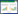中国科学院微生物研究所,中国微生物学会,中国菌物学会
文章信息
- 刘小宇, 陈敏. 2022
- LIU Xiaoyu, CHEN Min.
- 肺炎链球菌糖疫苗的研究进展
- Carbohydrate-based vaccines of Streptococcus pneumoniae
- 微生物学报, 62(2): 446-457
- Acta Microbiologica Sinica, 62(2): 446-457
-
文章历史
- 收稿日期:2021-04-30
- 修回日期:2021-08-18
- 网络出版日期:2021-11-15
肺炎链球菌是一种兼性厌氧革兰氏阳性菌,是上呼吸道共生菌群的一部分,可无症状地定殖于鼻咽[1–2]。若肺炎链球菌定殖鼻咽后没有被免疫系统清除,则会传播到下呼吸道以及其他器官和组织中[2],引起许多肺炎链球菌疾病,例如脑膜炎、菌血症、肺炎、急性中耳炎和鼻窦炎[3],其中侵袭性肺炎链球菌疾病(invasive pneumococcal disease,IPD)具有很高的致死率[4]。在美国,2018年约有31 400例(每10万人中9.6例)侵袭性肺炎链球菌病病例,其中3 480例直接导致死亡(疾病控制与预防中心,2018年),在世界范围内,每年发生的严重肺炎链球菌感染数量约为100–150万(世界卫生组织,2007年)[5]。肺炎链球菌是一种机会病原体,在两岁以下的儿童和老年人中的发病率更高[6]。
肺炎链球菌荚膜多糖(capsule polysaccharides,CPS)覆盖细菌整个表面,是主要毒力因子之一,主要作用机制是掩盖细胞表面并阻断针对细胞表面抗原的补体沉积,从而使细菌能够逃避吞噬作用并在宿主鼻咽处定殖[7–8]。至今已经鉴定出98种肺炎链球菌血清型[8–9],分型依据为CPS的化学结构。CPS上补体沉积及其与补体抑制蛋白(如H因子)结合的不同,导致了不同血清型侵袭性上的差异[10–11],这种差异体现在肺炎链球菌疾病发病机制的各个方面,包括对抗菌剂的敏感性和耐药性[10],血清型的比例也会随着时间的流逝和疫苗的使用而变化。同时CPS还具有免疫原性,可诱导产生抗肺炎链球菌感染的抗体,这也是目前研制肺炎球链菌疫苗的免疫学基础[12–13]。尽管当前市售的肺炎链球菌疫苗可预防其所包含的肺炎链球菌血清型感染,但越来越多的数据表明市售疫苗针对3型肺炎链球菌(Streptococcus pneumoniae serotype 3,ST3)的免疫效果不佳[14]。实际上,3型肺炎链球菌是成人IPD的第二大常见病原体,由其导致的IPD死亡率约为30%[5, 14],因此必须寻求新的策略来改进针对这种高侵入性肺炎链球菌血清型的疫苗[15]。
1 肺炎链球菌疫苗(3型)研究进展当前市面上有两类疫苗可用于预防肺炎链球菌感染,即肺炎链球菌多糖疫苗(pneumococcal polysaccharide vaccines,PPVs)和肺炎链球菌糖缀合物疫苗(pneumococcal protein-conjugate vaccine,PCVs)。这2类疫苗的制备都是利用了荚膜多糖的高免疫原性[16],例如23价肺炎链球菌多糖疫苗(PPV23)由23种常见的血清型菌株荚膜多糖的混合物制备而成。但细菌荚膜多糖属于非胸腺依赖性抗原(thymus independent antigen,TI-Ag)中的TI-2 Ag,用这种抗原进行免疫时只能通过交联B细胞受体(B-cell receptor,BCR)刺激成熟B细胞产生应答,而无法活化T细胞,也不能形成免疫记忆[17–19]。因此,PPV在婴幼儿、老年人和免疫缺陷患者等人群中的免疫反应低下。这种缺陷可以通过将多糖抗原与蛋白载体缀合来克服,已经证明这种糖缀合物可有效诱导胸腺依赖性免疫。目前应用最广的糖缀合物疫苗PCV13就是由13种血清型的CPS与无毒的白喉毒素突变体(CRM197)偶联制成。另外,还有一些以肺炎链球菌蛋白以及灭活菌体作为抗原的疫苗正处于临床阶段(表 1)。
| Vaccine | Antigen | Carrier protein | Current status |
| PPV23 (Merck) | Capsular polysaccharide (1, 2, 3, 4, 5, 6B, 7F, 8, 9N, 9V, 10A, 11A, 12F, 14, 15B, 17F, 18C, 19F, 19A, 20, 22F, 23F, 33F) | None | Licensed |
| PCV7 (Pfizer) | Capsular polysaccharide (4, 6B, 9V, 14, 18C, 19F, 23F) | CRM197 | Licensed |
| PCV10 (GSK Biologicals) | Capsular polysaccharide (1, 4, 5, 6B, 7F, 9V, 14, 18C, 19F, 23F) | D, TT, DT | Licensed |
| PCV13 (Pfizer) | Capsular polysaccharide (1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F, 23F) | CRM197 | Licensed |
| PCV15 (Merck) | Capsular polysaccharide (1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F, 22F, 23F, 33F) | CRM197 | Phase Ⅲ |
| PCV20 (Pfizer) | Capsular polysaccharide (1, 3, 4, 5, 6A, 6B, 7, 9V, 14, 18C, 19A, 19F, 23F, 8, 10A, 11A, 12F, 15BC, 22F, 33F) | CRM197 | Phase Ⅲ |
| Protein vaccine (Sanofi-Pasteur) | PcpA | None | PhaseⅠ |
| Protein vaccine (Sanofi-Pasteur) | PlyD1 | None | PhaseⅠ |
| Protein vaccine (Sanofi-Pasteur) | PcpA, PhtD | None | PhaseⅠ |
| Protein vaccine (Sanofi-Pasteur) | PcpA, PhtD, PlyD1 | None | PhaseⅠ |
| Protein vaccine (GSK Biologicals) | PD, Ply, PhtD | None | PhaseⅠ |
| PCV10+protein vaccine (GSK Biologicals) | PCV10, PlyD1, PhtD | D, TT, DT | Phase Ⅱ |
| PCV13+protein vaccine (GSK Biologicals) | PCV13, PhtD, dPly | None | Phase Ⅱ |
| Protein vaccine (GSK Biologicals) | PhtD | None | Phase Ⅱ |
| Protein vaccine (GNCA) | SP-2108, SP-0148, SP-1912 | None | Phase Ⅱ |
| Protein vaccine (Intercell AG) | PcsB, StkP, PsaA | None | Phase Ⅰ |
| Inactivated whole cell vaccine (PATH) | Inactivated Streptococcus pneumoniae Rx1 strain | None | Phase Ⅱ |
1.1 以多糖为抗原的疫苗
虽然肺炎链球菌来源的荚膜多糖可以直接作为抗原,但纯化成本太高,因此许多研究者开展了体外合成以及生物合成3型肺炎链球菌荚膜多糖(以下简称3型CPS)的探索以达到降低成本、提高安全性的目的。
1.1.1 3型肺炎链球菌荚膜多糖的合成机制在肺炎链球菌已鉴定出的98种CPS中,仅3型和37型CPS是通过合酶依赖性途径合成的[20]。3型CPS的基因座位于dexB和aliA之间(图 1A),其中编码UDP-葡萄糖(Glc)脱氢酶的基因ugd和编码3型荚膜多糖合成酶(CPS3S)的基因wchE是3型CPS合成所必需的基因[21]。CPS3S是一种相对较小的整合膜蛋白,属于连续型β-糖基转移酶,负责糖链的引发、聚合和转运。它的催化中心可以交替结合UDP-Glc和UDP-葡萄糖醛酸(GlcA),其供体特异性由受体末端非还原糖控制[22]。它通过将Glc从UDP-Glc转移至磷脂酰甘油(PG)受体上来启动合成,然后在生长链的非还原末端交替添加GlcA和Glc以实现糖链的合成[23–24]。在UDP-GlcA的浓度相对较高的条件下,寡糖可以达到8个糖的长度,这时CPS3S将多糖链转移到膜的外表面,并继续增加链长,而在UDP-GlcA浓度较低时,PG连接的寡糖与合酶结合松散,多糖链被释放并在膜上积累[21, 25]。从脂连接的寡糖到脂连接的多糖合成的过渡以及最终链长的控制取决于UDP糖的浓度[21] (图 1)。
1.1.2 多糖疫苗
研究者们成功地利用不同宿主实现了CPS3S的异源表达,在体内或体外实现3型CPS的合成,并证明其具有免疫原性。例如,Cartee等在大肠杆菌中实现了CPS3S的表达,并利用表达CPS3S的大肠杆菌细胞膜在体外合成了3型CPS[26]。但不同于在肺炎链球菌中表达,大肠杆菌中合成的CPS被脂质前体锚定在膜上而没有释放出来。Smith等和Gilbert等分别在烟草细胞和乳酸乳球菌中成功表达了CPS3S,并在体内合成了3型CPS,利用植物提取物或重组乳酸乳球菌作为抗原进行小鼠免疫实验,证明了异源CPS3S合成的CPS具有良好的免疫原性[27–28]。
1.1.3 糖缀合物疫苗由于多糖疫苗的种种局限,越来越多的研究者把注意力转移到糖缀合物疫苗的研究上。多糖-蛋白缀合物激活适应性免疫应答的机制如图 2所示,缀合物首先被树突状细胞吸收并转运到淋巴结中,B细胞进行识别并提取缀合物抗原,内化到内体中,蛋白质部分被蛋白酶加工成小肽,形成多糖-肽复合物[29];多糖-肽复合物与Ⅱ类组织相容性复合体(MHCⅡ)结合,将抗原表位暴露在B细胞表面从而被T细胞受体(T-cell receptor,TCR)识别[30]。关于TCR识别的表位有两种理论,之前公认的理论认为载体蛋白加工后产生的肽表位由MHCⅡ呈递并被肽特异性T细胞识别;而Avci等提出肽的作用是将糖表位锚定在MHCⅡ上,并将糖表位呈递至多糖特异性T细胞[30–31]。同时,B细胞表面CD80/CD86与T细胞表面CD28的相互作用提供了T细胞活化的第二信号,活化的T细胞释放细胞因子(如IL-4)以刺激B细胞成熟到记忆细胞,并诱导抗体类别从IgM转变为多糖特异性IgG,因此在暴露于相同的抗原后,会产生大量的高亲和力IgG抗体[32–34]。
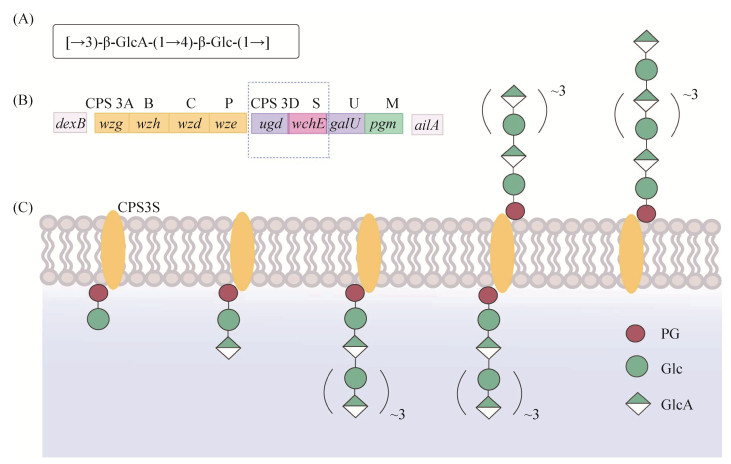
糖缀合物疫苗的研制需要将具有免疫原性的多糖与载体蛋白结合。最简单的方法就是通过化学法将多糖与载体蛋白直接结合(图 3A),但通过这种方法获得的糖缀合物通常具有复杂的交联结构。为了简化糖缀合物结构,可以先将CPS部分水解,再通过分级分离选择中等长度的糖链,然后在还原末端引入伯胺基用于插入二酯或双功能接头,以准备与蛋白质偶联(图 3B)[32–33]。张涛等就是将ST3的CPS进行部分水解之后再与蛋白CRM197通过化学方法偶联,制备成糖缀合物疫苗,并且在小鼠免疫实验中表现出了较高的免疫原性[35]。
|
| 图 3 多糖与载体蛋白的结合方式 Figure 3 Combination method of polysaccharide and carrier protein. A: direct binding; B: coupling with protein using linker after partial hydrolysis. |
1.2 以寡糖为抗原的疫苗——合成寡糖疫苗
糖缀合物疫苗大大提高了疫苗的免疫原性[17, 30, 36],然而其中所需的多糖抗原的分离和纯化仍然具有挑战性,可能会导致疫苗的异质组成和批次间的可变性。因此,基于合成的糖类抗原的糖缀合物疫苗的开发变得越来越重要,b型嗜血性流感杆菌的合成寡糖疫苗Quimi-Hib的成功就是最好的证明[37]。
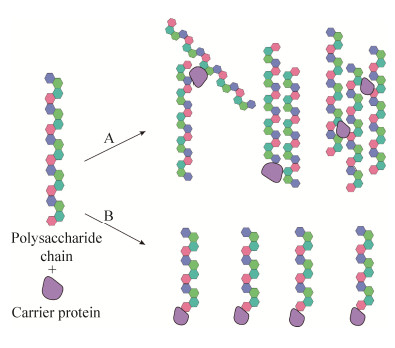
合成寡糖具有定义明确的组成、高度可重复的生物学特性和较高的安全性。另外,合成寡糖有助于阐明微生物多糖的最小结构,称为表位或抗原决定簇[38],可以确保产生足够数量的抗体以赋予宿主长期的保护性免疫力。因此,基于合成寡糖的糖缀合物疫苗现在正处于疫苗开发的最前沿,是一种有潜力的多糖疫苗的替代物[38–39]。我们期望降低合成抗原的复杂性以确保大规模合成的可行性,同时还要保留引起特异性免疫应答的能力。可以根据抗体的结构特征以及抗原/抗体结合特异性来设计抗原结构,以提高合成寡糖缀合物的免疫原性[39–40](图 4)。
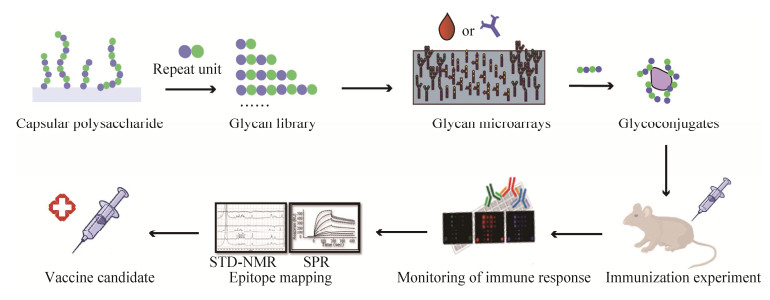
|
| 图 4 设计合成寡糖疫苗的主要步骤[39] Figure 4 Major steps involved in rational design of synthetic carbohydrate vaccines[39]. First, elucidate the structure of the polysaccharide repeat unit on the cell surface; obtain various structures of oligosaccharides by one-pot method, automation, chemical enzymatic methods, etc., to form a glycan library; use glycan microarrays to screen patient sera for antibodies against a synthetic antigen library; immunize mice after conjugated with protein carriers to prepare monoclonal antibodies; use glycan array analysis to monitor antibody titer; use X-ray crystallography, small-angle X-ray scattering, nuclear magnetic resonance transfer saturation difference spectroscopy, surface plasmon resonance, isothermal titration calorimetry, competitive ELISA and other methods to analyze the 3D structure of oligosaccharide-antibody conjugates and the interaction between the two effects[39–40]; finally get the optimized antigen design. |
通过对CD1-糖脂复合物的T细胞识别的结构研究[41]和糖肽识别的功能研究发现,只有1至4个碳水化合物单位的糖分子可以容纳在T细胞受体(TCR)的结合位点内[42–43],而B细胞受体位点理论上可以结合多达6个单位[44–45]。表 2中列出了近10年中针对ST3的合成寡糖疫苗的研究,结果表明合成寡糖(尤其是合成四糖和六糖)能够在小鼠中引起较强的免疫反应,因此未来可以通过添加合成四糖或六糖的缀合物代替ST3的低免疫原性CPS来改进商品化的糖缀合物疫苗[46]。
| Synthetic oligosaccharide antigen | Carrier protein | Immunogenicity | References |
| Tetrasaccharides | CRM197 | Tetrasaccharide-CRM197 can induce protective immunity and protect against pneumonia caused by ST3 in mice. | [15, 47–48] |
| Disaccharides, trisaccharides, tetrasaccharides | BSA | Tetrasaccharide-BSA has stronger immunogenicity and can bind to antibodies induced by natural CPS-CRM197. | [49] |
| Pentasaccharides, hexaose, heptaose, octasaccharide | TT | Pentasaccharide and hexasaccharide-TT have stronger immunogenicity; hexasaccharide-TT can cause long-term immune memory, which can effectively protect mice from ST3 infection and prolong survival. | [46] |
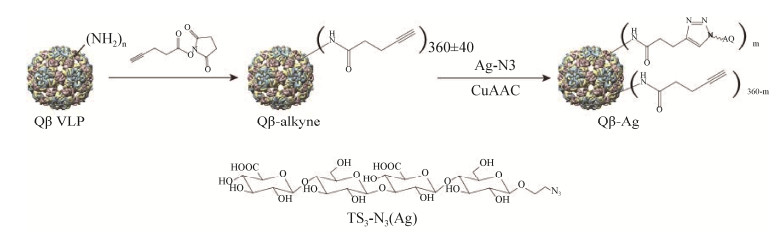
虽然用蛋白作为载体可以提高多糖抗原的免疫原性,但只能产生持续时间有限的中低亲和力免疫反应。为了生产更有效的结合疫苗,Polonskaya等将3型CPS的合成寡糖连接到了一种病毒样颗粒(VLP)噬菌体Qβ VLPs上(图 5),这样就可以将B细胞和T细胞的识别力集中在Qβ VLPs表面展示的单个寡糖抗原上,从而引发更加持久的高亲和力抗体反应。在预防性免疫模型中,经Qβ-TS3免疫的小鼠受到了有效保护(存活率95%)[45]。
|
| 图 5 Qβ-四糖缀合物的合成[45] Figure 5 Synthesis of Qβ-tetrasaccharide conjugates[45]. An azidoethanol linker was added at the end of the synthesized tetrasaccharide to introduce an orthogonal azide group, and then the oligosaccharide was coupled to the bacteriophage QβVLP through a copper-catalyzed azide-alkyne cycloaddition reaction. |
以上所述都是单价疫苗,合成寡糖也可以用于制备多价疫苗。例如,Kaplonek等制备了包含5种血清型抗原(ST2、ST3、ST5、ST8和ST14)的五价合成寡糖缀合物疫苗(sPCV5),sPCV5是通过将等量的5种寡糖缀合物吸附到明矾上来制备的,与在售的疫苗PCV13和PCV10相比,用sPCV5免疫的兔产生了更加强烈的特异性抗体反应[50]。
1.3 其他类型的疫苗虽然多糖疫苗和糖缀合物疫苗在降低肺炎链球菌疾病发病率方面取得了较大的成效,但也存在一些明显的局限。多糖疫苗缺乏针对幼儿和老人的免疫原性,对疫苗血清型鼻咽携带的影响很小,也不能诱导黏膜免疫[48, 51–53]。糖缀合物疫苗(包括合成寡糖疫苗)虽然克服了部分多糖疫苗的限制,但其保护性免疫仅限于给定PCV中包含的血清型,生产成本很高,并且在肺炎高发人群中的保护作用小[13, 54]。自从引入PCV以来,由非疫苗血清型引起的IPD的发生率在全世界范围内急剧增加[55–57],这种血清型替代现象表明需要开发非血清依赖型肺炎链球菌疫苗[58],因此出现了以肺炎链球菌蛋白作为抗原的新型疫苗,其中有许多蛋白抗原例如表面蛋白A (PsaA)、溶血素(Ply)等已经完成了Ⅰ期或Ⅱ期的临床检验,并证明具有免疫原性和安全性(表 1)。
新型疫苗开发策略已经达到了不同的发展阶段,主要包括以下几种类型:单独或组合的蛋白抗原;单独或组合的蛋白抗原作为多糖抗原的载体;肺炎链球菌蛋白抗原与传统载体结合;添加了肺炎链球菌蛋白的传统结合疫苗;重组肺炎链球菌融合蛋白;DNA疫苗;荚膜多糖低水平表达的减毒全细胞疫苗[13]等。
2 展望尽管糖缀合物疫苗能够保护人们免受其包含的血清型的侵害,是有效和安全的,但仍有一些问题需要解决。现有的肺炎链球菌疫苗并不能覆盖所有血清型,还需要进一步研究血清型的纳入或替代,对于像3型和19A型肺炎链球菌这样的疫苗有效性较低的血清型也需要不断改进。此外,糖缀合物疫苗中载体蛋白和接头本身也具有免疫原性,可能引起非特异性的免疫反应而抑制多糖特异性抗体的产生[45],因此需要设计和开发不含载体蛋白和接头的疫苗[59]。
糖化学的最新进展为基于合成寡糖的糖缀合物疫苗研制开辟了道路。寡糖抗原的合理设计和日益复杂的合成策略使制备高度复杂的微生物寡糖成为可能[60]。可以通过优化和精炼寡糖结构以及糖缀合物的配比,确定寡糖的最佳长度/大小和合适的糖/蛋白质摩尔比,以获得半合成和完全合成的疫苗候选物,从而有可能克服目前肺炎链球菌糖缀合物疫苗的局限性[15]。蛋白质的位点特异性糖基化可以精确控制合成的糖蛋白的表位密度,提高糖蛋白疫苗候选物的安全性和有效性,解决其可用性和供应问题[33]。此外,病毒样颗粒作为一种靶向递送载体可以替代传统蛋白载体与合成抗原结合,以期获得更高的免疫原性。关于肺炎链球菌合成寡糖疫苗的研究已取得了一定的进展,虽然没有进入临床,但同类型其他市售疫苗的成功昭示着此类疫苗具有巨大的潜力。
以肺炎链球菌蛋白和灭活细菌作为抗原的新型疫苗可以克服多糖类疫苗的多种限制,并且可以诱发更广泛的免疫应答,制作成本也更为低廉,但同样有许多问题需要解决,包括所需的剂量数和引起反应的持久性、是否需要佐剂、以及婴儿免疫时是否发生肺炎链球菌定殖等等[61]。
还有一些研究为解决肺炎链球菌疾病提供了除疫苗外的其他方法。例如ST3特异性糖苷水解酶(Pn3Pase)处理可降解3型CPS,从而减少ST3的鼻咽定殖并提高其对宿主免疫清除的敏感性,这是克服ST3的持久性和高死亡率的一种可行的替代方法[62–63]。不同类型的方法各有其优势,又能互补各自的不足,因此在不断研发新型肺炎疫苗和其他新疗法的同时也不能淘汰传统疫苗,而是应该继续优化多糖类疫苗的生产,降低其生产成本,这样才能提高应对全世界范围内肺炎链球菌感染的能力。
| [1] | Bogaert D, De Groot R, Hermans P. Streptococcus pneumoniae colonisation: the key to pneumococcal disease. The Lancet Infectious Diseases, 2004, 4(3): 144-154. DOI:10.1016/S1473-3099(04)00938-7 |
| [2] | Van Der Poll T, Opal SM. Pathogenesis, treatment, and prevention of pneumococcal pneumonia. Lancet, 2009, 374(9700): 1543-1556. DOI:10.1016/S0140-6736(09)61114-4 |
| [3] | Weiser JN, Ferreira DM, Paton JC. Streptococcus pneumoniae: transmission, colonization and invasion. Nature Reviews Microbiology, 2018, 16(6): 355-367. DOI:10.1038/s41579-018-0001-8 |
| [4] | Drijkoningen JJC, Rohde GGU. Pneumococcal infection in adults: burden of disease. Clinical Microbiology and Infection, 2014, 20(Suppl 5): 45-51. |
| [5] | Luck JN, Tettelin H, Orihuela CJ. Sugar-coated killer: serotype 3 pneumococcal disease. Frontiers in Cellular and Infection Microbiology, 2020, 10: 613287. DOI:10.3389/fcimb.2020.613287 |
| [6] | Brooks LRK, Mias GI. Streptococcus pneumonia's virulence and host immunity: aging, diagnostics, and prevention. Frontiers in Immunology, 2018, 9: 1366. DOI:10.3389/fimmu.2018.01366 |
| [7] | Steel HC, Cockeran R, Anderson R, Feldman C. Overview of community-acquired pneumonia and the role of inflammatory mechanisms in the immunopathogenesis of severe pneumococcal disease. Mediators of Inflammation, 2013, 2013: 490346. |
| [8] | Geno KA, Saad JS, Nahm MH. Discovery of novel pneumococcal serotype 35D, a natural WciG-deficient variant of serotype 35B. Journal of Clinical Microbiology, 2017, 55(5): 1416-1425. DOI:10.1128/JCM.00054-17 |
| [9] | Paton JC, Trappetti C. Streptococcus pneumoniae capsular polysaccharide. Microbiology Spectrum, 2019, 7(2). DOI:10.1128/microbiolspec.gpp3-0019-2018 |
| [10] | Weinberger DM, Trzciński K, Lu YJ, Bogaert D, Brandes A, Galagan J, Anderson PW, Malley R, Lipsitch M. Pneumococcal capsular polysaccharide structure predicts serotype prevalence. PLoS Pathogens, 2009, 5(6): e1000476. DOI:10.1371/journal.ppat.1000476 |
| [11] | McDaniel LS, Swiatlo E. Pneumococcal disease. Infectious Diseases in Clinical Practice, 2004, 12(2): 93-98. DOI:10.1097/01.idc.0000121024.62151.c9 |
| [12] | MacLeod CM, Hodges RG. Prevention of pneumococcal pneumonia by immunization with specific capsular polysaccharides. The Journal of Experimental Medicine, 1945, 82: 445-465. DOI:10.1084/jem.82.6.445 |
| [13] | Feldman C, Anderson R. Review: current and new generation pneumococcal vaccines. Journal of Infection, 2014, 69(4): 309-325. DOI:10.1016/j.jinf.2014.06.006 |
| [14] | Wantuch PL, Avci FY. Invasive pneumococcal disease in relation to vaccine type serotypes. Human Vaccines & Immunotherapeutics, 2019, 15(4): 874-875. |
| [15] | Parameswarappa SG, Reppe K, Geissner A, Ménová P, Govindan S, Calow ADJ, Wahlbrink A, Weishaupt MW, Monnanda BP, Bell RL, Pirofski LA, Suttorp N, Sander LE, Witzenrath M, Pereira CL, Anish C, Seeberger PH. A semi-synthetic oligosaccharide conjugate vaccine candidate confers protection against Streptococcus pneumoniae serotype 3 infection. Cell Chemical Biology, 2016, 23(11): 1407-1416. DOI:10.1016/j.chembiol.2016.09.016 |
| [16] | Principi N, Esposito S. Development of pneumococcal vaccines over the last 10 years. Expert Opinion on Biological Therapy, 2018, 18(1): 7-17. DOI:10.1080/14712598.2018.1384462 |
| [17] | Avci FY, Kasper DL. How bacterial carbohydrates influence the adaptive immune system. Annual Review of Immunology, 2010, 28: 107-130. DOI:10.1146/annurev-immunol-030409-101159 |
| [18] | Weintraub A. Immunology of bacterial polysaccharide antigens. Carbohydrate Research, 2003, 338(23): 2539-2547. DOI:10.1016/j.carres.2003.07.008 |
| [19] | De Gregorio E, Rappuoli R. From empiricism to rational design: a personal perspective of the evolution of vaccine development. Nature Reviews Immunology, 2014, 14(7): 505-514. DOI:10.1038/nri3694 |
| [20] | Bentley SD, Aanensen DM, Mavroidi A, Saunders D, Rabbinowitsch E, Collins M, Donohoe K, Harris D, Murphy L, Quail MA, Samuel G, Skovsted IC, Kaltoft MS, Barrell B, Reeves PR, Parkhill J, Spratt BG. Genetic analysis of the capsular biosynthetic locus from all 90 pneumococcal serotypes. PLoS Genetics, 2006, 2(3): e31. DOI:10.1371/journal.pgen.0020031 |
| [21] | Yother J. Capsules of Streptococcus pneumoniae and other bacteria: paradigms for polysaccharide biosynthesis and regulation. Annual Review of Microbiology, 2011, 65: 563-581. DOI:10.1146/annurev.micro.62.081307.162944 |
| [22] | Forsee WT, Cartee RT, Yother J. A kinetic model for chain length modulation of Streptococcus pneumoniae cellubiuronan capsular polysaccharide by nucleotide sugar donor concentrations. Journal of Biological Chemistry, 2009, 284(18): 11836-11844. DOI:10.1074/jbc.M900379200 |
| [23] | Cartee RT, Forsee WT, Schutzbach JS, Yother J. Mechanism of type 3 capsular polysaccharide synthesis in Streptococcus pneumoniae. Journal of Biological Chemistry, 2000, 275(6): 3907-3914. DOI:10.1074/jbc.275.6.3907 |
| [24] | Forsee WT, Cartee RT, Yother J. Characterization of the lipid linkage region and chain length of the cellubiuronic acid capsule of Streptococcus pneumoniae. The Journal of Biological Chemistry, 2009, 284(18): 11826-11835. DOI:10.1074/jbc.M900386200 |
| [25] | Geno KA, Gilbert GL, Song JY, Skovsted IC, Klugman KP, Jones C, Konradsen HB, Nahm MH. Pneumococcal capsules and their types: past, present, and future. Clinical Microbiology Reviews, 2015, 28(3): 871-899. DOI:10.1128/CMR.00024-15 |
| [26] | Cartee RT, Forsee WT, Jensen JW, Yother J. Expression of the Streptococcus pneumoniae type 3 synthase in Escherichia coli. Assembly of type 3 polysaccharide on a lipid primer. The Journal of Biological Chemistry, 2001, 276(52): 48831-48839. |
| [27] | Smith CM, Fry SC, Gough KC, Patel AJ, Glenn S, Goldrick M, Roberts IS, Whitelam GC, Andrew PW. Recombinant plants provide a new approach to the production of bacterial polysaccharide for vaccines. PLoS One, 2014, 9(2): e88144. DOI:10.1371/journal.pone.0088144 |
| [28] | Gilbert C, Robinson K, Le Page RW, Wells JM. Heterologous expression of an immunogenic pneumococcal type 3 capsular polysaccharide in Lactococcus lactis. Infection and Immunity, 2000, 68(6): 3251-3260. DOI:10.1128/IAI.68.6.3251-3260.2000 |
| [29] |
Cheng YH, Shen R, Qiao RJ. Advances on immune response mechanisms of bacterial glyco-conjugate vaccines. Progress in Microbiology and Immunology, 2018, 46(4): 81-86.
(in Chinese) 程亚慧, 沈荣, 乔瑞洁. 细菌性多糖蛋白结合疫苗免疫应答机制的研究进展. 微生物学免疫学进展, 2018, 46(4): 81-86. |
| [30] | Avci FY, Li XM, Tsuji M, Kasper DL. A mechanism for glycoconjugate vaccine activation of the adaptive immune system and its implications for vaccine design. Nature Medicine, 2011, 17(12): 1602-1609. DOI:10.1038/nm.2535 |
| [31] | Rappuoli R, De Gregorio E. A sweet T cell response. Nature Medicine, 2011, 17(12): 1551-1552. DOI:10.1038/nm.2587 |
| [32] | Zimmermann S, Lepenies B. Glycans as vaccine antigens and adjuvants: immunological considerations. Carbohydrate-Based Vaccines, 2015, 1331: 11-26. |
| [33] | Adamo R, Nilo A, Castagner B, Boutureira O, Berti F, Bernardes GJL. Synthetically defined glycoprotein vaccines: current status and future directions. Chemical Science, 2013, 4(8): 2995-3008. DOI:10.1039/c3sc50862e |
| [34] | Rappuoli R, De Gregorio E, Costantino P. On the mechanisms of conjugate vaccines. Proceedings of the National Academy of Sciences of the United States of America, 2019, 116(1): 14-16. DOI:10.1073/pnas.1819612116 |
| [35] |
Zhang T, Meng X, Zhu T, Liu Z, Zhang LP, Hao JQ. Preparation of Streptococcus pneumonia type 3 capsular polysaccharide conjugate vaccine. Chinese Journal of Immunology, 2015, 31(10): 1361-1365.
(in Chinese) 张涛, 孟欣, 朱涛, 刘正, 张立平, 郝杰清. 3型肺炎球菌荚膜多糖结合疫苗的研制. 中国免疫学杂志, 2015, 31(10): 1361-1365. DOI:10.3969/j.issn.1000-484X.2015.10.014 |
| [36] | Berti F, Adamo R. Recent mechanistic insights on glycoconjugate vaccines and future perspectives. ACS Chemical Biology, 2013, 8(8): 1653-1663. DOI:10.1021/cb400423g |
| [37] | Verez-Bencomo V, Fernández-Santana V, Hardy E, Toledo ME, Rodríguez MC, Heynngnezz L, Rodriguez A, Baly A, Herrera L, Izquierdo M, Villar A, Valdés Y, Cosme K, Deler ML, Montane M, Garcia E, Ramos A, Aguilar A, Medina E, Toraño G, Sosa I, Hernandez I, Martínez R, Muzachio A, Carmenates A, Costa L, Cardoso F, Campa C, Diaz M, Roy R. A synthetic conjugate polysaccharide vaccine against Haemophilus influenzae type B. Science, 2004, 305(5683): 522-525. DOI:10.1126/science.1095209 |
| [38] | Astronomo RD, Burton DR. Carbohydrate vaccines: developing sweet solutions to sticky situations?. Nature Reviews Drug Discovery, 2010, 9(4): 308-324. DOI:10.1038/nrd3012 |
| [39] | Anish C, Schumann B, Pereira CL, Seeberger PH. Chemical biology approaches to designing defined carbohydrate vaccines. Chemistry & Biology, 2014, 21(1): 38-50. |
| [40] | Morelli L, Poletti L, Lay L. Carbohydrates and immunology: synthetic oligosaccharide antigens for vaccine formulation. European Journal of Organic Chemistry, 2011, 2011(29): 5723-5777. DOI:10.1002/ejoc.201100296 |
| [41] | Pellicci DG, Clarke AJ, Patel O, Mallevaey T, Beddoe T, Le Nours J, Uldrich AP, McCluskey J, Besra GS, Porcelli SA, Gapin L, Godfrey DI, Rossjohn J. Recognition of β-linked self glycolipids mediated by natural killer T cell antigen receptors. Nature Immunology, 2011, 12(9): 827-833. DOI:10.1038/ni.2076 |
| [42] | Deck MB, Sjölin P, Unanue ER, Kihlberg J. MHC-restricted, glycopeptide-specific T cells show specificity for both carbohydrate and peptide residues. Journal of Immunology, 1999, 162(8): 4740-4744. |
| [43] | Mogemark M, Cirrito TP, Sjölin P, Unanue ER, Kihlberg J. Influence of saccharide size on the cellular immune response to glycopeptides. Organic & Biomolecular Chemistry, 2003, 1(12): 2063-2069. |
| [44] | Kabat EA. The upper limit for the size of the human antidextran combining site. Journal of Immunology, 1960, 84: 82-85. |
| [45] | Polonskaya Z, Deng SL, Sarkar A, Kain L, Comellas-Aragones M, McKay CS, Kaczanowska K, Holt M, McBride R, Palomo V, Self KM, Taylor S, Irimia A, Mehta SR, Dan JM, Brigger M, Crotty S, Schoenberger SP, Paulson JC, Wilson IA, Savage PB, Finn MG, Teyton L. T cells control the generation of nanomolar-affinity anti-glycan antibodies. The Journal of Clinical Investigation, 2017, 127(4): 1491-1504. DOI:10.1172/JCI91192 |
| [46] | Feng SJ, Xiong CH, Wang SB, Guo ZW, Gu GF. Semisynthetic glycoconjugate vaccines to elicit T cell-mediated immune responses and protection against Streptococcus pneumoniae serotype 3. ACS Infectious Diseases, 2019, 5(8): 1423-1432. DOI:10.1021/acsinfecdis.9b00103 |
| [47] | Benaissa-Trouw B, Lefeber DJ, Kamerling JP, Vliegenthart JFG, Kraaijeveld K, Snippe H. Synthetic polysaccharide type 3-related di-, tri-, and tetrasaccharide-CRM 197 conjugates induce protection against Streptococcus pneumoniae type 3 in mice. Infection and Immunity, 2001, 69(7): 4698-4701. DOI:10.1128/IAI.69.7.4698-4701.2001 |
| [48] | Musher DM. How effective is vaccination in preventing pneumococcal disease?. Infectious Disease Clinics of North America, 2013, 27(1): 229-241. DOI:10.1016/j.idc.2012.11.011 |
| [49] | Kurbatova EA, Akhmatova NK, Zaytsev AE, Akhmatova EA, Egorova NB, Yastrebova NE, Sukhova EV, Yashunsky DV, Tsvetkov YE, Nifantiev NE. Higher cytokine and opsonizing antibody production induced by bovine serum albumin (BSA)-conjugated tetrasaccharide related to Streptococcus pneumoniae type 3 capsular polysaccharide. Frontiers in Immunology, 2020, 11: 578019. DOI:10.3389/fimmu.2020.578019 |
| [50] | Kaplonek P, Khan N, Reppe K, Schumann B, Emmadi M, Lisboa MP, Xu FF, Calow ADJ, Parameswarappa SG, Witzenrath M, Pereira CL, Seeberger PH. Improving vaccines against Streptococcus pneumoniae using synthetic glycans. Proceedings of the National Academy of Sciences of the United States of America, 2018, 115(52): 13353-13358. DOI:10.1073/pnas.1811862115 |
| [51] | Falup-Pecurariu O. Lessons learnt after the introduction of the seven valent-pneumococcal conjugate vaccine toward broader spectrum conjugate vaccines. Biomedical Journal, 2012, 35(6): 450. DOI:10.4103/2319-4170.104409 |
| [52] | Postma DF, Van Werkhoven CH, Huijts SM, Bolkenbaas M, Oosterheert JJ, Bonten MJM. New trends in the prevention and management of community-acquired pneumonia. The Netherlands Journal of Medicine, 2012, 70(8): 337-348. |
| [53] | Miyaji EN, Oliveira MLS, Carvalho E, Ho PL. Serotype-independent pneumococcal vaccines. Cellular and Molecular Life Sciences, 2013, 70(18): 3303-3326. DOI:10.1007/s00018-012-1234-8 |
| [54] | Ginsburg AS, Alderson MR. New conjugate vaccines for the prevention of pneumococcal disease in developing countries. Drugs of Today, 2011, 47(3): 207. DOI:10.1358/dot.2011.47.3.1556471 |
| [55] | Balsells E, Guillot L, Nair H, Kyaw MH. Serotype distribution of Streptococcus pneumoniae causing invasive disease in children in the post-PCV era: a systematic review and meta-analysis. PLoS One, 2017, 12(5): e0177113. DOI:10.1371/journal.pone.0177113 |
| [56] | Hanquet G, Krizova P, Valentiner-Branth P, Ladhani SN, Nuorti JP, Lepoutre A, Mereckiene J, Knol M, Winje BA, Ciruela P, Ordobas M, Guevara M, McDonald E, Morfeldt E, Kozakova J, Slotved HC, Fry NK, Rinta-Kokko H, Varon E, Corcoran M, Van Der Ende A, Vestrheim DF, Munoz-Almagro C, Latasa P, Castilla J, Smith A, Henriques-Normark B, Whittaker R, Pastore Celentano L, Savulescu C, Group SM Pneumo. Effect of childhood pneumococcal conjugate vaccination on invasive disease in older adults of 10 European countries: implications for adult vaccination. Thorax, 2019, 74(5): 473-482. DOI:10.1136/thoraxjnl-2018-211767 |
| [57] | Savulescu C, Krizova P, Lepoutre A, Mereckiene J, Vestrheim DF, Ciruela P, Ordobas M, Guevara M, McDonald E, Morfeldt E, Kozakova J, Varon E, Cotter S, Winje BA, Munoz-Almagro C, Garcia L, Castilla J, Smith A, Hanquet G. Effect of high-valency pneumococcal conjugate vaccines on invasive pneumococcal disease in children in SpIDnet countries: an observational multicentre study. The Lancet Respiratory Medicine, 2017, 5(8): 648-656. DOI:10.1016/S2213-2600(17)30110-8 |
| [58] | Jiang HF, Meng Q, Liu XR, Chen HY, Zhu CQ, Chen YS. PspA diversity, serotype distribution and antimicrobial resistance of invasive pneumococcal isolates from paediatric patients in Shenzhen, China. Infection and Drug Resistance, 2021, 14: 49-58. DOI:10.2147/IDR.S286187 |
| [59] | Mettu R, Chen CY, Wu CY. Synthetic carbohydrate- based vaccines: challenges and opportunities. Journal of Biomedical Science, 2020, 27(1): 1-22. DOI:10.1186/s12929-019-0592-z |
| [60] | Colombo C, Pitirollo O, Lay L. Recent advances in the synthesis of glycoconjugates for vaccine development. Molecules, 2018, 23(7): 1712. DOI:10.3390/molecules23071712 |
| [61] | Moffitt K, Malley R. Rationale and prospects for novel pneumococcal vaccines. Human Vaccines & Immunotherapeutics, 2016, 12(2): 383-392. |
| [62] | Middleton DR, Paschall AV, Duke JA, Avci FY. Enzymatic hydrolysis of pneumococcal capsular polysaccharide renders the bacterium vulnerable to host defense. Infection and Immunity, 2018, 86(8): e00316-18. |
| [63] | Paschall AV, Middleton DR, Wantuch PL, Avci FY. Therapeutic activity of type 3 Streptococcus pneumoniae capsule degrading enzyme Pn3Pase. Pharmaceutical Research, 2020, 37(12): 1-9. DOI:10.1007/s11095-020-02960-3 |
 2022, Vol. 62
2022, Vol. 62