S, LERTWATTANARAK R, GARDUÑO JD, GALEANA JJ, LI JQ, ZAMARRIPA F, LANCASTER JL, MOHAN S, HUSSEY S, MUSI N. Elevated muscle TLR4 expression and metabolic endotoxemia in human aging[J]. The Journals of Gerontology Series A, Biological Sciences and Medical Sciences, 2015, 70(2): 232-246. |
| [78] | GROSICKI GJ, FIELDING RA, LUSTGARTEN MS. Gut microbiota contribute to age-related changes in skeletal muscle size, composition, and function: biological basis for a gut-muscle axis[J]. Calcified Tissue International, 2018, 102(4): 433-442. |
| [79] | RUBIO C, LIZÁRRAGA E, ÁLVAREZ-CILLEROS D, PÉREZ-PARDO P, SANMARTÍN-SALINAS P, TOLEDO-LOBO MV, ALVAREZ C, ESCRIVÁ F, FERNÁNDEZ-LOBATO M, GUIJARRO LG, VALVERDE AM, CARRASCOSA JM. Aging in male wistar rats associates with changes in intestinal microbiota, gut structure, and cholecystokinin-mediated gut-brain axis function[J]. The Journals of Gerontology: Series A, 2021, 76(11): 1915-1921. |
| [80] | DOYLE A, ZHANG GH, ABDEL FATTAH EA, EISSA NT, LI YP. Toll-like receptor 4 mediates lipopolysaccharide-induced muscle catabolism via coordinate activation of ubiquitin-proteasome and autophagy-lysosome pathways[J]. FASEB Journal: Official Publication of the Federation of American Societies for Experimental Biology, 2011, 25(1): 99-110. |
| [81] | LUO BB, XIANG D, NIEMAN DC, CHEN PJ. The effects of moderate exercise on chronic stress-induced intestinal barrier dysfunction and antimicrobial defense[J]. Brain, Behavior, and Immunity, 2014, 39: 99-106. |
| [82] | ELLINGSGAARD H, HAUSELMANN I, SCHULER B, HABIB AM, BAGGIO LL, MEIER DT, EPPLER E, BOUZAKRI K, WUEEST S, MULLER YD, HANSEN AMK, REINECKE M, KONRAD D, GASSMANN M, REIMANN F, HALBAN PA, GROMADA J, DRUCKER DJ, GRIBBLE FM, EHSES JA, et al. Interleukin-6 enhances insulin secretion by increasing glucagon-like peptide-1 secretion from L cells and alpha cells[J]. Nature Medicine, 2011, 17: 1481-1489. |
| [83] |
宋超, 张竞文, 薄海, 吉力立, 张勇. PGC-1α参与调节向心运动中骨骼肌白介素6的抗炎效应[J]. 中国运动医学杂志, 2015, 34(4): 329-333.
SONG C, ZHANG JW, BO H, JI LL, ZHANG Y. The effects of concentric exercise on the anti-inflammation of interleukin-6 in skeletal muscle[J]. Chinese Journal of Sports Medicine, 2015, 34(4): 329-333 (in Chinese). |
| [84] | WEBSTER JM, KEMPEN LJAP, HARDY RS, LANGEN RCJ. Inflammation and skeletal muscle wasting during Cachexia[J]. Frontiers in Physiology, 2020, 11: 597675. |
| [85] | SCRIVEN M, McSWEENEY A, O'CARROLL T, MORKL S, BUTLER MI. The muscle-gut-brain axis and psychiatric illness[J]. Advanced Biology, 2023, 7(6): e2200214. |
| [86] | KÖNIG J, WELLS J, CANI PD, GARCÍA-RÓDENAS CL, MacDONALD T, MERCENIER A, WHYTE J, TROOST F, BRUMMER RJ. Human intestinal barrier function in health and disease[J]. Clinical and Translational Gastroenterology, 2016, 7(10): e196. |
| [87] | ZHAO Y, DUA P, LUKIW WJ. Microbial sources of amyloid and relevance to amyloidogenesis and Alzheimer's disease (AD)[J]. Journal of Alzheimer's Disease & Parkinsonism, 2015, 5(1): 177. |
| [88] | RAPSINSKI GJ, WYNOSKY-DOLFI MA, OPPONG GO, TURSI SA, WILSON RP, BRODSKY IE, TÜKEL Ç. Toll-like receptor 2 and NLRP3 cooperate to recognize a functional bacterial amyloid, curli[J]. Infection and Immunity, 2015, 83(2): 693-701. |
| [89] | ZHAN XH, STAMOVA B, SHARP FR. Lipopolysaccharide associates with amyloid plaques, neurons and oligodendrocytes in Alzheimer's disease brain: a review[J]. Frontiers in Aging Neuroscience, 2018, 10: 42. |
| [90] | ZHAO YH, JABER V, LUKIW WJ. Secretory products of the human GI tract microbiome and their potential impact on Alzheimer's disease (AD): detection of lipopolysaccharide (LPS) in AD hippocampus[J]. Frontiers in Cellular and Infection Microbiology, 2017, 7: 318. |
| [91] | LEFEVRE C, BINDELS LB. Role of the gut microbiome in skeletal muscle physiology and pathophysiology[J]. Current Osteoporosis Reports, 2022, 20(6): 422-432. |
| [92] | DALLE S, ROSSMEISLOVA L, KOPPO K. The role of inflammation in age-related sarcopenia[J]. Frontiers in Physiology, 2017, 8: 1045. |
| [93] | ZHENG GH, QIU PT, XIA R, LIN HY, YE BZ, TAO J, CHEN LD. Effect of aerobic exercise on inflammatory markers in healthy middle-aged and older adults: a systematic review and meta-analysis of randomized controlled trials[J]. Frontiers in Aging Neuroscience, 2019, 11: 98. |
| [94] | SARGENT L, NALLS M, AMELLA EJ, SLATTUM PW, MUELLER M, BANDINELLI S, TIAN Q, SWIFT-SCANLAN T, LAGEMAN SK, SINGLETON A. Shared mechanisms for cognitive impairment and physical frailty: a model for complex systems[J]. Alzheimer's & Dementia, 2020, 6(1): e12027. |
 2024, Vol. 64
2024, Vol. 64