中国科学院微生物研究所,中国微生物学会
文章信息
- 曹雪峰, 彭练慈, 方仁东. 2023
- CAO Xuefeng, PENG Lianci, FANG Rendong.
- 细菌溶血磷脂的生物合成及生物学功能
- De novo biosynthetic pathway and biological functions of bacterial lysophospholipids
- 微生物学报, 63(12): 4482-4501
- Acta Microbiologica Sinica, 63(12): 4482-4501
-
文章历史
- 收稿日期:2023-04-19
- 网络出版日期:2023-07-06
2. 草食动物科学重庆市重点实验室, 重庆 400715
2. Chongqing Key Laboratory of Herbivore Science, Chongqing 400715, China
磷脂是一种在细胞膜表面常见的复合脂类物质,其最显著的特点是与细胞膜上的蛋白质、糖脂、胆固醇等物质共同形成脂质双分子层。溶血磷脂(lysophospholipid, LPL)是磷脂双分子层中的次要组分[1]。和二酰化的磷脂分子不同,LPL只有一条酰基化脂肪酸链与中心甘油(glycerol)分子相连[1]。在真核生物中,LPL是一类具有生物活性的脂类信号分子,能介导多种细胞生理反应[2-4]。常见的LPL包括溶血磷脂酸(lysophosphatidic acid, LysoPA)、溶血磷脂酰胆碱(lysophosphatidylcholine, LysoPC)、溶血磷脂酰乙醇胺(lysophosphatidylethanolamine, LysoPE)、溶血磷脂酰甘油(lysophosphatidylglycerol, LysoPG)、溶血磷脂酰丝氨酸(lysophosphatidylserine, LysoPS)和溶血磷脂酰肌醇(lysophosphatidylinositol, LysoPI)[5-7]。LPL通常作为细胞膜磷脂生物合成的中间产物或在磷脂水解过程中被生成[6]。其生物合成途径包括(图 1):(1) 特异性磷脂酶剪切磷脂的一条酰基链后,LPL作为水解产物被生成[8];(2) 在七酰化脂多糖或三酰化磷脂的形成过程中,位于革兰氏阴性菌外膜上的脂质A (lipid A)棕榈酰转移酶PagP将棕榈酸链从磷脂分子上转移到六酰化脂质A或另一种磷脂上后,LPL作为副产物被生成[9-11];(3) 在革兰氏阴性菌脂蛋白的成熟过程中,载脂蛋白N-酰基转移酶(apolipoprotein N-acyltransferase, Lnt)将磷脂分子中的一条脂肪酸链转移到脂蛋白前体上后,LPL作为副产物被生成[12-13]。在合成途径(1)中,根据磷脂酶在磷脂分子上作用靶点的不同,磷脂酶可被分为A、B、C、D这4种亚型。其中A型磷脂酶(phospholipase A, PldA)可在(Sn)-1或(Sn)-2位点剪切磷脂分子的一条酰基链,因此又可以被进一步分为可在(Sn)-1位点水解脂肪酰基酯键并生成(Sn)-1 LPL的PldA1,和在(Sn)-2位点剪切酯键形成(Sn)-2 LPL的PldA2[14-15]。
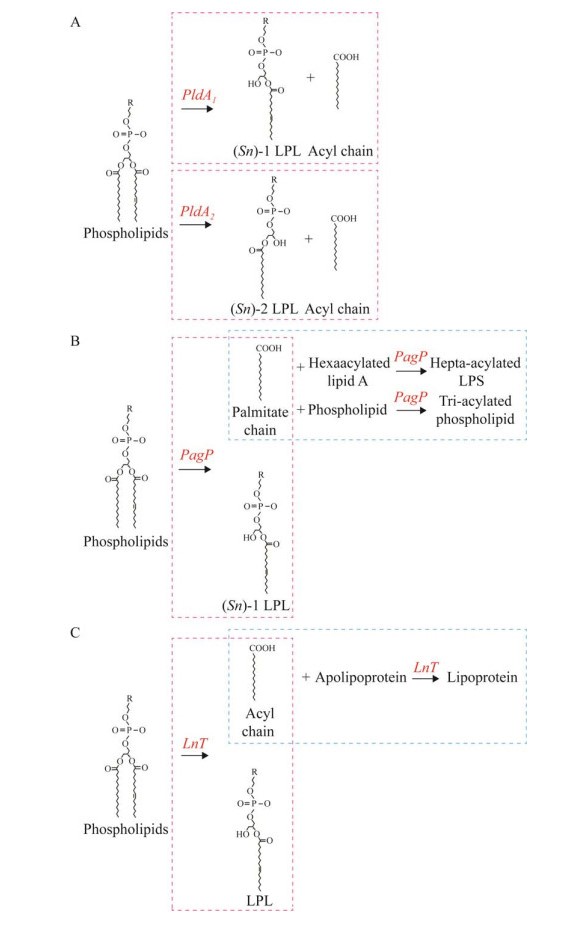
|
| 图 1 细菌LPL生物合成途径 Figure 1 The bacterial LPL biosynthesis pathways. A: LPL generated by phospholipase. B: LPL produced by PagP as by-product in the formation of hepta-acylated LPS and tri-acylated PG. C: LPL produced by Lnt as by-product in the lipoprotein de novo biosynthetic pathway. The pink dotted line represents the first step reaction, the blue dotted line represents the second step reaction. The involved enzymes and their cleavage sites are indicated in red. |
在真核细胞中,LPL的生物学功能已被广泛研究。除了作为细胞内脂质生物合成的中间前体外,LPL还是G蛋白偶联信号通路中重要的信使分子。通过募集、激活T细胞、B细胞和巨噬细胞,G蛋白偶联信号通路可调控动物机体内特定的免疫反应。此外,LPL还可以作为多功能细胞生长因子被人和动物利用[16-18]。LPL在通常在组织间液和血浆中大量存在,但在细胞中浓度很低。在人体中,体液中LPL的确切含量尚不清晰,但lysoPC被测定出是人体血浆中含量最丰富的LPL,其浓度约为200–300 μmol/L[19-21]。其他LPL在人体血浆中的浓度大约为:lysoPE (10–50 μmol/L)、lysoPI (1–15 μmol/L)、lysoPA (0.6–1.0 μmol/L)、lysoPG (0.4 μmol/L)、lysoPS (0.1 μmol/L)[20, 22-27]。
在细菌中,LPL的生物学功能少有被研究。细菌中的LPL通常会通过二酰化磷脂的酰化反应或磷脂酶B [也称为溶血磷脂酶,它可以在(Sn)-1和(Sn)-2任意位点切割两种LPL的脂肪酸链]被分解[28-30],因此含量普遍极低。这可能是因为LPL的结构特点不利于细菌细胞膜磷脂双分子层的形成和稳定。LPL是一种具有特殊非圆柱形几何结构的分子,只能在液体中形成胶束(微胶粒)结构,无法形成稳定的双层结构,因此LPL在细胞膜中的掺入会在磷脂双分子层中产生不稳定性,从而破坏细胞膜的屏障作用[31]。
之前的研究表明,细胞膜中的类LPL锥形分子结构将增加脂质双分子层的曲度,并改变整个细胞膜的横向压力分布,导致细胞膜中形成渗漏边界,和瞬时(或永久)的分子间隙,使细胞膜出现非均质性的结构缺陷[32]。同时,膜胶束化和膜分子间隙的产生会破坏细胞膜的生理结构并使细胞膜的通透性增加[5]。因此LPL的存在将会对细胞膜稳态带来巨大风险。基于以上原理,宿主体内可将细菌膜磷脂降解为LPL的外源性PldA2被认为是一种高效的抗菌剂[33]。虽然目前对细菌LPL的生物学功能还知之甚少,但已有研究指出,宿主分泌的LPL能有效的降低细菌在感染过程中的感染效率[4]。同时,在细菌感染过程中,LPL在宿主免疫细胞中的含量也被发现显著升高,但其作用机制还尚不清晰[34-35]。此外,在沙门氏菌(Salmonella)感染过程中,宿主源LPL还被发现能够刺激细菌释放促炎单体鞭毛蛋白,从而增强宿主对该细菌的先天免疫反应和炎症反应[36]。
由于LPL对细胞膜的潜在毒性作用,因此细菌细胞膜中通过内外源性PldA、PagP或Lnt依赖的酶促反应所产生的LPL需要迅速清除[37-38]。LplT (lysophospholipdis transporter)是存在于部分革兰氏阴性菌中的一种LPL转移系统。细菌能利用LplT将细胞外膜内外叶及细胞内膜外叶的lysoPE、lysoPG和lysoCL转移至细胞内膜内叶[37],随后通过酰基-酰基转移蛋白合成酶(又称为LPL酰基转移酶,LPL Aas) acyl-ACP synthetase被二次酰化后清除。因此LplT-Aas系统在保护革兰氏阴性菌免受外源PldA2攻击过程中具有不可替代的作用[28, 39]。另一方面,LPL对大多数革兰氏阳性菌有极强的细胞毒性,因此LPL在革兰氏阳性菌中几乎不存在[6, 40-41]。
通常情况下,LPL只占细菌磷脂总含量的不到1%,但环境压力会致使细菌细胞膜中LPL的含量显著升高[37, 42-44]。一些病原菌在遇到胆盐、高温和酸性等环境刺激时,其LPL的分泌和释放都会急剧增加[43, 45-48]。之前的研究表明,在低氧压力下空肠弯曲杆菌(Campylobacter jejuni)需要增加其细胞膜LPL的含量来维持细菌的运动活力[42]。此外,另一份研究[49]和其他学者[50]的相关研究还表明,细菌微摩尔浓度级的LPL就足以对真核细胞造成细胞毒性。但关于细菌在压力环境中为何以及如何积聚高含量的LPL还没有被清晰的阐释。目前已有的研究表明,当不同类型的磷脂,比如适于双分子层形成的磷脂酰甘油(phosphatidylglycerol, PG)与会给细胞膜双分子层带来结构压力的锥形不饱和磷脂酰乙醇胺(phosphatidylethanolamine, PE)混合时,细胞膜内会出现不稳定,此时以特定比例适当掺入LPL可以通过释放脂质双分子层的弹性弯曲压力来恢复细胞膜的稳定性[51]。因此LPL的累积可能可以帮助细胞维持其膜结构稳态。此外,LPL对细胞膜的曲度修饰可以改变某些膜蛋白的结构和功能[52-53],因此细胞膜LPL的累积也可能是细菌调控其环境应激反应的结构基础。另一方面,在环境压力条件下LPL在细菌中的增加还有可能是源于PldA活性的升高。当细菌受到环境压力时,细胞膜的完整性和不对称性会被破坏,导致细菌PldA的活性通过钙离子依赖的二聚化机制被增强[54-55],从而诱发磷脂分子生成LPL的效率提高[6, 37]。虽然目前对细菌LPL生物学功能的了解还存在大量的空白,但LPL被认为是细菌致病机制和炎性反应中被低估的重要因子。本文以下部分将重点分类描述细菌中主要LPL的生物合成通路及生物学功能。
1 不同种类LPL的生物学特性 1.1 溶血磷脂酸lysoPA 1.1.1 LysoPA的特点水溶性lysoPA是溶血甘油磷脂家族中结构最简单的分子。在真核细胞中,lysoPA是细胞免疫反应中重要的生物标志物,它能诱导包括细胞凋亡抑制、细胞趋化性、细胞因子和趋化因子分泌、血小板聚集和伤口愈合效率增强在内的多种免疫反应[1, 17, 56-58]。在细菌中,对lysoPA的了解仅限于它是磷脂酸(phosphatidic acid, PA)生物合成过程中的中间产物且在细胞中的含量极低[59]。
细菌lysoPA被认为是所有甘油磷脂生物合成的前体,可通过酰基-酰基转移蛋白合成酶依赖的3-磷酸甘油酰基转移酶被催化合成(图 2)[6, 60-61]。LysoPA的生物合成是在细胞溶质中发生的,由于缺乏相应的结合位点,lysoPA无法被LplT转移系统识别和转运到细菌细胞膜中[62]。但lysoPA能通过PldA介导的磷脂水解途径或在部分细菌和真核细胞中被具有溶血磷脂酶D活性的自体毒素(autotoxin, ATX)在细胞膜中催化生成(图 2)[1, 37, 63-65]。LysoPA是除了lysoPC外另一个能在真核细胞中由lysoPE、lysoPS或者lysoPI通过ATX介导的催化反应生成的LPL[66]。
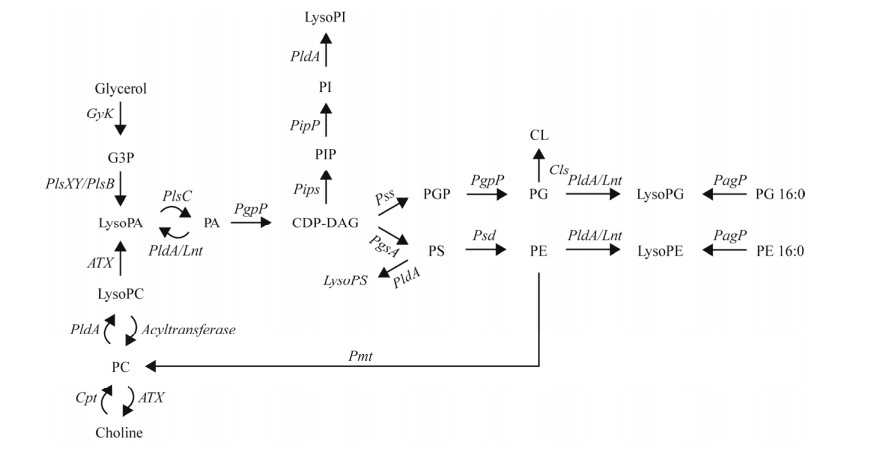
|
| 图 2 细菌细胞膜磷脂从头合成通路 Figure 2 The de novo biosynthetic pathway of bacterial membrane phospholipids. The enzymes involved in the different catalyzed reactions are indicated in the text. G3P: Glycerol-3-phosphate; CDP-DAG: Cytidine diphosphate-diacylglycerol; PGP: Phosphatidylglycerol-phosphate; PIP: Phosphatidylinositol phosphate; GyK: Glycerokinase; Pls: Acyltransferase; PldA: Phospholipase A; Lnt: Apolipoprotein N-acyltransferase; CdsA: Phosphatidate cytidylyltransferase; Pips: Phosphatidylinositolphosphate synthase; Pipp: Phosphatidyl-inositolphosphate phosphatase; Pss: Phosphatidylserine synthase; Psd: Phosphatidylserine decarboxylase; PgsA: Phosphatidylglycerolphosphate synthase; PgpP: Phosphatidylglycerol phosphate phosphatase; Cls: Cardiolipin synthase; PagP: Lipid A palmitoyltransferase; Pmt: Phospholipid N-methyltransferase; ATX: Autotoxin. |
1.1.2 LysoPA的功能
细菌lysoPA的生物生理学功能目前还未被清晰阐释。现有的研究表明,幽门螺旋杆菌(Helicobacter pylori)鞭毛的合成效率与lysoPA在菌体中的积累呈正相关[67]。
虽然目前对lysoPA功能的研究还相当匮乏,但细菌源lysoPA被认为与动物健康紧密相关。最新研究一方面表明,小鼠患结直肠肿癌后,其肠道菌群将发生显著改变,同时粪便中lysoPA和lysoPC含量也被发现显著升高[68];另一方面,在断奶仔猪日粮中添加植物乳酸杆菌(Lactobacillus plantarum)将增加泛菌属细菌的相对丰度,同时能通过促进1-棕榈酸lysoPA的产生显著提高仔猪对脂肪消化和吸收[69]。因此,来源于肠道菌群的lysoPA与动物肠道健康之间的相互平衡可能对维持肠道稳态十分重要。
1.1.3 宿主源lysoPA在细菌-宿主互作中的作用除了细菌源lysoPA,宿主源lysoPA也能影响细菌的感染过程。通过促进肠道对盐类的吸收及抑制肠道对阴离子的分泌,宿主自分泌的lysoPA能在缓解因为细菌感染而导致的急性腹泻中发挥重要作用[70-71]。在此过程中,宿主源lysoPA通过调控有丝分裂原活化蛋白激酶/细胞外信号通路调节激酶(MAPK/ERK)、G蛋白A (RhoA)和RhoA激酶、富脯氨酸酪氨酸激酶[72]、3型Na+/H+交换器[64]、囊性纤维化跨膜传导调节蛋白[73]、Cl–/OH–交换器[74]的分泌和功能来维持肠道离子稳态,从而发挥缓解腹泻的作用。此外,由于可以通过激活细胞外调节蛋白激酶(ERK1/2)、丝氨酸/苏氨酸磷酸酶和P13激酶信号通路抑制细菌内毒素诱导的炎性反应,lysoPA也被认为是细菌性肠炎的有效抗炎剂[71]。
1.1.4 LysoPA对宿主的作用LysoPA在真核细胞中的生理生物学功能主要是通过激活细胞表面的lysoPA受体来完成。通过受体的激活,lysoPA能介导上皮细胞凋亡、抑制细胞内抗肿瘤反应[60, 75]。将小鼠进行lysoPA受体缺失处理后,小鼠的肠通透性被发现显著升高,同时通过H2O2诱导产生的结肠紧密连接蛋白和黏附连接蛋白的表达量被检测到明显减少[39]。此外,小鼠lysoPA受体缺失骨髓源树突状细胞在受到LPS刺激时,TNF-α表达水平和IL-10分泌水平分别显著的升高和降低[76]。细菌lysoPA对宿主lysoPA受体的激活还和细菌溶血性有关。相关研究表明,由产气荚膜梭菌(Clostridium perfringens)引起的溶血是红细胞表面的lysoPA受体通过Ca2+通道被产气荚膜梭菌激活,从而造成红细胞裂解[77-79]。因此lysoPA被认为是调控宿主细胞生理功能的重要因子。
1.2 溶血磷脂酰胆碱lysoPC 1.2.1 LysoPC的特点LysoPC,又称为溶血卵磷脂,是一种磷脂酰胆碱(phosphatidylcholine, PC)的衍生物,在PC的一个脂肪酸链被磷脂酶切割后产生。LysoPC能进一步被催化生成lysoPA,或者通过PC生成胆碱(图 2)[6, 37, 80]。在大多数动物组织中,lysoPC是含量最丰富的LPL,也是部分生理和病理过程的核心分子[1, 81]。但目前只有少数原核生物被认为能够分泌lysoPC,其中包括根癌农杆菌(Agrobacterium tumefaciens)、铜绿假单胞菌(Pseudomonas aeruginosa)、肺炎衣原体(Chlamydia pneumonia)、约氏黄杆菌(Flavobacterium johnsoniae)和伯氏疏螺旋体(莱姆病病原) (Borrelia burgdorferi)等[61, 82]。
和lysoPA类似,lysoPC也不是LplT转移系统的可识别底物。因为其过大的胆碱头部基团会阻挡转运所必需的酶进入LplT的结合位点[6, 37]。虽然包括奈瑟氏淋球菌(Neisseria gonorrhoeae)、大肠杆菌(Escherichia coli)、霍乱弧菌(Vibrio cholera)、鼠伤寒沙门氏菌(Salmonella Typhimurium)、幽门螺旋杆菌(Helicobacter pylori)、空肠弯曲杆菌(Campylobacter jejuni)、多形拟杆菌(Bacteroides thetaiotaomicron)、枯草芽孢杆菌(Bacillus subtilis)、乳酸杆菌(Lactobacillus sp.)和产气荚膜梭菌(Clostridium perfringens)在内的多种细菌都无法分泌lysoPC,但这些细菌被认为可以利用宿主体内的lysoPC参与自身代谢[61]。在动物肠道中,食源性PC可被胰磷脂酶降解为lysoPC[6, 37, 83],间接表明lysoPC对大多数肠道菌相对无毒。
1.2.2 LysoPC对细菌的作用在真核细胞中,lysoPC的生物学功能已被充分研究。除了作为脂质代谢的前体,宿主体内的lysoPC还被认为是炎症严重程度的生物标志因子[6, 37]。最近的研究指出,宿主体内lysoPC水平的降低可能与宿主死亡风险的升高有所关联[84]。
在细菌中,对lysoPC生物生理学功能的了解目前还存在大量空白。在有限的研究中,内源性lysoPC被发现能调控细菌膜蛋白的功能[6]。假结核耶尔森菌(Yersinia pseudotuberculosis)分泌的内源lysoPC能通过调控外膜蛋白F的活性来选择性过滤胞内外的亲水性溶质[85]。大肠杆菌的大电导机械力敏感通道(mechanosensitive channel with very large conductance, MscL)可被内源lysoPC触发至完全开放状态,从而应对膜张力所带来的渗透压力[86]。全打开的MscL是一个大间隙的充水空,可被细菌用作紧急安全阀。霍乱弧菌能利用内源lysoPC作为营养物质来重塑细胞壁磷脂的结构和分布[87]。
外源性lysoPC对细菌的影响主要体现在改变抗菌素活力上。例如,外源lysoPC可以通过提高细菌细胞膜通透性迅速杀死革兰氏阳性耐甲氧西林金黄色葡萄球菌MRSA (methicillin- resistant Staphylococcus aureus)[88];还可以提高金黄色葡萄球菌S. aureus对部分抗生素(例如金霉素)的敏感性[88]。外源lysoPC对革兰氏阴性菌更多的是非直接作用,例如,lysoPC能提高多粘菌素B对鼠伤寒沙门氏菌,肺炎克雷伯菌(Klebsiella pneumonia)和铜绿假单胞菌(Pseudomonas aeruginosa)的杀菌效果[89-91]。这种非直接影响的机制可能是lysoPC通过提高细胞膜通透性使抗菌物质更容易进入菌体内[87]。
1.2.3 LysoPC在细菌致病中的作用除了可以改变细菌细胞膜的结构,外源lysoPC还在病原菌的细胞侵袭和炎症中发挥着重要作用[92]。沙门氏菌感染宿主后能利用宿主细胞释放的lysoPC促进其侵袭蛋白Sips和1型毒力岛转录调控因子HilA的表达,从而增强其对宿主细胞的侵袭效率[93]。在这个过程中,宿主源lysoPC还可以通过调控沙门氏菌的cAMP (cyclic adenosine 3, 5′-monophosphate)依赖性信号通路,诱导其鞭毛蛋白的合成和分泌,从而通过结合5型Toll样受体反向激活宿主的炎症和先天性免疫反应[36]。在结核分枝杆菌(Mycobacterium tuberculosis)感染过程中,虽然尚不清楚lysoPC在其中发挥的作用,但lysoPC在宿主巨噬细胞和吞噬体内的水平被发现显著升高[34-35]。外源lysoPC还对耻垢分枝杆菌(Mycobacterium smegmatis)原生质球(spheroplasts)有剧毒,因为lysoPC可以破坏和裂解原生质球的细胞膜。但lysoPC对完整的耻垢分枝杆菌并没有毒性作用,这可能是由于耻垢分枝杆菌细胞膜上的YhhN家族蛋白可以拮抗lysoPC对细胞的损伤[94]。
此外,细菌分泌的lysoPC还能影响宿主的部分生理学功能。来源于宿主肠道菌群的lysoPC能破坏肠道的屏障功能并与宿主炎性肠病的发生有密切关联[95]。埃希氏菌(Escherichia)、嗜胆菌(Bilophila)、肠杆菌(Enterorhabdus)和戈尔多尼巴氏菌(Gordonibacter)这4种革兰氏阴性菌与宿主粪便中lysoPC含量的增加有密切联系。高浓度的lysoPC在体内和体外均被证实能诱导宿主的免疫应答和破坏宿主细胞之间的紧密连接[95]。因此来源于肠道菌群的lysoPC被认为能损伤肠道上皮屏障,并且是引起宿主结肠炎的重要致病因子。
1.3 溶血磷脂酰乙醇胺lysoPE 1.3.1 LysoPE的特点LysoPE是细菌中最主要也是被研究的最为广泛的两性LPL。LysoPE主要存在于革兰氏阴性菌的外膜中,能够给细胞膜提供正向弯曲压力[1, 6, 96-97]。细菌源lysoPE能在细菌死亡后被释放到外界环境中,但目前还未发现活菌能够直接将lysoPE分泌到菌体外[4]。在人和动物中,lysoPE仅微量存在于组织之中,在血浆中含量略高(血清中含量第二的LPL;浓度为10–50 μmol/L,或血清总磷脂量的1%)[1]。
细菌lysoPE可通过内源PldA介导的PE水解反应或脂质A棕榈酰转移酶PagP介导的六酰化LPS、三酰化PG合成反应被产生(图 2)[6, 10-11, 37, 98]。由于lysoPE能给细胞膜带来正向弯曲压力和其自身的类洗涤剂特点,lysoPE被认为不适合掺入细胞膜脂质双分子结构中。因此通过细菌的脂质稳态调控,lysoPE通常会被快速转化为PE[6, 99-100]。但在一些细菌尤其是病原菌中,在特定压力条件下,其细胞膜中lysoPE的含量会显著增加[6, 37]。最近的研究表明,在空肠弯曲杆菌中,随着氧气浓度的上升,其细胞膜中lysoPE的含量最高可达细菌总磷脂含量的34%[42]。在其他研究中,环境中的可用葡萄糖、高温或者苯酚类杀虫剂可诱导假结核耶尔森菌(Yersinia pseudotuberculosis)中lysoPE的含量从约1%上升至16.3%[46, 101-102]。在8 ℃下生长的假结核分枝杆菌中,细菌生长稳定期细胞中lysoPE可达磷脂总含量的45%[103]。在霍乱弧菌中,胆盐可刺激其细胞膜lysoPE的含量从2%增加到30%[43]。在幽门螺旋杆菌中,酸性压力可以使其lysoPE的含量升高且同时诱导尿素酶细胞毒素A的释放[48, 104]。压力条件下细菌lysoPE含量的增加有可能是因为磷脂酶PldA活性的增强。噬菌体裂解[105]、大肠菌素释放[106]、乙二胺四乙酸(ethylenediamine tetraacetic acid, EDTA)处理[107]或者热刺激[47]均可提高大肠杆菌PldA的活性[48, 104]。
在大肠杆菌中,lysoPE酰基链的长度介于14–18个碳原子之间,其中以lysoPE 18:1和lysoPE 16:1最为常见[99, 108]。在空肠弯曲杆菌中,其lysoPE酰基链的长度被发现介于12–20个碳原子之间,且在各长度的脂肪酸链上均有检测到饱和、不饱和以及含环丙烷结构,其中以lysoPE 14:0和lysoPE 19:0c的含量最高[42, 109]。在霍乱弧菌中,有3种不同长度脂肪酸链的lysoPE (C16、C18和C20)被发现,其中lysoPE 16:0和lysoPE 18:1为最主要的亚类[43, 110]。在幽门螺旋杆菌中,目前仅检测出lysoPE 18:1[111]。
1.3.2 LysoPE对细菌的作用LysoPE被证实是真核细胞必需的生长因子和细胞外调节因子[112],也被发现具有分子伴侣的特点。例如,在尿素变性后,lysoPE能促进柠檬酸合成酶和α-葡萄糖苷酶的功能性折叠[46-47]。在细菌中,lysoPE被认为同时具有分子伴侣和化学伴侣的性质,可以直接影响细菌膜蛋白的结构和功能。LysoPE在细菌细胞膜中含量的升高一方面可促进额外的铁离子内流进入菌体内[99],但同时会破坏细菌细胞膜稳态对膜结构造成严重损伤,造成细菌生长抑制以及细胞间质的渗漏,极端情况下导致细胞溶解[113]。在大肠杆菌中,细胞膜中高含量的lysoPE会导致细胞外膜和内膜的界限消失,造成细胞质颗粒变性和空泡化[113]。LysoPE在细胞膜中的累积还会破坏膜脂质对称性,引起细胞外膜渗透屏障的破坏,从而使大肠菌素-铁复合体和万古霉素等物质更容易进入细胞周质,分别导致细菌复制增殖的促进和抑制[99]。
LysoPE还在细菌拮抗外界环境压力过程中发挥着重要作用。在幽门螺旋杆菌和假结核分枝杆菌中,lysoPE的积累是细菌适应酸性环境或抵御抗生素压力的必要条件[48, 102]。细菌抗酸可能涉及到的分子机制包括:(1) 通过改变细胞膜和外膜蛋白的构象阻断质子内流以及细胞周质、细胞质分子伴侣与其配体的识别、结合,从而调控细胞内pH[114];(2) 通过激活细胞膜上的氯离子传导通道(chloride channel, ClC),使质子以不带电的HCl形式进入菌体,随后解离为H+和Cl–,降低菌体内pH[115-116];(3) 通过重塑细胞内膜磷脂的组成,降低质子渗透性,从而拮抗酸性外环境[117]。细菌耐抗生素的机制可能是通过降低细菌膜孔蛋白通道的通透性,抑制抗生素的转运[102, 118]。目前已知lysoPE在细胞膜中的掺入可以导致膜局部曲度的改变,引起膜蛋白(包括孔蛋白)构象及功能的改变[53]。但lysoPE介导细菌耐酸及耐药的具体机制还有待进一步研究。
此外,压力条件下lysoPE在细胞内膜上的重分配会改变细胞膜双分子层的变形能,导致膜蛋白构象中自由能的改变[119],随后可反作用于蛋白,调控蛋白的分泌、扩散和嵌入[120]。因此,lysoPE在细菌膜上的精确分布为细菌必需膜蛋白的正确装配提供了结构基础[121-122]。
1.3.3 LysoPE的抗菌活性除了对细菌细胞膜结构的修饰作用之外,lysoPE还具有抗菌活性。家蝇(Musca domestica)分泌的lysoPE (16:1)被发现具有抗菌活力[123]。从拟杆菌门噬几丁质菌属(Chitinophaga spp.)细菌中分离纯化的lysoPE在浓度为4–16 μg/mL时具有抗革兰氏阴性卡他莫拉菌(Moraxella catarrhalis)的活性,在浓度为16–64 μg/mL时具有抗革兰氏阳性藤黄微球菌(Micrococcus luteus)的活性[124]。LysoPE的抗菌活性被认为和选择性抑制细菌钾离子转运系统有关[123]。细菌需要钾离子来完成许多细胞生理反应,包括维持细胞内酶的活性、pH稳态和膜电位调节等[125]。通过阻碍细菌对钾离子的摄取,lysoPE可对细菌造成致死性损伤[123]。但是该假设并不能完全解释为什么lysoPE不会影响大多数革兰氏阳性菌的生长,因为革兰氏阳性菌只有一个单操作性的钾离子转运通道,而革兰氏阴性菌具有多个此通道,因此选择性系统抑制被认为会对革兰氏阳性菌带来致命的影响[6, 126-127]。LysoPE的抗菌活性还有可能是因为外源lysoPE可以选择性掺入细菌细胞膜中,透化细胞膜。有报道表明,lysoPE水平在细胞膜中的升高会导致细菌细胞膜的瓦解并激活细菌的自溶机制,造成细胞壁成分和胞内物质(例如酶)的大量外流[104]。
1.3.4 LysoPE在细菌-宿主互作中的作用细菌分泌的LysoPE还能对宿主产生重要影响。尽管lysoPE在细菌中普遍存在,但长期以来被认为在调节宿主与微生物的相互作用中并不重要[128],目前这种观点正在被逐渐改变。海洋无脊椎水螅虫(Hydractinia echinata)幼虫的变态反应就依赖于细菌LPL。幼虫与LPL混合物(16:0/18:1 lysoPG、18:0 lysoPE和16:0 lysoPA)的物理接触被证实是幼虫向群体成虫阶段转化的必需条件[129]。人和动物体内共生菌群分泌的lysoPE被报道可以通过修复肠上皮层的完整性保护宿主肠道[4]。细菌释放的lysoPE还能通过修复H2O2引起的宿主紧密连接蛋白和黏附连接蛋白表达抑制来维持肠道的屏障功能[4, 130]。马岛噬冷菌(Algoriphagus machipongonensis)生成的lysoPE是促进原生动物领鞭毛虫(Salpingoeca rosetta)从单细胞结构发育成多细胞玫瑰花结结构的激活因子和协同增效因子。在此过程中lysoPE通过激活磺酰脂玫瑰花结诱导因子参与领鞭毛虫玫瑰花结发育的起始、稳定和成熟阶段的生物调控,帮助了其玫瑰花结完整结构的形成,并催化花结的成熟[128]。但另一方面,细菌分泌的lysoPE对宿主细胞有毒性作用。我们最新的研究表明,来源于空肠弯曲杆菌的lysoPE (尤其是短链lysoPE)能够通过激活宿主细胞的氧化应激反应透化宿主细胞的细胞膜[49]。而在嗜肺军团菌(Legionella pneumophila)感染过程中,宿主细胞线粒体膜中lysoPE和lysoPC的累积可通过激活线粒体细胞色素C的释放引起细胞凋亡[131]。因此lysoPE在细菌-宿主互作中具有双向调节作用。
但目前,lysoPE在宿主细胞上的特异性受体还未被识别。有报道表明溶血磷脂酸受体(lysophosphatidic acid receptor, LPAR)能被lysoPE直接识别或在真核细胞中通过自体毒素将lysoPE转化为lysoPA后被识别。在LPAR被lysoPE激活后会导致蛋白激酶K (protein kinase C, PKC)的活化,随后促进细胞紧密连接的形成和黏附连接蛋白的异位,增强上皮层的完整性[132-133]。
1.4 溶血磷脂酰甘油lysoPGLysoPG是细菌细胞膜中另一个次要LPL组分,能通过PldA2介导的磷脂酰甘油(phosphatidylglycerol, PG)水解反应或PagP催化的脂质A合成反应被生成[6, 37, 134]。和lysoPE类似,lysoPG能被革兰氏阴性菌的溶血磷脂转运系统(lysophospholipid transporter, LplT)从细菌细胞膜中转出,随后被内源酰基转移酶Aas酰化。在大肠杆菌中lysoPG的重塑效率是lysoPE的3倍[135]。由于LplT转运过程中对lysoPG和lysoPE的底物结合亲和力、转运速率相似[62],lysoPG的高重塑率可能是由于酰基转移酶Aas对lysoPG的亲和力更高。LysoPG在细菌细胞膜中仅极少量存在的原因也因此被认为是lysoPG极易被重酰化以补充膜磷脂所致[40]。但我们最近的研究结果表明,缺少LplT转运系统的空肠弯曲杆菌C. jejuni最高可储存约为总磷质含量27%的lysoPG在其细胞膜中[42, 109]。
目前对lysoPG的生物生理学功能的了解还相当匮乏。在大肠杆菌中,lysoPG可能在细胞外膜中扮演着荚膜多糖锚定物的角色[136]。在人和动物中,lysoPG的含量在出现急性冠脉综合征的组织中剧增,且被认为和心血管疾病的发病有关[20]。在恶性疟原虫(Plasmodium falciparum)中,其自身分泌的lysoPG有助于其在宿主红细胞内的生长发育[137]。但以上研究均未对lysoPG作用的具体通路或机制进行进一步的研究。
1.5 溶血磷脂酰丝氨酸lysoPS 1.5.1 LysoPS的特点LysoPS和其他LPL最大的不同是其头部结构是一个磷酸-l-丝氨酸分子。这个头部基团能与LPL甘油主链sn-3位置上的氢氧化物结合形成磷酸酯键,从而合成甘油磷酸-l-丝氨酸[6, 138]。分泌型PldA是lysoPS生物合成的关键酶(图 2)[1, 6]。在细胞内,lysoPS可被从C10的短链脂肪酸到C24的长链脂肪酸的多种脂质酰化而生成[139]。
1.5.2 LysoPS的功能在真核细胞中,lysoPS是一种重要的生物活性脂质,在包括巨噬细胞活化、肥大细胞脱颗粒、白细胞激活和调节性T细胞成熟等免疫反应过程中发挥重要作用[140]。在如曼氏血吸虫(Schistosoma mansoni)的寄生虫中,其体表膜中富含lysoPS和lysoPE,这两种LPL在调控寄生虫与宿主的互作中发挥重要作用[141]。和其他LPL类似,lysoPS存在于细菌细胞膜中,但仅微量存在于如脱硫弧菌属(Desulfovibrio sp.)的特定细菌中[61]。因此lysoPS在细菌中的生物学功能目前还尚不清晰。有报道表明,在悉生小鼠中定殖的大肠杆菌与在无菌小鼠中定殖的大肠杆菌相比具有更高浓度的lysoPS[142]。还有研究推测植物乳酸杆菌分泌的存在于胞外膜泡中的lysoPS可能是生物体内或体间信息传递的必需脂类介质[63]。但以上报道均未对lysoPS的具体作用机制进行进一步研究。最近有学者指出,肠道菌群分泌的lysoPS在克罗恩病(Crohn’s disease)中可诱发宿主体内辅助性T细胞(Th1)的免疫病理反应,包括在结肠中促进能产生IFN-γ的CD4+ T细胞的积累、增强Th1细胞的效应、调节Th1细胞的生物能量代谢和诱导Th1细胞表观遗传变异等[143]。因此,细菌lysoPS可能是细菌致病和肠道炎症发展过程中的一种重要生物活性脂类因子。
1.6 溶血磷脂酰肌醇lysoPI 1.6.1 LysoPI的特点lysoPI是内源溶血甘油磷脂的另一种亚类,其头部结构为肌醇分子。LysoPI是由PldA水解磷脂酰肌醇(phosphatidylinositol, PI)的一条脂肪酸链后被生成(图 2),其酰基链绝大多数以C16:0,C18:0或C24:0的形式存在[1, 6, 144]。通常来说,合成PI和lysoPI是真核细胞特有的代谢途径,但需氧放线菌(Actinomycetes)、粘球菌(Myxococcus)和部分δ-变形菌(Deltaproteobacteria)含有丰富的PI[61, 145]。
1.6.2 LysoPI的生物学功能在某些真核细胞系中,lysoPI具有影响细胞生长、分化和活力(精子获能)的能力[146-147]。但因为lysoPI在细菌中少有被检测到,因此对其生物学功能的了解仅停留在细菌-宿主互作上[61]。在一项最新的研究中,一种新型蜜蜂乳酸杆菌(Lactobacillus apis)被发现能够促进蓝蜂的lysoPI代谢,并且能够增强蜜蜂的记忆力,但其具体机制尚不清晰[148]。幽门螺旋杆菌也被报道能诱导动物组织上皮细胞中lysoPI的合成[149]。
在另一项研究中,lysoPI 18:0被发现在肺结核病人的血浆中含量升高[150],虽然这部分lysoPI被认为是与结核分枝杆菌(Mycobacterium tuberculosis)相关联,但目前的研究手段无法区分lysoPI是由细菌直接分泌还是由细菌刺激宿主分泌而产生。在人体内,对lysoPI的研究较为充分,lysoPI被发现是一种人内源性大麻素神经递质[151],能够诱导细胞增殖、迁移及肿瘤过程中内皮细胞的活化[152-153]。此外,人G蛋白偶联蛋白55 (G protein-coupled protein receptor 55, GPR55)被认为是lysoPI的受体[154]。总之,lysoPI在真核细胞中大量存在并在许多重要生物生理学过程中发挥重要作用,宿主体内的菌群很有可能通过参与lysoPI的分泌来调控宿主体内的部分生化反应。
2 总结细菌被膜(细胞壁、细胞膜)是保护细胞免受恶劣多变的外部环境影响的重要多层外部屏障结构。作为重要的生物活性信号分子,LPL能通过间接影响细菌细胞膜脂质层的完整性,改变细菌的细胞结构和功能。最近的相关研究指出LPL在细菌侵袭和环境压力适应过程中发挥着重要作用。但在这个快速发展的领域,细菌LPL对细胞带来的生理学影响还存在大量的未知。本综述阐释了LPL在细菌生理学以及在细菌-宿主互作过程中所发挥的功能。目前的研究表明,环境的改变和宿主的免疫反应是细菌长期面临的生存压力,细菌因此进化出了LPL调控机制来改变其被膜的物理结构以适应多种严苛的细胞外环境。分泌型LPL被认为不仅具有抗菌效力还具有细胞毒性。因此,一方面,细菌可以通过自身LPL的分泌在与其他细菌的竞争中取得生存优势和提高自身对宿主的感染效率;另一方面,细菌胞外的宿主源LPL能够作为碳源被细菌利用并储存,帮助病原体在感染宿主过程中存活[155-157]。综上所述,LPL是细菌中一种重要的生物分子,对LPL在细菌中发挥的多重生物学功能以及LPL如何影响细菌-宿主之间互作的更深入研究将有助于人类更进一步揭开细菌生存及感染的自然规律,同时也为传染病防控及公共卫生治理提供新的方向和思路。
| [1] | TAN ST, RAMESH T, TOH XR, NGUYEN LN. Emerging roles of lysophospholipids in health and disease[J]. Progress in Lipid Research, 2020, 80: 101068 DOI:10.1016/j.plipres.2020.101068. |
| [2] | LEE HJ, HONG WG, WOO Y, AHN JH, KO HJ, KIM H, MOON S, HAHN TW, JUNG YM, SONG DK, JUNG YJ. Lysophosphatidylcholine enhances bactericidal activity by promoting phagosome maturation via the activation of the NF-κB pathway during Salmonella infection in mouse macrophages[J]. Molecules and Cells, 2020, 43(12): 989-1001 DOI:10.14348/molcells.2020.0030. |
| [3] | YATOMI Y, KURANO M, IKEDA H, IGARASHI K, KANO K, AOKI J. Lysophospholipids in laboratory medicine[J]. Proceedings of the Japan Academy, Series B, 2018, 94(10): 373-389 DOI:10.2183/pjab.94.025. |
| [4] | ZOU DY, PEI JW, LAN JF, SANG H, CHEN HJ, YUAN HL, WU D, ZHANG YY, WANG YF, WANG DY, ZOU YJ, CHEN D, REN JN, GAO X, LIN ZY. A SNP of bacterial blc disturbs gut lysophospholipid homeostasis and induces inflammation through epithelial barrier disruption[J]. EBioMedicine, 2020, 52: 102652 DOI:10.1016/j.ebiom.2020.102652. |
| [5] | AROURI A, MOURITSEN OG. Membrane-perturbing effect of fatty acids and lysolipids[J]. Progress in Lipid Research, 2013, 52(1): 130-140 DOI:10.1016/j.plipres.2012.09.002. |
| [6] | CAO XF, van PUTTEN JPM, WÖSTEN MMSM. Biological functions of bacterial lysophospholipids[M]//Advances in Microbial Physiology. Amsterdam: Elsevier, 2023: 129-154. |
| [7] | D'ARRIGO P, SERVI S. Synthesis of lysophospholipids[J]. Molecules, 2010, 15(3): 1354-1377 DOI:10.3390/molecules15031354. |
| [8] | FILKIN SY, LIPKIN AV, FEDOROV AN. Phospholipase superfamily: structure, functions, and biotechnological applications[J]. Biochemistry (Moscow), 2020, 85(1): 177-195. |
| [9] | LUO Y, HUANG ZH, LIAO JL, LIU ZH, LI XP, YAO S, HE H, HU DJ, REN Z, ZENG HT, YAN QN, ZHAN H. Downregulated GTCPH I/BH4 pathway and decreased function of circulating endothelial progenitor cells and their relationship with endothelial dysfunction in overweight postmenopausal women[J]. Stem Cells International, 2018, 2018: 1-11. |
| [10] | DALEBROUX ZD, MATAMOUROS S, WHITTINGTON D, BISHOP RE, MILLER SI. PhoPQ regulates acidic glycerophospholipid content of the Salmonella Typhimurium outer membrane[J]. Proceedings of the National Academy of Sciences of the United States of America, 2014, 111(5): 1963-1968. |
| [11] | BISHOP RE, GIBBONS HS, GUINA T, TRENT MS, MILLER SI, RAETZ CR. Transfer of palmitate from phospholipids to lipid A in outer membranes of gram-negative bacteria[J]. The EMBO Journal, 2000, 19(19): 5071-5080 DOI:10.1093/emboj/19.19.5071. |
| [12] | HILLMANN F, ARGENTINI M, BUDDELMEIJER N. Kinetics and phospholipid specificity of apolipoprotein N-acyltransferase[J]. The Journal of Biological Chemistry, 2011, 286(32): 27936-27946 DOI:10.1074/jbc.M111.243519. |
| [13] | JACKOWSKI S, ROCK CO. Transfer of fatty acids from the 1-position of phosphatidylethanolamine to the major outer membrane lipoprotein of Escherichia coli[J]. Journal of Biological Chemistry, 1986, 261(24): 11328-11333 DOI:10.1016/S0021-9258(18)67387-9. |
| [14] | ISTIVAN TS, COLOE PJ. Phospholipase A in Gram-negative bacteria and its role in pathogenesis[J]. Microbiology, 2006, 152(5): 1263-1274 DOI:10.1099/mic.0.28609-0. |
| [15] | SCHMIEL DH, MILLER VL. Bacterial phospholipases and pathogenesis[J]. Microbes and Infection, 1999, 1(13): 1103-1112 DOI:10.1016/S1286-4579(99)00205-1. |
| [16] | GAIRE BP, CHOI JW. Critical roles of lysophospholipid receptors in activation of neuroglia and their neuroinflammatory responses[J]. International Journal of Molecular Sciences, 2021, 22(15): 7864 DOI:10.3390/ijms22157864. |
| [17] | GRÄLER M. Lysophospholipids and their G protein-coupled receptors in inflammation and immunity[J]. Biochimica et Biophysica Acta (BBA)-Molecular and Cell Biology of Lipids, 2002, 1582(1/2/3): 168-174. |
| [18] | PAKIET A, SIKORA K, KOBIELA J, ROSTKOWSKA O, MIKA A, SLEDZINSKI T. Alterations in complex lipids in tumor tissue of patients with colorectal cancer[J]. Lipids in Health and Disease, 2021, 20(1): 1-11 DOI:10.1186/s12944-020-01429-x. |
| [19] | DROBNIK W, LIEBISCH G, AUDEBERT FX, FRÖHLICH D, GLÜCK T, VOGEL P, ROTHE G, SCHMITZ G. Plasma ceramide and lysophosphatidylcholine inversely correlate with mortality in sepsis patients[J]. Journal of Lipid Research, 2003, 44(4): 754-761 DOI:10.1194/jlr.M200401-JLR200. |
| [20] | KURANO M, DOHI T, NOJIRI T, KOBAYASHI T, HIROWATARI Y, INOUE A, KANO K, MATSUMOTO H, IGARASHI K, NISHIKAWA M, MIYAUCHI K, DAIDA H, IKEDA H, AOKI J, YATOMI Y. Blood levels of serotonin are specifically correlated with plasma lysophosphatidylserine among the glycero-lysophospholipids[J]. BBA Clinical, 2015, 4: 92-98 DOI:10.1016/j.bbacli.2015.08.003. |
| [21] | TAYLOR LA, ARENDS J, HODINA AK, UNGER C, MASSING U. Plasma lyso-phosphatidylcholine concentration is decreased in cancer patients with weight loss and activated inflammatory status[J]. Lipids in Health and Disease, 2007, 6: 17 DOI:10.1186/1476-511X-6-17. |
| [22] | AOKI J. Structure and function of phosphatidylserine-specific phospholipase A1[J]. Biochimica et Biophysica Acta (BBA)-Molecular and Cell Biology of Lipids, 2002, 1582(1/2/3): 26-32. |
| [23] | KURANO M, KANO K, HARA M, TSUKAMOTO K, AOKI J, YATOMI Y. Regulation of plasma glycero-lysophospholipid levels by lipoprotein metabolism[J]. Biochemical Journal, 2019, 476(23): 3565-3581 DOI:10.1042/BCJ20190498. |
| [24] | MISRA UK. Isolation of lysophosphatidylethanolamine from human serum[J]. Biochimica et Biophysica Acta (BBA)-Lipids and Lipid Metabolism, 1965, 106(2): 371-378 DOI:10.1016/0005-2760(65)90045-7. |
| [25] | MORENO-NAVARRETE JM, CATALÁN V, WHYTE L, DÍAZ-ARTEAGA A, VÁZQUEZ-MARTÍNEZ R, ROTELLAR F, GUZMÁN R, GÓMEZ-AMBROSI J, PULIDO MR, RUSSELL WR, IMBERNÓN M, ROSS RA, MALAGÓN MM, DIEGUEZ C, FERNÁNDEZ- REAL JM, FRÜHBECK G, NOGUEIRAS R. The l-α-lysophosphatidylinositol/GPR55 system and its potential role in human obesity[J]. Diabetes, 2012, 61(2): 281-291 DOI:10.2337/db11-0649. |
| [26] | QUEHENBERGER O, ARMANDO AM, BROWN AH, MILNE SB, MYERS DS, MERRILL AH, BANDYOPADHYAY S, JONES KN, KELLY S, SHANER RL, SULLARDS CM, WANG E, MURPHY RC, BARKLEY RM, LEIKER TJ, RAETZ CRH, GUAN ZQ, LAIRD GM, SIX DA, RUSSELL DW, et al. Lipidomics reveals a remarkable diversity of lipids in human plasma[J]. Journal of Lipid Research, 2010, 51(11): 3299-3305 DOI:10.1194/jlr.M009449. |
| [27] | SALOUS AK, PANCHATCHARAM M, SUNKARA M, MUELLER P, DONG AP, WANG YH, GRAF GA, SMYTH SS, MORRIS AJ. Mechanism of rapid elimination of lysophosphatidic acid and related lipids from the circulation of mice[J]. Journal of Lipid Research, 2013, 54(10): 2775-2784 DOI:10.1194/jlr.M039685. |
| [28] | LIN Y, BOGDANOV M, LU S, GUAN Z, MARGOLIN W, WEISS J, ZHENG L. The phospholipid-repair system LplT/Aas in Gram-negative bacteria protects the bacterial membrane envelope from host phospholipase A2 attack[J]. Journal of Biological Chemistry, 2018, 293(9): 3386-3398 DOI:10.1074/jbc.RA117.001231. |
| [29] | RAMRAKHIANI L, CHAND S. Recent progress on phospholipases: different sources, assay methods, industrial potential and pathogenicity[J]. Applied Biochemistry and Biotechnology, 2011, 164(7): 991-1022 DOI:10.1007/s12010-011-9190-6. |
| [30] | SUTPHEN R, XU Y, WILBANKS GD, FIORICA J, GRENDYS EC Jr, LaPOLLA JP, ARANGO H, HOFFMAN MS, MARTINO M, WAKELEY K, GRIFFIN D, BLANCO RW, CANTOR AB, XIAO YJ, KRISCHER JP. Lysophospholipids are potential biomarkers of ovarian cancer[J]. Cancer Epidemiology, Biomarkers & Prevention: a Publication of the American Association for Cancer Research, Cosponsored by the American Society of Preventive Oncology, 2004, 13(7): 1185-1191. |
| [31] | HØYRUP P, DAVIDSEN J, JØRGENSEN K. Lipid membrane partitioning of lysolipids and fatty acids: effects of membrane phase structure and detergent chain length[J]. The Journal of Physical Chemistry B, 2001, 105(13): 2649-2657 DOI:10.1021/jp003631o. |
| [32] | MOURITSEN OG. Lipids, curvature, and nano-medicine[J]. European Journal of Lipid Science and Technology: EJLST, 2011, 113(10): 1174-1187 DOI:10.1002/ejlt.201100050. |
| [33] | WRIGHT GC, WEISS J, KIM KS, VERHEIJ H, ELSBACH P. Bacterial phospholipid hydrolysis enhances the destruction of Escherichia coli ingested by rabbit neutrophils. Role of cellular and extracellular phospholipases[J]. Journal of Clinical Investigation, 1990, 85(6): 1925-1935 DOI:10.1172/JCI114655. |
| [34] | DUAN L, GAN HX, ARM J, REMOLD HG. Cytosolic phospholipase A2 participates with TNF-α in the induction of apoptosis of human macrophages infected with Mycobacterium tuberculosis H37Ra[J]. The Journal of Immunology, 2001, 166(12): 7469-7476 DOI:10.4049/jimmunol.166.12.7469. |
| [35] | RAJARAM MVS, BROOKS MN, MORRIS JD, TORRELLES JB, AZAD AK, SCHLESINGER LS. Mycobacterium tuberculosis activates human macrophage peroxisome proliferator-activated receptor gamma linking mannose receptor recognition to regulation of immune responses[J]. Journal of Immunology (Baltimore, Md: 1950), 2010, 185(2): 929-942 DOI:10.4049/jimmunol.1000866. |
| [36] | SUBRAMANIAN N, QADRI A. Lysophospholipid sensing triggers secretion of flagellin from pathogenic salmonella[J]. Nature Immunology, 2006, 7(6): 583-589 DOI:10.1038/ni1336. |
| [37] | ZHENG L, LIN YB, LU S, ZHANG JZ, BOGDANOV M. Biogenesis, transport and remodeling of lysophospholipids in Gram-negative bacteria[J]. Biochimica et Biophysica Acta (BBA)-Molecular and Cell Biology of Lipids, 2017, 1862(11): 1404-1413. |
| [38] | DAVIDSEN J, MOURITSEN OG, JØRGENSEN K. Synergistic permeability enhancing effect of lysophospholipids and fatty acids on lipid membranes[J]. Biochimica et Biophysica Acta (BBA)- Biomembranes, 2002, 1564(1): 256-262 DOI:10.1016/S0005-2736(02)00461-3. |
| [39] | LIN SB, HAN YR, JENKIN K, LEE SJ, SASAKI M, KLAPPROTH JM, HE PJ, YUN CC. Lysophosphatidic acid receptor 1 is important for intestinal epithelial barrier function and susceptibility to colitis[J]. The American Journal of Pathology, 2018, 188(2): 353-366 DOI:10.1016/j.ajpath.2017.10.006. |
| [40] | FOREMAN-WYKERT AK, WEISS J, ELSBACH P. Phospholipid synthesis by Staphylococcus aureus during (Sub) lethal attack by mammalian 14-kilodalton group IIA phospholipase A2[J]. Infection and Immunity, 2000, 68(3): 1259-1264 DOI:10.1128/IAI.68.3.1259-1264.2000. |
| [41] | KONDO E, KANAI K. Mechanism of bactericidal activity of lysolecithin and its biological implication[J]. Japanese Journal of Medical Science and Biology, 1985, 38(4): 181-194 DOI:10.7883/yoken1952.38.181. |
| [42] | CAO XF, BROUWERS JFHM, van DIJK L, van de LEST CHA, PARKER CT, HUYNH S, van PUTTEN JPM, KELLY DJ, WÖSTEN MMSM. The unique phospholipidome of the enteric pathogen Campylobacter jejuni: lysophosholipids are required for motility at low oxygen availability[J]. Journal of Molecular Biology, 2020, 432(19): 5244-5258 DOI:10.1016/j.jmb.2020.07.012. |
| [43] | GILES DK, HANKINS JV, GUAN ZQ, STEPHEN TRENT M. Remodelling of the Vibrio cholerae membrane by incorporation of exogenous fatty acids from host and aquatic environments[J]. Molecular Microbiology, 2011, 79(3): 716-728 DOI:10.1111/j.1365-2958.2010.07476.x. |
| [44] | LÓPEZ-LARA IM, GEIGER O. Bacterial lipid diversity[J]. Biochimica et Biophysica Acta Molecular and Cell Biology of Lipids, 2017, 1862(11): 1287-1299 DOI:10.1016/j.bbalip.2016.10.007. |
| [45] | BUKHOLM G, TANNÆS T, NEDENSKOV P, ESBENSEN Y, GRAV HJ, HOVIG T, ARIANSEN S, GULDVOG I. Colony variation of Helicobacter pylori: pathogenic potential is correlated to cell wall lipid composition[J]. Scandinavian Journal of Gastroenterology, 1997, 32(5): 445-454 DOI:10.3109/00365529709025079. |
| [46] | DAVYDOVA L, BAKHOLDINA S, BARKINA M, VELANSKY P, BOGDANOV M, SANINA N. Effects of elevated growth temperature and heat shock on the lipid composition of the inner and outer membranes of Yersinia pseudotuberculosis[J]. Biochimie, 2016, 123: 103-109 DOI:10.1016/j.biochi.2016.02.004. |
| [47] | KERN R, JOSELEAU-PETIT D, CHATTOPADHYAY MK, RICHARME G. Chaperone-like properties of lysophospholipids[J]. Biochemical and Biophysical Research Communications, 2001, 289(5): 1268-1274 DOI:10.1006/bbrc.2001.6093. |
| [48] | TANNÆS T, DEKKER N, BUKHOLM G, BIJLSMA JJE, APPELMELK BJ. Phase variation in the Helicobacter pylori phospholipase A gene and its role in acid adaptation[J]. Infection and Immunity, 2001, 69(12): 7334-7340 DOI:10.1128/IAI.69.12.7334-7340.2001. |
| [49] | CAO XF, van de LEST CHA, HUANG LZX, van PUTTEN JPM, WÖSTEN MMSM. Campylobacter jejuni permeabilizes the host cell membrane by short chain lysophosphatidylethanolamines[J]. Gut Microbes, 2022, 14(1): 2091371 DOI:10.1080/19490976.2022.2091371. |
| [50] | COLLES SM, CHISOLM GM. Lysophosphatidylcholine- induced cellular injury in cultured fibroblasts involves oxidative events[J]. Journal of Lipid Research, 2000, 41(8): 1188-1198 DOI:10.1016/S0022-2275(20)33425-8. |
| [51] | BEN-ZEEV G, TELIAS M, NUSSINOVITCH I. Lysophospholipids modulate voltage-gated calcium channel currents in pituitary cells; effects of lipid stress[J]. Cell Calcium, 2010, 47(6): 514-524 DOI:10.1016/j.ceca.2010.04.006. |
| [52] | BAVI O, VOSSOUGHI M, NAGHDABADI R, JAMALI Y. The effect of local bending on gating of MscL using a representative volume element and finite element simulation[J]. Channels, 2014, 8(4): 344-349 DOI:10.4161/chan.29572. |
| [53] | VÁSQUEZ V, SOTOMAYOR M, CORDERO- MORALES J, SCHULTEN K, PEROZO E. A structural mechanism for MscS gating in lipid bilayers[J]. Science, 2008, 321(5893): 1210-1214 DOI:10.1126/science.1159674. |
| [54] | SNIJDER HJ, UBARRETXENA-BELANDIA I, BLAAUW M, KALK KH, VERHEIJ HM, EGMOND MR, DEKKER N, DIJKSTRA BW. Structural evidence for dimerization-regulated activation of an integral membrane phospholipase[J]. Nature, 1999, 401(6754): 717-721 DOI:10.1038/401717a0. |
| [55] | DEKKER N, TOMMASSEN J, LUSTIG A, ROSENBUSCH JP, VERHEIJ HM. Dimerization regulates the enzymatic activity of Escherichia coli outer membrane phospholipase A[J]. Journal of Biological Chemistry, 1997, 272(6): 3179-3184 DOI:10.1074/jbc.272.6.3179. |
| [56] | BALAZS L, OKOLICANY J, FERREBEE M, TOLLEY B, TIGYI G. Topical application of the phospholipid growth factor lysophosphatidic acid promotes wound healing in vivo[J]. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology, 2001, 280(2): R466-R472 DOI:10.1152/ajpregu.2001.280.2.R466. |
| [57] | GUEGUEN G, GAIGÉ B, GRÉVY JM, ROGALLE P, BELLAN J, WILSON M, KLAÉBÉ A, PONT F, SIMON MF, CHAP H. Structure-activity analysis of the effects of lysophosphatidic acid on platelet aggregation[J]. Biochemistry, 1999, 38(26): 8440-8450 DOI:10.1021/bi9816756. |
| [58] |
杨晋静, 陈海波, 王宪云, 范雪松, 丛祥凤, 陈曦. 溶血磷脂酸对心肌细胞缺氧/复氧损伤的保护作用及机制[J]. 中国循环杂志, 2013, 28(3): 222-225.
DOI:10.3969/j.issn.1000-3614.2013.03.018 YANG JJ, CHEN HB, WANG XY, FAN XS, CONG XF, CHEN X. The protective role with its mechanism of lysophosphatidic acid on hypoxia/re-oxygenation induced neonatal rat's H9c2 cardiomyocyte injury[J]. Chinese Circulation Journal, 2013, 28(3): 222-225 (in Chinese). |
| [59] | ZHANG YM, ROCK CO. Membrane lipid homeostasis in bacteria[J]. Nature Reviews Microbiology, 2008, 6(3): 222-233 DOI:10.1038/nrmicro1839. |
| [60] | MATHEW D, TORRES RM. Lysophosphatidic acid is an inflammatory lipid exploited by cancers for immune evasion via mechanisms similar and distinct from CTLA-4 and PD-1[J]. Frontiers in Immunology, 2021, 11: 531910 DOI:10.3389/fimmu.2020.531910. |
| [61] | SOHLENKAMP C, GEIGER O. Bacterial membrane lipids: diversity in structures and pathways[J]. FEMS Microbiology Reviews, 2016, 40(1): 133-159 DOI:10.1093/femsre/fuv008. |
| [62] | LIN Y, BOGDANOV M, TONG S, GUAN Z, ZHENG L. Substrate selectivity of lysophospholipid transporter LplT involved in membrane phospholipid remodeling in Escherichia coli[J]. Journal of Biological Chemistry, 2016, 291(5): 2136-2149 DOI:10.1074/jbc.M115.700419. |
| [63] | KIM H, KIM M, MYOUNG K, KIM W, KO J, KIM KP, CHO EG. Comparative lipidomic analysis of extracellular vesicles derived from Lactobacillus plantarum APsulloc 331261 living in green tea leaves using liquid chromatography-mass spectrometry[J]. International Journal of Molecular Sciences, 2020, 21(21): 8076 DOI:10.3390/ijms21218076. |
| [64] | LIN SB, YERUVA S, HE PJ, SINGH AK, ZHANG HC, CHEN MM, LAMPRECHT G, de JONGE HR, TSE M, DONOWITZ M, HOGEMA BM, CHUN J, SEIDLER U, YUN CC. Lysophosphatidic acid stimulates the intestinal brush border Na(+)/H(+) exchanger 3 and fluid absorption via LPA(5) and NHERF2[J]. Gastroenterology, 2010, 138(2): 649-658 DOI:10.1053/j.gastro.2009.09.055. |
| [65] | URANBILEG B, ITO N, KURANO M, SAIGUSA D, SAITO R, URUNO A, KANO K, IKEDA H, YAMADA Y, SUMITANI M, SEKIGUCHI M, AOKI J, YATOMI Y. Alteration of the lysophosphatidic acid and its precursor lysophosphatidylcholine levels in spinal cord stenosis: a study using a rat cauda equina compression model[J]. Scientific Reports, 2019, 9: 16578 DOI:10.1038/s41598-019-52999-5. |
| [66] | HERR DR, ONG JHJ, ONG WY. Potential therapeutic applications for inhibitors of autotaxin, a bioactive lipid-producing lysophospholipase D, in disorders affecting the nervous system[J]. ACS Chemical Neuroscience, 2018, 9(3): 398-400 DOI:10.1021/acschemneuro.8b00057. |
| [67] | KAO CY, KUO PY, LIAO HW. Untargeted microbial exometabolomics and metabolomics analysis of Helicobacter pylori J99 and jhp0106 mutant[J]. Metabolites, 2021, 11(12): 808 DOI:10.3390/metabo11120808. |
| [68] | YANG J, WEI H, ZHOU YF, SZETO CH, LI CG, LIN YF, COKER OO, LAU HCH, CHAN AWH, SUNG JJY, YU J. High-fat diet promotes colorectal tumorigenesis through modulating gut microbiota and metabolites[J]. Gastroenterology, 2022, 162(1): 135-149.e2 DOI:10.1053/j.gastro.2021.08.041. |
| [69] | GENG T, SU S, SUN K, ZHAO L, ZHAO Y, BAO N, PAN L, SUN H. Effects of feeding a Lactobacillus plantarum JL01 diet on caecal bacteria and metabolites of weaned piglets[J]. Letters in Applied Microbiology, 2021, 72(1): 24-35 DOI:10.1111/lam.13399. |
| [70] | HOQUE KM, CHAKRABORTY S, ALI SHEIKH I, WOODWARD OM. New advances in the pathophysiology of intestinal ion transport and barrier function in diarrhea and the impact on therapy[J]. Expert Review of Anti-Infective Therapy, 2012, 10(6): 687-699 DOI:10.1586/eri.12.47. |
| [71] | LIN ME, HERR DR, CHUN J. Lysophosphatidic acid (LPA) receptors: signaling properties and disease relevance[J]. Prostaglandins & Other Lipid Mediators, 2010, 91(3/4): 130-138. |
| [72] | DAS S, JAYARATNE R, BARRETT KE. The role of ion transporters in the pathophysiology of infectious diarrhea[J]. Cellular and Molecular Gastroenterology and Hepatology, 2018, 6(1): 33-45 DOI:10.1016/j.jcmgh.2018.02.009. |
| [73] | LI CY, DANDRIDGE KS, DI AK, MARRS KL, HARRIS EL, ROY K, JACKSON JS, MAKAROVA NV, FUJIWARA Y, FARRAR PL, NELSON DJ, TIGYI GJ, NAREN AP. Lysophosphatidic acid inhibits cholera toxin-induced secretory diarrhea through CFTR-dependent protein interactions[J]. The Journal of Experimental Medicine, 2005, 202(7): 975-986 DOI:10.1084/jem.20050421. |
| [74] | SINGLA A, DWIVEDI A, SAKSENA S, GILL RK, ALREFAI WA, RAMASWAMY K, DUDEJA PK. Mechanisms of lysophosphatidic acid (LPA) mediated stimulation of intestinal apical Cl–/OH– exchange[J]. American Journal of Physiology Gastrointestinal and Liver Physiology, 2010, 298(2): G182-G189 DOI:10.1152/ajpgi.00345.2009. |
| [75] |
陈荷丹, 林晓庆, 薛纪极, 朱坚胜. 溶血磷脂酸受体1与器官纤维化关系的研究进展[J]. 浙江医学, 2022, 44(12): 1337-1341.
DOI:10.12056/j.issn.1006-2785.2022.44.12.2021-2640 CHEN HD, LIN XQ, XUE JJ, ZHU JS. Research progress on the relationship between lysophosphatidic acid receptor 1 and organ fibrosis[J]. Zhejiang Medical Journal, 2022, 44(12): 1337-1341 (in Chinese). |
| [76] | EMO J, MEEDNU N, CHAPMAN TJ, REZAEE F, BALYS M, RANDALL T, RANGASAMY T, GEORAS SN. Lpa2 is a negative regulator of both dendritic cell activation and murine models of allergic lung inflammation[J]. Journal of Immunology (Baltimore, Md: 1950), 2012, 188(8): 3784-3790 DOI:10.4049/jimmunol.1102956. |
| [77] | OCHI S, ODA M, NAGAHAMA M, SAKURAI J. Clostridium perfringens alpha-toxin-induced hemolysis of horse erythrocytes is dependent on Ca2+ uptake[J]. Biochimica et Biophysica Acta (BBA)-Biomembranes, 2003, 1613(1/2): 79-86. |
| [78] | SAKURAI J, OCHI S, TANAKA H. Evidence for coupling of Clostridium perfringens alpha-toxin-induced hemolysis to stimulated phosphatidic acid formation in rabbit erythrocytes[J]. Infection and Immunity, 1993, 61(9): 3711-3718 DOI:10.1128/iai.61.9.3711-3718.1993. |
| [79] | WANG J, HERTZ L, RUPPENTHAL S, EL NEMER W, CONNES P, GOEDE JS, BOGDANOVA A, BIRNBAUMER L, KAESTNER L. Lysophosphatidic acid-activated calcium signaling is elevated in red cells from sickle cell disease patients[J]. Cells, 2021, 10(2): 456 DOI:10.3390/cells10020456. |
| [80] |
韩黎, 吴旭琴, 孟玉芬, 纪蕾, 陈世平. 李斯特菌诱导宿主细胞骨架重排与磷脂酶D[J]. 微生物学报, 2006, 46(5): 852-855.
DOI:10.3321/j.issn:0001-6209.2006.05.036 HAN L, WU XQ, MENG YF, JI L, CHEN SP. Listeria-induced host cellular actin cytoskeleton rearrangement and phospholipase D[J]. Acta Microbiologica Sinica, 2006, 46(5): 852-855 (in Chinese). |
| [81] | BOLDYREVA LV, MOROZOVA MV, SAYDAKOVA SS, KOZHEVNIKOVA EN. Fat of the gut: epithelial phospholipids in inflammatory bowel diseases[J]. International Journal of Molecular Sciences, 2021, 22(21): 11682 DOI:10.3390/ijms222111682. |
| [82] | MAKIDE K, KITAMURA H, SATO Y, OKUTANI M, AOKI J. Emerging lysophospholipid mediators, lysophosphatidylserine, lysophosphatidylthreonine, lysophosphatidylethanolamine and lysophosphatidylglycerol[J]. Prostaglandins & Other Lipid Mediators, 2009, 89(3/4): 135-139. |
| [83] | HUI DY. Intestinal phospholipid and lysophospholipid metabolism in cardiometabolic disease[J]. Current Opinion in Lipidology, 2016, 27(5): 507-512 DOI:10.1097/MOL.0000000000000334. |
| [84] | KNUPLEZ E, MARSCHE G. An updated review of pro- and anti-inflammatory properties of plasma lysophosphatidylcholines in the vascular system[J]. International Journal of Molecular Sciences, 2020, 21(12): 4501 DOI:10.3390/ijms21124501. |
| [85] | ROKITSKAYA TI, KOTOVA EA, NABEREZHNYKH GA, KHOMENKO VA, GORBACH VI, FIRSOV AM, ZELEPUGA EA, ANTONENKO YN, NOVIKOVA OD. Single channel activity of OmpF-like porin from Yersinia pseudotuberculosis[J]. Biochimica et Biophysica Acta (BBA)-Biomembranes, 2016, 1858(4): 883-891 DOI:10.1016/j.bbamem.2016.02.005. |
| [86] | PEROZO E, CORTES DM, SOMPORNPISUT P, KLODA A, MARTINAC B. Open channel structure of MscL and the gating mechanism of mechanosensitive channels[J]. Nature, 2002, 418(6901): 942-948 DOI:10.1038/nature00992. |
| [87] | VAARA M, PORRO M. Group of peptides that act synergistically with hydrophobic antibiotics against gram-negative enteric bacteria[J]. Antimicrobial Agents and Chemotherapy, 1996, 40(8): 1801-1805 DOI:10.1128/AAC.40.8.1801. |
| [88] | MIYAZAKI H, MIDORIKAWA N, FUJIMOTO S, MIYOSHI N, YOSHIDA H, MATSUMOTO T. Antimicrobial effects of lysophosphatidylcholine on methicillin-resistant Staphylococcus aureus[J]. Therapeutic Advances in Infectious Disease, 2017, 4(4): 89-94 DOI:10.1177/2049936117714920. |
| [89] | STEEL HC, COCKERAN R, ANDERSON R. Platelet-activating factor and lyso-PAF possess direct antimicrobial properties in vitro[J]. APMIS, 2002, 110(2): 158-164 DOI:10.1034/j.1600-0463.2002.100206.x. |
| [90] | YADAV J, ISMAEEL S, QADRI A. Lysophosphatidylcholine potentiates antibacterial activity of polymyxin B[J]. Antimicrobial Agents and Chemotherapy, 2020, 64(12): e01337-e01320. |
| [91] | YAN JJ, JUNG JS, LEE JE, LEE J, HUH SO, KIM HS, JUNG KC, CHO JY, NAM JS, SUH HW, KIM YH, SONG DK. Therapeutic effects of lysophosphatidylcholine in experimental sepsis[J]. Nature Medicine, 2004, 10(2): 161-167 DOI:10.1038/nm989. |
| [92] | SHIVCHARAN S, YADAV J, QADRI A. Host lipid sensing promotes invasion of cells with pathogenic Salmonella[J]. Scientific Reports, 2018, 8: 15501 DOI:10.1038/s41598-018-33319-9. |
| [93] | LOU LX, ZHANG P, PIAO RL, WANG Y. Salmonella pathogenicity island 1 (SPI-1) and its complex regulatory network[J]. Frontiers in Cellular and Infection Microbiology, 2019, 9: 270 DOI:10.3389/fcimb.2019.00270. |
| [94] | JURKOWITZ MS, AZAD AK, MONSMA PC, KEISER TL, KANYO J, LAM TT, BELL CE, SCHLESINGER LS. Mycobacterium tuberculosis encodes a YhhN family membrane protein with lysoplasmalogenase activity that protects against toxic host lysolipids[J]. The Journal of Biological Chemistry, 2022, 298(5): 101849 DOI:10.1016/j.jbc.2022.101849. |
| [95] | TANG XL, WANG WJ, HONG GC, DUAN CH, ZHU SR, TIAN YE, HAN CQ, QIAN W, LIN R, HOU XH. Gut microbiota-mediated lysophosphatidylcholine generation promotes colitis in intestinal epithelium-specific Fut2 deficiency[J]. Journal of Biomedical Science, 2021, 28(1): 20 DOI:10.1186/s12929-021-00711-z. |
| [96] | AILTE I, LINGELEM ABD, KAVALIAUSKIENE S, BERGAN J, KVALVAAG AS, MYRANN AG, SKOTLAND T, SANDVIG K. Addition of lysophospholipids with large head groups to cells inhibits Shiga toxin binding[J]. Scientific Reports, 2016, 6: 30336 DOI:10.1038/srep30336. |
| [97] | OLIVER JD, COLWELL RR. Extractable lipids of gram-negative marine bacteria: phospholipid composition[J]. Journal of Bacteriology, 1973, 114(3): 897-908 DOI:10.1128/jb.114.3.897-908.1973. |
| [98] | CESARI AB, PAULUCCI NS, BIASUTTI MA, MORALES GM, DARDANELLI MS. Changes in the lipid composition of Bradyrhizobium cell envelope reveal a rapid response to water deficit involving lysophosphatidylethanolamine synthesis from phosphatidylethanolamine in outer membrane[J]. Research in Microbiology, 2018, 169(6): 303-312 DOI:10.1016/j.resmic.2018.05.008. |
| [99] | QIU N, MISRA R. Overcoming iron deficiency of an Escherichia coli tonB mutant by increasing outer membrane permeability[J]. Journal of Bacteriology, 2019, 201(17): e00340. |
| [100] | FULLER N, RAND RP. The influence of lysolipids on the spontaneous curvature and bending elasticity of phospholipid membranes[J]. Biophysical Journal, 2001, 81(1): 243-254 DOI:10.1016/S0006-3495(01)75695-0. |
| [101] | NINA S, LUDMILA D, SVETLANA B, OLGA N, OLGA P, TAMARA S, VALERY S, MIKHAIL B. Effect of phenol-induced changes in lipid composition on conformation of OmpF-like porin of Yersinia pseudotuberculosis[J]. FEBS Letters, 2013, 587(14): 2260-2265 DOI:10.1016/j.febslet.2013.05.056. |
| [102] | SANINA N, POMAZENKOVA L, BAKHOLDINA S, CHOPENKO N, ZABOLOTNAYA A, REUTOV V, STENKOVA A, BYSTRITSKAYA E, BOGDANOV M. Relationship between adaptive changing of lysophosphatidylethanolamine content in the bacterial envelope and ampicillin sensitivity of Yersinia pseudotuberculosis[J]. Microbial Physiology, 2019, 28(5): 236-239. |
| [103] | BAKHOLDINA SI, SANINA NM, SHUBIN FN, POPOVA OB, SOLOV'EVA TF. Thermotropic behavior of lipids and the morphology of Yersinia pseudotuberculosis cells with a high content of lysophosphatidylethanolamine[J]. Microbiology, 2007, 76(3): 280-286 DOI:10.1134/S0026261707030046. |
| [104] | TANNAES T, BUKHOLM IK, BUKHOLM G. High relative content of lysophospholipids of Helicobacter pylori mediates increased risk for ulcer disease[J]. FEMS Immunology and Medical Microbiology, 2005, 44(1): 17-23 DOI:10.1016/j.femsim.2004.10.003. |
| [105] | CRONAN JE JR, WULFF DL. A role for phospholipid hydrolysis in the lysis of Escherichia coli infected with bacteriophage T4[J]. Virology, 1969, 38(2): 241-246 DOI:10.1016/0042-6822(69)90365-1. |
| [106] | PUGSLEY AP, SCHWARTZ M. Colicin E2 release: lysis, leakage or secretion? Possible role of a phospholipase[J]. The EMBO Journal, 1984, 3(10): 2393-2397 DOI:10.1002/j.1460-2075.1984.tb02145.x. |
| [107] | HARDAWAY KL, BULLER CS. Effect of ethylenediaminetetraacetate on phospholipids and outer membrane function in Escherichia coli[J]. Journal of Bacteriology, 1979, 137(1): 62-68 DOI:10.1128/jb.137.1.62-68.1979. |
| [108] | MICHEL GPF, STARKA J. Origin and fate of the lysophosphatidylethanolamine in a chain-forming mutant (envC) of Escherichia coli[J]. Microbiology, 1984, 130(6): 1391-1398 DOI:10.1099/00221287-130-6-1391. |
| [109] | CAO XF, BROUWERS JFHM, van DIJK L, van de LEST CHA, PARKER CT, HUYNH S, van PUTTEN JPH, KELLY DJ, WÖSTEN MMSM. Dataset of the phospholipidome and transcriptome of Campylobacter jejuni under different growth conditions[J]. Data in Brief, 2020, 33: 106349 DOI:10.1016/j.dib.2020.106349. |
| [110] | VANHOVE AS, HANG SY, VIJAYAKUMAR V, WONG AC, ASARA JM, WATNICK PI. Vibrio cholerae ensures function of host proteins required for virulence through consumption of luminal methionine sulfoxide[J]. PLoS Pathogens, 2017, 13(6): e1006428 DOI:10.1371/journal.ppat.1006428. |
| [111] | LIN AS, SHUMAN JHB, KOTNALA A, SHAW JA, BECKETT AC, HARVEY JL, TUCK M, DIXON BREA, REYZER ML, ALGOOD HMS, SCHEY KL, PIAZUELO MB, COVER TL. Loss of corpus-specific lipids in Helicobacter pylori-induced atrophic gastritis[J]. mSphere, 2021, 6(6): e00826. |
| [112] | LI RS. Lysophospholipids and their G protein-coupled receptors in atherosclerosis[J]. Frontiers in Bioscience, 2016, 21(1): 70-88 DOI:10.2741/4377. |
| [113] | LIU YY, ZHU Y, WICKREMASINGHE H, BERGEN PJ, LU J, ZHU XQ, ZHOU QL, AZAD M, NANG SC, HAN ML, LEI T, LI J, LIU JH. Metabolic perturbations caused by the over-expression of mcr-1 in Escherichia coli[J]. Frontiers in Microbiology, 2020, 11: 588658 DOI:10.3389/fmicb.2020.588658. |
| [114] | KANJEE U, HOURY WA. Mechanisms of acid resistance in Escherichia coli[J]. Annual Review of Microbiology, 2013, 67: 65-81 DOI:10.1146/annurev-micro-092412-155708. |
| [115] | FOSTER JW. Escherichia coli acid resistance: tales of an amateur acidophile[J]. Nature Reviews Microbiology, 2004, 2(11): 898-907 DOI:10.1038/nrmicro1021. |
| [116] | POROCA DR, PELIS RM, CHAPPE VM. ClC channels and transporters: structure, physiological functions, and implications in human chloride channelopathies[J]. Frontiers in Pharmacology, 2017, 8: 151. |
| [117] | LUND P, TRAMONTI A, de BIASE D. Coping with low pH: molecular strategies in neutralophilic bacteria[J]. FEMS Microbiology Reviews, 2014, 38(6): 1091-1125 DOI:10.1111/1574-6976.12076. |
| [118] | GHAI I, GHAI S. Exploring bacterial outer membrane barrier to combat bad bugs[J]. Infection and Drug Resistance, 2017, 10: 261-273 DOI:10.2147/IDR.S144299. |
| [119] | ANDERSEN OS, KOEPPE RE II. Bilayer thickness and membrane protein function: an energetic perspective[J]. Annual Review of Biophysics and Biomolecular Structure, 2007, 36: 107-130 DOI:10.1146/annurev.biophys.36.040306.132643. |
| [120] | LUNDBÆK JA, BIRN P, GIRSHMAN J, HANSEN AJ, ANDERSEN OS. Membrane stiffness and channel function[J]. Biochemistry, 1996, 35(12): 3825-3830 DOI:10.1021/bi952250b. |
| [121] | LI BY, YIN F, ZHAO XY, GUO YX, WANG WQ, WANG PX, ZHU HH, YIN YS, WANG XX. Colistin resistance gene mcr-1 mediates cell permeability and resistance to hydrophobic antibiotics[J]. Frontiers in Microbiology, 2020, 10: 3015 DOI:10.3389/fmicb.2019.03015. |
| [122] | YANG QE, LI M, SPILLER OB, ANDREY DO, HINCHLIFFE P, LI H, MACLEAN C, NIUMSUP P, POWELL L, PRITCHARD M, PAPKOU A, SHEN YB, PORTAL E, SANDS K, SPENCER J, TANSAWAI U, THOMAS D, WANG SL, WANG Y, SHEN JZ, et al. Balancing mcr-1 expression and bacterial survival is a delicate equilibrium between essential cellular defence mechanisms[J]. Nature Communications, 2017, 8: 2054 DOI:10.1038/s41467-017-02149-0. |
| [123] | MEYLAERS K, CLYNEN E, DALOZE D, DELOOF A, SCHOOFS L. Identification of 1-lysophosphatidylethanolamine (C16: 1) as an antimicrobial compound in the housefly, Musca domestica[J]. Insect Biochemistry and Molecular Biology, 2004, 34(1): 43-49 DOI:10.1016/j.ibmb.2003.09.001. |
| [124] | BILL MK, BRINKMANN S, OBERPAUL M, PATRAS MA, LEIS B, MARNER M, MAITRE MP, HAMMANN PE, VILCINSKAS A, SCHULER SMM, SCHÄBERLE TF. Novel glycerophospholipid, lipo- and N-acyl amino acids from bacteroidetes: isolation, structure elucidation and bioactivity[J]. Molecules, 2021, 26(17): 5195 DOI:10.3390/molecules26175195. |
| [125] | GRÜNDLING A. Potassium uptake systems in Staphylococcus aureus: new stories about ancient systems[J]. mBio, 2013, 4(5): e00784. |
| [126] | KAWANO M, ABUKI R, IGARASHI K, KAKINUMA Y. Potassium uptake with low affinity and high rate in Enterococcus hirae at alkaline pH[J]. Archives of Microbiology, 2001, 175(1): 41-45 DOI:10.1007/s002030000234. |
| [127] | KAKINUMA Y, YASUMURA K, IGARASHI K. Potassium/proton antiport system is dispensable for growth of Enterococcus hirae at low pH[J]. Bioscience, Biotechnology, and Biochemistry, 1999, 63(5): 875-878 DOI:10.1271/bbb.63.875. |
| [128] | WOZNICA A, CANTLEY AM, BEEMELMANNS C, FREINKMAN E, CLARDY J, KING N. Bacterial lipids activate, synergize, and inhibit a developmental switch in choanoflagellates[J]. Proceedings of the National Academy of Sciences of the United States of America, 2016, 113(28): 7894-7899. |
| [129] | GUO HJ, RISCHER M, WESTERMANN M, BEEMELMANNS C. Two distinct bacterial biofilm components trigger metamorphosis in the colonial hydrozoan Hydractinia echinata[J]. mBio, 2021, 12(3): e00401. |
| [130] | SUZUKI T. Regulation of intestinal epithelial permeability by tight junctions[J]. Cellular and Molecular Life Sciences, 2013, 70(4): 631-659 DOI:10.1007/s00018-012-1070-x. |
| [131] | ZHU WH, HAMMAD LA, HSU F, MAO YX, LUO ZQ. Induction of caspase 3 activation by multiple Legionella pneumophila Dot/icm substrates[J]. Cellular Microbiology, 2013, 15(11): 1783-1795. |
| [132] | BALDA MS, GONZALEZ-MARISCAL L, MATTER K, CEREIJIDO M, ANDERSON JM. Assembly of the tight junction: the role of diacylglycerol[J]. Journal of Cell Biology, 1993, 123(2): 293-302 DOI:10.1083/jcb.123.2.293. |
| [133] | RHIM JH, JANG IS, YEO EJ, SONG KY, PARK SC. Role of protein kinase C-dependent A-kinase anchoring proteins in lysophosphatidic acid-induced cAMP signaling in human diploid fibroblasts[J]. Aging Cell, 2006, 5(6): 451-461 DOI:10.1111/j.1474-9726.2006.00239.x. |
| [134] | HITE RD, SEEDS MC, SAFTA AM, JACINTO RB, GYVES JI, BASS DA, WAITE BM. Lysophospholipid generation and phosphatidylglycerol depletion in phospholipase A2-mediated surfactant dysfunction[J]. American Journal of Physiology-Lung Cellular and Molecular Physiology, 2005, 288(4): L618-L624 DOI:10.1152/ajplung.00274.2004. |
| [135] | HARVAT EM, ZHANG YM, TRAN CV, ZHANG ZG, FRANK MW, ROCK CO, SAIER MH JR. Lysophospholipid flipping across the Escherichia coli inner membrane catalyzed by a transporter (LplT) belonging to the major facilitator superfamily[J]. Journal of Biological Chemistry, 2005, 280(12): 12028-12034 DOI:10.1074/jbc.M414368200. |
| [136] | PHANPHAK S, GEORGIADES P, LI RH, KING J, ROBERTS IS, WAIGH TA. Super-resolution fluorescence microscopy study of the production of K1 capsules by Escherichia coli: evidence for the differential distribution of the capsule at the poles and the equator of the cell[J]. Langmuir, 2019, 35(16): 5635-5646 DOI:10.1021/acs.langmuir.8b04122. |
| [137] | TEWARI SG, SWIFT RP, REIFMAN J, PRIGGE ST, WALLQVIST A. Metabolic alterations in the erythrocyte during blood-stage development of the malaria parasite[J]. Malaria Journal, 2020, 19(1): 1-18 DOI:10.1186/s12936-019-3075-5. |
| [138] | FAHY E, SUD M, COTTER D, SUBRAMANIAM S. LIPID MAPS online tools for lipid research[J]. Nucleic Acids Research, 2007, 35(suppl_2): W606-W612. |
| [139] | BARNES MJ, LI CM, XU Y, AN JP, HUANG Y, CYSTER JG. The lysophosphatidylserine receptor GPR174 constrains regulatory T cell development and function[J]. The Journal of Experimental Medicine, 2015, 212(7): 1011-1020 DOI:10.1084/jem.20141827. |
| [140] | SHANBHAG K, MHETRE A, KHANDELWAL N, KAMAT SS. The lysophosphatidylserines-an emerging class of signalling lysophospholipids[J]. The Journal of Membrane Biology, 2020, 253(5): 381-397 DOI:10.1007/s00232-020-00133-2. |
| [141] | RETRA K, de WALICK S, SCHMITZ M, YAZDANBAKHSH M, TIELENS AGM, BROUWERS JFHM, van HELLEMOND JJ. The tegumental surface membranes of Schistosoma mansoni are enriched in parasite-specific phospholipid species[J]. International Journal for Parasitology, 2015, 45(9/10): 629-636. |
| [142] | CHAKRABARTI A, MEMBREZ M, MORIN- RIVRON D, SIDDHARTH J, CHOU CJ, HENRY H, BRUCE S, METAIRON S, RAYMOND F, BETRISEY B, LOYER C, PARKINSON SJ, MASOODI M. Transcriptomics-driven lipidomics (TDL) identifies the microbiome-regulated targets of ileal lipid metabolism[J]. Npj Systems Biology and Applications, 2017, 3: 33 DOI:10.1038/s41540-017-0033-0. |
| [143] | OTAKE-KASAMOTO Y, KAYAMA H, KISHIKAWA T, SHINZAKI S, TASHIRO T, AMANO T, TANI M, YOSHIHARA T, LI B, TANI H, LIU L, HAYASHI A, OKUZAKI D, MOTOOKA D, NAKAMURA S, OKADA Y, IIJIMA H, TAKEDA K, TAKEHARA T. Lysophosphatidylserines derived from microbiota in Crohn's disease elicit pathological Th1 response[J]. The Journal of Experimental Medicine, 2022, 219(7): e20211291 DOI:10.1084/jem.20211291. |
| [144] | BONDARENKO A, WALDECK-WEIERMAIR M, NAGHDI S, POTESER M, MALLI R, GRAIER WF. GPR55-dependent and -independent ion signalling in response to lysophosphatidylinositol in endothelial cells[J]. British Journal of Pharmacology, 2010, 161(2): 308-320 DOI:10.1111/j.1476-5381.2010.00744.x. |
| [145] | SANDOVAL-CALDERÓN M, GUAN ZQ, SOHLENKAMP C. Knowns and unknowns of membrane lipid synthesis in streptomycetes[J]. Biochimie, 2017, 141: 21-29 DOI:10.1016/j.biochi.2017.05.008. |
| [146] | PIÑEIRO R, FALASCA M. Lysophosphatidylinositol signalling: new wine from an old bottle[J]. Biochimica et Biophysica Acta (BBA)-Molecular and Cell Biology of Lipids, 2012, 1821(4): 694-705. |
| [147] | WHEELER MB, SEIDEL GE JR. Capacitation of bovine spermatozoa by lysophospholipids and trypsin[J]. Gamete Research, 1989, 22(2): 193-204 DOI:10.1002/mrd.1120220207. |
| [148] | LI L, SOLVI C, ZHANG F, QI ZY, CHITTKA L, ZHAO W. Gut microbiome drives individual memory variation in bumblebees[J]. Nature Communications, 2021, 12: 6588 DOI:10.1038/s41467-021-26833-4. |
| [149] | POMORSKI T, MEYER TF, NAUMANN M. Helicobacter pylori-induced prostaglandin E2 synthesis involves activation of cytosolic phospholipase A2 in epithelial cells[J]. Journal of Biological Chemistry, 2001, 276(1): 804-810 DOI:10.1074/jbc.M003819200. |
| [150] | COLLINS JM, WALKER DI, JONES DP, TUKVADZE N, LIU KH, TRAN VT, UPPAL K, FREDIANI JK, EASLEY KA, SHENVI N, KHADKA M, ORTLUND EA, KEMPKER RR, BLUMBERG HM, ZIEGLER TR. High-resolution plasma metabolomics analysis to detect Mycobacterium tuberculosis- associated metabolites that distinguish active pulmonary tuberculosis in humans[J]. PLoS One, 2018, 13(10): e0205398 DOI:10.1371/journal.pone.0205398. |
| [151] | LI X, WANG L, FANG P, SUN Y, JIANG X, WANG H, YANG XF. Lysophospholipids induce innate immune transdifferentiation of endothelial cells, resulting in prolonged endothelial activation[J]. Journal of Biological Chemistry, 2018, 293(28): 11033-11045 DOI:10.1074/jbc.RA118.002752. |
| [152] | ALHOUAYEK M, MASQUELIER J, MUCCIOLI GG. Lysophosphatidylinositols, from cell membrane constituents to GPR55 ligands[J]. Trends in Pharmacological Sciences, 2018, 39(6): 586-604 DOI:10.1016/j.tips.2018.02.011. |
| [153] | XU KM, SHAO Y, SAAOUD F, GILLESPIE A, DRUMMER C IV, LIU L, LU YF, SUN Y, XI H, TÜKEL Ç, PRATICO D, QIN XB, SUN JX, CHOI ET, JIANG XH, WANG H, YANG XF. Novel knowledge-based transcriptomic profiling of lipid lysophosphatidylinositol-induced endothelial cell activation[J]. Frontiers in Cardiovascular Medicine, 2021, 8: 773473 DOI:10.3389/fcvm.2021.773473. |
| [154] | OKA S, NAKAJIMA K, YAMASHITA A, KISHIMOTO S, SUGIURA T. Identification of GPR55 as a lysophosphatidylinositol receptor[J]. Biochemical and Biophysical Research Communications, 2007, 362(4): 928-934 DOI:10.1016/j.bbrc.2007.08.078. |
| [155] | JANG KB, PURVIS JM, KIM SW. Supplemental effects of dietary lysophospholipids in lactation diets on sow performance, milk composition, gut health, and gut-associated microbiome of offspring[J]. Journal of Animal Science, 2020, 98(8): skaa227 DOI:10.1093/jas/skaa227. |
| [156] | LEE C, MORRIS DL, COPELIN JE, HETTICK JM, KWON IH. Effects of lysophospholipids on short-term production, nitrogen utilization, and rumen fermentation and bacterial population in lactating dairy cows[J]. Journal of Dairy Science, 2019, 102(4): 3110-3120 DOI:10.3168/jds.2018-15777. |
| [157] | PRIDE AC, HERRERA CM, GUAN ZQ, GILES DK, STEPHEN TRENT M. The outer surface lipoprotein VolA mediates utilization of exogenous lipids by Vibrio cholerae[J]. mBio, 2013, 4(3): e00305. |
 2023, Vol. 63
2023, Vol. 63




