中国科学院微生物研究所,中国微生物学会
文章信息
- 张向菲, 刘畅, 胡冰, 王瑞娟, 秦建如, 王建华. 2022
- ZHANG Xiangfei, LIU Chang, HU Bing, WANG Ruijuan, QIN Jianru, WANG Jianhua.
- EV71抗病毒药物及疫苗研究进展
- Research progress of drugs and vaccines against enterovirus 71
- 微生物学报, 62(4): 1216-1230
- Acta Microbiologica Sinica, 62(4): 1216-1230
-
文章历史
- 收稿日期:2021-08-02
- 修回日期:2021-09-07
- 网络出版日期:2022-01-14
2. 中国科学院广州生物医药与健康研究院, 广东 广州 510530
2. Guangzhou Institutes of Biomedicine and Health, Chinese Academy of Sciences, Guangzhou 510530, Guangdong, China
手足口病(hand-foot and mouth disease,HFMD)是由肠道病毒71型(enterovirus 71,EV71)、柯萨奇病毒A组16型(coxsackievirus A16,CVA16)、柯萨奇A组10型(coxsackievirus A10,CVA10)以及柯萨奇A组6型(coxsackievirus A6,CVA6)等多种肠道病毒引发的传染病,流行同时病毒内部可通过亚基因型的改变和重组而变异[1–4]。该病人群普遍易感,患病人群中88.97%为5周岁及以下儿童,隐性感染率高,可通过飞沫、接触和饮食等方式传播,居住环境、生活方式、空间和气候等因素均影响发病率[5–7]。患者感染初期临床特征为发热、口腔溃疡、手脚部位会出现丘疹性水疱、斑疹等[8–10]。多数患儿7 d内自愈,但也有部分患儿会在几天后出现脑炎、脑膜炎、肺水肿和急性弛缓性麻痹等并发症,严重情况下会导致死亡[11]。
EV71是手足口病的主要病原体之一,1969年于美国加州福尼亚患有中枢神经系统疾病婴儿的粪便内首次分离出来,1998年于我国台湾地区首次流行[12–13]。EV71为单股正链RNA病毒,小核糖核酸病毒科,肠病毒属,正二十面体结构,直径约23–30 nm,有A、B、C这3种基因型[14–15]。P1、P2、P3是EV71的3种前体蛋白,可被自身酶系切割为结构蛋白(VP1、VP2、VP3和VP4)和非结构蛋白,其编码区基因重组会引起变种[16–17]。EV71具有嗜神经性,会在骨骼肌中复制,通过感染神经肌肉连接处的运动神经元,达到中枢神经系统,通过上下调节细胞因子、非蛋白编码基因的异常来诱导神经细胞凋亡和自噬[18–22]。侵入宿主后,先天免疫激活,病毒通过抑制TLR信号网络[23]、诱导宿主细胞代谢重编程[24]、刺激细胞因子信号转导蛋白(SOCS)的表达[25]、切割参与免疫的蛋白[26–27]等来规避免疫反应。由于目前仍无特效的抗病毒药物,为降低HFMD暴发的隐患,现针对EV71的药物研发及疫苗的研究进展综述如下[28]。
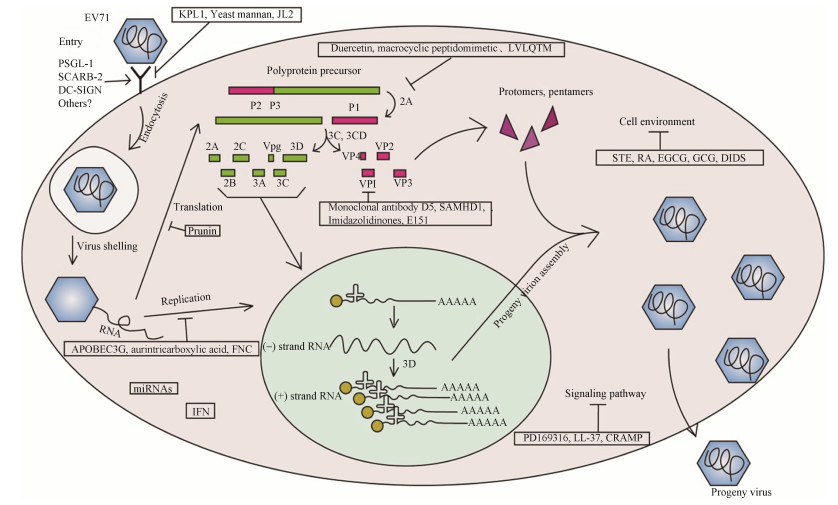
1 抗病毒药物目前临床上通过静脉注射利巴韦林(ribavirin),同时口服硝苯地平(nifedipine)、雷米普利(ramipril)来治疗由EV71引起的手足口病,患者7 d内完全康复[29–30]。利巴韦林可通过亚精胺-精胺N1-乙酰转移酶(SAT1)诱导多胺分解代谢,多胺耗竭限制病毒复制[31–32]。但是利巴韦林治疗过程中部分患者出现疲劳、贫血和头痛等不良反应[33],因此研发特效的抗病毒药物是极其重要的(图 1,表 1)。
|
| 图 1 EV71细胞内感染示意图和抗病毒药物概述 Figure 1 Schematic illustration of EV71 intracellular infection and summary of the antiviral agents. |
| Antivirals | Action site/mode | Strain type | In vitro cell type | In vivo mouse model | References |
| Blocking virus entry | |||||
| Monoclonal antibody KPL1 | PSGL-1 | EV71-G08-2 | RD, Jurkat T | [36] | |
| Yeast mannan | DC-SIGN | EV71 (HQ891927) | HEK293T | [39] | |
| Monoclonal antibody JL2 | The apical region of SCARB2 | EV71 (0804232Y) | HEK293T | [42] | |
| Inhibition of virus translation | |||||
| Prunin | EV71 IRES | EV71-41/H/B5/C4 | RD | One-day-old suckling BALB/c mice | [47] |
| Inhibition of viral multiprotein processing | |||||
| Duercetin | The substrate binding pocket of EV71 3Cpro | EV71 (SK-EV006) | RD | [51] | |
| Macrocyclic peptidomimetic | The substrate binding pocket of EV71 3Cpro | EV71 (Shenzhen/120F1/09) | RD | [52] | |
| LVLQTM | The active site of EV71 2Apro | EV71 (AEF32490) | HeLa | [58] | |
| Inhibition of replication of virus | |||||
| Anti-3Dpol monoclonal antibody | 1–250 amino acids of EV71 3Dpol | EV71-MAV-VR | Vero | BALB/c aged 6–8 wks and pregnant ICR mice | [62] |
| APOBEC3G | EV71 3Dpol | EV71-H (VR-1432) | Vero | [63] | |
| Aurintricarboxylic acid | EV71 3Dpol | EV71 (TW/4643/98) | Vero | [64] | |
| FNC | EV71 3Dpol | EV71-CC063 | RD | One-day-old ICR neonatal mice | [65] |
| Targeting virus capsid protein | |||||
| Monoclonal antibody D5 (mouse) | The surface exposed GH loop of VP1 | EV71-G082 | RD | [69] | |
| Monoclonal antibody D5 (plant) | SP70 peptide of the VP1 on EV71 | EV71 (MA V-W) | RD | Five-day-old ICR mice | [70] |
| SAMHD1 | Domain in VP1 that binds to VP2 of EV71 | EV71 | HEK293T | [71] | |
| Imidazolidinones (compound 27) | The hydrophobic pocket of VP1 | EV71 | RD | [72] | |
| E151 | The 5-fold axis of EV71 | EV71-B2 | RD | 14-day-old AG129 mice | [73] |
| Regulation of host cell environment | |||||
| STE. | EV71 (CA-BrCr-70) | RD, Vero | Seven-day-old ICR mice | [75] | |
| RA | EV71 (CA-BrCr-70) | RD | [76] | ||
| EGCG | EV71-BrCr | Vero | [77] | ||
| GCG | EV71-BrCr | Vero | [77] | ||
| DIDS | Blocking the current mediated by EV71 2B | EV71-SZ98 | RD | [79] | |
| Targeting MAPK signaling pathway | |||||
| PD169316 | EV71-GDV103 | RD, HeLa, Vero | Seven-day-old specific pathogen free mice | [81] | |
| Cathelicidin (LL-37) | Enhance host immune response | EV71 (Fuyang 0805) | Vero | Newborn ICR mice (day 2–3, n=5) | [82] |
| Cathelicidin (CRAMP) | Enhance host immune response | EV71 (Fuyang 0805) | Vero | Newborn ICR mice (day 2–3, n=5) | [82] |
| Other | |||||
| Rheum emodin | Regulating CDK2 and cyclin A2 expression | EV71 (Changchun077) | Vero | [94] | |
| Allophycocyanin | EV71-2231-TW | RD | [95] | ||
| Curcumin | Inhibit PKC δ phosphorylation | EV71 (Tainan/4643/98) | HT29 | [96] | |
| Ginsenoside Rb1 | Stimulating immune response | EV71-695F | RD | Two-day-old suckling mice | [97] |
| PML | EV71 VP1 | EV71-BrCr-TR | Vero, RD, HeLa | Three-day-old ICR mice | [98] |
| Acarbose | Blocking EV71 surface receptor binding sites | EV71-SK-EV006 | DLD1, FHC CCD18-Co | One-day-old ICR suckling mice | [99] |
| RD: rhabdomyosarcoma cells; Vero: African green monkey kidney cells; HeLa: henrietta lacks cells; SK-N-SH: Henrietta Lacks cells; Jurkat: human T lymphocytes cells; HEK293 T: human embryonic kidney cells; HT29: human colon cancer cell; DLD1: human colorectal adenocarcinoma epithelial cells; FHC: human normal colorectal mucosa cells; CCD18-Co: normal human colon fibroblast adherent cells. | |||||
1.1 针对病毒进入的方式 1.1.1 靶向PSGL-1位点
P-选择素糖蛋白配体1 (P-selectin glycoprotein ligand 1,PSGL-1)是EV71的主要功能性受体,表达于骨髓细胞及白细胞表面,其N端残基能够介导EV71的结合和感染,基因多态性显著影响宿主对病毒的易感性[34]。研究发现,经横纹肌肉瘤(rhabdomyosarcoma,RD)细胞传代后的EV71 VP1残基突变株,仍与PSGL-1有极强结合能力[35]。因此靶向PSGL-1成为了抑制病毒结合的主要选择。Ren等[36]对PSGL-1介导的病毒入侵作用进行了阐述,并发现靶向PSGL-1的单克隆抗体KPL1可以阻断EV71与PSGL-1的结合,减少被感细胞的死亡,为靶向治疗EV71打下基础。
1.1.2 靶向DC-SIGN位点树突状细胞特异性细胞间黏附分子-3结合非整合素(dendritic cell specific intercellular adhesion molecule-3 grabbing non integrin,DC-SIGN)作为EV71受体,与PSGL-1为协同作用,广泛分布于树突状细胞。研究发现,重症患者中DC-SIGN含量明显高于健康组,其单核苷酸多态性与EV71严重程度相关[37]。Ren等[38]通过利用小干扰RNA (small interfering RNA,siRNA)特异性敲除DC-SIGN后显著降低了病毒与细胞的结合率。酵母甘露聚糖也可阻断DC-SIGN的表达,降低细胞中EV71病毒量及VP1蛋白含量[39]。证实了DC-SIGN作为靶位点的有效性。
1.1.3 靶向SCARB2位点清道夫受体B2 (scavenger receptor class B member 2,SCARB2)作为EV71另一种功能性受体,广泛表达于人的各种组织,如胃底腺、肠黏膜上皮、支气管、肺细胞以及中枢神经系统的神经元等,其主要通过152–163(α5)和183–193(α7)螺旋与EV71 VP1的GH和VP2的EF环相互作用来介导病毒感染[40–41]。Zhang等[42]构建出SCARB2的单克隆抗体JL2,观察到其可与SCARB2的2、5和14α螺旋相互作用,阻止EV71与SCARB2的结合,从而抑制细胞病变。
1.2 针对病毒核酸翻译的方式抑制IRES的活性:EV71翻译的起始依赖内部核糖体进入位点(IRES)[43–44]。EV71 IRES依赖性翻译需要RNA解旋酶DDX3X,其通过与截短的真核翻译起始因子4G (eIF4G)的相互作用,解开IRES结构域Ⅵ的二级结构促进核糖体进入[45]。因此靶向抑制EV71 IRES元件的活性,可以抑制EV71 RNA翻译的起始,阻碍蛋白合成,从而抑制成熟病毒的产生以及装配[46]。
Gunaseelan等[47]筛选类黄酮化合物文库,发现樱桃苷(prunin)有效降低EV71感染BALB/c小鼠的临床症状和死亡率,明显降低RD细胞中EV71 RNA的含量,半数有效浓度(EC50)=0.115 3 µmol/L,半数致死浓度(CC50)=2.715 µmol/L。发现EV71经prunin处理连续传代后于第13代产生了抗性突变体,该突变允许其通过差异调节IRES反式作用因子Sam68和核内不均一性核糖核蛋白K (hnRNP K)的募集来克服抗病毒作用,但该突变体可用第二药物进行治疗。这些研究确立了prunin可作为EV71治疗剂进一步开发为候选药物。
1.3 针对病毒多蛋白加工的方式 1.3.1 抑制3Cpro的活性EV71 3C蛋白是半胱氨酸蛋白酶,通过蛋白加工、切割宿主蛋白来促进病毒复制、抑制Ⅰ型干扰素反应来逃避先天免疫、激活caspase诱导宿主细胞凋亡等,其基因多态性与临床严重程度和病毒复制有关[48]。此前发现EV71 3Cpro可通过切割端粒结合蛋白PinX1促进细胞凋亡[49]。近年发现EV71感染或异位表达3Cpro可切割核内不均一性核糖核蛋白A1 (hnRNP A1),消除其与Apaf-1 IRES的结合,导致Apaf-1的IRES依赖性合成、caspase-3的激活与细胞凋亡,进而释放病毒颗粒[50]。这些结果证实了EV71 3Cpro在病毒增殖过程中的重要性以及其作为靶位点的有效性。
Yao等[51]发现槲皮素(duercetin)可以插入EV71 3Cpro的底物结合区域中,阻断底物识别,抑制EV71 3Cpro的活性,但并不影响蛋白酶2Apro或RNA聚合酶3Dpol的活性,在RD细胞中,EC50=12.1 µmol/L,CC50>200.0 µmol/L。此外Li等[52]就针对EV71 3Cpro的晶体结构,用山口酯化反应代替典型的钌催化的烯烃复分解反应,设计出了具有确定构象的大环拟肽(macrocyclic peptidomimetic),此新型药物可准确靶向于3Cpro,抑制其活性,EC50=4.5 µmol/L。
1.3.2 抑制2Apro的活性EV71 2A蛋白是含有150个氨基酸残基,具有半胱氨酸蛋白酶活性的蛋白酶,主要参与多蛋白的加工、抑制宿主蛋白合成、逃避先天免疫和诱导细胞死亡等[53–54]。2Apro可诱导TXNIP介导的凋亡,调节METTL3的亚细胞位置来放大其自身基因表达的内部机制,参与病毒RNA的修饰[55–56];还可通过切割eIF4GI来诱导非典型应激颗粒aSG的形成,以隔离细胞的基因,促进病毒的翻译[57]。研究发现,六氨基酸肽LVLQTM是EV71 2Apro的有效底物类似物,可与其直接相互作用,结合于2Apro的活性位点,抑制2Apro的eIF4G切割活性以及细胞中的EV71的复制,是直接靶向2Apro、抑制EV71复制的一个有效选择[58]。
1.4 针对病毒核酸复制的方式抑制3Dpol的活性:病毒侵入宿主细胞后,以正链RNA作为模板,并在病毒自身RNA聚合酶的指导下合成负链RNA进行扩增,过程主要由RNA依赖的RNA聚合酶(3Dpol)指导[59–60]。EV71 3Dpol也参与caspase的激活,抑制MDA-5介导干扰素β (interferon β,IFN-β)启动子的激活,具有拮抗宿主的作用[61]。
Li等[62]将3Dpol作为抗病毒研究的靶位点,构建出该位点的单克隆抗体(3A12和2A10),直接干扰3Dpol活性,显著抑制了病毒在体外的复制,且在应用浓度下细胞损伤小。Wang等[63]发现胞苷脱氨酶(APOBEC3G,A3G)不仅能够抑制乙型肝炎病毒和丙型肝炎病毒复制,也可以与EV71 3Dpol及病毒RNA相互作用,包装到子代病毒中以降低其传染性,其异位表达抑制了EV71的复制。除金三羧酸(aurintricarboxylic acid)[64]外,Xu等[65]发现抑制艾滋病的小核苷类似物抑制剂FNC在EV71和CA16感染新生小鼠模型中,每2天以1 mg/kg体重进行FNC治疗,成功保护小鼠免受EV71和CA16病毒的致命攻击,并降低了各种组织中的病毒载量,EC50= 0.016 87 µmol/L,CC50=3.238 µmol/L。
1.5 针对病毒衣壳蛋白的方式结构蛋白VP1是EV71的衣壳蛋白,决定着病毒的基因型,极易发生氨基酸序列改变,是病毒的毒力决定簇,也是导致患者出现肺水肿的直接原因[66–67]。当VP1氨基酸重组时会出现病毒复制以及神经细胞自噬水平的不同[68]。鼠抗EV71单克隆抗体D5,其可与VP1 GH环上的SP70肽特异性结合,阻断病毒附着和内化,以二价结合模式稳定病毒,有效中和EV71感染,半数抑制浓度IC50=0.324 μg/mL[69]。在此基础上以烟草花叶作为抗体生产系统,构建出D5单克隆抗体也可直接靶向表位VP1 GH环的SP70肽,中和EV71,IC50=1.53 µg/mL,这有效降低了药物研发成本[70]。
组氨酸-天冬氨酸结构域蛋白1 (SAMHD1)是宿主限制性因子,可竞争作用于EV71 VP1与VP2的结合位点,阻断病毒颗粒的组装[71]。咪唑烷酮(imidazolidinones)作为杂环化合物,其化合物27不仅对HIV有效,还可以通过靶向EV71衣壳蛋白VP1,抑制病毒吸附和RNA脱膜[72]。磺化偶氮染料-精黑BN (E151)作为食品添加剂,可与EV71 VP1形成5重轴顶点相互作用,阻止病毒进入,在附着后阶段显著抑制EV71,IC50= 10.10 µmol/L[73]。
1.6 针对宿主细胞环境的方式 1.6.1 抗氧化剂氧化还原稳态是决定传染病预后的重要宿主因素,应激能够促进病毒复制。EV71感染细胞上调了线粒体的生物生成,子代病毒诱导宿主细胞氧化应激促进病毒复制,因此抑制活性氧(ROS)也成为抗病毒复制的有效方法。
荆芥(schizonepeta tenuifolia Briq.,STE)是一种天然唇形科植物,目前用于银翘汤中治疗轻症疾病[74]。Chen等发现STE不仅能够减少病毒的吸附和入侵,抑制EV71 2A蛋白酶切割eIF4G,还可以抑制病毒诱导活性氧(ROS)形成以及核内不均一核糖核蛋白A1 (hnRNP A1)从细胞核重新定位到细胞质,减少细胞病变效应,可作为保健食品或者潜在抗病毒药物进行开发[75]。
迷迭香酸(rosmarinic acid,RA)是山茱萸提取物(melissaofficinalis,MO),其抗病毒作用机制与STE类似,通过抑制ROS介导的p38激酶激活,以及诸如hnRNP A1易位和EPS15等下游分子调节EV71感染细胞的膜转运过程,降低EV71的感染,IC50=45.92±1.05 µg/mL,可作为治疗和预防EV71感染的候选药物[76]。
绿茶中的天然抗氧化剂表没食子儿茶素没食子酸酯(epigallocatechin gallate,EGCG)和没食子儿茶素没食子酸酯(gallocatechin gallate,GCG),可以减少ROS的产生,并且可逆转在葡萄糖-6-磷酸脱氢酶(G-6-PD)缺乏的细胞中EV71复制的增强作用[77]。
1.6.2 离子通道阻断剂柯萨奇病毒B组三型(CVB3)的蛋白质2B可以使游离细胞溶质Ca2+的浓度增加,以此促进病毒的释放[78]。Xie等[79]将EV71 2B蛋白与CVB3 2B蛋白进行基因序列比较,并通过亚细胞定位分析、双电极电压钳记录膜电流、离子置换实验等方法,发现EV71中的2B蛋白也具有离子通道属性,可介导氯离子依赖性电流,促进病毒释放。同时发现DIDS (4, 4′-diisothiocyano- 2, 2′-stilbenedisulfonic acid)作为离子通道阻断剂,明显抑制RD细胞中EV71病毒的产生,细胞病变效应也验证了这一结果,为抗病毒治疗提供新角度。
1.7 针对信号通路的疗法靶向MAPK信号通路:EV71感染后,丝裂原活化蛋白激酶(mitogen-activated protein kinase,MAPK)信号通路被激活,刺激炎性细胞因子的释放,有利于EV71的感染[80]。Zhang等[81]发现,PD169316作为p38-MAPK信号通路的特定p38抑制剂,不仅可抑制EV71复制,还减少了EV71诱导的细胞凋亡。动物实验表明,PD169316能够减少组织损伤并抑制炎性细胞因子的释放,减轻由EV71引起的乳鼠疾病。
人LL-37和小鼠CRAMP是内源性抗菌多肽(cathelicidin),对囊膜病毒具有杀灭作用。当新生ICR小鼠感染了非囊膜病毒EV71后不同组织中的CRAMP表达显著上调,LL-37或CRIMP能够上调IFN-β的表达及IRF3的磷酸化,下调IL-6及MAPK的活性直接或间接抑制病毒复制,为抗EV71感染的肽药物开发提供了有效的候选药物[82]。
1.8 其他方式 1.8.1 微小核糖核酸(miRNAs)miRNAs作为细胞内各种信号通路重要的调节因子,参与基因的表达调控,在病毒感染过程中发挥着不可或缺的作用[83]。
Li等[84]发现,EV71感染会降低miR-9的表达,同时诱导促炎因子(TNF-α、IL-6和IL-1)和干扰素(IFN-a和IFN-b)的高表达。如果诱导miR-9的过表达就能够降低VP1蛋白的表达和促炎因子的释放,通过介导RIG-Ⅰ信号通路的NF-κB活性在细胞和小鼠模型中发挥抗EV71作用。
Wang等[85]发现,EV71感染后,miR-30a会被外泌体包裹,以将其功能转移至受体巨噬细胞,通过靶向髓样分化因子88 (MyD88)抑制Ⅰ型干扰素反应,促进病毒复制。miR-146a也通过外泌体介导转移,抑制Ⅰ型干扰素反应,促进EV71感染,为反向抗病毒研究提供新思路[83]。
除此之外,还有多种miRNA在EV71感染过程中发挥重要调控作用。如EV71通过上调miRNA启动子的甲基化抑制miR-17-92簇[86]。宿主miR-494-3p通过直接靶向PTEN促进EV71复制[87]。敲除miR-876-5p后可减少细胞中病毒RNA,miR-27a可通过靶向EGFR mRNA,抑制病毒复制[88–89]。miR-103/miR-107通过调节SOCS3/STAT3途径抑制肠病毒71复制并促进Ⅰ型干扰素应答[90]。这些均证实了miRNA在抗病毒治疗过程中的重要性,以及其在病毒感染过程中的调控能力,由于miRNA靶向位点的多样、调节通路复杂、易降解等特性,采用miRNA进行EV71感染的治疗仍有许多困难需要攻克。
1.8.2 干扰素干扰素(interferon,IFN)是免疫调节的重要物质,已经应用于肿瘤及其他疾病的临床治疗。EV71侵入宿主细胞后,单核细胞和T淋巴细胞便会分泌干扰素发挥抗病毒效应[91]。Liu等[92]通过体外实验发现,在小鼠接种EV71前注射试剂上调Ⅰ型干扰素表达量,能够明显减少病毒含量,降低小鼠死亡率。Su等[93]在新生C57BL/6J小鼠腹膜内注射重组IFN-λ2后发现其抑制EV71复制并保护小鼠免受病毒攻击。这些结果均证实了干扰素的抗病毒作用,合理地利用可以发挥最优的抗病毒效果。
1.8.3 天然物质大黄素(rheum emodin)是从中药大黄中提取的一种活性成分,EV71感染人肺成纤维细胞系MRC5,经大黄素治疗后,病毒基因组水平降低了5.34倍,病毒蛋白表达降低了近30倍,EV71毒力降低了0.331 07倍,显著减少了MRC5细胞在S期的细胞周期停滞,具有抗EV71的效果[94]。
别蓝藻素(allophycocyanin)是从螺旋藻中提取的一种红色荧光蛋白,能够在病毒作用宿主细胞前后发挥作用,降低病毒的RNA合成、抑制病毒复制、延缓凋亡进程和减少宿主细胞的病变,IC50=(0.045±0.012) µmol/L[95]。
姜黄素(curcumin)作为抗癌活性物质,Huang等[96]发现姜黄素并不是如此前所说通过调节病毒吸附或者凋亡来发挥抗病毒作用,而是通过降低蛋白激酶Cδ (PKCδ)的磷酸化,减少肠道上皮细胞中的病毒翻译作用,来增加宿主细胞的生存能力,抑制肠道病毒感染。
人参皂苷Rb1 (ginsenoside Rb1)是西洋参中含量最丰富的三萜皂苷,Kang等[97]最新发现,Rb1能够以剂量依赖的方式降低EV71感染RD细胞的CPE (cytopathic effect)及病毒VP1的表达,有助于增强Ⅰ型IFN的表达,在感染的乳鼠中表现出比广谱抗病毒药物更强的抗病毒活性, 可以作为一种免疫增强剂,EC50=27.64 µmol/L。
PML (polysaccharide from Monostroma latissimum)是在绿藻单基质中分离出的硫酸鼠李糖(sulfated rhamnan),Wang等[98]发现在病毒吸附前或期间,PML可靶向衣壳蛋白VP1来抑制病毒复制,还可通过调节表皮生长因子受体(EGFR)/磷酸肌醇3-激酶(PI3K)/蛋白激酶B (Akt)途径的信号来抑制病毒吸附后感染的早期步骤,IC50=(0.5±0.3) µg/mL,无细胞毒性。
Feng等[99]给乳鼠口服易感染小鼠神经细胞的GFP-EV71后,检测病毒在体内的动态分布及EV71在体内从肠到外周组织运输的动态途径,发现阿卡波糖(acarbose)可能通过阻断EV71病毒粒子表面上的受体结合位点或抑制细胞表面上的各种乙醇受体来减少EV71从肠到全身的动态转移。阿卡波糖及其类似物可能是预防EV71感染的潜在药物。
2 EV71疫苗发展现状为防止手足口病流行,疫苗接种是目前有效预防疾病的唯一措施[100–101]。在面对疫苗株选取、中和抗体检测、疫苗抗原定量、动物模型和临床验证等难点,各类疫苗的研发从未停止。以下就灭活疫苗、病毒样颗粒疫苗和肽疫苗研究进展进行总结。
2.1 灭活全病毒疫苗灭活全病毒疫苗是指通过一定手段处理病原体使其失活,得到的无免疫原性的病原体制备的疫苗。自2015年我国成功研制出EV71全病毒灭活疫苗,于2016年投入使用,该疫苗在后期临床调查中均表现出无毒性、有限期长、安全性高和免疫原性强等特点,接种后可刺激免疫系统,上调干扰素、白介素以及IgG等含量[100–102]。但是仍有部分问题如少许接种者出现发热、过敏性皮疹、接种EV71疫苗后仍发展为脑炎患者的现象,并且当应急接种时,针对EV71的中和抗体的短期动态变化是未知的[103–105]。
Fan等[106]研发了一种二价灭活的EV71/ CA16疫苗,通过皮内途径注射BALB/c小鼠,在接种疫苗的局部上皮组织中检测到免疫信号分子的mRNA,同时免疫相关趋化因子、干扰素上调,28 d进行二次免疫后,成年小鼠会引发中和抗体和特异性T细胞反应,后代小鼠具有抵御病毒的能力。
由于疫苗制备的相似性以及患病的重叠性,联合疫苗的想法也由此诞生,用一种疫苗预防两种疾病,甲型肝炎(HAV)疫苗是中国国家免疫计划的一部分,是联合疫苗优选。Yang等[107]将HAV灭活疫苗和EV71灭活疫苗进行联合制备了HAV-EV71灭活疫苗,在单剂量接种后,大鼠未引发不良反应,且观察到双抗体的产生,3次接种后体重等生理指标未发生变化,无明显过敏反应。联合疫苗或将成为未来疫苗研发的新趋势。
2.2 病毒样颗粒疫苗病毒样颗粒疫苗是指去除遗传物质,仅存在病毒衣壳的具有免疫原性的蛋白制备的疫苗,在免疫原性评估中引发了可与灭活疫苗媲美的高而持久的中和抗体反应[108–109]。此前Wang等[110]利用昆虫表达系统,构建出gag-VP1 VLP组装体,通过体外小鼠实验,发现实验小鼠的体液免疫以及细胞免疫应答水平均高于对照组,并从后代体内检测出EV71抗体。
最近Luo等[111]通过共表达EV71 P1 (在多角体蛋白启动子下)和3CD (在CMV-IE启动子下)蛋白的重组杆状病毒(Bac-P1-3CD)制备EV71病毒样颗粒及其嵌合体,构建出显示保守的柯萨奇病毒A16表位的嵌合EV71-VLP,结果显示实验母鼠、用免疫小鼠的血清被动转移的新生小鼠完全免受致命EV71攻击时,部分免受致命的CA16感染。
为高效研发疫苗,Yang等[112]研发了产率高、工艺简单的重组技术,其在毕赤酵母中构建和表达了EV71的P1和3C基因,基于密码子优化的P1和3C基因,EV71-VLPs在毕赤酵母系统中高效表达,表达量达到270 mg/L。这些发现为手足口病今后的预防提供了一些可行的治疗方案,对疫苗的完善提供了有价值的参考。
2.3 肽疫苗除灭活疫苗、病毒样颗粒疫苗外,肽疫苗的研发也取得了可观进展。Lei等[113]从患者体内分离出EV71菌株,从VP1蛋白截断的20种合成肽中,筛选出了免疫原性较强的3种肽(肽2、肽4和肽8)制备了肽疫苗,结果显示肽疫苗改善了炎症,降低了肌肉和小肠中的病毒颗粒水平,并保护脑组织免受EV71感染,虽然其免疫效果不如灭活疫苗,但损害较小,可用于研究抗EV71疫苗的具体机制。
此外Liu等利用融合PCR技术扩增了EV71衣壳蛋白(VP2 N端180个氨基酸,VP3 N端120个氨基酸,VP1 C端131个氨基酸)的DNA片段,在T7启动子的控制下,将3个片段连接在一起,形成48 kDA融合蛋白;这种肽疫苗组装后类似病毒颗粒,接种后可观察新生小鼠的主动免疫,此外其诱导产生的特异性血清抗体可使患病小鼠对EV71产生有效抵抗,具有安全和生产优势[114]。但是即使有辅助剂,肽疫苗免疫效果仍较弱,为增强肽疫苗的免疫强度,克服其局限性,Kim等利用纳米技术将EV71-VP1表位肽和间隔交联剂偶联到长链脂肪酸的N端,开发了一种在生理pH值(pH 7.4)下自组装成纳米纤维的肽两亲物PA (peptide amphiphile),结果显示PA组表现出比肽组更高的免疫反应,有效地增强了对EV71感染的免疫反应,克服了肽疫苗的局限性[115]。
3 展望回顾手足口病暴发至今,随着致病机理的不断剖析,治疗手段也在不断完善。虽然目前仍未开发出特效抗病毒药物,但临床上已经通过广谱抗病毒药物联合对症治疗来对抗手足口病。EV71灭活疫苗的成功研发、中小学对疾病预防的重视,大幅降低了手足口病的暴发几率。
但是仍有一些问题需要克服:(1) EV71具有嗜神经性,机理并不清楚,严重程度下造成的神经系统疾病能否避免。(2) 包括严重急性呼吸系统综合征冠状病毒-2 (SARS-CoV-2)在内,均具有逃避先天免疫的能力,能否克服也是一个难点。(3) 病毒具有潜伏期,大多数发病时已经错过最佳治疗时期,如果能在病毒潜伏期就检测、杜绝病毒,不仅降低了治疗成本,也降低了对患者的损害。(4) 抗病毒药物是治疗的另一重要手段,能够从根本上扼杀病毒,因此其研发是必不可少的。(5) 虽然已经批准3种灭活EV71疫苗,但大部分农村地区疫苗覆盖率较低,家长接种意愿不高,因此应该加大宣传力度,重视疾病防御[116]。
| [1] | Fu XM, Wan ZZ, Li YP, Hu YH, Jin X, Zhang CY. National epidemiology and evolutionary history of four hand, foot and mouth disease-related enteroviruses in China from 2008 to 2016. Virologica Sinica, 2020, 35(1): 21-33. DOI:10.1007/s12250-019-00169-2 |
| [2] | Yu FY, Zhu RN, Jia LP, Song QW, Deng J, Liu LY, Zhao LQ, Qian Y. Sub-genotype change and recombination of coxsackievirus A6s may be the cause of it being the predominant pathogen for HFMD in children in Beijing, as revealed by analysis of complete genome sequences. International Journal of Infectious Diseases, 2020, 99: 156-162. DOI:10.1016/j.ijid.2020.07.010 |
| [3] | Bian LL, Wang YP, Yao X, Mao QY, Xu M, Liang ZL. Coxsackievirus A6: a new emerging pathogen causing hand, foot and mouth disease outbreaks worldwide. Expert Review of Anti-Infective Therapy, 2015, 13(9): 1061-1071. DOI:10.1586/14787210.2015.1058156 |
| [4] | Lim CTK, Jiang L, Ma S, James L, Ang LW. Basic reproduction number of coxsackievirus type A6 and A16 and enterovirus 71: estimates from outbreaks of hand, foot and mouth disease in Singapore, a tropical city-state. Epidemiology and Infection, 2016, 144(5): 1028-1034. DOI:10.1017/S0950268815002137 |
| [5] | Kou ZQ, Jia J, Liu XH, Luo TT, Xin XL, Gong JL, Zhang JF, Sun DP, Jiang FC, Gao RQ. Epidemiological characteristics and spatial-temporal clusters of hand, foot and mouth disease in Qingdao City, China, 2013-2018. PLoS One, 2020, 15(6): e0233914. DOI:10.1371/journal.pone.0233914 |
| [6] | Qian HK, Huo D, Wang XL, Jia L, Li XT, Li J, Gao ZY, Liu BW, Tian Y, Wu XN, Wang QY. Detecting spatial-temporal cluster of hand foot and mouth disease in Beijing, China, 2009-2014. BMC Infectious Diseases, 2016, 16: 206. DOI:10.1186/s12879-016-1547-6 |
| [7] | Jiang FC, Yang F, Chen L, Jia J, Han YL, Hao B, Cao GW. Meteorological factors affect the hand, foot, and mouth disease epidemic in Qingdao, China, 2007-2014. Epidemiology and Infection, 2016, 144(11): 2354-2362. DOI:10.1017/S0950268816000601 |
| [8] | Woodland DL. Hand, foot, and mouth disease. Viral Immunology, 2019, 32(4): 159. DOI:10.1089/vim.2019.29037.dlw |
| [9] | Wang XM, Zhu CF, Bao WG, Zhao K, Niu JQ, Yu XF, Zhang WY. Characterization of full-length enterovirus 71 strains from severe and mild disease patients in northeastern China. PLoS One, 2012, 7(3): e32405. DOI:10.1371/journal.pone.0032405 |
| [10] | Wang JY, Teng Z, Cui XQ, Li CS, Pan H, Zheng YX, Mao SH, Yang YY, Wu LM, Guo XK, Zhang X, Zhu YZ. Epidemiological and serological surveillance of hand-foot-and-mouth disease in Shanghai, China, 2012-2016. Emerging Microbes & Infections, 2018, 7(1): 1-12. |
| [11] |
Zhou LX, Li YN, Mai ZG, Qiang XH, Wang SZ, Yu TO, Fang B, Wen WB. Clinical feature of severe hand, foot and mouth disease with acute pulmonary edema in pediatric patients. Chinese Critical Care Medicine, 2015(7): 563-567.
(in Chinese) 周立新, 李轶男, 麦志广, 强新华, 汪首振, 誉铁鸥, 方滨, 温伟标. 危重型手足口病合并急性肺水肿患儿的临床特点. 中华危重病急救医学, 2015(7): 563-567. DOI:10.3760/cma.j.issn.2095-4352.2015.07.005 |
| [12] | Schmidt NJ, Lennette EH, Ho HH. An apparently new enterovirus isolated from patients with disease of the central nervous system. The Journal of Infectious Diseases, 1974, 129(3): 304-309. DOI:10.1093/infdis/129.3.304 |
| [13] | Shen WC, Chiu HH, Chow KC, Tsai CH. MR imaging findings of enteroviral encephaloymelitis: an outbreak in Taiwan. AJNR American Journal of Neuroradiology, 1999, 20(10): 1889-1895. |
| [14] | Chen MM, Ju Y, Chen M, Xie ZG, Zhou KJ, Tan Y, Mo JJ. Epidemiological and genetic characteristics of EV71 in hand, foot, and mouth disease in Guangxi, Southern China, from 2010 to 2015. PLoS One, 2017, 12(12): e0188640. DOI:10.1371/journal.pone.0188640 |
| [15] | McMinn PC. Recent advances in the molecular epidemiology and control of human enterovirus 71 infection. Current Opinion in Virology, 2012, 2(2): 199-205. DOI:10.1016/j.coviro.2012.02.009 |
| [16] | Yi LN, Lu J, Kung HF, He ML. The virology and developments toward control of human enterovirus 71. Critical Reviews in Microbiology, 2011, 37(4): 313-327. DOI:10.3109/1040841X.2011.580723 |
| [17] | Xu LZ, Qi MD, Ma CL, Yang MM, Huang P, Sun J, Shi JD, Hu YZ. Natural intertypic and intratypic recombinants of enterovirus 71 from mainland China during 2009-2018: a complete genome analysis. Virus Genes, 2021, 57(2): 172-180. DOI:10.1007/s11262-021-01830-3 |
| [18] | Too IHK, Yeo H, Sessions OM, Yan B, Libau EA, Howe JLC, Lim ZQ, Suku-Maran S, Ong WY, Chua KB, Wong BS, Chow VTK, Alonso S. Enterovirus 71 infection of motor neuron-like NSC-34 cells undergoes a non-lytic exit pathway. Scientific Reports, 2016, 6: 36983. DOI:10.1038/srep36983 |
| [19] | Chang CY, Li JR, Ou YC, Chen WY, Liao SL, Raung SL, Hsiao AL, Chen CJ. Enterovirus 71 infection caused neuronal cell death and cytokine expression in cultured rat neural cells. IUBMB Life, 2015, 67(10): 789-800. DOI:10.1002/iub.1434 |
| [20] | Luo Z, Su R, Wang WB, Liang YC, Zeng XF, Shereen MA, Bashir N, Zhang Q, Zhao L, Wu KL, Liu YL, Wu JG. EV71 infection induces neurodegeneration via activating TLR7 signaling and IL-6 production. PLoS Pathogens, 2019, 15(11): e1008142. DOI:10.1371/journal.ppat.1008142 |
| [21] | You L, Chen JB, Liu WY, Xiang Q, Luo Z, Wang WB, Xu W, Wu KL, Zhang Q, Liu YL, Wu JG. Enterovirus 71 induces neural cell apoptosis and autophagy through promoting ACOX1 downregulation and ROS generation. Virulence, 2020, 11(1): 537-553. DOI:10.1080/21505594.2020.1766790 |
| [22] | Hu YJ, Xu YY, Huang ZM, Deng Z, Fan JY, Yang RA, Ma HY, Song J, Zhang YH. Transcriptome sequencing analysis of SH-SY5Y cells infected with EV71 reveals the potential neuropathic mechanisms. Virus Research, 2020, 282: 197945. DOI:10.1016/j.virusres.2020.197945 |
| [23] | Shang J, Zheng Y, Mo JY, Wang WB, Luo Z, Li YK, Chen XL, Zhang QW, Wu KL, Liu WY, Wu JG. Sox4 represses host innate immunity to facilitate pathogen infection by hijacking the TLR signaling networks. Virulence, 2021, 12(1): 704-722. DOI:10.1080/21505594.2021.1882775 |
| [24] | Cheng ML, Chien KY, Lai CH, Li GJ, Lin JF, Ho HY. Metabolic reprogramming of host cells in response to enteroviral infection. Cells, 2020, 9(2): 473. DOI:10.3390/cells9020473 |
| [25] | Gao WY, Hou M, Liu X, Li ZL, Yang YJ, Zhang WY. Induction of SOCS expression by EV71 infection promotes EV71 replication. BioMed Research International, 2020, 2020: 2430640. |
| [26] | Du HW, Yin PQ, Yang XJ, Zhang LL, Jin Q, Zhu GF. Enterovirus 71 2C protein inhibits NF-κB activation by binding to RelA(p65). Scientific Reports, 2015, 5: 14302. DOI:10.1038/srep14302 |
| [27] | Wen WH, Qi ZX, Wang J. The function and mechanism of enterovirus 71 (EV71) 3C protease. Current Microbiology, 2020, 77(9): 1968-1975. DOI:10.1007/s00284-020-02082-4 |
| [28] | Gunaseelan S, Chu JJH. Identifying novel antiviral targets against enterovirus 71: where are we?. Future Virology, 2017, 12(4): 171-191. |
| [29] | Wang B, Li JL, Wang Y, Du N, Sun LY, Xiao HM, Zhao Y, Bao WG, Zhang WY. Understanding the epidemiological characteristics of EV71 and CVA16 infection to aid the diagnosis and treatment of hand, foot, and mouth disease. Journal of Medical Virology, 2019, 91(2): 201-207. DOI:10.1002/jmv.25282 |
| [30] | Xu Y, Wu YF, Luo HH, Zhang DD, Wu Y, Hu P. Acute kidney injury secondary to severe hand, foot and mouth disease caused by enterovirus-A71: hypertension is a common. Journal of Tropical Pediatrics, 2018, 65(5): 510-513. |
| [31] | Casaos J, Gorelick NL, Huq S, Choi J, Xia YX, Serra R, Felder R, Lott T, Kast RE, Suk I, Brem H, Tyler B, Skuli N. The use of ribavirin as an anticancer therapeutic: will it go viral?. Molecular Cancer Therapeutics, 2019, 18(7): 1185-1194. DOI:10.1158/1535-7163.MCT-18-0666 |
| [32] | Tate PM, Mastrodomenico V, Mounce BC. Ribavirin induces polyamine depletion via nucleotide depletion to limit virus replication. Cell Reports, 2019, 28(10): 2620-2633.e4. DOI:10.1016/j.celrep.2019.07.099 |
| [33] | Cursino CN, Monteiro P, Duarte G, Vieira TBQ, Crisante VC, Giordani F, Xavier AR, De Almeida RMVR, Calil-Elias S. Predictors of adverse drug reactions associated with ribavirin in direct-acting antiviral therapies for chronic hepatitis C. Pharmacoepidemiology and Drug Safety, 2019, 28(12): 1601-1608. DOI:10.1002/pds.4904 |
| [34] | Yen TY, Shih WL, Huang YC, Lee JT, Huang LM, Chang LY. Polymorphisms in enterovirus 71 receptors associated with susceptibility and clinical severity. PLoS One, 2018, 13(11): e0206769. DOI:10.1371/journal.pone.0206769 |
| [35] | Chang CK, Wu SR, Chen YC, Lee KJ, Chung NH, Lu YJ, Yu SL, Liu CC, Chow YH. Mutations in VP1 and 5′-UTR affect enterovirus 71 virulence. Scientific Reports, 2018, 8: 6688. DOI:10.1038/s41598-018-25091-7 |
| [36] | Ren XX, Li C, Xiong SD, Huang Z, Wang JH, Wang HB. Antibodies to P-selectin glycoprotein ligand-1 block dendritic cell-mediated enterovirus 71 transmission and prevent virus-induced cells death. Virulence, 2015, 6(8): 802-808. DOI:10.1080/21505594.2015.1094605 |
| [37] | Li YP, Wang MQ, Liu CR, Deng HL, Wu Y, Dang SS, Xu LH. Polymorphisms in the DC-SIGN gene and their association with the severity of hand, foot, and mouth disease caused by enterovirus 71. Archives of Virology, 2021, 166(4): 1133-1140. DOI:10.1007/s00705-021-04991-6 |
| [38] | Ren XX, Ma L, Liu QW, Li C, Huang Z, Wu L, Xiong SD, Wang JH, Wang HB. The molecule of DC-SIGN captures enterovirus 71 and confers dendritic cell-mediated viral trans-infection. Virology Journal, 2014, 11: 47. DOI:10.1186/1743-422X-11-47 |
| [39] |
Zhang L, Xu Z, Shen DH, Zhang JF. DC-SIGN molecules promote EV71 infection in dendritic cells in vitro. Chinese Journal of Clinical Laboratory Science, 2019, 37(4): 274-277.
(in Chinese) 张莉, 许中, 沈东华, 张剑峰. DC-SIGN受体促进肠道病毒71型体外感染树突状细胞. 临床检验杂志, 2019, 37(4): 274-277. |
| [40] | Zhou DM, Zhao YG, Kotecha A, Fry EE, Kelly JT, Wang XX, Rao ZH, Rowlands DJ, Ren JS, Stuart DI. Unexpected mode of engagement between enterovirus 71 and its receptor SCARB2. Nature Microbiology, 2019, 4(3): 414-419. DOI:10.1038/s41564-018-0319-z |
| [41] | Jin YF, Sun TT, Zhou GY, Li D, Chen SY, Zhang WG, Li XY, Zhang RG, Yang HY, Duan GC. Pathogenesis study of enterovirus 71 using a novel human SCARB2 knock-in mouse model. mSphere, 2021, 6(2): e0104820. DOI:10.1128/mSphere.01048-20 |
| [42] | Zhang XY, Yang P, Wang N, Zhang JL, Li JY, Guo H, Yin XY, Rao ZH, Wang XX, Zhang LG. The binding of a monoclonal antibody to the apical region of SCARB2 blocks EV71 infection. Protein & Cell, 2017, 8(8): 590-600. |
| [43] | Martínez-Salas E. The impact of RNA structure on picornavirus IRES activity. Trends in Microbiology, 2008, 16(5): 230-237. DOI:10.1016/j.tim.2008.01.013 |
| [44] | Xi JM, Ye F, Wang GZ, Han W, Wei ZZ, Yin B, Yuan JG, Qiang BQ, Peng XZ. Polypyrimidine tract-binding protein regulates enterovirus 71 translation through interaction with the internal ribosomal entry site. Virologica Sinica, 2019, 34(1): 66-77. DOI:10.1007/s12250-019-00089-1 |
| [45] | Su YS, Tsai AH, Ho YF, Huang SY, Liu YC, Hwang LH. Stimulation of the internal ribosome entry site (IRES)-dependent translation of enterovirus 71 by DDX3X RNA helicase and viral 2A and 3C proteases. Frontiers in Microbiology, 2018, 9: 1324. DOI:10.3389/fmicb.2018.01324 |
| [46] | Davila-Calderon J, Patwardhan NN, Chiu LY, Sugarman A, Cai ZG, Penutmutchu SR, Li ML, Brewer G, Hargrove AE, Tolbert BS. IRES-targeting small molecule inhibits enterovirus 71 replication via allosteric stabilization of a ternary complex. Nature Communications, 2020, 11: 4775. DOI:10.1038/s41467-020-18594-3 |
| [47] | Gunaseelan S, Wong KZ, Min N, Sun JL, Ismail NKBM, Tan YJ, Lee RCH, Chu JJH. Prunin suppresses viral IRES activity and is a potential candidate for treating enterovirus A71 infection. Science Translational Medicine, 2019, 11(516): eaar5759. DOI:10.1126/scitranslmed.aar5759 |
| [48] | Ma HY, Lu CY, Tsao KC, Shih HM, Cheng AL, Huang LM, Chang LY. Association of EV71 3C polymorphisms with clinical severity. Journal of Microbiology, Immunology and Infection, 2018, 51(5): 608-613. DOI:10.1016/j.jmii.2016.12.006 |
| [49] | Li J, Yao YF, Chen Y, Xu X, Lin YQ, Yang ZL, Qiao WT, Tan J. Enterovirus 71 3C promotes apoptosis through cleavage of PinX1, a telomere binding protein. Journal of Virology, 2017, 91(2): e0201616. DOI:10.1128/JVI.02016-16 |
| [50] | Li ML, Lin JY, Chen BS, Weng KF, Shih SR, Calderon JD, Tolbert BS, Brewer G. EV71 3C protease induces apoptosis by cleavage of hnRNP A1 to promote apaf-1 translation. PLoS One, 2019, 14(9): e0221048. DOI:10.1371/journal.pone.0221048 |
| [51] | Yao CG, Xi CL, Hu KH, Gao W, Cai XF, Qin JL, Lv SY, Du CH, Wei YH. Inhibition of enterovirus 71 replication and viral 3C protease by quercetin. Virology Journal, 2018, 15(1): 116. DOI:10.1186/s12985-018-1023-6 |
| [52] | Li P, Wu SQ, Xiao T, Li YL, Su ZM, Wei W, Hao F, Hu GP, Lin FS, Chen XS, Gu ZX, Lin TW, He HY, Li J, Chen SH. Design, synthesis, and evaluation of a novel macrocyclic anti-EV71 agent. Bioorganic & Medicinal Chemistry, 2020, 28(12): 115551. |
| [53] | Li C, Qiao Q, Hao SB, Dong Z, Zhao L, Ji J, Wang ZY, Wen HL. Nonstructural protein 2A modulates replication and virulence of enterovirus 71. Virus Research, 2018, 244: 262-269. DOI:10.1016/j.virusres.2017.11.023 |
| [54] | Bai JJ, Chen XX, Liu QQ, Zhou X, Long JE. Characteristics of enterovirus 71-induced cell death and genome scanning to identify viral genes involved in virus-induced cell apoptosis. Virus Research, 2019, 265: 104-114. DOI:10.1016/j.virusres.2019.03.017 |
| [55] | Yao M, Dong YC, Wang Y, Liu H, Ma HW, Zhang H, Zhang L, Cheng LF, Lv X, Xu ZK, Zhang FL, Lei YF, Ye W. N6-methyladenosine modifications enhance enterovirus 71 ORF translation through METTL3 cytoplasmic distribution. Biochemical and Biophysical Research Communications, 2020, 527(1): 297-304. DOI:10.1016/j.bbrc.2020.04.088 |
| [56] | Yao CG, Hu KH, Xi CL, Li N, Wei YH. Transcriptomic analysis of cells in response to EV71 infection and 2Apro as a trigger for apoptosis via TXNIP gene. Genes & Genomics, 2019, 41(3): 343-357. |
| [57] | Yang XD, Hu ZL, Fan SS, Zhang Q, Zhong Y, Guo D, Qin YL, Chen MZ. Picornavirus 2A protease regulates stress granule formation to facilitate viral translation. PLoS Pathogens, 2018, 14(2): e1006901. DOI:10.1371/journal.ppat.1006901 |
| [58] | Falah N, Montserret R, Lelogeais V, Schuffenecker I, Lina B, Cortay JC, Violot S. Blocking human enterovirus 71 replication by targeting viral 2A protease. Journal of Antimicrobial Chemotherapy, 2012, 67(12): 2865-2869. DOI:10.1093/jac/dks304 |
| [59] |
Xu X, Yao YF, Li J, Chai KL, Qiao WT, Tan J. Identification of the transcriptional activity domain of EV71 3Dpol. Chinese Journal of Virology, 2016, 32(5): 560-565.
(in Chinese) 徐骁, 姚云芳, 李靖, 柴克莉, 乔文涛, 谈娟. 肠道病毒71型3D聚合酶转录激活域的界定. 病毒学报, 2016, 32(5): 560-565. |
| [60] | Shi W, Ye HQ, Deng CL, Li R, Zhang B, Gong P. A nucleobase-binding pocket in a viral RNA-dependent RNA polymerase contributes to elongation complex stability. Nucleic Acids Research, 2019, 48(3): 1392-1405. |
| [61] | Kuo RL, Chen CJ, Wang RYL, Huang HI, Lin YH, Tam EH, Tu WJ, Wu SG, Shih SR. Role of enteroviral RNA-dependent RNA polymerase in regulation of MDA5-mediated beta interferon activation. Journal of Virology, 2019, 93(10): e0013219. DOI:10.1128/JVI.00132-19 |
| [62] | Li YM, Yu J, Qi XW, Yan HM. Monoclonal antibody against EV71 3Dpol inhibits the polymerase activity of RdRp and virus replication. BMC Immunology, 2019, 20(1): 6. DOI:10.1186/s12865-019-0288-x |
| [63] | Wang HQ, Zhong M, Li YP, Li K, Wu S, Guo TT, Cen S, Jiang JD, Li ZR, Li YH. APOBEC3G is a restriction factor of EV71 and mediator of IMB-Z antiviral activity. Antiviral Research, 2019, 165: 23-33. DOI:10.1016/j.antiviral.2019.03.005 |
| [64] | Hung HC, Chen TC, Fang MY, Yen KJ, Shih SR, Hsu JTA, Tseng CP. Inhibition of enterovirus 71 replication and the viral 3D polymerase by aurintricarboxylic acid. Journal of Antimicrobial Chemotherapy, 2010, 65(4): 676-683. DOI:10.1093/jac/dkp502 |
| [65] | Xu N, Yang J, Zheng BS, Zhang Y, Cao YM, Huan C, Wang SQ, Chang JB, Zhang WY. The pyrimidine analog FNC potently inhibits the replication of multiple enteroviruses. Journal of Virology, 2020, 94(9): e0020420. DOI:10.1128/JVI.00204-20 |
| [66] | Sun HY, Gao M, Cui DW. Molecular characteristics of the VP1 region of enterovirus 71 strains in China. Gut Pathogens, 2020, 12: 38. DOI:10.1186/s13099-020-00377-2 |
| [67] | Wang N, Yang XF, Sun JD, Sun ZX, Ma QY, Wang ZX, Chen ZQ, Wang ZB, Hu F, Wang HJ, Zhou LF, Zhang MS, Xu J. Neutrophil extracellular traps induced by VP1 contribute to pulmonary edema during EV71 infection. Cell Death Discovery, 2019, 5: 111. DOI:10.1038/s41420-019-0193-3 |
| [68] | Liu ZW, Zhuang ZC, Chen R, Wang XR, Zhang HL, Li SH, Wang ZY, Wen HL. Enterovirus 71 VP1 protein regulates viral replication in SH-SY5Y cells via the mTOR autophagy signaling pathway. Viruses, 2019, 12(1): 11. DOI:10.3390/v12010011 |
| [69] | Ku ZQ, Ye XH, Shi JP, Wang XL, Liu QW, Huang Z. Single neutralizing monoclonal antibodies targeting the VP1 GH loop of enterovirus 71 inhibit both virus attachment and internalization during viral entry. Journal of Virology, 2015, 89(23): 12084-12095. DOI:10.1128/JVI.02189-15 |
| [70] | Rattanapisit K, Chao Z, Siriwattananon K, Huang Z, Phoolcharoen W. Plant-produced anti-enterovirus 71 (EV71) monoclonal antibody efficiently protects mice against EV71 infection. Plants: Basel, Switzerland, 2019, 8(12): 560. |
| [71] | Zhao ZL, Li ZL, Huan C, Liu X, Zhang WY. SAMHD1 inhibits multiple enteroviruses by interfering with the interaction between VP1 and VP2 proteins. Journal of Virology, 2021, 95(13): e0062021. DOI:10.1128/JVI.00620-21 |
| [72] | Swain SP, Mohanty S. Imidazolidinones and imidazolidine-2, 4-diones as antiviral agents. ChemMedChem, 2019, 14(3): 291-302. DOI:10.1002/cmdc.201800686 |
| [73] | Meng T, Jia Q, Wong SM, Chua KB. In vitro and in vivo inhibition of the infectivity of human enterovirus 71 by a sulfonated food azo dye, brilliant black BN. Journal of Virology, 2019, 93(17): e0006119. DOI:10.1128/JVI.00061-19 |
| [74] |
Wu ZQ, Liu GH, Yan LJ, Nan CH, Yue ZJ, Wang XF. Experimental study on anti-influenza virus infection with Yinqiao-decoction by orthogonal design. Chinese Journal of Experimental and Clinical Virology, 2010, 24(6): 427-429.
(in Chinese) 吴振起, 刘光华, 闫丽娟, 南春红, 岳志军, 王雪峰. 正交设计银翘散抗流感病毒作用的实验研究. 中华实验和临床病毒学杂志, 2010, 24(6): 427-429. |
| [75] | Chen SG, Cheng ML, Chen KH, Horng JT, Liu CC, Wang SM, Sakurai H, Leu YL, Wang SD, Ho HY. Antiviral activities of Schizonepeta tenuifolia Briq. against enterovirus 71 in vitro and in vivo. Scientific Reports, 2017, 7: 935. DOI:10.1038/s41598-017-01110-x |
| [76] | Chen SG, Leu YL, Cheng ML, Ting SC, Liu CC, Wang SD, Yang CH, Hung CY, Sakurai H, Chen KH, Ho HY. Anti-enterovirus 71 activities of Melissa officinalis extract and its biologically active constituent rosmarinic acid. Scientific Reports, 2017, 7: 12264. DOI:10.1038/s41598-017-12388-2 |
| [77] | Ho HY, Cheng ML, Weng SF, Leu YL, Chiu DTY. Antiviral effect of epigallocatechin gallate on enterovirus 71. Journal of Agricultural and Food Chemistry, 2009, 57(14): 6140-6147. DOI:10.1021/jf901128u |
| [78] | Van Kuppeveld FJ, Hoenderop JG, Smeets RL, Willems PH, Dijkman HB, Galama JM, Melchers WJ. Coxsackievirus protein 2B modifies endoplasmic reticulum membrane and plasma membrane permeability and facilitates virus release. The EMBO Journal, 1997, 16(12): 3519-3532. DOI:10.1093/emboj/16.12.3519 |
| [79] | Xie SQ, Wang K, Yu WJ, Lu W, Xu K, Wang JW, Ye B, Schwarz W, Jin Q, Sun B. DIDS blocks a chloride-dependent current that is mediated by the 2B protein of enterovirus 71. Cell Research, 2011, 21(8): 1271-1275. DOI:10.1038/cr.2011.112 |
| [80] | Peng HJ, Shi M, Zhang L, Li YY, Sun J, Zhang LR, Wang XH, Xu XP, Zhang XL, Mao YJ, Ji Y, Jiang JT, Shi WF. Activation of JNK1/2 and p38 MAPK signaling pathways promotes enterovirus 71 infection in immature dendritic cells. BMC Microbiology, 2014, 14: 147. DOI:10.1186/1471-2180-14-147 |
| [81] | Zhang Z, Wang BS, Wu SP, Wen YB, Wang XY, Song XH, Zhang JL, Hou LH, Chen W. PD169316, a specific p38 inhibitor, shows antiviral activity against enterovirus 71. Virology, 2017, 508: 150-158. DOI:10.1016/j.virol.2017.05.012 |
| [82] | Yu J, Dai Y, Fu YX, Wang KZ, Yang Y, Li M, Xu W, Wei L. Cathelicidin antimicrobial peptides suppress EV71 infection via regulating antiviral response and inhibiting viral binding. Antiviral Research, 2021, 187: 105021. DOI:10.1016/j.antiviral.2021.105021 |
| [83] | Fu YX, Zhang L, Zhang F, Tang T, Zhou Q, Feng CH, Jin Y, Wu ZW. Exosome-mediated miR-146a transfer suppresses type Ⅰ interferon response and facilitates EV71 infection. PLoS Pathogens, 2017, 13(9): e1006611. DOI:10.1371/journal.ppat.1006611 |
| [84] | Li B, Zheng JQ. MicroR-9-5p suppresses EV71 replication through targeting NF-κB of the RIG-Ⅰ-mediated innate immune response. FEBS Open Bio, 2018, 8(9): 1457-1470. DOI:10.1002/2211-5463.12490 |
| [85] | Wang Y, Zhang ST, Song WJ, Zhang WX, Li JS, Li CX, Qiu YY, Fang YC, Jiang Q, Li X, Yan B. Exosomes from EV71-infected oral epithelial cells can transfer miR-30a to promote EV71 infection. Oral Diseases, 2020, 26(4): 778-788. DOI:10.1111/odi.13283 |
| [86] | Fu YX, Zhang L, Zhang R, Xu SJ, Wang HR, Jin Y, Wu ZW. Enterovirus 71 suppresses miR-17-92 cluster through up-regulating methylation of the miRNA promoter. Frontiers in Microbiology, 2019, 10: 625. DOI:10.3389/fmicb.2019.00625 |
| [87] | Zhao Q, Xiong Y, Xu JR, Chen S, Li P, Huang Y, Wang YY, Chen WX, Wang B. Host microRNA hsa-miR-494-3p promotes EV71 replication by directly targeting PTEN. Frontiers in Cellular and Infection Microbiology, 2018, 8: 278. DOI:10.3389/fcimb.2018.00278 |
| [88] | Wang RYL, Weng KF, Huang YC, Chen CJ. Elevated expression of circulating miR876-5p is a specific response to severe EV71 infections. Scientific Reports, 2016, 6: 24149. DOI:10.1038/srep24149 |
| [89] | Zhang LL, Chen X, Shi YY, Zhou BF, Du C, Liu YJ, Han S, Yin J, Peng BW, He XH, Liu WH. miR-27a suppresses EV71 replication by directly targeting EGFR. Virus Genes, 2014, 49(3): 373-382. DOI:10.1007/s11262-014-1114-4 |
| [90] | Huang BZ, Chen HP, Zheng YB. MiR-103/miR-107 inhibits enterovirus 71 replication and facilitates type Ⅰ interferon response by regulating SOCS/STAT3 pathway. Biotechnology Letters, 2021, 43(7): 1357-1369. DOI:10.1007/s10529-021-03115-z |
| [91] | Zhang WJ, Huang ZG, Huang MY, Zeng JC. Predicting severe enterovirus 71-infected hand, foot, and mouth disease: cytokines and chemokines. Mediators of Inflammation, 2020, 2020: 9273241. |
| [92] | Liu ML, Lee YP, Wang YF, Lei HY, Liu CC, Wang SM, Su IJ, Wang JR, Yeh TM, Chen SH, Yu CK. Type Ⅰ interferons protect mice against enterovirus 71 infection. Journal of General Virology, 2005, 86(12): 3263-3269. DOI:10.1099/vir.0.81195-0 |
| [93] | Su R, Shereen MA, Zeng XF, Liang YC, Li W, Ruan ZH, Li YK, Liu WY, Liu YL, Wu KL, Luo Z, Wu JG. The TLR3/IRF1/type Ⅲ IFN axis facilitates antiviral responses against enterovirus infections in the intestine. mBio, 2020, 11(6): e0254020. DOI:10.1128/mBio.02540-20 |
| [94] | Zhong T, Zhang LY, Wang ZY, Wang Y, Song FM, Zhang YH, Yu JH. Rheum emodin inhibits enterovirus 71 viral replication and affects the host cell cycle environment. Acta Pharmacologica Sinica, 2017, 38(3): 392-401. DOI:10.1038/aps.2016.110 |
| [95] | Shih SR, Tsai KN, Li YS, Chueh CC, Chan EC. Inhibition of enterovirus 71-induced apoptosis by allophycocyanin isolated from a blue-green alga Spirulina platensis. Journal of Medical Virology, 2003, 70(1): 119-125. DOI:10.1002/jmv.10363 |
| [96] | Huang HI, Chio CC, Lin JY. Inhibition of EV71 by curcumin in intestinal epithelial cells. PLoS One, 2018, 13(1): e0191617. DOI:10.1371/journal.pone.0191617 |
| [97] | Kang NX, Gao HW, He L, Liu YL, Fan HD, Xu QM, Yang SL. Ginsenoside Rb1 is an immune-stimulatory agent with antiviral activity against enterovirus 71. Journal of Ethnopharmacology, 2021, 266: 113401. DOI:10.1016/j.jep.2020.113401 |
| [98] | Wang SY, Wang W, Hao C, Yu YJ, Qin L, He MJ, Mao WJ. Antiviral activity against Enterovirus 71 of sulfated rhamnan isolated from the green alga Monostroma latissimum. Carbohydrate Polymers, 2018, 200: 43-53. DOI:10.1016/j.carbpol.2018.07.067 |
| [99] | Feng QY, Zhou HT, Zhang XY, Liu X, Wang J, Zhang CP, Ma XJ, Quan CJ, Zheng ZL. Acarbose, as a potential drug, effectively blocked the dynamic metastasis of EV71 from the intestine to the whole body. Infection, Genetics and Evolution, 2020, 81: 104210. DOI:10.1016/j.meegid.2020.104210 |
| [100] | Gao J, Tang FY, Wang ZG, Yu J, Hu R, Liu L, Kang GD. Post-marketing safety surveillance for inactivated enterovirus 71 vaccines in Jiangsu, China from 2017 to 2019. Vaccine, 2021, 39(9): 1415-1419. DOI:10.1016/j.vaccine.2021.01.048 |
| [101] | Mao QY, Wang YP, Bian LL, Xu M, Liang ZL. EV71 vaccine, a new tool to control outbreaks of hand, foot and mouth disease (HFMD). Expert Review of Vaccines, 2016, 15(5): 599-606. DOI:10.1586/14760584.2016.1138862 |
| [102] | Du ZC, Huang Y, Bloom MS, Zhang ZB, Yang ZC, Lu JY, Xu JX, Hao YT. Assessing the vaccine effectiveness for hand, foot, and mouth disease in Guangzhou, China: a time-series analysis. Human Vaccines & Immunotherapeutics, 2021, 17(1): 217-223. |
| [103] | Li J, Yin XZ, Lin AW, Nie XZ, Liu LY, Liu SH, Li N, Wang P, Song SS, Wang SN, Xu DY. EV71 vaccination impact on the incidence of encephalitis in patients with hand, foot and mouth disease. Human Vaccines & Immunotherapeutics, 2021, 17(7): 2097-2100. |
| [104] | Li ZQ, Qin ZQ, Tan HF, Zhang CH, Xu JX, Chen J, Ni LH, Yun XX, Cui M, Huang Y, Wang W, Zhang ZB. Analysis of the coverage of inactivated enterovirus 71 (EV71) vaccine and adverse events following immunization with the EV71 vaccine among children from 2016 to 2019 in Guangzhou. Expert Review of Vaccines, 2021, 20(7): 907-918. DOI:10.1080/14760584.2021.1933451 |
| [105] | Wang SY, Zeng J, Zhang XP, Gan ZK, Fan JQ, Chen YP, Liang ZZ, Hu XS, Zeng G, Lv HK. Short-term dynamic changes in neutralizing antibodies against enterovirus 71 after vaccination. Human Vaccines & Immunotherapeutics, 2020, 16(7): 1595-1601. |
| [106] | Fan ST, Liao Y, Jiang GR, Wang LC, Zhao H, Yu L, Xu XL, Li DD, Zhang Y, Li QH. Efficacy of an inactivated bivalent vaccine for enterovirus 71 and coxsackievirus A16 in mice immunized intradermally. Vaccine, 2021, 39(3): 596-604. DOI:10.1016/j.vaccine.2020.11.070 |
| [107] | Yang T, Liu BF, Yue L, Xie TH, Li H, Shao MX, Yang R, Luo FY, Long RX, Xie ZP. Preclinical safety assessment of a combined vaccine against hepatitis A virus and enterovirus 71. Vaccine, 2021, 39(29): 3952-3963. DOI:10.1016/j.vaccine.2021.05.058 |
| [108] | Wang ZY, Zhou CL, Gao F, Zhu QJ, Jiang YX, Ma XX, Hu YL, Shi LK, Wang XL, Zhang C, Liu BF, Shen LZ, Mao QY, Liu G. Preclinical evaluation of recombinant HFMD vaccine based on enterovirus 71 (EV71) virus-like particles (VLP): immunogenicity, efficacy and toxicology. Vaccine, 2021, 39(31): 4296-4305. DOI:10.1016/j.vaccine.2021.06.031 |
| [109] | Kim HJ, Son HS, Lee SW, Yoon Y, Hyeon JY, Chung GT, Lee JW, Yoo JS. Efficient expression of enterovirus 71 based on virus-like particles vaccine. PLoS One, 2019, 14(3): e0210477. DOI:10.1371/journal.pone.0210477 |
| [110] | Wang X, Dong K, Long M, Lin F, Gao ZW, Wang L, Zhang Z, Chen X, Dai Y, Wang HP, Zhang HZ. Induction of a high-titered antibody response using HIV gag-EV71 VP1-based virus-like particles with the capacity to protect newborn mice challenged with a lethal dose of Enterovirus 71. Archives of Virology, 2018, 163(7): 1851-1861. DOI:10.1007/s00705-018-3797-7 |
| [111] | Luo J, Huo CL, Qin H, Hu JH, Lei L, Pan ZS. Chimeric enterovirus 71 virus-like particle displaying conserved coxsackievirus A16 epitopes elicits potent immune responses and protects mice against lethal EV71 and CA16 infection. Vaccine, 2021, 39(30): 4135-4143. DOI:10.1016/j.vaccine.2021.05.093 |
| [112] | Yang ZJ, Gao F, Wang XL, Shi LK, Zhou Z, Jiang YX, Ma XX, Zhang C, Zhou CL, Zeng XF, Liu G, Fan J, Mao QY, Shi L. Development and characterization of an enterovirus 71 (EV71) virus-like particles (VLPs) vaccine produced in Pichia pastoris. Human Vaccines & Immunotherapeutics, 2020, 16(7): 1602-1610. |
| [113] | Lei L, Li Q, Xu SH, Tian MY, Zheng XH, Bi YX, Huang B. Transplantation of enterovirus 71 virion protein particle vaccine protects against enterovirus 71 infection in a neonatal mouse model. Annals of Transplantation, 2021, 26: e924461. |
| [114] | Liu JN, Zhao BB, Xue L, Wu J, Xu YF, Liu YD, Qin C. Immunization with a fusion protein vaccine candidate generated from truncated peptides of human enterovirus 71 protects mice from lethal enterovirus 71 infections. Virology Journal, 2020, 17(1): 58. DOI:10.1186/s12985-020-01328-8 |
| [115] | Kim YG, Lee YS, Jung JW, Jin HE. Epitope peptide amphiphile-based nanofiber as an effective vaccine for viral infectious diseases. Journal of Nanoscience and Nanotechnology, 2020, 20(9): 5329-5332. DOI:10.1166/jnn.2020.17655 |
| [116] | Wang YY, Meng FY, Li JX, Li GF, Hu JL, Cao JQ, Yu QF, Liang Q, Zhu FC. Willingness of parents to vaccinate their 6-60-month-old children with EV71 vaccines: a cross-sectional study in rural areas of northern Jiangsu province. Human Vaccines & Immunotherapeutics, 2020, 16(7): 1579-1585. |
 2022, Vol. 62
2022, Vol. 62




