中国科学院微生物研究所,中国微生物学会
文章信息
- 王雨晴, 张贤, 李闯, 薛正莲, 李翔飞. 2024
- WANG Yuqing, ZHANG Xian, LI Chuang, XUE Zhenglian, LI Xiangfei.
- 阿卡波糖代谢途径、酶系及发酵菌种改造的研究进展
- Advances in biosynthetic pathways, enzymes, and fermentation strain modification of acarbose
- 微生物学报, 64(12): 4760-4773
- Acta Microbiologica Sinica, 64(12): 4760-4773
-
文章历史
- 收稿日期:2024-07-10
- 网络出版日期:2024-09-25
2. 安徽省工业微生物分子育种工程实验室, 安徽 芜湖 241000
2. Anhui Engineering Laboratory for Industrial Microbiology Molecular Breeding, Wuhu 241000, Anhui, China
糖尿病(diabetes mellitus)是一种多发性的内分泌疾病,我国当前的患者数量已超过1.4亿,其中Ⅱ型糖尿病患者约占总发病人数的90%[1-2]。视网膜、神经元、心血管系统和肝肾功能障碍等并发症不仅严重危害民众的生命健康,还带来了巨大的医疗负担,可见糖尿病的防治具有重要的社会与经济意义[3]。
阿卡波糖(acarbose)是一种主要由放线菌(Actinoplanes sp.)发酵获得的α-糖苷酶抑制剂,广泛用于Ⅱ型糖尿病的治疗,其通过竞争性抑制人小肠α-糖苷酶的活性调节患者的餐后血糖水平[4-5]。阿卡波糖是一种假四糖化合物,分子结构由阿卡维基(acarviose)和麦芽糖基两部分组成,阿卡维基又由C7-环醇和4-氨基-4,6-双脱氧葡萄糖通过N-糖苷键的氨基桥连接而成,该部分竞争性占据α-糖苷酶的活性位点以抑制该酶的活性,从而降低肠道内糖的水解与吸收速率[6]。本文将结合近年的研究成果,总结分析阿卡波糖的代谢途径、酶系以及发酵菌株分子改造等方面的研究进展,为进一步完善阿卡波糖的代谢途径、深入研究相关酶的催化机制和发酵菌株的改造提供参考,以期促进阿卡波糖产量提升与杂质控制。
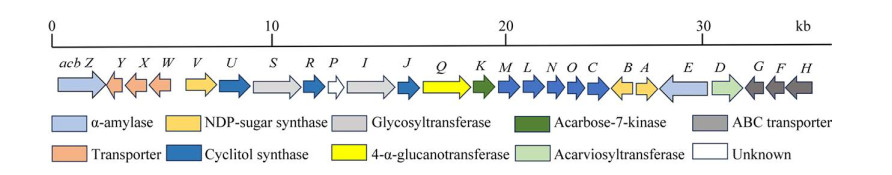
1 阿卡波糖的生物代谢途径2003年,Wehmeier[7]发现Actinoplanes sp. SE50/110全基因组中含有阿卡波糖代谢基因簇(acb基因簇)。2011年,Schwientek等[8]通过焦磷酸测序技术对Actinoplanes sp. SE50/110进行全基因组测序,证实其基因组中含有完整的acb基因簇。随后,该团队首次在NCBI上公开了Actinoplanes sp.的全基因组信息(GenBank登录号为CP003170)[9]。2015年,Wendler等[10]对Actinoplanes sp. SE50/110展开全面的蛋白质组学分析,明确了acb基因簇在全基因组中的定位。目前,acb基因簇已基本清晰,包括合成相关基因acb CMOLNUJRSIVBA、胞外阿卡波糖代谢相关基因acb DEZ、跨膜转运蛋白复合体基因acb WXY和acb FGH、胞外阿卡波糖激酶基因acb K以及胞内4-α-葡萄糖基转移酶基因acb Q[11-14] (图 1)。
|
| 图 1 Actinoplanes sp. SE50/110的acb基因簇及表达产物 Figure 1 acb gene cluster and the corresponding expression products of Actinoplanes sp. SE50/110. |
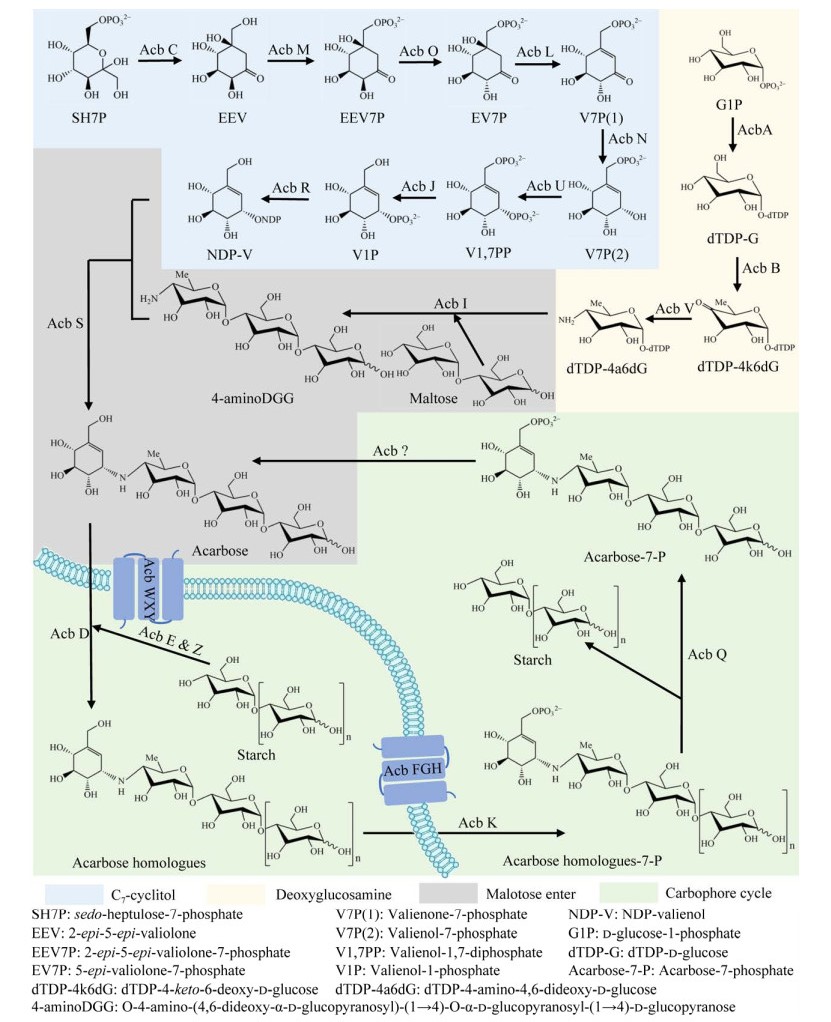
阿卡波糖的代谢途径最早在2002年由Zhang等[15]提出,后由Rockser等[16]于2009年更新。由于该途径的复杂性,研究者们通过基因组学、代谢组学和同位素掺入等手段仅证明了双脱氧氨基葡萄糖的合成和C7-环醇合成的前3步反应,对于C7-环醇合成的后续反应仅依据已公布的基因簇推测各基因编码的蛋白活性建立。直到2022年,Tsunoda等[17]用实验验证了C7-环醇合成路径的后续部分,纠正并完善了阿卡波糖的代谢途径,本文将介绍由Tsunoda等更新后的途径(表 1)。阿卡波糖代谢途径主要分为4个模块:C7-环醇的合成、双脱氧氨基脱氧葡萄糖的合成、麦芽糖的整合进入和胞外阿卡波糖及其同系物的转运(Carbophore循环)[14]。
| Biosynthetic module | Research content |
| C7-cyclitol synthesis | The process of conversion from precursor to C7-cyclitol was studied by testing the reaction mechanism of various enzymes. During the process, an ATP-dependent phosphotransferase activity of 2-epi-5-epi-valiolone was identified, which was later verified by experiments to be derived from the protein Acb M. It is speculated that Acb M may catalyze the first step of acarbose synthesis pathway, and the functions of Acb O and Acb K are preliminarily explored[15] |
| By cloning the DNA fragment containing the conserved gene of dTDP-D-glucose-4,6-dehydratase (acb B), two genes involved in acarbose synthesis, acb A and acb C, were identified, and their functions were subsequently identified by heterologous expression. Based on the analysis of 3-dehydroquinate synthase in eukaryotes, a possible five-step reaction mechanism for Acb C-catalyzed cyclization was proposed[18] | |
| Acb M was found to act on 2-epi-5-epi-valiolone-7-phosphate by enzymatic reaction in vitro[19] | |
| This paper summarized the physiology, genetics and enzymology of acarbose biosynthesis, and put forward a novel pathway and metabolic cycle called “carbonation group”, which provided new clues for the further development of acarbose biotechnology[20] | |
| The researchers found that 1-epi-valienol and valienol did not directly participate in the biosynthesis of acarbose, but played a diversion role, and the diversion products had an impact on the synthesis module of deoxyhexose. Strategies to minimize the flux of the diversion products and maximize the partial supply of aminodeoxyhexose can increase the yield of acarbose[21] | |
| The researchers experimentally verified the reactions after the first three steps of the C7-cyclitol synthesis pathway, correcting and perfecting the entire acarbose biosynthesis pathway[17] | |
| Deoxyglucosamine synthesis | In this paper, the biosynthetic pathways and mechanisms of several new deoxysugars in bacteria were summarized, aiming to provide reference for the synthesis mechanism of deoxysugar unit in acarbose[22] |
| The researchers explored the differences in the synthetic pathways of acarbose and validamycin A, laying the foundation for revealing the unique coupling mechanism of the two components of acarbose and validamycin A via nitrogen bridge[23] | |
| Based on the comparative analysis of gene functions, acb A, acb B, and acb V were the genes involved in the synthesis of dideoxyglucosamine units. Then, the deletion and replacement of these three genes proved that they were all essential genes for acarbose biosynthesis, and the in vitro enzymatic reaction of Acb A, Acb B, and Acb V successfully revealed the synthesis pathway of dideoxyglucosamine[24] | |
| Maltose integration | In order to determine the origin of maltose units in acarbose, the resting cells of Actinoplanes sp. SN223/29 were fed with 3H-or 2H-labeled maltose or maltotriose, and maltotriose was found to be an effective precursor of acarbose and maltose[6] |
| On the basis of excluding the participation of dTDP-acarviosin-7-P in the biosynthesis of acarbose, the catalytic functions of Acb I and Acb S were explored, and the specific reaction steps of maltose integration were finally determined[17, 25] | |
| Carbophore cycle | An ATP-dependent phosphotransferase, Acb K, was isolated from Actinoplanes. The function of Acb K was preliminarily identified by enzymatic reaction in vitro, and it was found to be related to phosphorylation of acarbose and its homologues[26] |
| An enzyme activity was found in the supernatant of acarbose fermentation broth, which was identified to be encoded by acb D gene. The functional difference between Acb D and CGTase was analyzed, and it was found to be related to the formation of acarbose and its homologues by radiolabel-based catalytic experiments[27] | |
| The researchers constructed recombinant strains associated with acb D, acb E and acb Z by gene knockout and replacement, respectively, and found that deletion of the acb D gene had a negative effect on acarbose synthesis, while deletion of the acb E and acb Z genes did not affect the synthesis of acarbose[28] | |
| In this study, the researchers analyzed the specific function of Acb Q by reacting with dextran of different polymerization degrees in vitro, and determined that Acb Q was related to the formation of acarbose homologues[29] |
1.1 C7-环醇的合成
C7-环醇模块的起始化合物为2-epi-5-epi-valiolone (EEV),该化合物由磷酸戊糖途径的一个中间化合物sedo-heptulose-7-phosphate (SH7P)经Acb C催化而来[18]。接着,EEV被Acb M磷酸化形成2-epi-5-epi-valiolone-7-phosphate (EEV7P),随后又被Acb O差向异构化生成5-epi-valiolone-7-phosphate (EV7P),该产物已得到质谱和核磁共振验证[19]。EV7P在环脱水酶Acb L的作用下脱水形成Valienone-7-phosphate [V7P(1)],再经NADPH-依赖的氧化还原酶Acb N催化生成Valienol-7-phosphate [V7P(2)],后经磷酸激酶Acb U转化为Valienol-1, 7-diphosphate (V1, 7PP),再由acb J编码的磷酸酶脱磷酸化生成Valienol-1-phosphate (V1P),最后在Acb R的作用下将其核苷酸化生成NDP-valienol (NDP-V)[17]。
1.2 双脱氧氨基葡萄糖的合成脱氧氨基葡萄糖合成模块的起始化合物为D-glucose-1-phosphate (G1P),首先,在Acb A的催化作用下G1P被核苷酸化为dTDP-D-glucose (dTDP-G)。随后,在Acb B的催化下脱水生成dTDP-4-keto-6-deoxy-D-glucose (dTDP-4k6dG),最终在氨基转移酶Acb V的介导下,dTDP-4k6dG转化为dTDP-4-amino-4,6-dideoxy-D-glucose (dTDP-4a6dG),即双脱氧氨基葡萄糖。
1.3 麦芽糖的整合进入dTDP-4a6dG首先在假糖基转移酶Acb I的催化下与细胞内的麦芽糖结合形成复合物O-4-amino-(4,6-dideoxy-α-D-glucopyranosy)-(1→4)-O-α-D-glucopyranosyl-(1→4)-D-glucopyranose (4-aminoDGG),随后在Acb S催化下与来自C7-环醇模块的NDP-V结合生成阿卡波糖。
1.4 Carbophore循环Carbophore循环模块的途径主要包括:胞外淀粉由淀粉水解酶Acb E和Acb Z催化形成不同聚合度的寡糖,再经阿卡维基转移酶Acb D的转糖基作用与阿卡维基(主要来自阿卡波糖)连接形成阿卡波糖同系物,而后借助转运蛋白复合体Acb FGH转至胞内,由Acb K催化C7位磷酸化,再由4-α-葡萄糖糖基转移酶Acb Q催化脱去所结合的糖基生成阿卡波糖-7-磷酸,最后通过另一种转运蛋白复合体Acb WXY转运至胞外形成阿卡波糖,从而完成1个循环,该循环与胞内碳源的利用相关联[30]。由于与胞外代谢相关的3个酶中仅有Acb D的功能得到了验证,Acb E和Acb Z的功能尚未明晰,因此“Carbophore循环”尚处于假说阶段,完整代谢途径见图 2。
|
| 图 2 阿卡波糖生物代谢途径 Figure 2 The biosynthetic pathways of acarbose. |
2 阿卡波糖生物代谢途径中的酶系
阿卡波糖的代谢途径错综复杂,但归根结底仍是一系列存在级联关系的酶促反应过程的统合,本文仅介绍功能获得鉴定的相关酶或蛋白质(表 2)。
| Biosynthetic pathways of acarbose | Acb protein | Name or function |
| C7-cyclitol synthesis | Acb C | 2-epi-5-epi-valiolone synthase |
| Acb M | 2-epi-5-epi-valiolone-7-kinase | |
| Acb O | 2-epi-5-epi-valiolone-7-phosphate-2-epimerase | |
| Acb L | 2-epi-valiolone-7-phosphate-1-reductase | |
| Acb N | Cyclitol oxidoreductase | |
| Acb U | Valienol-7-phosphate-1-kinase | |
| Acb J | HAD family hydrolase | |
| Acb R | Nucleoside transferase | |
| Deoxyglucosamine synthesis | Acb A | D-glucose-1-phosphate-thymidylyltransferase |
| Acb B | dTDP-D-glucose-4,6-dehydratase | |
| Acb V | dTDP-4-amino-4,6-dideoxy-D-glucose transaminase | |
| Maltose integration | Acb I | Glycosyltransferase |
| Acb S | Preglycosyltransferase | |
| Carbophore cycle | Acb Z | α-amylase |
| Acb E | Acarbose resistant α-amylase | |
| Acb Q | 4-α-glucanotransferase | |
| Acb K | Acarbose-7-kinase | |
| Acb D | Acarviosyltransferase | |
| Transmembrane transport | Acb W | ABC transporter permease |
| Acb X | ABC transporter permease | |
| Acb Y | ABC transporter ATP-binding protein | |
| Acb H | ABC transporter ATP-binding protein | |
| Acb F | ABC transporter permease | |
| Acb G | ABC transporter permease | |
| Unknown | Acb P | NUDIX hydrolase (putative) |
2.1 C7-环醇模块中的关键酶 2.1.1 2-epi-5-epi-valiolone合成酶(Acb C)
1999年,Stratmann等[18]以灰色链霉菌(Streptomyces griseu)中dTDP-D-葡萄糖-4,6-脱水酶的编码基因str E为探针,在Actinoplanes sp. SE50/110基因组中钓取出str E的同源基因acb B和acb B两侧的基因acb C与acb A,随后用变铅青链霉菌(Streptomyces lividans) 66对acb C异源表达,通过酶促反应证实了酶Acb C的作用是催化SH7P产生C7-环醇前体物质EEV。Acb C属于脱氢奎宁酸合酶DHQS家族,其与该家族中的3-脱氢奎宁酸合酶(AroB)在氨基酸序列上有很高的相似性;DHQS参与莽草酸生物合成途径,几乎存在于所有微生物和植物中[31]。2019年,Schaffert等[32]利用PSET152中的强启动子过表达了acb C,但过表达菌株的阿卡波糖合成量却略有减少,推测可能的原因之一是存在催化中间体的负反馈抑制。
2.1.2 2-epi-5-epi-valiolone-7-激酶(Acb M)和差向异构酶(Acb O)严格来说,Acb M才是催化C7-环醇模块代谢途径的第1种酶。2002年,Zhang等[15]通过对脱水、环化、磷酸化等酶反应机理的测试,研究了从前体物质EEV经多步反应最终转化为C7-环醇的过程,期间鉴定出一种ATP依赖的EEV磷酸转移酶活性,后将基因acb M在Streptomyces lividans TK64中异源表达和纯化,通过实验证明了该活性来源于表达产物Acb M。Acb M是己糖-6-激酶家族的成员,与枯草芽孢杆菌(Bacillus subtilis)的Glck以及其他微生物的葡萄糖激酶有很大相似性,葡萄糖激酶几乎存在于所有微生物中,与生物体内的糖代谢密切相关[33]。近期有研究表明,糖尿病患者体内葡萄糖激酶的活性很高,葡萄糖激酶抑制剂的使用可能会为糖尿病的预防和控制提供新思路[34]。2003年,Zhang等[19]再次用Streptomyces lividans TK64异源表达基因acb M和acb O,其中Acb O的催化产物经质谱和核磁共振确定为EV7P,由此证实Acb O与Acb M在催化关系上直接偶联。值得一提的是,Acb O的氨基酸序列与已知的蛋白无显著的相似性且不需要任何辅因子参与反应,是一种新的只作用于碳水化合物及其衍生物的差向异构酶[19, 33]。
2.1.3 环醇脱氢酶(Acb L)和环醇氧化还原酶(Acb N)Acb L是一种NADPH依赖的环醇脱氢酶,其氨基酸序列与Zn2+依赖的环醇脱氢酶高度相似。之前普遍认为Acb L催化EV7P形成5-epi-valiolol-7-P,之后再依次由Acb N、Acb U、Acb J和Acb R介导的级联反应最终生成NDP-1, 1-epi-valienol-7-P,从而合成C7-环醇[15]。为验证该推测,Zhao等[21]通过代谢分析、化学掺入和生化实验证实了Acb L和Acb N的催化功能;首先,他们以EEV为底物,利用Acb M和Acb O依次催化获得了EV7P,接着在NADPH存在下加入纯化的Acb L进行反应,产物经HPLC-TOF/MS验证为V7P(1)而不是5-epi-valiolol-7-P,由此证明V7P(1)是参与该代谢模块的一个中间产物。
Acb N是短链脱氢酶家族中的一种依赖NADPH的氧化还原酶,推测其功能是用于还原V7P(1)。为验证这一假设,Zhao等[21]将V7P(1)和NADPH一起孵育,反应产物由核磁共振验证为V7P(2);接着,又将Acb N、V7P(2)和NAD+共同孵育却未检测到产物,这表明Acb N不催化该反应的逆反应。至此,C7-环醇代谢模块中从SH7P到V7P(2)的5个步骤已通过实验证明。
2.1.4 Valienol-7-P-1-激酶(Acb U)2020年,Zhao等[21]发现V7P(2)是C7-环醇代谢模块中的一个中间体,而Acb U被认为可能作用于V7P(2)。2年后,Tsunoda等[17]通过实验证明了V1, 7PP参与阿卡波糖的生物合成,该物质正是由激酶Acb U催化V7P(2)所生成,但是在ATP存在的环境下将Acb U与V1P孵育却无任何产物的生成,这表明Acb U只催化1-OH的磷酸化。此外,在无底物的情况下,Acb U也可以和ATP反应产生ADP,这说明Acb U是一种具有ATPase活性的双功能酶。
2.1.5 HAD家族水解酶(Acb J)和核苷转移酶(Acb R)Acb J和HAD超家族的水解酶具有很高的序列相似性,Zhao等[21]采用同框缺失和回补的方法表征其功能,发现Acb J能够将V7P(2)和1-epi-valienol-7-P去磷酸化生成valienol和1-epi-valienol;然而,这2种化合物并不参与阿卡波糖的合成,只起到分流作用。Tsunoda等[17]为了验证Acb J是否能将V1, 7PP去磷酸化,将基因acb J导入Streptomyces lividans TK24中异源表达,后续的催化实验证实了Acb J可以将V1, 7PP去磷酸化生成V1P,同时发现Acb J只能使C7位磷酸基团去磷酸化,因为该酶与V1P反应未检测出新生产物。
Acb R与环醇核苷酸转移酶VldB有63%的一致性,后者在有效霉素合成过程中负责将V1P转化为GDP-valienol,因此推测Acb R也是一种核苷转移酶[20]。为验证C7-环醇合成模块的后续反应,Tsunoda等[17]将重组Acb R与V1P在ATP、UTP、GTP、CTP或dTTP存在下反应,通过使用无机焦磷酸酶、嘌呤核苷磷酸化酶和7-甲基-6-硫代鸟苷的偶联实验,确定Acb R可以高效率地将V1P转化为NDP-V,且在5种核苷三磷酸中GTP是首选底物。虽然Acb R也可以将1-epi-V1P、V1, 7PP和1-epi-V1, 7PP转化为NDP衍生物,但转化率显著低于V1P与GTP,表明V1P是Acb R最可能的底物。至此,C7-环醇模块的代谢途径经实验完整验证。
2.2 双脱氧氨基葡萄糖模块中的关键酶(Acb A、Acb B和Acb V)结构多样性的脱氧糖存在于多种次级代谢产物中,其合成途径一般都以G1P为前体,经D-葡萄糖-1-P-胸腺嘧啶转移酶和dTDP-D-葡萄糖-4,6-脱水酶催化形成dTDP-4k6dG,最后再经转氨、异构化等修饰作用形成多样化的脱氧糖,为各合成通路提供原料[22]。Maumud等[23]首先发现阿卡波糖的脱氧己糖部分由葡萄糖产生,dTDP-4k6dG是一个类似中间体。张丹等[24, 35]在比对分析基因功能的基础上推测acb A、acb B和acb V是参与双脱氧氨基葡萄糖单元合成的基因,首先通过基因敲除和回补实验证实了这3个基因在阿卡波糖生物合成中不可或缺,接着借助体外酶促反应实验成功揭示了Acb A、Acb B和Acb V在双脱氧氨基葡萄糖合成模块中的具体作用,最后确定了Acb A的氨基酸序列与铜绿假单胞菌(Pseudomonas aeruginosa)的RmlA有55.0%的相似性,属于葡萄糖-1-磷酸胸腺嘧啶转移酶家族,催化dTDP-4a6dG合成的第1步反应,需Mg2+作为辅因子;Acb B的氨基酸序列与P. aeruginosa的RmlB有45.7%的相似性,是dTDP-D-葡萄糖-4,6-脱水酶,它的功能是使dTDP-G分子内脱水,需要NAD+作辅因子;Acb V氨基酸序列与转氨酶的相似性较高,是一种氨基转移酶,功能是将谷氨酸的氨基转移到dTDP-4k6dG的C4位产生dTDP-4a6dG。
2.3 麦芽糖的整合进入和Carbophore循环模块中的关键酶 2.3.1 糖基转移酶(Acb I)和假糖基转移酶(Acb S)最开始推测形成阿卡波糖的最后一步反应是由中间体dTDP-acarviosin-7-P或者dTDP-acarviosin和麦芽糖经Acb J、Acb I、Acb S和Acb Q中的一种酶催化形成,dTDP-acarviosin-7-P和dTDP-acarviosin则被推测是dTDP-4a6dG分别与GDP-valienol-7-P和GDP-V偶联反应所生成,该反应由一种与糖基转移酶(Gts)高度相似的假糖基转移酶(PsGts)介导[20, 25];由于已经证实Acb R能高效催化V1P生成GDP-V,因此排除dTDP-acarviosin-7-P参与阿卡波糖的合成。为了验证dTDP-4a6dG是否能偶联GDP-V产生dTDP-acarviosin,研究者们在大肠杆菌中异源表达了acb基因簇中可能为GT基因的acb I和acb S,但将重组Acb I和Acb S与dTDP-4a6dG和GDP-V一起孵育却无任何新生物,这表明dTDP-acarviosin也不参与阿卡波糖的生物合成[17]。为验证阿卡波糖合成路径的最后一部分,根据早期研究者的推测,4-aminoDGG可能是由dTDP-4a6dG和麦芽糖或低聚麦芽糖通过转糖基反应偶联形成,Tsunoda等[17]最终以实验证明了Acb I能够催化dTDP-4a6dG和麦芽糖反应生成复合物4-aminoDGG,但用低聚麦芽糖代替麦芽糖反应时却未产生任何新物质,这表明Acb I具有严格的底物专一性。
Acb I的功能明确之后,Acb S被推测是最有可能参与阿卡波糖合成的最终步骤的酶。Tsunoda等[17]又将4-aminoDGG与NDP-valienol同Acb S一起孵育的产物经ESI-MS/MS检测,确定产物为阿卡波糖。Acb S属于淀粉合成酶(GTs family 5),虽与同为淀粉合成酶的Acb I有42.5%的一致性,但二者的催化类型迥异,前者催化非糖苷键的形成,而后者催化糖苷键的形成。
2.3.2 阿卡波糖-7-激酶(Acb K)1999年,Drepper等[26]在Actinoplanes sp.中分离出一种ATP依赖的阿卡波糖磷酸转移酶,即Acb K。研究发现,放线菌胞质中含有一种麦芽糖酶,阿卡波糖会严重抑制麦芽糖酶的活性,而Acb K的产物阿卡波糖-7-磷酸对该酶的抑制作用显著削弱,这说明阿卡波糖-7-磷酸对菌体有一定保护作用。此外,还推测Acb K与阿卡波糖合成过程中麦芽糖和低聚麦芽糖等碳源物质的利用相关。为进一步探究该酶的催化特性和生理功能,Zhang等[15]在2002年借助S. lividans菌株表达Acb K,发现其与磷酸转移酶的核糖激酶家族成员有较高相似性,并且与acb M存在于同一个多顺反子;底物特异性分析表明,Acb K只作用于阿卡波糖及其同系物,因此该酶被认为在Carbophore循环中起作用。2009年,Rcokser等[16]发现Acb K与一个未知蛋白质的氨基酸序列有57.9%相似性,该蛋白质的编码基因与链霉菌(Streptomyces sp.)基因组中α-淀粉酶的编码基因相邻。
2.3.3 阿卡维基转移酶(Acb D)Hemker等[27]在阿卡波糖发酵液的上清中发现了一种酶活性,经鉴定该酶是由acb D基因编码;Acb D属于α-淀粉酶glycoside hydrolases 13超家族,具有α-糖苷酶的所有结构特征,并与该家族中的环糊精糖基转移酶(cyclodextrin glycosyltransferase, CGTase)有46%的一致性,但Acb D却未表现出CGTase的功能;基于放射性标记法的催化实验表明,Acb D能以多种阿卡波糖及其同系物作为阿卡维基供体和多种糖类及衍生物作为糖基受体,具体功能是将阿卡维基转移到糖基受体分子上形成阿卡波糖及其同系物。Leemhuis等[36]通过定点突变将Acb D保守序列中的第140位氨基酸突变为His或者去掉CGTase的His140,发现二者的催化活性可以部分互换。许贤恩[37]从放线菌胞外提取物中分离纯化出Acb D,用其催化阿卡波糖和寡糖混合物确定了阿卡波糖同系物4b (acarviosy-1,4-glucose-1,4-glucose-1,4-glucose)的形成机制。张欣等[28]构建acb D基因的缺失菌株,导致阿卡波糖合成量下降42%,通过该菌株的基因回补,其合成能力又恢复正常,证明了Acb D在阿卡波糖的代谢过程中发挥着重要作用。薛正莲等[38]在大肠杆菌(Escherichia coli) BL21(DE3)中实现了Acb D的可溶性表达,研究了反应时间、温度和pH对重组Acb D催化反应的影响,探索了Acb D在21种不同糖基供体下催化活性的差异,为进一步明确Acb D在阿卡波糖代谢循环中的作用提供了参考。
2.3.4 4-α-葡萄糖基转移酶(Acb Q)Acb Q属于糖基水解酶家族,是一种4-α-葡聚糖转移酶,与来自流感嗜血杆菌(Haemophilus influenzae)的淀粉麦芽糖酶MalQ有最高相似性[20]。MalQ几乎参与所有生物体细胞内的麦芽糖和麦芽糊精的代谢,其具体功能是水解直链葡聚糖的α-1,4-糖苷键,并将从还原端释放的葡萄糖残基转移到α-1,4-葡聚糖受体的非还原端[39]。Tsunoda等[17]通过实验证明Acb Q不参与阿卡波糖的生物合成,推测其可能与阿卡波糖的修饰相关。Nölting等[29]在E. coli中异源表达了acb Q并分析其功能,发现葡萄糖聚合度不高于7的α-1,4-葡聚糖均可作为Acb Q的底物,其中麦芽三糖为首选底物,但Acb Q不识别含有α-1, 1-和α-1, 2-糖苷的底物。不同的是,MalQ只作用于主链长度不少于2个葡萄糖分子的糖苷且最佳底物为麦芽四糖[40]。阿卡波糖是Acb Q葡聚糖转糖基反应的特异性受体,但阿卡波糖不能作为单一底物,其可在Acb Q存在的环境中接受不同长度的麦芽寡糖形成多种阿卡波糖同系物,这点与Acb D的功能相似。在阿卡波糖合成过程中,Acb Q与其他Acb蛋白相比具有最高的蛋白稳定性,其稳定性受翻译后影响,这表明该蛋白与阿卡波糖的代谢高度相关[29]。进一步的实验证明,Acb Q还可作用于磷酸化的阿卡波糖同系物,因此推测Acb Q在细胞内的底物主要为阿卡波糖同系物-7-磷酸[26, 39]。
3 阿卡波糖生产菌株的分子改造利用游动放线菌发酵生产阿卡波糖时所生成的阿卡波糖同系物(组分A、B、C、D、4a、4b、4c和假阿卡波糖等,见表 3)[41]将增加下游分离纯化工作的难度,得益于Actinoplanes sp. SE50/110全基因组信息的注释与公布,阿卡波糖发酵菌株的分子改造取得了很大的进展。近年来,研究者们多通过对相关基因敲除[13, 21, 42-44]、回补[28, 45]、过表达[46]和基因整合[47-49]来改造阿卡波糖的发酵菌株以提高阿卡波糖的产量和质量。
| Name | Structure |
| Acarbose | Ac-1,4-Glc-1,4-Glc |
| Homologue A | Ac-1,4-Glc-1,4-Fru |
| Homologue B | Ac-1,4-Glc-1,4-(1-epi-vlienol) |
| Homologue C | Ac-1,4-Glc-1, 1-Glc |
| Homologue D | Ac-1,4-Glc-1,4-Man |
| Homologue 4a | Ac-1,4-Glc-1,4-Glc-1,4-Fru |
| Homologue 4b | Ac-1,4-Glc-1,4-Glc-1,4-Glc |
| Homologue 4c | Ac-1,4-Glc-1,4-Glc-1, 1-Glc |
| Pseudoacarbose | Acarviosyl-1-4-(6-desoxy) Glc-1-4-Glc |
| Ac: Acarviose; Glc: Glucose; Fru: Fructose; Man: Mannose. | |
基因敲除方面,2015年,余贞等[42]首次报道了E. coli ET2567和游动放线菌之间的结合转移体系,采用同源重组技术对tre Y基因进行敲除,成功使发酵液中杂质组分C的含量减少到敲除前的10%。2016年,Wolf等[43]基于pKC1139质粒构建,利用CRIPSR/Cas9基因编辑技术敲除Actinoplanes sp. SE50/110基因组中酪氨酸酶的编码基因MelC,使发酵液中黑色素的含量显著降低,证明了MelC与发酵液中黑色素的形成相关。2019年,Xie等[13]通过对比Actinoplanes sp. SE50与其衍生菌株Actinoplanes sp. SE50/110的acb基因转录水平,将Actinoplanes sp. SE50基因组上发生突变的8个基因分别敲除验证,发现CWT_4325基因的缺失可使阿卡波糖的产量提高25%,推测CWT_4325的敲除直接导致细胞内乙酰辅酶A和乙醇的累积,进而导致了C7-环醇前体物质7-磷酸-景天庚酮糖(SH7P)的含量增高。同年,解慧欣等[44]通过检测不同麦芽糖浓度下阿卡波糖合成相关基因的转录水平,发现麦芽糖浓度的提高显著促进基因acb W的转录,敲除acb W基因就不再产生阿卡波糖,证明了acb W在阿卡波糖的合成中不可或缺;分别将基因acb W和acb WXY过表达,阿卡波糖合成量提高了6.4%和8%。2020年,Zhao等[21]通过生物实验证明了发酵液中存在2种分流产物,并认为分流产物的形成与磷酸水解酶有着很大关系。为减少分流产物的形成,敲除水解酶基因ACPL_8310和ACPL_2834,并将acb J基因的启动子替换为弱启动子WVp和kasOp*,同时最大限度增加氨基脱氧己糖部分的供应,使阿卡波糖的合成量达到了7.4 g/L。
基因回补方面,2022年,张欣等[28]分别构建基因acb E、acb Z和acb D的缺失菌株,发现acb E和acb Z基因的缺失对阿卡波糖的合成量无影响,acb D基因的缺失导致阿卡波糖的合成量下降42%,将acb D基因回补后,菌株又可正常生产阿卡波糖,证实了acb D在阿卡波糖生物合成中的重要作用。同年,Li等[45]通过对Tet R家族转录调控因子的基因TetR1敲除和回补,证实其可以促进acb B和acb D基因的表达,对阿卡波糖的生物合成有正向作用。
过表达方面,2017年,Zhao等[46]调整了受体和供体细胞的数量及比例,优化了孵育时间和培养基中MgCl2的浓度,使用甲氧苄啶代替萘啶酮酸来抑制大肠杆菌的生长,提高了种属间接合转移的效率,利用高效的基因操作方法将acb过表达使阿卡波糖的产量提升了35%。
基因整合方面,2023年,周健等[47]基于酶标仪检测方法,建立了与孔板发酵相结合的游动放线菌产阿卡波糖的高通量筛选模型,并通过CRISPR/Cas9技术,将假糖基转移酶基因acb S和酪氨酸酶基因melC整合,使重组菌株的阿卡波糖合成量提高了22%[48]。薛正莲等[49]通过比较转录组学分析挖掘得到了一种来源于游动放线菌的转录因子AcaR (ACPL_RS31540),其有调控阿卡波糖合成的能力;基于此,利用分子生物学手段将AcaR整合至Actinoplanes sp. ZFAC得到重组菌株Actinoplanes sp. ZFAC/AcaR,该重组菌株经发酵后可生产阿卡波糖4.86 g/L,相比于出发菌株,重组菌株的阿卡波糖合成量提高了20.8%。
4 展望阿卡波糖是一种主要由Actinoplanes sp.发酵获得的α-糖苷酶抑制剂,广泛用于II型糖尿病的治疗中。阿卡波糖的代谢路径庞大复杂,且涉及多种Acb酶催化的级联反应体系,随着基因组学、代谢组学和转录组学等技术的发展,阿卡波糖代谢通路的基本框架正逐步明确,相关酶系的研究和发酵菌株的改造也取得了一定的进展,但以下3个方面还需推进研究。(1) 目前,Acb P、Acb E和Acb Z在代谢途径中具体功能尚未被证实,这使得Carbophore循环仍处于假说阶段,因此通过实验证实上述酶的催化功能,进一步完善阿卡波糖的代谢路径是未来研究的大方向。(2) 已鉴定的若干关键酶有必要深入研究,如Acb D、Acb Q等与阿卡波糖及其同系物的合成密切相关,明确其催化机制和催化特性可对阿卡波糖杂质组分的控制起重要作用。(3) 由于阿卡波糖的代谢路径是多个酶协作的复杂网络系统,相关调控因子的功能也尤为重要,但目前关于调控因子方面的研究较为薄弱。因此,基于调控因子的研究,以提高阿卡波糖产量与质量为目的持续性改造阿卡波糖发酵菌株将成为未来十分有效的手段。
| [1] | SUN H, SAEEDI P, KARURANGA S, PINKEPANK M, OGURTSOVA K, DUNCAN BB, STEIN C, BASIT A, CHAN JCN, MBANYA JC, PAVKOV ME, RAMACHANDARAN A, WILD SH, JAMES S, HERMAN WH, ZHANG P, BOMMER C, KUO S, BOYKO EJ, MAGLIANO DJ. IDF Diabetes Atlas: global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045[J]. Diabetes Research and Clinical Practice, 2022, 183: 109119 DOI:10.1016/j.diabres.2021.109119. |
| [2] |
李兰英, 孙曙光. 我国糖尿病健康教育模式的研究现状及展望[J]. 中国医学创新, 2022, 19(17): 173-178.
LI LY, SUN SG. Research status and prospect of health education model of diabetes mellitus in China[J]. Medical Innovation of China, 2022, 19(17): 173-178 (in Chinese). |
| [3] | DEMIR S, NAWROTH PP, HERZIG S, ÜSTÜNEL BE. Emerging targets in type 2 diabetes and diabetic complications[J]. Advanced Science, 2021, 8(18): e2100275 DOI:10.1002/advs.202100275. |
| [4] | CAMPBELL LK, WHITE JR, CAMPBELL RK. Acarbose: its role in the treatment of diabetes mellitus[J]. The Annals of Pharmacotherapy, 1996, 30(11): 1255-1262 DOI:10.1177/106002809603001110. |
| [5] | DALSGAARD NB, GASBJERG LS, HANSEN LS, HANSEN NL, STENSEN S, HARTMANN B, REHFELD JF, HOLST JJ, VILSBØLL T, KNOP FK. The role of GLP-1 in the postprandial effects of acarbose in type 2 diabetes[J]. European Journal of Endocrinology, 2021, 184(3): 383-394 DOI:10.1530/EJE-20-1121. |
| [6] | LEE S, SAUERBREI B, NIGGEMANN J, EGELKROUT E. Biosynthetic studies on the alpha-glucosidase inhibitor acarbose in Actinoplanes sp.: source of the maltose unit[J]. The Journal of Antibiotics, 1997, 50(11): 954-960 DOI:10.7164/antibiotics.50.954. |
| [7] | WEHMEIER UF. The biosynthesis and metabolism of acarbose in Actinoplanes sp. SE50/110: a progress report[J]. Biocatalysis and Biotransformation, 2003, 21(4/5): 279-284. |
| [8] | SCHWIENTEK P, SZCZEPANOWSKI R, RÜCKERT C, STOYE J, PÜHLER A. Sequencing of high G+C microbial genomes using the ultrafast pyrosequencing technology[J]. Journal of Biotechnology, 2011, 155(1): 68-77 DOI:10.1016/j.jbiotec.2011.04.010. |
| [9] | SCHWIENTEK P, SZCZEPANOWSKI R, RÜCKERT C, KALINOWSKI J, KLEIN A, SELBER K, WEHMEIER UF, STOYE J, PÜHLER A. The complete genome sequence of the acarbose producer Actinoplanes sp. SE50/110[J]. BMC Genomics, 2012, 13: 112 DOI:10.1186/1471-2164-13-112. |
| [10] | WENDLER S, OTTO A, ORTSEIFEN V, BONN F, NESHAT A, SCHNEIKER-BEKEL S, WALTER F, WOLF T, ZEMKE T, WEHMEIER UF, HECKER M, KALINOWSKI J, BECHER D, PÜHLER A. Comprehensive proteome analysis of Actinoplanes sp. SE50/110 highlighting the location of proteins encoded by the acarbose and the pyochelin biosynthesis gene cluster[J]. Journal of Proteomics, 2015, 125: 1-16 DOI:10.1016/j.jprot.2015.04.013. |
| [11] | WENDLER S, HÜRTGEN D, KALINOWSKI J, KLEIN A, NIEHAUS K, SCHULTE F, SCHWIENTEK P, WEHLMANN H, WEHMEIER UF, PÜHLER A. The cytosolic and extracellular proteomes of Actinoplanes sp. SE50/110 led to the identification of gene products involved in acarbose metabolism[J]. Journal of Biotechnology, 2013, 167(2): 178-189 DOI:10.1016/j.jbiotec.2012.08.011. |
| [12] |
汪雪梅, 翁倩卉, 赵芹芹, 白林泉. 阿卡波糖生物合成调控蛋白的钓取与功能解析[J]. 微生物学报, 2021, 61(11): 3667-3685.
WANG XM, WENG QH, ZHAO QQ, BAI LQ. Mining and function studies of regulators for acarbose biosynthesis in Actinoplanes sp. SE50/110[J]. Acta Microbiologica Sinica, 2021, 61(11): 3667-3685 (in Chinese). |
| [13] | XIE HX, ZHAO QQ, ZHANG X, KANG QJ, BAI LQ. Comparative functional genomics of the acarbose producers reveals potential targets for metabolic engineering[J]. Synthetic and Systems Biotechnology, 2019, 4(1): 49-56 DOI:10.1016/j.synbio.2019.01.001. |
| [14] |
王远山, 戴科磊, 谢卡茜, 翁春跃. 阿卡波糖及其结构类似杂质生物合成与调控的研究进展[J]. 生物工程学报, 2022, 38(2): 605-619.
WANG YS, DAI KL, XIE KX, WENG CY. Biosynthesis and regulatory mechanism of acarbose and its structural analogs: a review[J]. Chinese Journal of Biotechnology, 2022, 38(2): 605-619 (in Chinese). |
| [15] | ZHANG CS, STRATMANN A, BLOCK O, BRÜCKNER R, PODESCHWA M, ALTENBACH HJ, WEHMEIER UF, PIEPERSBERG W. Biosynthesis of the C(7)-cyclitol moiety of acarbose in Actinoplanes species SE50/110.7-O-phosphorylation of the initial cyclitol precursor leads to proposal of a new biosynthetic pathway[J]. The Journal of Biological Chemistry, 2002, 277(25): 22853-22862 DOI:10.1074/jbc.M202375200. |
| [16] | ROCKSER Y, WEHMEIER UF. The gac-gene cluster for the production of acarbose from Streptomyces glaucescens GLA. O-identification, isolation and characterization[J]. Journal of Biotechnology, 2009, 140(1/2): 114-123. |
| [17] | TSUNODA T, SAMADI A, BURADE S, MAHMUD T. Complete biosynthetic pathway to the antidiabetic drug acarbose[J]. Nature Communications, 2022, 13(1): 3455 DOI:10.1038/s41467-022-31232-4. |
| [18] | STRATMANN A, MAHMUD T, LEE S, DISTLER J, FLOSS HG, PIEPERSBERG W. The AcbC protein from Actinoplanes species is a C7-cyclitol synthase related to 3-dehydroquinate synthases and is involved in the biosynthesis of the α-glucosidase inhibitor acarbose[J]. Journal of Biological Chemistry, 1999, 274(16): 10889-10896 DOI:10.1074/jbc.274.16.10889. |
| [19] | ZHANG CS, PODESCHWA M, ALTENBACH HJ, PIEPERSBERG W, WEHMEIER UF. The acarbose-biosynthetic enzyme AcbO from Actinoplanes sp. SE 50/110 is a 2-epi-5-epi-valiolone-7-phosphate 2-epimerase[J]. FEBS Letters, 2003, 540(1/2/3): 47-52. |
| [20] | WEHMEIER UF, PIEPERSBERG W. Biotechnology and molecular biology of the alpha-glucosidase inhibitor acarbose[J]. Applied Microbiology and Biotechnology, 2004, 63(6): 613-625 DOI:10.1007/s00253-003-1477-2. |
| [21] | ZHAO QQ, LUO YC, ZHANG X, KANG QJ, ZHANG D, ZHANG LL, BAI LQ, DENG ZX. A severe leakage of intermediates to shunt products in acarbose biosynthesis[J]. Nature Communications, 2020, 11(1): 1468 DOI:10.1038/s41467-020-15234-8. |
| [22] | LIU HW, THORSON JS. Pathways and mechanisms in the biogenesis of novel deoxysugars by bacteria[J]. Annual Review of Microbiology, 1994, 48: 223-256 DOI:10.1146/annurev.mi.48.100194.001255. |
| [23] | MAHMUD T, LEE S, FLOSS HG. The biosynthesis of acarbose and validamycin[J]. Chemical Record, 2001, 1(4): 300-310 DOI:10.1002/tcr.1015. |
| [24] |
张丹, 赵芹芹, 蒋明, 康前进, 白林泉. 游动放线菌Actinoplanes sp. SE50/110中阿卡波糖脱氧氨基糖单元的生物合成[J]. 微生物学报, 2020, 60(1): 118-134.
ZHANG D, ZHAO QQ, JIANG M, KANG QJ, BAI LQ. Biosynthetic pathway of deoxyaminosugar moiety in acarbose from Actinoplanes sp. SE50/110[J]. Acta Microbiologica Sinica, 2020, 60(1): 118-134 (in Chinese). |
| [25] | TSUNODA T, ABUELIZZ HA, SAMADI A, WONG CP, AWAKAWA T, BRUMSTED CJ, ABE I, MAHMUD T. Catalytic mechanism of nonglycosidic C-N bond formation by the pseudoglycosyltransferase enzyme VldE[J]. ACS Catalysis, 2023, 13(20): 13369-13382 DOI:10.1021/acscatal.3c02404. |
| [26] | DREPPER A, PAPE H. Acarbose 7-phosphotransferase from Actinoplanes sp.: purification, properties, and possible physiological function[J]. The Journal of Antibiotics, 1996, 49(7): 664-668 DOI:10.7164/antibiotics.49.664. |
| [27] | HEMKER M, STRATMANN A, GOEKE K, SCHRÖDER W, LENZ J, PIEPERSBERG W, PAPE H. Identification, cloning, expression, and characterization of the extracellular acarbose-modifying glycosyltransferase, AcbD, from Actinoplanes sp. strain SE50[J]. Journal of Bacteriology, 2001, 183(15): 4484-4492 DOI:10.1128/JB.183.15.4484-4492.2001. |
| [28] |
张欣, 赵芹芹, 白林泉. 游动放线菌Actinoplanes sp. SE50/110阿卡波糖胞外代谢相关基因功能的初步研究[J]. 基因组学与应用生物学, 2022, 41(5): 1006-1016.
ZHANG X, ZHAO QQ, BAI LQ. Functions of genes involved in extracellular acarbose metabolic pathway from Actinoplanes sp. SE50/110[J]. Genomics and Applied Biology, 2022, 41(5): 1006-1016 (in Chinese). |
| [29] | NÖLTING S, MÄRZ C, JACOB L, PERSICKE M, SCHNEIKER-BEKEL S, KALINOWSKI J. The 4-α-glucanotransferase AcbQ is involved in acarbose modification in Actinoplanes sp. SE50/110[J]. Microorganisms, 2023, 11(4): 848 DOI:10.3390/microorganisms11040848. |
| [30] |
冯志华, 王远山, 郑裕国. 阿卡波糖的生物合成途径研究进展[J]. 生物技术通报, 2011(8): 60-67.
FENG ZH, WANG YS, ZHENG YG. Progress in biosynthesis pathway of acarbose[J]. Biotechnology Bulletin, 2011(8): 60-67 (in Chinese). |
| [31] | CARPENTER EP, HAWKINS AR, FROST JW, BROWN KA. Structure of dehydroquinate synthase reveals an active site capable of multistep catalysis[J]. Nature, 1998, 394(6690): 299-302 DOI:10.1038/28431. |
| [32] | SCHAFFERT L, MÄRZ C, BURKHARDT L, DROSTE J, BRANDT D, BUSCHE T, ROSEN W, SCHNEIKER-BEKEL S, PERSICKE M, PÜHLER A, KALINOWSKI J. Evaluation of vector systems and promoters for overexpression of the acarbose biosynthesis gene acbC in Actinoplanes sp. SE50/110[J]. Microbial Cell Factories, 2019, 18(1): 114 DOI:10.1186/s12934-019-1162-5. |
| [33] | ROMERO-RODRÍGUEZ A, RUIZ-VILLAFÁN B, ROCHA-MENDOZA D, MANZO-RUIZ M, SÁNCHEZ S. Biochemistry and regulatory functions of bacterial glucose kinases[J]. Archives of Biochemistry and Biophysics, 2015, 577: 1-10. |
| [34] | ASHCROFT FM, LLOYD M, HAYTHORNE EA. Glucokinase activity in diabetes: too much of a good thing?[J]. Trends in Endocrinology & Metabolism, 2023, 34(2): 119-130. |
| [35] |
ZHANG D. Analysis of biosynthesis of C7-cyclol and dideoxysugar modules in acarbose[D]. Shanghai: Master's Thesis of Shanghai Jiao Tong University, 2019 (in Chinese). 张丹. 解析阿卡波糖中C7-环醇和双脱氧糖模块的生物合成[D]. 上海: 上海交通大学硕士学位论文, 2019. |
| [36] | LEEMHUIS H, WEHMEIER UF, DIJKHUIZEN L. Single amino acid mutations interchange the reaction specificities of cyclodextrin glycosyltransferase and the acarbose-modifying enzyme acarviosyl transferase[J]. Biochemistry, 2004, 43(41): 13204-13213 DOI:10.1021/bi049015q. |
| [37] |
XU XN. Actinoplanes sp. ZJB03852 enzymological basis of formation and fermentation control of structurally similar impurity components of acarbose[D]. Hangzhou: Master's Thesis of Zhejiang University of Technology, 2014 (in Chinese). 许贤恩. Actinoplanes sp. ZJB03852阿卡波糖结构类似杂质组分形成酶学基础及其发酵控制研究[D]. 杭州: 浙江工业大学硕士学位论文, 2014. |
| [38] |
XUE ZL, WANG YQ, LI C, LI DD, ZHU SB, LI XF. Heterologous expression and transglycosylation of acarviosyltransferase[J/OL]. Food Science, 2024. https://link.cnki.net/urlid/11.2206.TS.20240821.1515.060 (in Chinese). 薛正莲, 王雨晴, 李闯, 李丹丹, 朱司宝, 李翔飞. 阿卡维基转移酶的异源表达及转糖基作用[J/OL]. 食品科学, 2024. https://link.cnki.net/urlid/11.2206.TS.20240821.1515.060. |
| [39] | NGUYEN DHD, PARK SH, TRAN PL, KIM JW, LE QT, BOOS W, PARK JT. Characterization of the transglycosylation reaction of 4-α-glucanotransferase (MalQ) and its role in glycogen breakdown in Escherichia coli[J]. Journal of Microbiology and Biotechnology, 2019, 29(3): 357-366 DOI:10.4014/jmb.1811.11051. |
| [40] | KIM JE, TRAN PL, KO JM, KIM SR, KIM JH, PARK JT. Comparison of catalyzing properties of bacterial 4-α-glucanotransferases focusing on their cyclizing activity[J]. Journal of Microbiology and Biotechnology, 2021, 31(1): 43-50 DOI:10.4014/jmb.2009.09016. |
| [41] | LEISTNER A, HOLZGRABE U. Alternative methods to assess the impurity profile of a monographed API using acarbose as an example[J]. Journal of Pharmaceutical and Biomedical Analysis, 2022, 221: 115063 DOI:10.1016/j.jpba.2022.115063. |
| [42] |
余贞, 李美红, 李娜, 郑玲辉, 黄隽. 游动放线菌8-22中treY基因敲除对于降低阿卡波糖C组分的作用[J]. 微生物学通报, 2015, 42(7): 1301-1306.
YU Z, LI MH, LI N, ZHENG LH, HUANG J. Effect of treY gene inactivation on reducing of acarbose component C in Actinoplanes 8-22[J]. Microbiology China, 2015, 42(7): 1301-1306 (in Chinese). |
| [43] | WOLF T, GREN T, THIEME E, WIBBERG D, ZEMKE T, PÜHLER A, KALINOWSKI J. Targeted genome editing in the rare actinomycete Actinoplanes sp. SE50/110 by using the CRISPR/Cas9 System[J]. Journal of Biotechnology, 2016, 231: 122-128 DOI:10.1016/j.jbiotec.2016.05.039. |
| [44] |
解慧欣, 赵芹芹, 白林泉. 麦芽糖及转运蛋白对游动放线菌生产阿卡波糖的影响[J]. 基因组学与应用生物学, 2019, 38(10): 4536-4544.
XIE HX, ZHAO QQ, BAI LQ. Maltose and transporter's effects on acarbose production in Actinoplanes[J]. Genomics and Applied Biology, 2019, 38(10): 4536-4544 (in Chinese). |
| [45] | LI ZX, YANG SB, ZHANG ZY, WU YJ, TANG JW, WANG LJ, CHEN SX. Enhancement of acarbose production by genetic engineering and fed-batch fermentation strategy in Actinoplanes sp. SIPI12-34[J]. Microbial Cell Factories, 2022, 21(1): 240 DOI:10.1186/s12934-022-01969-0. |
| [46] | ZHAO QQ, XIE HX, PENG Y, WANG XR, BAI LQ. Improving acarbose production and eliminating the by-product component C with an efficient genetic manipulation system of Actinoplanes sp. SE50/110[J]. Synthetic and Systems Biotechnology, 2017, 2(4): 302-309 DOI:10.1016/j.synbio.2017.11.005. |
| [47] |
周健, 李翔飞, 李闯, 刘坤, 赵世光, 薛正莲. 游动放线菌产阿卡波糖高通量筛选模型的建立及优化[J]. 食品与发酵工业, 2023, 49(20): 73-80.
ZHOU J, LI XF, LI C, LIU K, ZHAO SG, XUE ZL. Establishment and optimization of high-throughput screening model for acarbose production by Actinoplanes sp. SE50[J]. Food and Fermentation Industries, 2023, 49(20): 73-80 (in Chinese). |
| [48] |
ZHOU J. Breeding and molecular modification of acarbose high-yielding strains[D]. Wuhu: Master's Thesis of Anhui Polytechnic University, 2023 (in Chinese). 周健. 阿卡波糖高产菌株选育及分子改造[D]. 芜湖: 安徽工程大学硕士学位论文, 2023. |
| [49] |
XUE ZL, LI XF, HAN RM, LIU K, ZHAO M, LIU Y, LIU RH, QIN YF, LI WQ. The invention relates to transcription factor AcaR, gene, recombinant bacterium derived from swimming actinomycetes, preparation method and application thereof: CN117843740A[P]. 2024-04-09. 薛正莲, 李翔飞, 韩如梦, 周健, 刘坤, 赵明, 刘艳, 刘瑞华, 秦艳飞, 李为全. 一种游动放线菌来源的转录因子AcaR、基因、重组菌及其制备方法和应用: CN117843740A[P]. 2024-04-09. |
 2024, Vol. 64
2024, Vol. 64