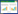中国科学院微生物研究所,中国微生物学会,中国菌物学会
文章信息
- 江晗, 谢碧波, 赵思思, 尹卫国, 赵飞骏. 2022
- Han Jiang, Bibo XIE, Sisi ZHAO, Weiguo YIN, Feijun ZHAO. 2022
- 梅毒螺旋体的营养物质转运及能量合成相关代谢机制研究
- Metabolic mechanisms of nutrients transport and energy synthesis of Treponema pallidum
- 微生物学报, 62(1): 57-64
- Acta Microbiologica Sinica, 62(1): 57-64
-
文章历史
- 收稿日期:2021-03-24
- 修回日期:2021-06-28
- 网络出版日期:2021-07-07
2. 广东省清远市人民医院分子诊断中心, 广东 清远 511518
2. Molecular Diagnostic Center, People's Hospital of Qingyuan City, Qingyuan 511518, Guangdong, China
梅毒严重危害人类身心健康,全球梅毒发病率一直居高不下[1]。其病原体梅毒螺旋体(Treponema pallidum,Tp)极具侵袭力,可通过血液、淋巴系统从感染部位迅速扩散至宿主其他多个远端组织器官,并逃避机体免疫系统的清除,可“隐匿性”引发机体持续性、系统性、多组织器官慢性损伤[2-3]。Tp是一种人体专性寄生菌,基因组序列分析表明其DNA复制、转录、翻译和修复系统完整,但缺乏许多编码新陈代谢的基因,生物合成能力有限,需要从宿主摄取能量和多种营养成分,故代谢和生物合成活性减至最低[4]。Tp包含一个功能齐全的糖酵解途径,但缺乏脂肪酸β氧化相关基因,葡萄糖缺乏时Tp运动能力丧失,恢复葡萄糖供应时运动能力迅速恢复,葡萄糖可能是Tp新陈代谢的主要能量来源,机体血液和组织液中葡萄糖可满足Tp对糖酵解起始物的需求。
尽管近年来Tp体外细胞培养有一定突破[5-7],但目前仍无法用培养基对Tp进行人工培养,更无法对其进行基因操作研究,从而严重阻碍对Tp新陈代谢机理、致病机制[8-10]及疫苗相关的研究[11-13]。本文主要针对Tp的葡萄糖摄取、糖酵解途径及代谢产物去路等代谢机制进行阐述和整合,结合最新的研究依据和观点,以期唤起科研工作者重新关注并进一步探索Tp尚未明了的代谢机能,突破Tp体外人工培养的瓶颈,为进一步阐明Tp可能的致病机制、寻找新的临床治疗靶点及疫苗的研发提供参考借鉴。
1 Tp的营养物质转运Tp外膜上的非选择性通道和胞质膜上的ABC转运蛋白、共转运蛋白共同介导了营养物质从外部环境向胞内转运的过程[3]。营养物质通过非选择性通道时为被动扩散,无需消耗能量。环境中高浓度的葡萄糖可经Tp非选择性孔蛋白(如TprC,又称Tp0117)被动扩散,通过Tp菌体外膜转运至Tp细胞周质[14-15]。ABC (ATP-binding cassette)转运蛋白是蛋白质的超家族,可通过细胞膜转运营养物质和次生代谢产物,通过ABC转运蛋白时需消耗ATP,通过同向转运体需要H+或Na+的化学梯度差协同支持[16]。如长链脂肪酸(LCFA)经多聚脂蛋白复合物(TatT-TatP,又称Tp0956-Tp0957)的转运需要H+、Na+梯度差协同作用[17-18],此外还有天冬氨酸和谷氨酸协同转运体(Tp0555和Tp0934),丙氨酸和甘氨酸协同转运体(Tp0414和Tp0998)以及支链氨基酸的协同转运体(Tp0265)[3]。用于摄取蛋氨酸的MetI-MetN-MetQ是一种ABC转运蛋白[19],虽然Tp的基因组编码了寡肽转运蛋白的底物结合蛋白(OppA,也称为Tp0585)、组氨酸转运蛋白的底物结合蛋白(HisJ,也称为Tp0308)和极性氨基酸转运蛋白的底物结合蛋白(Tp0309),但并未发现它们的转运蛋白的通透酶和ATP结合蛋白[4]。Tp对葡萄糖的转运同样至关重要,葡萄糖经高亲和力转运载体MglB-2 ABC转运蛋白(由Tp0545、Tp0684、Tp0685和Tp0686共同编码组成的蛋白复合体)跨越细胞质膜进入菌体细胞质[20-22]。此外,生物信息学预测Tp还可能通过一种糖类ABC渗透酶(Tp0075-Tp0076)转运葡萄糖,其有两个不同的底物结合蛋白(UgpB和MsmE,又称Tp0074和Tp0737)和一个ATP结合亚基(Tp0804)[4]。有趣的是,Tp的外膜蛋白(OMP)允许营养吸收和最终代谢产物输出,并且几种特定的ABC转运蛋白催化糖的摄取,这被认为是Tp寄生生活方式的关键[23]。这些研究表明,ABC转运蛋白、ATP的生成和化学渗透梯度的维持对Tp摄取葡萄糖等营养物质来说至关重要,然而相关的生理机制仍有待进一步阐明。
2 Tp能量合成的主要途径——糖酵解途径近期,越来越多的文献证实了Tp仅依靠葡萄糖分解代谢(糖酵解)来产生ATP[3-4]。进入Tp胞内的葡萄糖经糖酵解途径,又称EMP (Embden Meyerhof-Parmas pathway)途径,降解为丙酮酸并伴随着ATP生成。常规糖酵解过程中有3个不可逆的反应,催化这3步反应的酶(己糖激酶、磷酸果糖激酶和丙酮酸激酶)均为糖酵解途径限速酶[24]。Tp基因组中存在糖酵解途径所需所有酶的编码基因,但与通常ATP依赖糖酵解途径不同的是Tp使用焦磷酸(PPi)依赖性磷酸果糖激酶和丙酮酸磷酸二激酶替换了糖酵解途径中的磷酸果糖激酶和丙酮酸激酶,这种改变使得Tp可以从底物水平磷酸化中获得更多的ATP (图 1)。
|
| 图 1 Tp糖酵解途径能量产生,氨基酸、脂质生物合成和NAD+的再生[3] Figure 1 Tp glycolysis pathway energy production, amino acid, lipid biosynthesis and NAD+ regeneration. 1, 3-DPG: 1, 3-diphosphoglyceric acid; ABC: ATP-binding cassette; Acetyl-P: acetyl phosphate; AckA: acetate kinase; AsnA: aspartate-ammonia ligase; CoA: coenzyme A; DHAP: dihydroxyacetone phosphate; flavodoxinox: oxidized flavodoxin; flavodoxinred: reduced flavodoxin; Fructose-1, 6-P2: fructose 1, 6-bisphosphate; Fructose-6-P: fructose 6-phosphate; Glyceraldehyde-3-P: glyceraldehyde 3-phosphate; LDH: D-lactate dehydrogenase; Mgl: methylgalactoside; NOX2: NADH oxidase 2; OAD: oxaloacetate decarboxylase; PFOR: pyruvate-flavodoxin oxidoreductase; PG: phosphatidyl glycerol; Pi: inorganic phosphate; PPDK: pyruvate phosphate dikinase; PPFK: phosphofructokinase; PPi: inorganic pyrophosphate; Pta: phosphate acetyl transferase; TpaaT: aspartate aminotransferase. |
Tp的糖酵解途径共经历10个主要化学反应(图 1)。①进入Tp胞内的葡萄糖首先被己糖激酶(Tp0505)催化生成6-磷酸葡萄糖,磷酸根由ATP供给。由于Tp中PPi依赖性磷酸果糖激酶和丙酮酸磷酸二激酶催化的反应为可逆性,且缺乏磷酸烯醇式丙酮酸(PEP)依赖性磷酸转移酶系统(PTS)通透酶[17, 25],Tp主要通过己糖激酶来调节葡萄糖的摄取。②6-磷酸葡萄糖经葡萄糖-6磷酸异构酶(Tp0475)催化转变为6-磷酸果糖。③Tp使用PPi依赖性磷酸果糖激酶(PPi-PFK)代替ATP依赖性磷酸果糖激酶(ATP-PFK)催化6-磷酸果糖磷酸化生成1, 6-二磷酸果糖,磷酸根由PPi供给[17-18, 26]。ATP-PFK催化的反应是不可逆的,是ATP依赖糖酵解重要的调节酶,但PPi-PFK催化的反应是可逆的[17-18]。Tp存在两个假定的PPi-PFK基因,一个编码62.4 kDa的PPi-PFK β亚基(TP0542),另一个编码50.2 kDa的PPi-PFK (TP0108),目前已证实TP0542编码的PPi-PFK具有较大的活性[4, 17-18]。④1, 6-二磷酸果糖在果糖-二磷酸醛缩酶(Tp0662)催化下生成磷酸二羟丙酮和3-磷酸甘油醛。⑤磷酸二羟丙酮也可经磷酸丙糖异构酶(Tp0537)进一步转变为3-磷酸甘油醛。至此1分子葡萄糖生成2分子3-磷酸甘油醛,ATP依赖糖酵解途径通过两次磷酸化作用消耗2分子ATP,Tp糖酵解途径磷酸根一次由ATP供给,一次由焦磷酸供给,这就减少了糖酵解途径ATP的消耗(表 1)。中间产物磷酸二羟丙酮还可经3-磷酸甘油脱氢酶(Tp1009)和NADH转化为3-磷酸甘油和NAD+[26]。3-磷酸甘油是脂质合成底物之一,在磷脂酰甘油磷酸合成酶(PgsA,又称Tp0256)催化下可生成磷脂酰甘油(PG),磷脂酰甘油是生物膜的重要组成部分。⑥3-磷酸甘油醛在3-磷酸甘油醛脱氢酶(Tp0844)催化下进一步氧化脱氢生成1, 3-二磷酸甘油酸,脱下的氢和电子转给脱氢酶的辅酶NAD+生成NADH。⑦在磷酸甘油酸激酶(Tp0538)催化下,1, 3-二磷酸甘油酸生成3-磷酸甘油酸,磷酸根转移给ADP生成ATP。⑧在磷酸甘油酸变位酶(Tp0168)催化下,3-磷酸甘油酸C3位上的磷酸基转变到C2位上生成2-磷酸甘油酸。⑨2-磷酸甘油酸由烯醇化酶(Tp0817)催化,脱水生成磷酸烯醇式丙酮酸(phosphoenolpyruvate,PEP)。⑩磷酸烯醇式丙酮酸在丙酮酸磷酸二激酶(PPDK,Tp0746编码)催化下生成丙酮酸。催化反应中一个磷酸基团从磷酸烯醇式丙酮酸转移,另一个磷酸基团从PPi转移,AMP被磷酸化两次形成ATP [18],由于PPi的部分化学能被利用,与腺苷酸激酶(ADK,也称为TP0595)偶联时,PPDK催化的反应产生4个ATP。ATP依赖糖酵解中,在丙酮酸激酶催化下磷酸烯醇式丙酮酸(PEP)高能磷酸基团转移给ADP生成ATP,产生2个ATP[18] (表 1,图 2)。
| ATP | ATP dependent glycolysis pathway | Treponema pallidum PPi-related glycolysis pathway |
| Consumption | G+ATP→G-6-P+ADP exokinase | G+ATP→G-6-P+ADP exokinase (Tp0505) |
| F-6-P+ATP→F-1, 6-P2+ADP ATP-PPFK | F-6-P+PPi↔F-1, 6-P2+Pi PPi-PPFK (Tp0542) | |
| Production | 1, 3-DPG+ADP↔3-phosphoglycerate+ATP phosphoglycerate kinase | 1, 3-DPG+ADP↔3-phosphoglycerate+ATP phosphoglycerate kinase (Tp0538) |
| PEP+ADP→Pyruvate+ATP pyruvate kinase | PEP+AMP+PPi↔Pyruvate+ATP+Pi PPDK (Tp0746) |
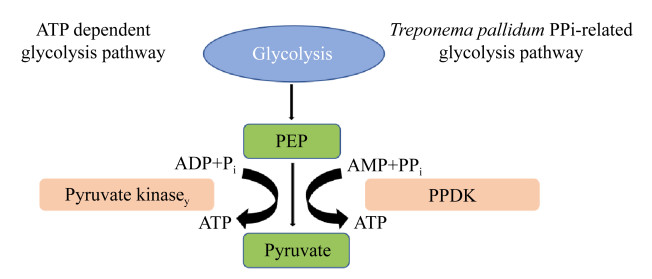
|
| 图 2 丙酮酸磷酸二激酶(PPDK)催化反应生成ATP (包含腺苷酸激酶反应)与丙酮酸激酶催化反应生成ATP对比 Figure 2 Comparision of ATP generation catalyzed by pyruvate kinase and pyruvate phosphate dikinase (PPDK) (including adenylate kinase reaction). |
真核细胞中一分子葡萄糖经糖酵解途径净生成2分子ATP (不计算NADH和FADH产生能量),Tp由于利用了PPi部分化学能,一分子葡萄糖经糖酵解途径可净生成5分子ATP。比起ATP依赖的糖酵解途径,Tp能从自身独特的糖酵解途径中获得更多的能量。这或许是因为Tp没有编码三羧酸循环和氧化磷酸化相关蛋白基因,生成的NADH和乙酰辅酶A无法从中获得能量(一分子NADH经氧化磷酸化生成2.5分子ATP,一分子乙酰辅酶A经三羧酸循环可生成30或32分子ATP)。因此,由于Tp最终可生成ATP的能力有限,而其营养物质的转运又多依赖ATP,这样独特的生物学特性在很大程度上限制了Tp营养物质的获取和新陈代谢的速率。
3 Tp的丙酮酸去路和NAD+再生丙酮酸是糖酵解的最终产物,其是对真核生物和人类代谢的许多方面至关重要的基石分子[27]。在有氧环境中,丙酮酸与辅酶A在丙酮酸-黄素氧化还原酶(Tp0939)催化下生成乙酰辅酶A和二氧化碳,电子从丙酮酸转移到黄素氧化还原蛋白(Tp0925),黄素氧化还原蛋白由氧化型转化为还原型;还原型黄素氧化还原蛋白在重新氧化过程中推动Na+/H+-Rnf复合体(Tp0151、Tp0152)将H+或Na+逆浓度排出膜外产生电化学梯度,这一过程伴随NAD+的还原[28-29]。因此,核黄素摄取和黄素利用对Tp代谢和能量产生十分重要,TpN38 (也称为Tp0298)的X射线结构显示它是核黄素转运蛋白(RfuABCD)的底物结合蛋白[30],其与黄素腺嘌呤二核苷酸(FAD)、核黄素-5-磷酸(FMN)转移酶(Ftp,又称Tp0796)协同作用[31],以满足Tp对黄素辅助因子的巨大需求。Tp中还存在2种V型H+-ATP酶,V型H+-ATP酶可消耗ATP逆浓度梯度将H+泵出细胞外,形成跨膜电化学势梯度[32],膜外H+顺梯度差流入膜内,该过程耦合ATP合酶生成ATP。此外,乙酰辅酶A在乙酸激酶(AckA,又称Tp0476)和磷酸乙酰转移酶(Pta,又称Tp0094)作用下转化为乙酸,产生ATP,乙酸是大多数兼性和严格厌氧微生物能量代谢的末端产物之一。Tp使用NADH氧化酶2 (NOX2,又称Tp0921)将分子氧还原为水,NADH作为电子供体再生为NAD+。氧供应不足时丙酮酸接受糖酵解途径中产生的NADH,使NADH重新氧化为NAD+,D-乳酸转化为丙酮酸最终代谢为乙酸,该反应由D-乳酸脱氢酶(LdhD,又称Tp0037)催化[33]。该反应中的ATP通过底物水平磷酸化产生,该途径可以为Tp的生理活动产生额外的ATP。
Tp还可以通过丙酮酸和草酰乙酸进行有限的氨基酸代谢(图 1)。Tp通过磷酸烯醇式丙酮酸羧化激酶(PckA,又称Tp0122)和草酰乙酸脱羧酶(OadA-OadB,又称Tp0056-Tp0057)使磷酸烯醇式丙酮酸和草酰乙酸、丙酮酸和草酰乙酸相互转换。以谷氨酸作为氨基供体,草酰乙酸通过天冬氨酸氨基转移酶(AST,又称Tp0223)转化为天冬氨酸,随后天冬氨酸通过天冬酰胺合成酶(AsnA,又称Tp0556)转化为天冬酰胺[34]。这些研究表明,Tp通过丙酮酸和其相关分子对代谢发挥重要的作用。
4 问题与展望长期以来,在梅毒螺旋体中存在一个难题,即高侵染性病原体-梅毒螺旋体如何产生足够的能量来完成人类感染过程中复杂的发病机理。几十年来,已经假定梅毒螺旋体仅依靠葡萄糖分解代谢(糖酵解)来产生ATP[3-4]。最近,越来越多的研究表明Tp外膜上的非选择性通道和胞质膜上的ABC转运蛋白共同介导了葡萄糖从外部环境向胞内的转运。此外,Tp通过糖酵解途径在底物水平磷酸化中利用PPi,生成了更多的高能磷酸基团,增加了ATP的生成。有趣的是,Tp还可以通过丙酮酸介导能量产生、氨基酸、脂质生物合成和NAD+的再生。但螺旋体的运动性是关键的毒力因子,对宿主的侵袭和传播至关重要,细菌运动通常取决于丰富的能量产生[35]。这与TP仅依赖糖酵解的前提有一定的矛盾,因为糖酵解是一种从葡萄糖中产生ATP严重受限的途径。ATP的生成和化学渗透梯度的维持对Tp的营养摄取来说至关重要,然而相关的生理机制仍有待进一步阐明。
Tp的营养来源极度依赖于宿主,依靠其提供的核酸碱基、脂肪酸、大多数氨基酸和葡萄糖作为能源,以及在温度、渗透压、氧气和CO2含量和pH值等方面用于维持Tp微环境的稳态条件[7]。并且Tp代谢能力相对其他病原体仍十分有限,为了逃避宿主的免疫清除,Tp限制了菌体表面稀有外膜蛋白的种类和数量,目前已明确的外膜蛋白也只有Tp92[8-9]、Tpr家族蛋白、黏附素蛋白等寥寥几类[36],这也在一定程度上影响了Tp营养物质的获取和新陈代谢的速率,从而导致Tp的增殖速度很慢(约30 h)。进一步了解Tp相关的代谢机制,寻找Tp生理活动过程中存在的关键蛋白及必需成分,可能为Tp的体外人工培养配制适宜培养基、Tp的持续性感染机制[8-10]及疫苗研发[11-13]新靶标的探寻提供参考借鉴。
| [1] | Smolak A, Rowley J, Nagelkerke N, Kassebaum NJ, Chico RM, Korenromp EL, Abu-Raddad LJ. Trends and predictors of syphilis prevalence in the general population: global pooled analyses of 1103 prevalence measures including 136 million syphilis tests. Clinical Infectious Diseases, 2017, 66(8): 1184-1191. |
| [2] | Peeling RW, Mabey DCW. Focus: syphilis. Nature Reviews Microbiology, 2004, 2(6): 448. |
| [3] | Radolf JD, Deka RK, Anand A, Šmajs D, Norgard MV, Yang XF. Treponema pallidum, the syphilis spirochete: making a living as a stealth pathogen. Nature Reviews Microbiology, 2016, 14(12): 744-759. DOI:10.1038/nrmicro.2016.141 |
| [4] | Fraser CM, Norris SJ, Weinstock GM, White O, Sutton GG, Dodson R, Gwinn M, Hickey EK, Clayton R, Ketchum KA, Sodergren E, Hardham JM, McLeod MP, Salzberg S, Peterson J, Khalak H, Richardson D, Howell JK, Chidambaram M, Utterback T, McDonald L, Artiach P, Bowman C, Cotton MD, Fujii C, Garland S, Hatch B, Horst K, Roberts K, Sandusky M, Weidman J, Smith HO, Venter JC. Complete genome sequence of Treponema pallidum, the syphilis spirochete. Science, 1998, 281(5375): 375-388. DOI:10.1126/science.281.5375.375 |
| [5] | Edmondson DG, Norris SJ. In vitro cultivation of the syphilis spirochete Treponema pallidum. Current Protocols, 2021, 1(2): e44. |
| [6] | Edmondson DG, DeLay BD, Kowis LE, Norris SJ. Parameters affecting continuous in vitro culture of Treponema pallidum strains. mBio, 2021, 12(1): e03536-e03520. |
| [7] | Edmondson DG, Hu B, Norris SJ. Long-term in vitro culture of the syphilis spirochete Treponema pallidum subsp. pallidum. mBio, 2018, 9(3): e01153-e01118. |
| [8] | Luo X, Zhang XH, Gan L, Zhou CL, Zhao T, Zeng TB, Liu SQ, Xiao YJ, Yu J, Zhao FJ. The outer membrane protein Tp92 of Treponema pallidum induces human mononuclear cell death and IL-8 secretion. Journal of Cellular and Molecular Medicine, 2018, 22(12): 6039-6054. DOI:10.1111/jcmm.13879 |
| [9] | Luo X, Zhang XH, Zhao T, Zeng TB, Liu W, Deng MX, Zhao FJ. A preliminary study on the proinflammatory mechanisms of Treponema pallidum outer membrane protein Tp92 in human macrophages and HMEC-1 cells. Microbial Pathogenesis, 2017, 110: 176-183. DOI:10.1016/j.micpath.2017.06.046 |
| [10] | Liu W, Deng MX, Zhang XH, Yin WG, Zhao T, Zeng TB, Liu SQ, Xiao YJ, Zhang L, Luo X, Zhao FJ. Performance of novel infection phase-dependent antigens in syphilis serodiagnosis and treatment efficacy determination. Clinica Chimica Acta, 2019, 488: 13-19. DOI:10.1016/j.cca.2018.10.017 |
| [11] | Duan JX, Zhao Y, Zhang XH, Jiang H, Xie BB, Zhao T, Zhao FJ. Research status and perspectives for pathogenic spirochete vaccines. Clinica Chimica Acta, 2020, 507: 117-124. DOI:10.1016/j.cca.2020.04.002 |
| [12] | Zhao FJ, Liu SQ, Zhang XH, Yu J, Zeng TB, Gu WM, Cao XY, Chen X, Wu YM. CpG adjuvant enhances the mucosal immunogenicity and efficacy of a Treponema pallidum DNA vaccine in rabbits. Human Vaccines & Immunotherapeutics, 2013, 9(4): 753-760. |
| [13] | Zhao FJ, Zhang XH, Liu SQ, Gu WM, Yu J, Zeng TB, Zhang YJ, Chen X, Wu YM. Treponema pallidum Gpd DNA vaccine adjuvanted with IL-2 and coated by chitosan nanoparticles attenuates syphilitic lesion development in the rabbit model. Science China-Life Sciences, 2013, 56(2): 174-180. DOI:10.1007/s11427-012-4434-4 |
| [14] | Anand A, LeDoyt M, Karanian C, Luthra A, Koszelak-Rosenblum M, Malkowski MG, Puthenveetil R, Vinogradova O, Radolf JD. Bipartite topology of Treponema pallidum repeat proteins C/D and I. Journal of Biological Chemistry, 2015, 290(19): 12313-12331. DOI:10.1074/jbc.M114.629188 |
| [15] | Anand A, Luthra A, Dunham-Ems S, Caimano MJ, Karanian C, LeDoyt M, Cruz AR, Salazar JC, Radolf JD. TprC/D (Tp0117/131), a trimeric, pore-forming rare outer membrane protein of Treponema pallidum, has a bipartite domain structure. Journal of Bacteriology, 2012, 194(9): 2321-2333. DOI:10.1128/JB.00101-12 |
| [16] | Liu H, Cheng M, Zhao SS, Lin CY, Song JZ, Yang Q. ATP-binding cassette transporter regulates N, N'-diacetylchitobiose transportation and chitinase production in Trichoderma asperellum T4. International Journal of Molecular Sciences, 2019, 20(10): 2412. DOI:10.3390/ijms20102412 |
| [17] | Roberson RS, Ronimus RS, Gephard S, Morgan HW. Biochemical characterization of an active pyrophosphate-dependent phosphofructokinase from Treponema pallidum. FEMS Microbiology Letters, 2001, 194(2): 257-260. DOI:10.1111/j.1574-6968.2001.tb09479.x |
| [18] | Mertens E. ATP versus pyrophosphate: glycolysis revisited in parasitic protists. Parasitology Today, 1993, 9(4): 122-126. DOI:10.1016/0169-4758(93)90169-G |
| [19] | Deka RK, Neil L, Hagman KE, Machius M, Tomchick DR, Brautigam CA, Norgard MV. Structural evidence that the 32-kilodalton lipoprotein (Tp32) of Treponema pallidum is an l-methionine-binding protein. Journal of Biological Chemistry, 2004, 279(53): 55644-55650. DOI:10.1074/jbc.M409263200 |
| [20] | Deka RK, Goldberg MS, Hagman KE, Norgard MV. The Tp38(TpMglB-2) lipoprotein binds glucose in a manner consistent with receptor function in Treponema pallidum. Journal of Bacteriology, 2004, 186(8): 2303-2308. DOI:10.1128/JB.186.8.2303-2308.2004 |
| [21] | Brautigam CA, Deka RK, Liu WZ, Norgard MV. The Tp0684(MglB-2) lipoprotein of Treponema pallidum: a glucose-binding protein with divergent topology. PLoS One, 2016, 11(8): e0161022. DOI:10.1371/journal.pone.0161022 |
| [22] | Brautigam CA, Deka RK, Liu WZ, Norgard MV. Crystal stuctures of MglB-2(TP0684), a topologically variant d-glucose-binding protein from Treponema pallidum, reveal a ligand-induced conformational change. Protein Science, 2018, 27(4): 880-885. DOI:10.1002/pro.3373 |
| [23] | Buyuktimkin B, Zafar H, Saier MH Jr. Comparative genomics of the transportome of ten Treponema species. Microbial Pathogenesis, 2019, 132: 87-99. DOI:10.1016/j.micpath.2019.04.034 |
| [24] | Wilson DF, Matschinsky FM. Metabolic homeostasis in life as we know it: its origin and thermodynamic basis. Frontiers in Physiology, 2021, 12: 658997. DOI:10.3389/fphys.2021.658997 |
| [25] | Gonzalez CF, Stonestrom AJ, Lorca GL, Saier MH Jr. Biochemical characterization of phosphoryl transfer involving HPr of the phosphoenolpyruvate-dependent phosphotransferase system in Treponema denticola, an organism that lacks PTS permeases. Biochemistry, 2005, 44(2): 598-608. DOI:10.1021/bi048412y |
| [26] | Schwan TG, Battisti JM, Porcella SF, Raffel SJ, Schrumpf ME, Fischer ER, Carroll JA, Stewart PE, Rosa P, Somerville GA. Glycerol-3-phosphate acquisition in spirochetes: distribution and biological activity of glycerophosphodiester phosphodiesterase (GlpQ) among Borrelia species. Journal of Bacteriology, 2003, 185(4): 1346-1356. DOI:10.1128/JB.185.4.1346-1356.2003 |
| [27] | Gray LR, Tompkins SC, Taylor EB. Regulation of pyruvate metabolism and human disease. Cellular and Molecular Life Sciences: CMLS, 2014, 71(14): 2577-2604. DOI:10.1007/s00018-013-1539-2 |
| [28] | Deka RK, Brautigam CA, Liu WZ, Tomchick DR, Norgard MV. Evidence for posttranslational protein flavinylation in the syphilis spirochete Treponema pallidum: structural and biochemical insights from the catalytic core of a periplasmic flavin-trafficking protein. mBio, 2015, 6(3): e00519-e00515. |
| [29] | Mayer F, Müller V. Adaptations of anaerobic Archaea to life under extreme energy limitation. FEMS Microbiology Reviews, 2014, 38(3): 449-472. DOI:10.1111/1574-6976.12043 |
| [30] | Deka RK, Brautigam CA, Biddy BA, Liu WZ, Norgard MV. Evidence for an ABC-type riboflavin transporter system in pathogenic spirochetes. mBio, 2013, 4(1): e00615-e00612. |
| [31] | Deka RK, Brautigam CA, Liu WZ, Tomchick DR, Norgard MV. The TP0796 lipoprotein of Treponema pallidum is a bimetal-dependent FAD pyrophosphatase with a potential role in flavin homeostasis. Journal of Biological Chemistry, 2013, 288(16): 11106-11121. DOI:10.1074/jbc.M113.449975 |
| [32] | Saier MH Jr, Paulsen IT. Whole genome analyses of transporters in spirochetes: borrelia burgdorferi and Treponema pallidum. Journal of Molecular Microbiology and Biotechnology, 2000, 2(4): 393-399. |
| [33] | Deka RK, Liu WZ, Norgard MV, Brautigam CA. Biophysical and biochemical characterization of TP0037, a d-lactate dehydrogenase, supports an acetogenic energy conservation pathway in Treponema pallidum. mBio, 2020, 11(5): e02249-e02220. |
| [34] | McGill MA, Edmondson DG, Carroll JA, Cook RG, Orkiszewski RS, Norris SJ. Characterization and serologic analysis of the Treponema pallidum proteome. Infection and Immunity, 2010, 78(6): 2631-2643. DOI:10.1128/IAI.00173-10 |
| [35] | Mitchell JG, Kogure K. Bacterial motility: links to the environment and a driving force for microbial physics. FEMS Microbiology Ecology, 2006, 55(1): 3-16. DOI:10.1111/j.1574-6941.2005.00003.x |
| [36] | Radolf JD, Kumar S. The Treponema pallidum outer membrane. Current Topics in Microbiology and Immunology. Cham: Springer International Publishing, 2017: 1-38. |
 2022, Vol. 62
2022, Vol. 62