中国科学院微生物研究所,中国微生物学会
文章信息
- 谭玲, 王建新, 王慧. 2022
- TAN Ling, WANG Jianxin, WANG Hui.
- 肉毒神经毒素抑制剂的研究进展
- Research advances in inhibitors of botulinum neurotoxins
- 微生物学报, 62(4): 1270-1285
- Acta Microbiologica Sinica, 62(4): 1270-1285
-
文章历史
- 收稿日期:2021-08-11
- 修回日期:2021-10-17
- 网络出版日期:2021-11-03
肉毒神经毒素(botulinum neurotoxins,BoNTs)是梭状芽孢杆菌属(C. botulinum、C. argentinense、C. baratii、C. butyricum和C. sporogenes)在厌氧条件下分泌的神经毒素,误食极微量(低至ng/kg)肉毒毒素感染的食物即可导致人中毒死亡,是目前世界上已知的毒性最强的生物类毒素[1]。由于BoNTs具有易于制备及强毒性的特点,已被美国疾病与控制预防中心(center for disease control and prevention,CDC)列为A类生物恐怖制剂[2]。肉毒毒素可分为7种血清型(A–G)[3–4],其中能引起人类肉毒中毒的有A、B、E和F 4种血清型。在这4种血清型中,A型的毒性最强,B、E和F型次之;并且由A型导致的肉毒毒素中毒的病例最为常见,其次是B型,E和F型导致的中毒较为少见[5–7]。
肉毒毒素引起人类中毒的方式可分为食源性中毒、婴儿肠道中毒、成人肠道中毒、伤口感染、医源性和吸入性中毒[8–10]。在欧洲,2015年至2019年间,欧盟/欧洲经济区累计报告499例确诊病例和16例死亡病例,平均每年99例,其中食源性中毒最为常见[11–12];其次,由婴儿肠道中毒引起的肉毒中毒也居高不下,2013年至2017年间,美国CDC累计报告确诊病例900例,婴儿肉毒中毒病例695例,占美国总病例的77%[13];此外,由于肉毒毒素独特的生物学效应,A和B型肉毒毒素已在临床上用于多种疾病如斜视、偏头痛、肌肉痉挛等及医疗美容,医源性引发的肉毒中毒也时有发生[14–15]。
无论是哪一种方式导致的肉毒中毒,当毒素进入神经元胞质后,会选择性地结合并切割与神经信号传递有关的蛋白,从而导致神经信号传递的失败,引发神经麻痹症状,严重者将因呼吸衰竭而死亡。目前,临床上应对肉毒中毒的药物治疗主要是注射马源性抗血清,但必须依赖于病情的早期诊断[5, 16]。此外,抗血清具有副作用大、不易储存,并且对进入神经元的毒素没有很好中和效果等缺点[17–19]。一旦毒素进入神经元胞内,可用的治疗手段仅是使用机械性通气来维持生命,但支持性治疗持续时间较长且费用昂贵,往往会给患者带来巨大的精神和经济负担。因此,需要研究者们进一步研发出更为有效的肉毒毒素抑制剂,以防止不必要的BoNTs中毒。而小分子抑制剂具有相对稳定、易于开发且膜通透性较好等特点,可作为潜在的毒素中毒的医疗干预手段。因此,本文重点关注了近年来报道的肉毒毒素小分子抑制剂的研究进展。
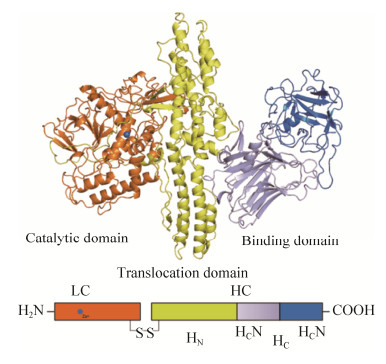
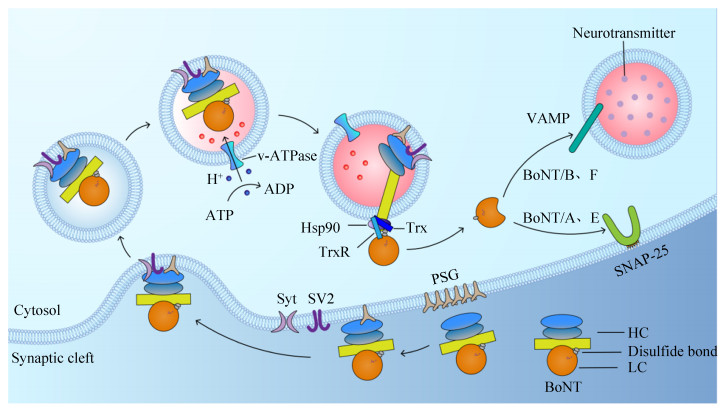
1 BoNTs的结构与中毒机制目前肉毒毒素的结构已研究得较为明确,由一条50 kDa的轻链(light chain,LC)和一条100 kDa的重链(heavy chain,HC)组成,并通过二硫键连接[20]。完整的肉毒毒素包含3个功能结构域(图 1)。其中HC作为运输载体,其羧基端(HC)为受体结合区(receptor binding domain,RBD),由受体结合区C端(HCC)和受体结合区N端(HCN)两个子域组成,负责与特定的神经元表面受体结合,从而启动毒素的转运功能。而HC的氨基端(HN)为跨膜转运结构域(translocation domain,TD),负责将LC转运至神经元胞内;LC作为毒素的催化活性中心,具有锌依赖性蛋白酶活性[21–24]。由于肉毒毒素序列的保守性,目前肉毒毒素的中毒机制已研究得较为详细,主要通过结合、内化、转运和酶切4个步骤发挥神经毒性作用(图 2)。
|
| 图 2 BoNTs的作用机制 Figure 2 The mechanism of action of BoNTs. |
1.1 BoNTs与细胞表面的受体结合
研究表明肉毒毒素在外周神经末梢的特异性结合仅与其HC片段相关[21, 25]。毒素HC上存在着2个重要的结合位点:乳糖结合位点和唾液酸结合位点[26]。乳糖结合位点为毒素与神经节苷脂结合的重要位点;唾液酸结合位点与突触小泡蛋白受体的结合具有血清型特异性[27–28]。当BoNTs感染人体后会通过循环系统到达突触前运动神经末梢,此时HC的乳糖结合位点与神经细胞表面的多聚唾液酸神经节苷脂(polysialogangliosides,PSG)以不同的亲和力迅速结合,大量的PSG将BoNTs各血清型聚积在神经元细胞表面[22, 25]。然后不同血清型的毒素在PSG的介导下通过唾液酸结合位点与低密度的突触结合蛋白(synaptotagmin,Syt)或突触囊泡蛋白2 (synaptic vesicle protein 2,SV2)发生特异性结合,Sty和SV2是突触囊泡膜上介导内吞小泡形成所必需的整合蛋白[29]。其中A、E和F型的蛋白受体为SV2;B型的蛋白受体为Syt Ⅰ和Syt Ⅱ[29–32]。
1.2 受体介导毒素的内化BoNTs与神经细胞表面的受体结合后,HC介导形成的通道会通过温度与能量依赖的方式将毒素内化到囊泡腔中。若给予一定的神经刺激可加快内化这一过程。Colasante等的研究表明,与BoNT/A结合的SV2受体决定了每个囊泡吸收的毒素数量,而不是多聚神经节苷脂受体;并且每个囊泡吸收的毒素分子不超过2个[33]。
1.3 BoNTs的跨膜转运当毒素内化到囊泡腔后会形成胞内体。在酸性(pH < 6)条件下,毒素HN结构域发生变化并嵌入囊泡膜而形成一个易位通道,部分展开的LC会从突触囊泡管腔侧易位到胞质侧,此时的LC仍然不具有催化活性[7, 20, 34]。当毒素的链间二硫键在裂解酶如热休克蛋白90 (Hsp90)和硫氧还蛋白-硫氧还蛋白还原酶系统(thioredoxin- thioredoxin reductase,Trx-TrxR)的作用下断开,LC与HC分离并重新折叠而形成新的三维结构,LC通过末端修饰而形成具有内切酶活性的蛋白[35–36]。
1.4 BoNTs的内切酶活性正常情况下,神经递质在突触前膜以胞吐的方式释放。突触前神经元中的可溶性N-乙酰马来酰胺敏感因子附着蛋白受体复合物(soluble N-ethylmaleimide sensitive factor attachment protein receptors,SNARE),包括突触相关蛋白-25 (synaptosomal-associated protein-25,SNAP-25)、突触融合蛋白(syntaxin)和囊泡相关膜蛋白2 (vesicle-associated membrane proteins 2,VAMP2)以及一些胞质蛋白组成的复合物,可介导转运小泡与突触前膜的锚定和融合,在去离子化的作用下使Ca2+通道打开,利用Ca2+内流形成的胞吐作用释放神经递质[37–40]。目前已知的毒素LC均具有金属蛋白酶活性。当胞质中的LC被激活后,会通过特异性识别并切割SNARE蛋白,其中A和E型切割SNAP-25,B和F型切割VAMP[7],将乙酰胆碱囊泡滞留在突触前膜。肉毒毒素通过抑制突触囊泡中神经递质的释放,阻断神经递质的传递而引起肌肉弛缓性麻痹[41]。
2 BoNTs小分子抑制剂的研究进展随着对BoNTs中毒机制的详细了解,越来越多的机制靶点被发现并用于开发相关抑制剂。近年来,研究者们根据肉毒毒素的结构定向设计药物或通过高通量筛选不同的小分子文库发现多种针对肉毒中毒的潜在抑制剂,并以先导化合物为基础不断改进及合成类似的化合物以进一步提高药效。
2.1 BoNTs的结合抑制剂BoNTs与突触前膜上的相应受体结合是其进入胞内发挥作用必不可少的一步,针对BoNTs的结合位点寻找有效的抑制剂,使大部分毒素无法进入胞内发挥作用,可减少毒素带来的弛缓性麻痹。研究发现一些抑制毒素与受体结合的化合物,如10 mmol/L的奎宁酸(quinic acid)可竞争性抑制BoNT/A与PSG结合[42]。多柔比星(doxorubicin),一种DNA嵌入剂,临床上多用于治疗乳腺、膀胱和肾脏等癌症及某些类型的白血病,在细胞表面与BoNTs的结合位点相同,可竞争性地结合PSG[43]。此外,来源于黄蛞蝓(limax flavus)和栽培小麦(triticum vulgaris)的凝集素对唾液酸具有亲和力,可作为BoNTs的广谱抑制剂。研究者对BoNT/B的高亲和力位点进行检测时,黄蛞蝓和栽培小麦凝集素的Ki值分别为0.31 μmol/L和0.37 μmol/L[44]。虽然神经细胞表面的受体可作为设计毒素抑制剂的有效靶点,但是它们的作用类似于抗血清疗法,治疗窗口有限。当毒素进入胞内,基于受体的药物治疗作用将会受限。
2.2 BoNTs的内化抑制剂BoNTs结合上相应受体后会通过内化途径进入胞内。Harper等发现,BoNT/A主要通过动力依赖的方式进入神经元,首次强调了动力蛋白在神经元和运动神经末梢上介导BoNT内化的作用[45],为肉毒中毒提供了一个新的治疗靶点。此外,他们发现Dyngo-40a和dynasore可阻止BoNT/A的内化,使SNAP25不被裂解;Dyngo-4a可阻止BoNT/A诱导的大鼠膈肌麻痹,使小鼠肉毒中毒的时间推迟30%以上,动力蛋白抑制剂的发现为抗毒素的使用延长了有效作用时间。2015年,Seki等发现Dyngo-4a分子具有BoNT/A的双重抑制活性,不但可以阻断毒素进入胞内的内化途径,而且可以在蛋白水解酶上发挥抑制作用[46]。
2.3 BoNTs的转运抑制剂BoNTs形成胞内体后会在转运过程将轻链释放,并通过重折叠形成活性结构而发挥酶切作用。BoNTs的转运包括转运小体的酸化、HC通道的形成及轻链的折叠释放。研究表明氯喹、氯化铵和盐酸甲胺可通过阻止胞内体酸化而抑制肉毒毒素的结合或内化过程,具有广谱抗毒
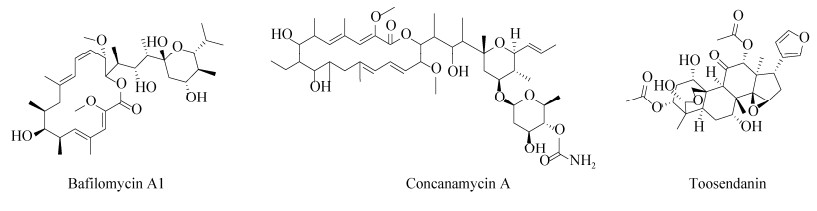
素的作用,但由于这些药物本身作为神经肌肉阻断剂具有一定毒性,不适用于在体内通过增加胞内体的pH值而发挥拮抗作用[5, 47–48]。此外,纳摩尔浓度的莫能菌素(sodium monensin)和尼日利亚菌素(nigericin)等离子载体药物可增加阳离子如Na+、H+、K+的膜通透性,通过充当H+分流器来阻止囊泡酸化,从而延迟BoNTs的神经毒性[49–50]。然而,高浓度的离子载体药物会直接抑制突触的活性,所以此类药物只能在很小的浓度范围内延迟BoNTs的麻痹作用。ATP酶在胞内体的酸化过程中同样扮演着重要的角色,抑制H+-ATPase可导致转运小体无法酸化,从而起到抑制毒素释放的作用。巴弗洛霉素A1 (bafilomycin A1)和刀豆霉素A (concanamycin A)作为H+-ATPase抑制剂,可抑制BoNTs易位进入神经元胞质,保护SNARE蛋白免受毒素的影响[51] (图 3)。然而,在小鼠膈神经半横膈膜和鸡腹侧脊髓细胞试验中发现,巴弗洛霉素A1的疗效会随着使用时间的推迟而减弱[52–53]。
一些已用于临床上治疗其他疾病的药物在抑制肉毒毒素上也初见效果,如川楝素(toosendanin,TSN),一种从中药中提取的三萜衍生物,对毒素的金属内肽酶活性没有影响,但能阻止LC通过HC通道的转位进入胞质接近其酶底物(图 3)。研究表明,TSN在200 nmol/L的浓度下可有效抑制神经元中的BoNT/A的生物活性,在低至8 nmol/L的浓度下也可观察到部分抑制[54–56]。TSN可影响离子通道的活性,抑制K+的外流并增加Ca2+内流从而促进乙酰胆碱的释放,从而改善肉毒中毒症状,虽然川楝素具有明显的毒性,但在有效剂量范围内对小鼠无明显毒性[57–58]。此外,川楝素四氢呋喃类似物可在亚纳摩尔(约10–10)浓度时稳定HC通道的封闭构象,从而阻止肉毒中毒过程中的LC易位[57]。
尽管BoNTs存在不同的蛋白受体和细胞内底物,但是它们都需要在转运小体酸化的条件下将LC释放入胞质中才能进一步形成活性结构。Trx-TrxR系统主要负责断开毒素HC和LC间的二硫键,使各血清型毒素LC易位进入胞质[36]。因此,Trx-TrxR系统可作为抑制肉毒中毒的靶标。研究发现,PX-12 (1-methyl propyl 2-imidazolyl disulfide)作为Trx抑制剂,可通过干扰链间二硫键的还原来阻止金属蛋白酶在胞质中的释放[35, 59]。金诺芬(auranofin,AF),一种硫氧还蛋白还原酶的特异性抑制剂,常用于治疗多种癌症、艾滋病感染及人类类风湿性关节炎等疾病[60–61]。研究发现AF可抑制小脑颗粒神经元中的A、B和E型肉毒中毒[62]。此外,依布硒(ebselen),一种具有谷胱甘肽过氧化物酶活性的硒有机化合物,是Trx和TrxR的广谱抑制剂,可有效保护小鼠免受A、B、E和F型的肉毒毒素致死性剂量中毒[63–64]。Seki等根据结构活性关系(structure activity relationship,SAR)的基本原理生成了一系列的N-羟基琥珀酰亚胺抑制剂,它们可通过抑制TrxR而阻断BoNT/A诱导的SNAP-25的裂解[65]。此外研究发现,黄酮类化合物如杨梅素(myricetin)和槲皮素(quercetin),其IC50分别为0.62 μmol/L和0.97 μmol/L,可与TrxR活性部位的硒原子发生不可逆结合,从而起到抑制作用[66]。而姜黄素,一种来自植物的多酚,可通过形成共价加合物而不可逆的抑制TrxR活性,其在体内外的IC50分别为3.6 μmol/L和15 μmol/L,具有时间和剂量依赖性[67]。
毒素LC在胞内特异性切割底物前,必须要经过重折叠而获得其天然结构,而Hsp90是介导LC重折叠的主要蛋白[36]。Azarnia Tehran等研究发现格尔德霉素(geldanamycin,GA),一种具有苯醌结构的抑制剂,可选择性与Hsp90的ATP结合域结合,从而阻止毒素LC易位后的重折叠[35]。此外,他们还发现GA与PX-12具有很强的协同作用,二者联合使用的抑制浓度比单一抑制剂低,并且能增加抑制效率。通过抑制BoNT进入细胞的过程,可抑制所有血清型的BoNTs。针对转运过程中相关靶点的药物虽然特异性较强,但它们对毒素的金属蛋白酶活性无影响;一旦患者出现中毒症状,此类抑制剂只能阻止循环系统中的毒素在胞内激活,而不能抑制已经在胞内发挥作用的毒素;并且,调节转运小体的酸化过程对于正常的细胞生理功能可能会产生不良的影响。
2.4 BoNTs的酶活性抑制剂毒素释放活性LC,发挥酶切作用是肉毒中毒的最后一个步骤,也是当前研发抑制剂重点关注的靶点。针对毒素LC的抑制剂设计主要集中于金属内肽酶的活性位点、α-exosite和β-exosite。目前已知的肉毒毒素LC的活性位点中心均包含1个HEXXH (X为任意氨基酸)的锌离子结合基序,而β-exosite位于活性位点附近,α-exosite则位于相对面[68]。近年来,小分子抑制剂被认为是潜在肉毒毒素抑制剂,并被相继报道。其中,研究最多的主要为喹啉醇类抑制剂和以羟肟酸为母体合成的多种羟肟酸类抑制剂,其他抑制如苯醌类和苄基环戊二酮等也有报道[50]。
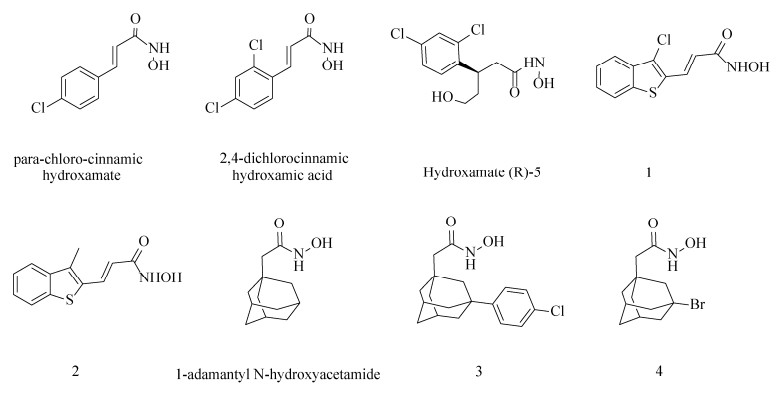
2.4.1 靶向BoNTs活性位点的抑制剂以羟肟酸为母体合成的多种羟肟酸类化合物是目前研究较多的小分子抑制剂,该类化合物能螯合毒素分子中的锌离子而抑制LC的催化活性。Janda团队在近20年间发现了许多可逆转肉毒中毒的小分子抑制剂。他们最初在SNAP-25和Cys蛋白水解位点两侧的氨基酸Gln和Arg的启发下合成了BoNT/A羟肟酸抑制剂,使其具有螯合锌的功能。虽然测试的系列抑制剂效果不佳,但却标志着将羟肟酸首次用作BoNT/A LC的金属结合抑制剂[69–71]。然后,他们将羟肟酸作为BoNT/A LC的金属结合抑制剂,对羟肟酸类化合物库进行筛选并优化先导化合物对氯肉桂异羟肟酸(para-chloro-cinnamic hydroxamte)(IC50=15 μmol/L)的结构,制备出了2, 4-二氯肉桂异羟肟酸(2, 4-dichlorocinnamic hydroxamic acid)(图 4),其IC50=(0.41±0.03) μmol/L,Ki=(300±12) nmol/L;在动物实验中,该化合物在BoNT/A致死剂量的小鼠中起到显著的保护作用,是最有效的A型肉毒毒素抑制剂之一[72–73]。同时,2, 4-二氯肉桂异羟肟酸也是第一个与BoNT/A LC共结晶的小分子抑制剂,由此产生的X射线结构为之后设计更为有效的手性化合物提供基础,如hydroxamate(R)-5 (异羟肟酸(R)-5) (图 4),其IC50=1 μmol/L,Ki=(0.16±0.02) μmol/L,大约是在此之前最好的小分子非肽类BoNT/A抑制剂的两倍[74–75]。在2, 4-二氯肉桂异羟肟酸为母体的基础上,Čapková等通过在其邻位上引入溴、三氟甲基和甲基取代基而使抑制活性增加,所产生的3种新的小分子抑制剂效力与母体分子相当[76]。
2, 4-二氯肉桂异羟肟酸自发现以来已被用作多项研究的先导分子,通过改变苯环上的取代基或修饰苯环与异羟肟酸之间的连接基团来提高抑制活性。但其本身的2, 4-二氯苯环不仅影响了自身的溶解度,而且降低了酶活性位点潜在的亲水性相互作用。Capek等使用杂环支架取代2, 4-二氯苯环衍生出的化合物3-(3-chloro- benzo[b]thiophen-2-yl)-N-hydroxy-acrylamide (1)和N-hydroxy-3-(3-methyl-benzo[b] thiophen-2-yl)- acrylamide (2)具有较好的抑制效力,其IC50分别为(0.34±0.023) μmol/L和(0.63±0.035) μmol/L[77]。此外,Čapková等将金刚烷片段取代2, 4-二氯肉桂异羟肟酸的芳香族,可得到Ki=(0.46±0.08) μmol/L的化合物1-金刚烷基N-羟基乙酰胺(1-adamantyl N-hydroxyacetamide)[78] (图 4)。并在后者的基础上进一步制备出了更为有效的酶抑制剂[3-(4-chlorophenyl)-1-adamantyl]acetohydroxamic acid (3)和[3-(4-bromophenyl)-1-adamantyl] acetohydroxamic acid (4),两种新化合物的效力提高了约17倍,其Ki分别为(27±4) nmol/L和(27± 2) nmol/L,IC50分别为0.03 μmol/L和0.04 μmol/L,但是此类化合物在20 μmol/L时会表现出细胞毒性,并且在较低浓度下对A型肉毒中毒未显示出保护作用[79]。
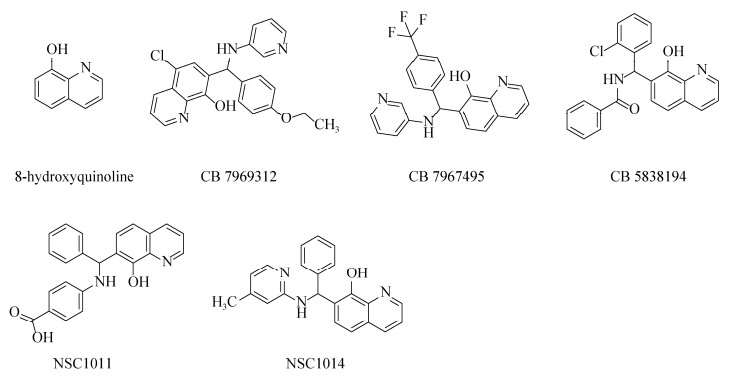
喹啉醇与传统的锌螯合异羟肟酸相比的一个明显优势是亲脂性增加,从而具有相对较强的细胞膜穿透能力。近年来,越来越多的以喹啉为基础的化合物在临床中被作为潜在的候选药物。其中,8-羟基喹啉(8-hydroxyquinoline,8-HQ)作为最简单的化合物,仅带有螯合基序的喹啉主链,是药物化学应用中最常见的支架[80] (图 5)。研究发现8-HQ的5位和7位被取代后所得的衍生物具有抑制金属蛋白酶-2(MMP-2)的作用,而2位或4位的取代则显示无效作用[81]。8-HQ作为重要的金属离子螯合剂之一,可用于临床上批准使用的氯碘羟喹(clioquinol)和二氯羟喹(chloroxine)的分子设计用于抑制肉毒中毒。2009年,Roxas-Duncan等对美国国立癌症研究所(national cancer institute,NCI)对化合物库进行虚拟筛选,选择5种毒性较低且能有效抑制全长和截短(1–425) BoNT/A LC的羟基喹啉类似物,其中CB7969312和CB7967495分别在0.5 μmol/L和5 μmol/L时可中和等量的BoNT/A,是活性较高的BoNT/A小分子抑制剂(图 5);而在分子模拟研究中,CB7967495比CB7969312效率更高[82]。Montgomery等在此研究的基础上选择CB7969312和CB7967495进行体外实验,2种化合物在各实验中表现出抑制其他血清型毒素的作用,首次报道了小分子化合物可抑制BoNT/B、E和F[83]。此外,Bompiani等对3个化合物库(ChemBridge,ChemDiv和life chemicals)进行高通量筛选,鉴定出4种对BoNT/A有抑制作用的化合物支架;通过对8-HQ化合物进行二次筛选后,18个化合物的IC50 < 10 μmol/L,其中CB7637950的IC50低至1.6 μmol/L[84]。Harrell等以8-HQ为支架合成了36种化合物,其中5-氯取代的化合物的活性最强,其IC50低至0.6 μmol/L,是非常有效的BoNT/A抑制剂[85]。Caglič等通过2次筛选已市售的喹啉衍生物发现一种抑制剂CB5838194 (图 4),其IC50为(0.8±0.1) μmol/L,在细胞水平上显示较好的抑制活性并且毒性较低[86]。而Bremer等通过筛选磺酰胺文库,发现N-芳基磺胺类药物表现出的抑制作用最强,并报道了5种新设计的化合物,在体外实验中显示出抑制活性[87]。此外,Chauhan等研究发现化合物NSC1011和NSC1014 (图 5),其IC50分别为(30.47±6.24) μmol/L和(14.91±2.49) μmol/L,在小鼠实验中可有效抑制BoNT/F毒性,可作为BoNT/F中毒的候选药物,其潜力比之前报道的化合物更高[88]。
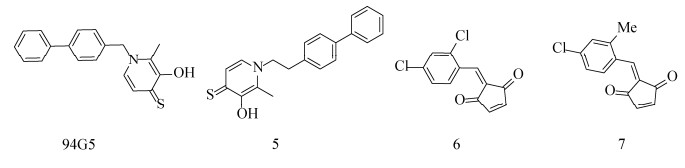
以8-HQ为支架的喹啉类化合物一直在不断的研发中,但8-HQ的溶解性是一个需要解决的问题,近年来的许多研究试图在核心结构上进行修饰或取代以解决这一问题。Agrawal等通过筛选多种金属结合基团的螯合剂片段文库发现,3-羟基-2-甲基-4H-吡喃-4-硫酮(3, 4-HOPTO)片段94G5可抑制多种金属酶[89] (图 6)。Lin等通过筛选螯合剂片段文库(CFL-1)发现,几种含有3, 4-HOPTO片段的化合物在测试浓度下使BoNT/A LC的活性降低了95%以上。其中,化合物1-(2-([1, 1'-biphenyl]-4-yl) ethyl)-3-hydroxy- 2-methylpyridine-4(1H)-thione (5)对SNAP-25的裂解具有最佳的保护效果;以3, 4-HOPTO为支架衍生出的一些抑制剂表现出良好的细胞活性,良好的细胞渗透性及水溶性让3, 4-HOPTO支架有望发展为抑制BoNT/A LC的候选材料[90]。此外,BoNT/A较长的半衰期也是需要解决的一个问题。Čapková等以2, 4-二氯肉桂异羟肟酸为先导分子,用环戊二酮弹头取代羟肟酸来产生相对有效的亲和标签,通过改造得到了3种苄基环戊二酮衍生物,其中2-(2, 4- dichlorobenzylidene) cyclopent-4-ene-1, 3-dione(6)和2-(4-chloro-2-methylbenzylidene) cyclopent-4- ene-1, 3-dione (7),两者具有类似的亚微摩尔(约10–7)抑制[91]。此外,他们还认为N-乙基马来酰亚胺的抑制活性是由于其环状结构,因此他们通过筛选亲电子片段并进行SNAPtide测定,结果显示1, 4-苯醌(1, 4-benzoquinones,BQs)和1, 4-萘醌(1, 4-naphthoquinones,NQs)为BoNT/A的有效抑制剂,并且两者的抑制活性具有时间依赖性[92]。
Kumar等基于蛋白结构虚拟筛选NCI化合物数据库发现一类针对BoNT/E蛋白酶活性的芴类化合物。其中NSC-77053 [Ki=(1.29±0.26) μmol/L,IC50=(48.5±6.5) μmol/L]在体外实验低浓度下显示出显著的抑制作用,是首个靶向BoNT/E催化位点的小分子非肽抑制剂[93]。2016年,他们基于NSC77053进行相似性搜索,发现36种具有不同接头和官能团的NSC77053类似物并进行测试,确定了5种新的化合物,其中NSC75271表现出最为有效的BoNT/E内肽酶活性抑制作用,其IC50为(2.4±0.4) μmol/L[94]。同年,Zhou等发现抑制剂C562-1101,其IC50值为(14.2±1.7) μmol/L,在同等测试条件下比NSC77053的抑制效率高出约3倍[95]。
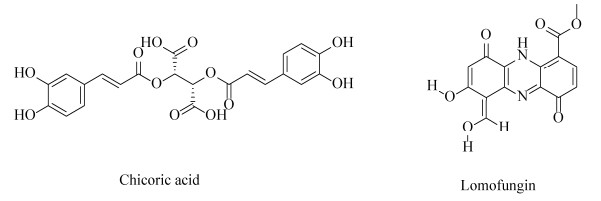
2.4.2 靶向BoNTs轻链exosite的抑制剂目前,除了靶向毒素LC的金属内肽酶活性位点的抑制剂外,LC活性位点周围的α-exosite和β-exosite也是研究抑制剂的靶标。菊苣酸(chicoric acid,CA),一种二咖啡酰基酒石酸,其非天然异构体L-CA是一种研究较为透彻的HIV整合酶抑制剂[96] (图 7)。Salzameda等发现D-CA (Ki=0.7 μmol/L)是α-exosite的有效抑制剂,可与外泌体结合位点结合,表现出非竞争性抑制,并且其在与竞争性活性位点抑制剂联合使用时具有协同作用[97–98]。此外,洛蒙真菌素(lomofungin),其Ki=(6.7±0.7) μmol/L,可与BoNT/A LC蛋白上的β-exosite非竞争性结合,在小鼠神经元细胞中,洛蒙真菌素能够抑制BoNT/A LC引起的SNAP-25被切割[99]。但由于洛蒙真菌素所含有醛、酯和多种酚等反应性官能团会降低其在体内的稳定性,并不是一个最佳的β-exosite结合抑制剂,仍需要进一步改造其结构或寻找其他更为有效的靶向抑制剂。
2.5 其他
由于毒素可通过内化机制形成胞内体,从而在跨膜转运后到达胞质发挥作用。Gillespie等筛选出一种可抑制致死毒素的中毒并阻止多种pH依赖性的细菌毒素和病毒进入哺乳动物细胞的化合物——4-bromobenzaldehyde N- (2, 6-dimethylphenyl)semicarbazone (EGA)[100]。Tehran等将EGA作为抗肉毒毒素的潜在化合物,在体外实验中,EGA可减轻A型肉毒毒素导致的小鼠中毒症状,并显著降低BoNT/B的致死率[101]。虽然EGA可有效减少肉毒毒素导致的神经毒性,但它并未干扰细胞中毒的特定步骤,目前对EGA作用的特定细胞靶点仍然未知,还有待进一步研究。
正常细胞的稳态需要部分激酶和磷酸激酶来调节,研究表明毒素LC为Src激酶的底物,毒素磷酸化后可能会影响其活性及半衰期[102],Kiris等报道了靶向宿主信号通路的抑制剂可有效削弱多种BoNTs血清型的活性,为减轻肉毒中毒提供新的途径[103]。同时,他们发现了几种小分子Scr家族激酶(SFK)抑制剂以剂量依赖的方式显示出拮抗A、B和E型的作用,较为有效的抑制剂有KX2-391、saracatinib、bosutinib和dasatinib,而小分子抑制剂的联合疗法作用于同一靶标的多个位点可能会带来额外的疗效。同年,他们以小鼠胚胎干细胞源性运动神经元(embryonic stem cell-derived motor neurons, ES-MNs)为基础,通过筛选一个小型的磷酸酶抑制剂库,发现了4种广谱抗毒素抑制剂(NSC663284、pentamidine、BVT948和9, 10- phenanthrenequinone),它们在人类ES-MN中以剂量依赖的方式拮抗毒素的多种血清型[104]。
3 结语与展望近年来,随着对肉毒毒素的结构与功能及其在宿主神经末梢的作用机制的深入研究,研究人员发现更多的靶点可用于开发新型的抑制剂。目前,研究者们根据肉毒中毒的作用机制正在研发一系列抑制剂,主要有抑制毒素与神经元表面受体结合、抑制毒素内化和转运以及抑制毒素金属内肽酶活性。当毒素感染人体后会通过循环系统到达突触前神经末梢,并与相应受体结合,但由于毒素与受体结合的这一过程非常迅速,因此毒素结合抑制剂的治疗窗口较为有限;毒素内化和转运过程是毒素释放活性LC进入胞质中发挥毒性作用的关键步骤,相关研究报道了许多抑制剂阻断这一过程,如动力蛋白抑制剂Dyngo-40a、H+-ATPase抑制剂巴弗洛霉素A1等,但由于此类抑制剂作用靶细胞广泛,可能会给机体带来较大的副作用。由于肉毒毒素导致的神经毒性与金属内肽酶直接相关,当毒素LC进入神经细胞体内后,会持续存在并不断切割其特异性底物。因此,抑制LC的酶活作用能够阻遏正在发生的肉毒中毒,缓解中毒症状。近年来多数研究团队将研发抑制剂的重点放在了毒素LC,通过靶向金属内肽酶的活性位点、α-exosite和β-exosite设计抑制剂以加速毒素降解。此外,Tsai等在分析A和E型毒素的胞内持留性差异时发现,泛素依赖性降解的敏感性差异可能是毒素持久性差异的基础,并且可通过靶向泛素化蛋白酶系统来加速毒素的降解,从而缩短其影响的持续时间[105]。这为提高抑制剂的有效性和靶向性提供了一个新的方向。虽然已报道了大量潜在的候选药物,但由于各类抑制剂在生物体内的利用度较低,只有部分药物在小鼠模型中显示保护作用,并且均未进入临床试验阶段。因此,已报道的许多小分子抑制剂仍需在先导化合物的基础上不断改进以获得更为有效的体内抑制特性或是采用特殊的运输载体将药物递送至靶点发挥作用。未来,在各种新兴技术的不断发展下,研究者们有望更快地发现各类更为有效的小分子抑制剂,在肉毒毒素抑制剂研究上取得突破性进展。
| [1] | Rasetti-Escargueil, Popoff. Antibodies and vaccines against botulinum toxins: available measures and novel approaches. Toxins, 2019, 11(9): 528. DOI:10.3390/toxins11090528 |
| [2] | Arnon SS, Schechter R, Inglesby TV, Henderson DA, Bartlett JG, Ascher MS, Eitzen E, Fine AD, Hauer J, Layton M, Lillibridge S, Osterholm MT, O'Toole T, Parker G, Perl TM, Russell PK, Swerdlow DL, Tonat K, Biodefense WGOC. Botulinum toxin as a biological weapon: medical and public health management. JAMA, 2001, 285(8): 1059-1070. DOI:10.1001/jama.285.8.1059 |
| [3] | Hill KK, Smith TJ. Genetic diversity Within Clostridium botulinum serotypes, botulinum neurotoxin gene clusters and toxin subtypes. Current topics in microbiology and immunology, 2013, 364: 1-20. |
| [4] | Barash JR, Arnon SS. A novel strain of Clostridium botulinum that produces type B and type H botulinum toxins. The Journal of Infectious Diseases, 2014, 209(2): 183-191. DOI:10.1093/infdis/jit449 |
| [5] | Pirazzini M, Rossetto O. Challenges in searching for therapeutics against botulinum neurotoxins. Expert Opinion on Drug Discovery, 2017, 12(5): 497-510. DOI:10.1080/17460441.2017.1303476 |
| [6] | Dembek ZF, Smith LA, Rusnak JM. Botulism: cause, effects, diagnosis, clinical and laboratory identification, and treatment modalities. Disaster Medicine and Public Health Preparedness, 2007, 1(2): 122-134. DOI:10.1097/DMP.0b013e318158c5fd |
| [7] | Rossetto O, Pirazzini M, Montecucco C. Botulinum neurotoxins: genetic, structural and mechanistic insights. Nature Reviews Microbiology, 2014, 12(8): 535-549. |
| [8] | Johnson EA. Clostridium botulinum and the most poisonous poison. Foodborne Pathogens, 2017, 7(8): 617-619. |
| [9] | Antonucci L, Locci C, Schettini L, Clemente MG, Antonucci R. Infant botulism: an underestimated threat. Infectious Diseases: London, England, 2021, 53(9): 647-660. DOI:10.1080/23744235.2021.1919753 |
| [10] | Cenciarelli O, Riley PW, Baka A. Biosecurity threat posed by botulinum toxin. Toxins, 2019, 11(12): 681. DOI:10.3390/toxins11120681 |
| [11] | European Centre for Disease Prevention and Control. Botulism. In: ECDC. Annual epidemiological report for 2015. Stockholm: ECDC; 2018 |
| [12] | European Centre for Disease Prevention and Control (ECDC). Surveillance Atlas of Infectious Diseases. Available from: https://atlas.ecdc.europa.eu/public/index.aspx. Accessed year: 2021/06/08 |
| [13] | Centers for Disease Control and Prevention (CDC). Botulism annual summaries. Available from: https://www.cdc.gov/botulism/surveillance.html. Accessed year: 2021/06/08 |
| [14] | Bai LL, Peng XB, Liu YQ, Sun YW, Wang X, Wang XW, Lin GD, Zhang P, Wan K, Qiu ZW. Clinical analysis of 86 botulism cases caused by cosmetic injection of botulinum toxin (BoNT). Medicine, 2018, 97(34): e10659. DOI:10.1097/MD.0000000000010659 |
| [15] | Alam M, Geisler A, Warycha M, Paghdal K, Roongpisuthipong W, Schlessinger DI, Chen BR, Reynolds KA, West DP, Poon E. Effect of postinjection facial exercise on time of onset of botulinum toxin for glabella and forehead wrinkles: a randomized, controlled, crossover clinical trial. Journal of the American Academy of Dermatology, 2019, 80(4): 1144-1147. DOI:10.1016/j.jaad.2018.10.013 |
| [16] | Hill SE, Iqbal R, Cadiz CL, Le J. Foodborne botulism treated with heptavalent botulism antitoxin. The Annals of Pharmacotherapy, 2013, 47(2): e12. DOI:10.1345/aph.1R646 |
| [17] | Chalk CH, Benstead TJ, Pound JD, Keezer MR. Medical treatment for botulism. The Cochrane Database of Systematic Reviews, 2019, 4: CD008123. |
| [18] | Choudhury S, Baker MR, Chatterjee S, Kumar H. Botulinum toxin: an update on pharmacology and newer products in development. Toxins, 2021, 13(1): 58. DOI:10.3390/toxins13010058 |
| [19] | Lam KH, Perry K, Shoemaker CB, Jin R. Two VHH antibodies neutralize botulinum neurotoxin E1 by blocking its membrane translocation in host cells. Toxins, 2020, 12(10): 616. DOI:10.3390/toxins12100616 |
| [20] | Montal M. Botulinum neurotoxin: a marvel of protein design. Annual Review of Biochemistry, 2010, 79: 591-617. DOI:10.1146/annurev.biochem.051908.125345 |
| [21] | Binz T, Rummel A. Cell entry strategy of clostridial neurotoxins. Journal of Neurochemistry, 2009, 109(6): 1584-1595. DOI:10.1111/j.1471-4159.2009.06093.x |
| [22] | Benson MA, Fu ZJ, Kim JJP, Baldwin MR. Unique ganglioside recognition strategies for clostridial neurotoxins. Journal of Biological Chemistry, 2011, 286(39): 34015-34022. DOI:10.1074/jbc.M111.272054 |
| [23] | Swaminathan S. Molecular structures and functional relationships in clostridial neurotoxins. The FEBS Journal, 2011, 278(23): 4467-4485. DOI:10.1111/j.1742-4658.2011.08183.x |
| [24] | Lacy DB, Tepp W, Cohen AC, DasGupta BR, Stevens RC. Crystal structure of botulinum neurotoxin type A and implications for toxicity. Nature Structural Biology, 1998, 5(10): 898-902. DOI:10.1038/2338 |
| [25] | Rummel A. Double receptor anchorage of botulinum neurotoxins accounts for their exquisite neurospecificity. Current topics in microbiology and immunology, 2013, 364: 61-90. |
| [26] | Rummel A, Bade S, Alves J, Bigalke H, Binz T. Two carbohydrate binding sites in the HCC-domain of tetanus neurotoxin are required for toxicity. Journal of Molecular Biology, 2003, 326(3): 835-847. DOI:10.1016/S0022-2836(02)01403-1 |
| [27] | Emsley P, Fotinou C, Black I, Fairweather NF, Charles IG, Watts C, Hewitt E, Isaacs NW. The structures of the HC fragment of tetanus toxin with carbohydrate subunit complexes provide insight into ganglioside binding. Journal of Biological Chemistry, 2000, 275(12): 8889-8894. DOI:10.1074/jbc.275.12.8889 |
| [28] | Rossetto O, Pirazzini M, Bolognese P, Rigoni M, Montecucco C. An update on the mechanism of action of tetanus and botulinum neurotoxins. Acta Chimica Slovenica, 2011, 58(4): 702-707. |
| [29] | Ahnert-Hilger G, Münster-Wandowski A, Höltje M. Synaptic vesicle proteins: targets and routes for botulinum neurotoxins. Current topics in microbiology and immunology, 2013, 364: 159-177. |
| [30] | Rummel A. Two feet on the membrane: uptake of clostridial neurotoxins. Uptake and Trafficking of Protein Toxins, 2017, 406: 1-37. |
| [31] | Rummel A. The long journey of botulinum neurotoxins into the synapse. Toxicon, 2015, 107: 9-24. DOI:10.1016/j.toxicon.2015.09.009 |
| [32] | Dong M, Richards DA, Goodnough MC, Tepp WH, Johnson EA, Chapman ER. Synaptotagmins Ⅰ and Ⅱ mediate entry of botulinum neurotoxin B into cells. Journal of Cell Biology, 2003, 162(7): 1293-1303. DOI:10.1083/jcb.200305098 |
| [33] | Colasante C, Rossetto O, Morbiato L, Pirazzini M, Molgó J, Montecucco C. Botulinum neurotoxin type A is internalized and translocated from small synaptic vesicles at the neuromuscular junction. Molecular Neurobiology, 2013, 48(1): 120-127. DOI:10.1007/s12035-013-8423-9 |
| [34] | Koriazova LK, Montal M. Translocation of botulinum neurotoxin light chain protease through the heavy chain channel. Nature Structural Biology, 2003, 10(1): 13-18. DOI:10.1038/nsb879 |
| [35] | Azarnia Tehran D, Pirazzini M, Leka O, Mattarei A, Lista F, Binz T, Rossetto O, Montecucco C. Hsp90 is involved in the entry of clostridial neurotoxins into the cytosol of nerve terminals. Cellular Microbiology, 2017, 19(2): e12647. DOI:10.1111/cmi.12647 |
| [36] | Pirazzini M, Azarnia Tehran D, Zanetti G, Rossetto O, Montecucco C. Hsp90 and thioredoxin-thioredoxin reductase enable the catalytic activity of clostridial neurotoxins inside nerve terminals. Toxicon, 2018, 147: 32-37. DOI:10.1016/j.toxicon.2017.10.028 |
| [37] | Söllner T, Bennett MK, Whiteheart SW, Scheller RH, Rothman JE. A protein assembly-disassembly pathway in vitro that may correspond to sequential steps of synaptic vesicle docking, activation, and fusion. Cell, 1993, 75(3): 409-418. DOI:10.1016/0092-8674(93)90376-2 |
| [38] | Wang TL, Li LC, Hong WJ. SNARE proteins in membrane trafficking. Traffic: Copenhagen, Denmark, 2017, 18(12): 767-775. DOI:10.1111/tra.12524 |
| [39] | Han J, Pluhackova K, Böckmann RA. The multifaceted role of SNARE proteins in membrane fusion. Frontiers in Physiology, 2017, 8: 5. |
| [40] | Banerjee A, Kowalchyk JA, DasGupta BR, Martin TFJ. SNAP-25 is required for a late postdocking step in Ca2+-dependent exocytosis. Journal of Biological Chemistry, 1996, 271(34): 20227-20230. DOI:10.1074/jbc.271.34.20227 |
| [41] | Shoemaker CB, Oyler GA. Persistence of botulinum neurotoxin inactivation of nerve function. Current topics in microbiology and immunology, 2013, 364: 179-196. |
| [42] | Cai S, Singh BR. Strategies to design inhibitors of Clostridium botulinum neurotoxins. Infectious Disorders Drug Targets, 2007, 7(1): 47-57. DOI:10.2174/187152607780090667 |
| [43] | Eswaramoorthy S, Kumaran D, Swaminathan S. Crystallographic evidence for doxorubicin binding to the receptor-binding site in Clostridium botulinum neurotoxin B. Acta Crystallographica Section D, 2001, 57(11): 1743-1746. DOI:10.1107/S0907444901013531 |
| [44] | Bakry N, Kamata Y, Simpson LL. Lectins from Triticum vulgaris and Limax flavus are universal antagonists of botulinum neurotoxin and tetanus toxin. The Journal of Pharmacology and Experimental Therapeutics, 1991, 258(3): 830-836. |
| [45] | Harper CB, Martin S, Nguyen TH, Daniels SJ, Lavidis NA, Popoff MR, Hadzic G, Mariana A, Chau N, McCluskey A, Robinson PJ, Meunier FA. Dynamin inhibition blocks botulinum neurotoxin type A endocytosis in neurons and delays botulism. Journal of Biological Chemistry, 2011, 286(41): 35966-35976. DOI:10.1074/jbc.M111.283879 |
| [46] | Seki H, Xue S, Hixon MS, Pellett S, Remes M, Johnson EA, Janda KD. Toward the discovery of dual inhibitors for botulinum neurotoxin A: concomitant targeting of endocytosis and light chain protease activity. Chemical Communications: Cambridge, England, 2015, 51(28): 6226-6229. DOI:10.1039/C5CC00677E |
| [47] | Simpson LL. The interaction between aminoquinolines and presynaptically acting neurotoxins. The Journal of Pharmacology and Experimental Therapeutics, 1982, 222(1): 43-48. |
| [48] | Simpson LL. Ammonium chloride and methylamine hydrochloride antagonize clostridial neurotoxins. The Journal of Pharmacology and Experimental Therapeutics, 1983, 225(3): 546-552. |
| [49] | Sheridan RE. Protonophore antagonism of botulinum toxin in mouse muscle. Toxicon, 1996, 34(8): 849-855. DOI:10.1016/0041-0101(96)00040-2 |
| [50] | Kiris E, Burnett JC, Kane CD, Bavari S. Recent advances in botulinum neurotoxin inhibitor development. Current Topics in Medicinal Chemistry, 2014, 14(18): 2044-2061. DOI:10.2174/1568026614666141022093350 |
| [51] | Woo DH, Hur YN, Jang MW, Justin Lee C, Park M. Inhibitors of synaptic vesicle exocytosis reduce surface expression of postsynaptic glutamate receptors. Animal Cells and Systems, 2020, 24(6): 341-348. DOI:10.1080/19768354.2020.1838607 |
| [52] | Simpson LL, Coffield JA, Bakry N. Inhibition of vacuolar adenosine triphosphatase antagonizes the effects of clostridial neurotoxins but not phospholipase A2 neurotoxins. The Journal of Pharmacology and Experimental Therapeutics, 1994, 269(1): 256-262. |
| [53] | Ruthel G, Burnett JC, Nuss JE, Wanner LM, Tressler LE, Torres-Melendez E, Sandwick SJ, Retterer CJ, Bavari S. Post-intoxication inhibition of botulinum neurotoxin serotype A within neurons by small-molecule, non-peptidic inhibitors. Toxins, 2011, 3(3): 207-217. DOI:10.3390/toxins3030207 |
| [54] | Li MF, Shi YL. Toosendanin interferes with pore formation of botulinum toxin type A in PC12 cell membrane. Acta Pharmacologica Sinica, 2006, 27(1): 66-70. DOI:10.1111/j.1745-7254.2006.00236.x |
| [55] | Shi YL, Wang ZF. Cure of experimental botulism and antibotulismic effect of toosendanin. Acta Pharmacologica Sinica, 2004, 25(6): 839-848. |
| [56] | Nakai YY, Pellett S, Tepp WH, Johnson EA, Janda KD. Toosendanin: synthesis of the AB-ring and investigations of its anti-botulinum properties (Part Ⅱ). Bioorganic & Medicinal Chemistry, 2010, 18(3): 1280-1287. |
| [57] | Fischer A, Nakai YY, Eubanks LM, Clancy CM, Tepp WH, Pellett S, Dickerson TJ, Johnson EA, Janda KD, Montal M. Bimodal modulation of the botulinum neurotoxin protein-conducting channel. Proceedings of the National Academy of Sciences of the United States of America, 2009, 106(5): 1330-1335. DOI:10.1073/pnas.0812839106 |
| [58] | Sun SH, Suresh S, Liu HS, Tepp WH, Johnson EA, Edwardson JM, Chapman ER. Receptor binding enables botulinum neurotoxin B to sense low pH for translocation channel assembly. Cell Host & Microbe, 2011, 10(3): 237-247. |
| [59] | Kirkpatrick DL, Kuperus M, Dowdeswell M, Potier N, Donald LJ, Kunkel M, Berggren M, Angulo M, Powis G. Mechanisms of inhibition of the thioredoxin growth factor system by antitumor 2-imidazolyl disulfides. Biochemical Pharmacology, 1998, 55(7): 987-994. DOI:10.1016/S0006-2952(97)00597-2 |
| [60] | Tonissen KF, Di Trapani G. Thioredoxin system inhibitors as mediators of apoptosis for cancer therapy. Molecular Nutrition & Food Research, 2009, 53(1): 87-103. |
| [61] | Lewis MG, DaFonseca S, Chomont N, Palamara AT, Tardugno M, Mai A, Collins M, Wagner WL, Yalley-Ogunro J, Greenhouse J, Chirullo B, Norelli S, Garaci E, Savarino A. Gold drug auranofin restricts the viral reservoir in the monkey AIDS model and induces containment of viral load following ART suspension. AIDS: London, England, 2011, 25(11): 1347-1356. DOI:10.1097/QAD.0b013e328347bd77 |
| [62] | Zuverink M, Chen C, Przedpelski A, Blum FC, Barbieri JT. A heterologous reporter defines the role of the tetanus toxin interchain disulfide in light-chain translocation. Infection and Immunity, 2015, 83(7): 2714-2724. DOI:10.1128/IAI.00477-15 |
| [63] | Zhao R, Masayasu H, Holmgren A. Ebselen: a substrate for human thioredoxin reductase strongly stimulating its hydroperoxide reductase activity and a superfast thioredoxin oxidant. Proceedings of the National Academy of Sciences of the United States of America, 2002, 99(13): 8579-8584. DOI:10.1073/pnas.122061399 |
| [64] | Pirazzini M, Azarnia Tehran D, Zanetti G, Megighian A, Scorzeto M, Fillo S, Shone CC, Binz T, Rossetto O, Lista F, Montecucco C. Thioredoxin and its reductase are present on synaptic vesicles, and their inhibition prevents the paralysis induced by botulinum neurotoxins. Cell Reports, 2014, 8(6): 1870-1878. DOI:10.1016/j.celrep.2014.08.017 |
| [65] | Seki H, Xue S, Pellett S, Šilhár P, Johnson EA, Janda KD. Cellular protection of SNAP-25 against botulinum neurotoxin/A: inhibition of thioredoxin reductase through a suicide substrate mechanism. Journal of the American Chemical Society, 2016, 138(17): 5568-5575. DOI:10.1021/jacs.5b12929 |
| [66] | Lu J, Papp LV, Fang JG, Rodriguez-Nieto S, Zhivotovsky B, Holmgren A. Inhibition of mammalian thioredoxin reductase by some flavonoids: implications for myricetin and quercetin anticancer activity. Cancer Research, 2006, 66(8): 4410-4418. DOI:10.1158/0008-5472.CAN-05-3310 |
| [67] | Fang JG, Lu J, Holmgren A. Thioredoxin reductase is irreversibly modified by curcumin: a novel molecular mechanism for its anticancer activity. Journal of Biological Chemistry, 2005, 280(26): 25284-25290. DOI:10.1074/jbc.M414645200 |
| [68] | Breidenbach MA, Brunger AT. Substrate recognition strategy for botulinum neurotoxin serotype A. Nature, 2004, 432(7019): 925-929. DOI:10.1038/nature03123 |
| [69] | Boldt GE, Kennedy JP, Hixon MS, McAllister LA, Barbieri JT, Tzipori S, Janda KD. Synthesis, characterization and development of a high-throughput methodology for the discovery of botulinum neurotoxin a inhibitors. Journal of Combinatorial Chemistry, 2006, 8(4): 513-521. DOI:10.1021/cc060010h |
| [70] | Lin L, Olson ME, Eubanks LM, Janda KD. Strategies to counteract botulinum neurotoxin A: nature's deadliest biomolecule. Accounts of Chemical Research, 2019, 52(8): 2322-2331. DOI:10.1021/acs.accounts.9b00261 |
| [71] | Rao BG. Recent developments in the design of specific matrix metalloproteinase inhibitors aided by structural and computational studies. Current Pharmaceutical Design, 2005, 11(3): 295-322. DOI:10.2174/1381612053382115 |
| [72] | Boldt GE, Kennedy JP, Janda KD. Identification of a potent botulinum neurotoxin a protease inhibitor using in situ lead identification chemistry. Organic Letters, 2006, 8(8): 1729-1732. DOI:10.1021/ol0603211 |
| [73] | Eubanks LM, Hixon MS, Jin W, Hong S, Clancy CM, Tepp WH, Baldwin MR, Malizio CJ, Goodnough MC, Barbieri JT, Johnson EA, Boger DL, Dickerson TJ, Janda KD. An in vitro and in vivo disconnect uncovered through high-throughput identification of botulinum neurotoxin A antagonists. Proceedings of the National Academy of Sciences of the United States of America, 2007, 104(8): 2602-2607. DOI:10.1073/pnas.0611213104 |
| [74] | Silvaggi NR, Boldt GE, Hixon MS, Kennedy JP, Tzipori S, Janda KD, Allen KN. Structures of Clostridium botulinum neurotoxin serotype A light chain complexed with small-molecule inhibitors highlight active-site flexibility. Chemistry & Biology, 2007, 14(5): 533-542. |
| [75] | Stowe GN, Silhár P, Hixon MS, Silvaggi NR, Allen KN, Moe ST, Jacobson AR, Barbieri JT, Janda KD. Chirality holds the key for potent inhibition of the botulinum neurotoxin serotype a protease. Organic Letters, 2010, 12(4): 756-759. DOI:10.1021/ol902820z |
| [76] | Čapková K, Yoneda Y, Dickerson TJ, Janda KD. Synthesis and structure-activity relationships of second-generation hydroxamate botulinum neurotoxin A protease inhibitors. Bioorganic & Medicinal Chemistry Letters, 2007, 17(23): 6463-6466. |
| [77] | Capek P, Zhang Y, Barlow DJ, Houseknecht KL, Smith GR, Dickerson TJ. Enhancing the pharmacokinetic properties of botulinum neurotoxin serotype A protease inhibitors through rational design. ACS Chemical Neuroscience, 2011, 2(6): 288-293. DOI:10.1021/cn200021q |
| [78] | Čapková K, Hixon MS, McAllister LA, Janda KD. Toward the discovery of potent inhibitors of botulinum neurotoxin A: development of a robust LC MS based assay operational from low to subnanomolar enzyme concentrations. Chemical Communications: Cambridge, England, 2008(30): 3525-3527. |
| [79] | Šilhár P, Silvaggi NR, Pellett S, Čapková K, Johnson EA, Allen KN, Janda KD. Evaluation of adamantane hydroxamates as botulinum neurotoxin inhibitors: synthesis, crystallography, modeling, kinetic and cellular based studies. Bioorganic & Medicinal Chemistry, 2013, 21(5): 1344-1348. |
| [80] | Chauhan R, Chauhan V, Sonkar P, Dhaked RK. Identification of inhibitors against botulinum neurotoxins: 8-hydroxyquinolines hold promise. Mini Reviews in Medicinal Chemistry, 2019, 19(20): 1694-1706. DOI:10.2174/1389557519666190906120228 |
| [81] | Jacobsen JA, Fullagar JL, Miller MT, Cohen SM. Identifying chelators for metalloprotein inhibitors using a fragment-based approach. Journal of Medicinal Chemistry, 2011, 54(2): 591-602. DOI:10.1021/jm101266s |
| [82] | Roxas-Duncan V, Enyedy I, Montgomery VA, Eccard VS, Carrington MA, Lai HG, Gul N, Yang DCH, Smith LA. Identification and biochemical characterization of small-molecule inhibitors of Clostridium botulinum neurotoxin serotype A. Antimicrobial Agents and Chemotherapy, 2009, 53(8): 3478-3486. DOI:10.1128/AAC.00141-09 |
| [83] | Montgomery VA, Ahmed SA, Olson MA, Mizanur RM, Stafford RG, Roxas-Duncan VI, Smith LA. Ex vivo inhibition of Clostridium botulinum neurotoxin types B, C, E, and F by small molecular weight inhibitors. Toxicon, 2015, 98: 12-19. DOI:10.1016/j.toxicon.2015.02.012 |
| [84] | Bompiani KM, Caglič D, Krutein MC, Benoni G, Hrones M, Lairson LL, Bian HY, Smith GR, Dickerson TJ. High-throughput screening uncovers novel botulinum neurotoxin inhibitor chemotypes. ACS Combinatorial Science, 2016, 18(8): 461-474. DOI:10.1021/acscombsci.6b00033 |
| [85] | Harrell WA Jr, Vieira RC Jr, Ensel SM Jr, Montgomery V Jr, Guernieri R Jr, Eccard VS Jr, Campbell Y Jr, Roxas-Duncan V Jr, Cardellina JH II, Webb RP II, Smith LA II. A matrix-focused structure-activity and binding site flexibility study of quinolinol inhibitors of botulinum neurotoxin serotype A. Bioorganic & Medicinal Chemistry Letters, 2017, 27(3): 675-678. |
| [86] | Caglič D, Krutein MC, Bompiani KM, Barlow DJ, Benoni G, Pelletier JC, Reitz AB, Lairson LL, Houseknecht KL, Smith GR, Dickerson TJ. Identification of clinically viable quinolinol inhibitors of botulinum neurotoxin A light chain. Journal of Medicinal Chemistry, 2014, 57(3): 669-676. DOI:10.1021/jm4012164 |
| [87] | Bremer PT, Adler M, Phung CH, Singh AK, Janda KD. Newly designed quinolinol inhibitors mitigate the effects of botulinum neurotoxin A in enzymatic, cell-based, and ex vivo assays. Journal of Medicinal Chemistry, 2017, 60(1): 338-348. DOI:10.1021/acs.jmedchem.6b01393 |
| [88] | Chauhan R, Chauhan V, Sonkar P, Vimal M, Dhaked RK. Targeted 8-hydroxyquinoline fragment based small molecule drug discovery against neglected botulinum neurotoxin type F. Bioorganic Chemistry, 2019, 92: 103297. DOI:10.1016/j.bioorg.2019.103297 |
| [89] | Agrawal A, Johnson S, Jacobsen J, Miller M, Chen LH, Pellecchia M, Cohen S. Chelator fragment libraries for targeting metalloproteinases. ChemMedChem, 2010, 5(2): 195-199. DOI:10.1002/cmdc.200900516 |
| [90] | Lin L, Turner LD, Šilhár P, Pellett S, Johnson EA, Janda KD. Identification of 3-hydroxy-1, 2-dimethylpyridine-4(1H)-thione as a metal-binding motif for the inhibition of botulinum neurotoxin A. RSC Medicinal Chemistry, 2021, 12(1): 137-143. DOI:10.1039/D0MD00320D |
| [91] | Čapková K, Hixon MS, Pellett S, Barbieri JT, Johnson EA, Janda KD. Benzylidene cyclopentenediones: first irreversible inhibitors against botulinum neurotoxin A's zinc endopeptidase. Bioorganic & Medicinal Chemistry Letters, 2010, 20(1): 206-208. |
| [92] | Bremer PT, Hixon MS, Janda KD. Benzoquinones as inhibitors of botulinum neurotoxin serotype A. Bioorganic & Medicinal Chemistry, 2014, 22(15): 3971-3981. |
| [93] | Kumar G, Agarwal R, Swaminathan S. Discovery of a fluorene class of compounds as inhibitors of botulinum neurotoxin serotype E by virtual screening. Chemical Communications: Cambridge, England, 2012, 48(18): 2412-2414. DOI:10.1039/c2cc17158a |
| [94] | Kumar G, Agarwal R, Swaminathan S. Small molecule non-peptide inhibitors of botulinum neurotoxin serotype E: structure-activity relationship and a pharmacophore model. Bioorganic & Medicinal Chemistry, 2016, 24(18): 3978-3985. |
| [95] | Zhou YC, McGillick BE, Teng YHG, Haranahalli K, Ojima I, Swaminathan S, Rizzo RC. Identification of small molecule inhibitors of botulinum neurotoxin serotype E via footprint similarity. Bioorganic & Medicinal Chemistry, 2016, 24(20): 4875-4889. |
| [96] | Reinke RA, Lee DJ, McDougall BR, King PJ, Victoria J, Mao YQ, Lei XY, Reinecke MG, Robinson WE Jr. L-chicoric acid inhibits human immunodeficiency virus type 1 integration in vivo and is a noncompetitive but reversible inhibitor of HIV-1 integrase in vitro. Virology, 2004, 326(2): 203-219. DOI:10.1016/j.virol.2004.06.005 |
| [97] | Salzameda NT, Eubanks LM, Zakhari JS, Tsuchikama K, DeNunzio NJ, Allen KN, Hixon MS, Janda KD. A cross-over inhibitor of the botulinum neurotoxin light chain B: a natural product implicating an exosite mechanism of action. Chemical Communications: Cambridge, England, 2011, 47(6): 1713-1715. DOI:10.1039/c0cc04078a |
| [98] | Silhár P, Capková K, Salzameda NT, Barbieri JT, Hixon MS, Janda KD. Botulinum neurotoxin A protease: discovery of natural product exosite inhibitors. Journal of the American Chemical Society, 2010, 132(9): 2868-2869. DOI:10.1021/ja910761y |
| [99] | Eubanks LM, Silhár P, Salzameda NT, Zakhari JS, Feng XC, Barbieri JT, Shoemaker CB, Hixon MS, Janda KD. Identification of a natural product antagonist against the botulinum neurotoxin light chain protease. ACS Medicinal Chemistry Letters, 2010, 1(6): 268-272. DOI:10.1021/ml100074s |
| [100] | Gillespie EJ, Ho CLC, Balaji K, Clemens DL, Deng G, Wang YE, Elsaesser HJ, Tamilselvam B, Gargi A, Dixon SD, France B, Chamberlain BT, Blanke SR, Cheng GH, De La Torre JC, Brooks DG, Jung ME, Colicelli J, Damoiseaux R, Bradley KA. Selective inhibitor of endosomal trafficking pathways exploited by multiple toxins and viruses. Proceedings of the National Academy of Sciences of the United States of America, 2013, 110(50): E4904-E4912. |
| [101] | Tehran DA, Zanetti G, Leka O, Lista F, Fillo S, Binz T, Shone CC, Rossetto O, Montecucco C, Paradisi C, Mattarei A, Pirazzini M. A novel inhibitor prevents the peripheral neuroparalysis of botulinum neurotoxins. Scientific Reports, 2015, 5: 17513. DOI:10.1038/srep17513 |
| [102] | Toth S, Brueggmann EE, Oyler GA, Smith LA, Hines HB, Ahmed SA. Tyrosine phosphorylation of botulinum neurotoxin protease domains. Frontiers in Pharmacology, 2012, 3: 102. |
| [103] | Kiris E, Burnett JC, Nuss JE, Wanner LM, Peyser BD, Du HT, Gomba GY, Kota KP, Panchal RG, Gussio R, Kane CD, Tessarollo L, Bavari S. Src family kinase inhibitors antagonize the toxicity of multiple serotypes of botulinum neurotoxin in human embryonic stem cell-derived motor neurons. Neurotoxicity Research, 2015, 27(4): 384-398. DOI:10.1007/s12640-015-9526-z |
| [104] | Kiris E, Nuss JE, Stanford SM, Wanner LM, Cazares L, Maestre MF, Du HT, Gomba GY, Burnett JC, Gussio R, Bottini N, Panchal RG, Kane CD, Tessarollo L, Bavari S. Phosphatase inhibitors function as novel, broad spectrum botulinum neurotoxin antagonists in mouse and human embryonic stem cell-derived motor neuron- based assays. PLoS One, 2015, 10(6): e0129264. DOI:10.1371/journal.pone.0129264 |
| [105] | Tsai YC, Maditz R, Kuo CL, Fishman PS, Shoemaker CB, Oyler GA, Weissman AM. Targeting botulinum neurotoxin persistence by the ubiquitin-proteasome system. Proceedings of the National Academy of Sciences of the United States of America, 2010, 107(38): 16554-16559. DOI:10.1073/pnas.1008302107 |
 2022, Vol. 62
2022, Vol. 62