中国科学院微生物研究所,中国微生物学会,中国菌物学会
文章信息
- 钱新杰, 李一昊, 曾颃, 殷晗杰, 王瑜欣, 黄豪圣, 巩倩雯, 李德志, 薛峰, 汤芳, 戴建君. 2021
- Xinjie Qian, Yihao Li, Hang Zeng, Hanjie Yin, Yuxin Wang, Haosheng Huang, Qianwen Gong, Dezhi Li, Feng Xue, Fang Tang, Jianjun Dai. 2021
- EAEC噬菌体PNJ1809-11和PNJ1809-13作为环境消毒剂的杀菌效果评估
- Evaluation of sterilization efficiency of EAEC phage PNJ1809-11 and PNJ1809-13 as environmental disinfectants
- 微生物学报, 61(7): 2018-2030
- Acta Microbiologica Sinica, 61(7): 2018-2030
-
文章历史
- 收稿日期:2020-07-23
- 修回日期:2020-09-27
- 网络出版日期:2020-12-02
常见的致泻型大肠杆菌分为以下6种类型:肠致病性大肠杆菌(EPEC)、产肠毒素性大肠杆菌(ETEC)、肠出血性大肠杆菌(EHEC)、肠侵袭性大肠杆菌(EIEC)、弥散黏附性大肠杆菌(DAEC)和肠聚集性大肠杆菌(EAEC)[1]。其中EAEC可通过粪口传播引起炎症性腹泻,以特有的“聚集样”或“砖堆样”的方式黏附于上皮细胞株HEp-2[2-3]。EAEC多引起发展中国家和工业化国家断奶幼儿和旅行者的急性或持续性腹泻和溶血性尿毒症,而常见的养殖动物如鸡、猪、牛、羊也是其重要宿主,其中牛的带菌率可高达16%[4]。环境中残留的细菌对现代养殖企业的效益产生重要影响,企业多使用化学制剂通过熏蒸或喷洒的方式对环境进行消毒,如过硫酸氢钾、戊二醛和二氧化氯等,但这些消毒剂在使用过程中产生的药物残留会对工作人员的健康造成一定伤害,或被动物误吸误食之后对动物的健康造成威胁[5-6]。
1921年,Bruynoghe和Maisin首次将噬菌体应用于葡萄球菌感染的治疗[7],此后,由于噬菌体在自然界的丰度及其较好的宿主特异性,特别是噬菌体在杀菌过程中的安全性以及对多重耐药菌的杀灭效果,使噬菌体在“生物防治”方面具有较大的发展潜力。
本试验对实验室保存的2株EAEC噬菌体PNJ180911、PNJ180913进行生物学特性分析,研究其对宿主菌CVCC232生物被膜的裂解效果,并探索了噬菌体在模拟密闭空间、养殖温度、粪便pH和阳光直射等环境中对宿主菌的杀灭效果,为研制新型杀菌剂奠定了基础,对EAEC的防控具有重要意义。
1 材料和方法 1.1 培养基与试剂琼脂粉、胰蛋白胨、酵母提取物、氯化钠、HCl、NaOH、甲醇、冰醋酸、草酸结晶紫、SM缓冲液(NaCl,5.5 g;MgSO4·7H2O,2 g;1 mol/L pH 7.5 Tris-HCl,50 mL;明胶,0.1 g,ddH2O定容至1000 mL)。
1.2 菌株与培养条件肠聚集性大肠杆菌CVCC232购自中国兽药监察所菌种保藏中心,分离自上海某猪场腹泻仔猪。大肠杆菌在液体Luria-Bertain(LB)培养基或固体LB平板上培养。噬菌体PNJ180911、PNJ180913均由本实验室分离并保存。
1.3 噬菌体形态的观察将10 μL噬菌体悬液滴于铜网上,静置10 min,用滤纸吸去边缘多余的液体,滴加10 μL 2%磷钨酸,复染2 min,自然风干后,用透射电镜(TEM)观察噬菌体的形态。
1.4 噬菌体宿主谱的测定用平板点样法测定噬菌体的宿主谱,选取本实验室保存的167株不同动物源的大肠杆菌测定2株噬菌体的宿主谱,观察平板上噬菌斑的形成情况。
1.5 噬菌体最佳感染复数(MOI)的测定将CVCC232培养至对数期,浓度为1× 108 CFU/mL。按照感染复数为0.001、0.01、0.1、1、10和100的比例分别将噬菌体与细菌等量混合,静置15 min,10000 r/min离心10 min,弃上清,沉淀用5 mL液体LB重悬,37 ℃摇床培养5 h后10000 r/min离心10 min,取上清液,双层琼脂平板法测效价,以产生最高效价的比例为最佳感染复数。
1.6 噬菌体一步生长曲线的测定将噬菌体与宿主菌CVCC232以最佳感染复数均匀混合,37 ℃静置15 min,10000 r/min离心10 min,弃上清,将沉淀用37 ℃预热的5 mL液体LB重悬,取200 μL,12000 r/min离心30 s,取上清测效价,以此时间计为零时,置于37 ℃摇床培养,前20 min每5 min取样测效价,20 min后每10 min取样并测效价,共持续120 min。
1.7 噬菌体对pH和温度的耐受性检测噬菌体对pH的耐受性检测:用HCl、NaOH溶液将SM缓冲液调节至pH 2、3、4、5、6、7、8、9、10、11、12,以不同pH的SM缓冲液稀释噬菌体,37 ℃水浴锅温育,分别于0.5 h、1 h后取样,双层琼脂平板法测定其效价。
噬菌体对温度的耐受性检测:将噬菌体分别放置于40、50、60、70、80 ℃水浴锅中温育,分别于0.5、1 h后取样,双层琼脂平板法测效价。
1.8 噬菌体体外杀菌效果的测定将新鲜菌液(1×108 CFU/mL)与噬菌体分别以MOI=0.1、1、10、100的比例均匀混合后加入96孔板中,阳性对照孔加入200 μL浓度为1×107 CFU/mL的菌液,设3个复孔。将96孔板置于酶标仪中37 ℃振荡培养,每小时测量OD600处的吸光值,共测量16 h。
1.9 模拟养殖环境中噬菌体制剂的杀菌效果 1.9.1 模拟封闭装置中噬菌体制剂的杀菌效果:在已灭菌的透明密封箱底部放置若干无盖的固体LB平板,使用无菌喷壶将5 mL CVCC232 (5× 105 CFU/mL)均匀喷洒在箱子内部,室温静置,使细菌自然沉降到箱子底部(图 1)。

|
| 图 1 模拟封闭空间装置 Figure 1 Simulated closed space. |
将噬菌体PNJ1809-11、PNJ1809-13和噬菌体鸡尾酒经雾化和喷雾处理后,测定其对宿主菌的杀灭效果。
(1) 雾化法:将30 mL噬菌体悬液(109 PFU/mL)装至超声雾化器中,连接箱体,使噬菌体雾化液充满整个箱体。
(2) 喷雾法:将5 mL噬菌体悬液装入喷壶中,均匀喷洒至箱体。
雾化或喷雾结束后,分别于30 min、1 h、2 h后各取出3块平板,倒置,37 ℃孵育14 h,对照组使用无菌SM缓冲液雾化或喷雾,然后进行菌落计数。
1.9.2 噬菌体喷雾对地面的杀菌效果:使用切割后的水泥地板(15 cm×15 cm)模拟水泥地面。使用喷壶喷洒2.5 mL菌液(5×105 CFU/mL)至水泥板完全浸湿,10 min后喷洒等量的噬菌体或者SM液,2 h后用无菌脱脂棉球蘸取PBS缓冲液擦拭水泥板,然后将棉球浸入5 mL PBS中,4 ℃静置24 h,平板计数法测菌落数。
1.9.3 噬菌体喷雾对粪便的杀菌效果:将20 g高压灭菌后的猪粪与10 mL CVCC232均匀混合后倒入无菌平皿(直径90 mm、高12 mm)中,使其充满整个平皿,24 h后喷洒5.0 mL噬菌体制剂,每日2次,共喷洒2 d,对照组喷洒SM缓冲液。最后一次喷洒2 h后,分别称取表面和距表面0.5 cm处的粪便各1 g,分别加入5 mL PBS,平板计数法测菌落数。
1.10 噬菌体对细菌生物被膜的裂解作用参考欧阳凤菊的方法[8],采用微孔板法对CVCC232生物被膜形成能力进行检测,判定CVCC232形成被膜的最佳时长。在96微孔板中培养出生物被膜态的细菌,弃培养液,每孔加入200 μL噬菌体悬液,对照组加入等量SM缓冲液,37 ℃培养,24 h后观察被膜裂解情况。
1.11 噬菌体在养殖环境中的耐受性测定 1.11.1 养殖温度对噬菌体活性的影响:某鸡场全年测温数据显示,鸡场内最高温度不超过30 ℃,动物俯卧休息的地方与体温相近,约为37 ℃,而养殖场的温度一般控制在26 ℃[9-10]。本试验探索了26 ℃和37 ℃条件下噬菌体效价在7 d内的变化。将噬菌体悬浮液分别放入26 ℃和37 ℃恒温培养箱内连续培养7 d,每天于相同时间点取样测效价。
1.11.2 粪便pH对噬菌体活性的影响:畜禽粪便的pH范围是6.0-8.5[11-12],故将噬菌体悬液pH分别调为6.0和8.5,置于4 ℃,于每天相同时间点取样测效价,共测量7 d。
1.11.3 阳光直射对噬菌体活性的影响:噬菌体作为环境杀菌剂在白天使用时,阳光中紫外线可影响噬菌体的活性。本试验直接采用阳光照射的方法测定其对噬菌体效价的影响。将噬菌体装入透明试管内置于阳光下照射6 h,每2 h测一次效价。
1.12 宿主菌抗性突变率的检测将CVCC232培养至对数期,以最佳感染复数将噬菌体和菌液混合,用平板计数法检测该体系中CVCC232的原始菌落数,37 ℃摇床培养5 h至澄清(此时即认定所有非抗性菌株已被噬菌体裂解),8000 r/min、10 min离心混合液,弃上清,沉淀用PBS重悬,用平板计数法测定抗性菌落数。菌株的抗性突变率=抗性菌落数/原始菌落数[13]。
1.13 数据及分析本研究中所有实验数据均为3个独立重复实验的平均值±标准偏差(mean±SD)。采用GraphPad Prism version 5.0软件进行数据分析和图表制作。
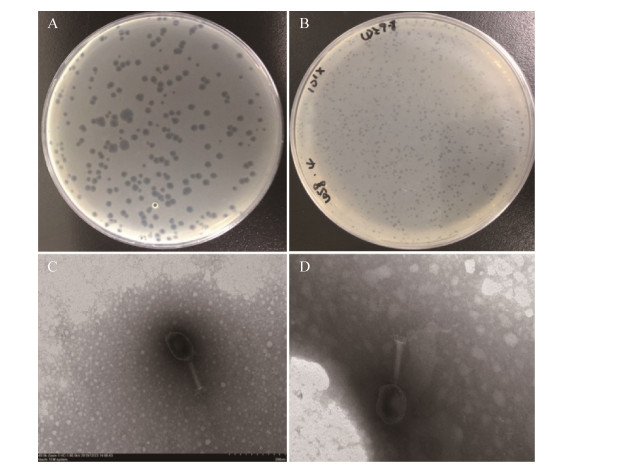
2 结果和分析 2.1 噬菌体的形态噬菌体PNJ1809-11在双层琼脂板上形成的噬菌斑直径约为3 mm,有晕环,噬菌斑边缘透亮、整齐(图 2-A);PNJ1809-13在双层琼脂平板上形成的噬菌斑直径约为1 mm,无晕环,噬菌斑边缘透亮、整齐(图 2-B)。
|
| 图 2 噬菌体的形态 Figure 2 Shapes of phages. A: plaque shape of PNJ1809-11; B: plaque shape of PNJ1809-13; C: transmission electron micrograph of PNJ1809-11; D: transmission electron micrograph of PNJ1809-13. |
透射电镜下噬菌体PNJ1809-11头部呈狭长的六面体,直径约118 nm×73 nm,有一个可伸缩的尾部,长约103 nm,并可见伸展的尾丝(图 2-C);PNJ1809-13头部呈六面体,直径约113 nm×65 nm,有一个可收缩的尾部,长约104 nm,并可见伸展的尾丝,两株噬菌体均为肌尾病毒科噬菌体(图 2-D)。
2.2 噬菌体的裂解谱噬菌体裂解谱测定结果显示,PNJ1809-11可裂解155株大肠杆菌,裂解率为92.82%;PNJ1809- 13可以裂解46株大肠杆菌,裂解率为27.54% (附表 1)。
| Bacteria number | Animal sources | Origin | PNJ1809-11 | PNJ1809-13 |
| Q167 | goat | Qinghai | + | + |
| Q159 | goat | Haiyan County, Qinghai | + | + |
| Q155 | goat | Tianjun County, Qinghai | + | + |
| Q152 | goat | Huzhu County, Qinghai | + | + |
| Q151 | goat | Huzhu County, Qinghai | + | + |
| Q150 | goat | Huzhu County, Qinghai | + | + |
| Q149 | goat | Huzhu County, Qinghai | + | + |
| Q148 | goat | Huzhu County, Qinghai | + | - |
| Q147 | goat | Huzhu County, Qinghai | + | + |
| Q145 | goat | Huzhu County, Qinghai | + | – |
| Q143 | goat | Huzhu County, Qinghai | + | + |
| Q129 | goat | Haiyan County, Qinghai | + | + |
| Q122 | goat | Tianjun County, Qinghai | + | – |
| Q121 | goat | Tianjun County, Qinghai | + | + |
| Q113 | goat | Huzhu County, Qinghai | + | – |
| Q110 | goat | Tianjun County, Qinghai | + | + |
| Q107 | goat | Tianjun County, Qinghai | + | + |
| Q106 | goat | Tianjun County, Qinghai | + | – |
| Q100 | goat | Tianjun County, Qinghai | + | + |
| Q099 | goat | Tianjun County, Qinghai | + | – |
| Q098 | goat | Tianjun County, Qinghai | – | + |
| Q096 | goat | Tianjun County, Qinghai | – | + |
| Q095 | goat | Tianjun County, Qinghai | + | – |
| Q094 | goat | Tianjun County, Qinghai | – | + |
| Q091 | goat | Tianjun County, Qinghai | + | – |
| Q089 | goat | Tianjun County, Qinghai | – | + |
| Q088 | goat | Tianjun County, Qinghai | – | + |
| Q085 | goat | Tianjun County, Qinghai | + | – |
| Q084 | goat | Tianjun County, Qinghai | – | + |
| Q075 | goat | Tianjun County, Qinghai | + | – |
| Q069 | goat | Tianjun County, Qinghai | + | – |
| Q063 | goat | Tianjun County, Qinghai | + | + |
| Q038 | goat | Hudong Breeding Farm, Qinghai | + | – |
| Q035 | goat | Hudong Breeding Farm, Qinghai | + | – |
| Q034 | goat | Hudong Breeding Farm, Qinghai | + | – |
| Q017 | goat | Hudong Breeding Farm, Qinghai | + | – |
| Q010 | goat | Qinhai | + | – |
| DE427 | duck | Chu County, Chuzhou, Anhui | + | – |
| DE425 | duck | Hengxi, Jiangning, Jiangsu | + | – |
| DE420 | duck | Hengxi, Jiangning, Jiangsu | + | – |
| DE419 | duck | Hengxi, Jiangning, Jiangsu | + | – |
| DE414 | duck | Hengxi, Jiangning, Jiangsu | + | – |
| DE407 | duck | Hengxi, Jiangning, Jiangsu | + | – |
| DE405 | duck | Hengxi, Jiangning, Jiangsu | + | – |
| DE382 | duck | Hengxi, Jiangning, Jiangsu | + | – |
| DE366 | duck | Hengxi, Jiangning, Jiangsu | + | – |
| DE365 | duck | Hengxi, Jiangning, Jiangsu | + | – |
| DE363 | duck | Hengxi, Jiangning, Jiangsu | + | – |
| DE360 | duck | Hengxi, Jiangning, Jiangsu | + | – |
| DE358 | duck | Hengxi, Jiangning, Jiangsu | + | – |
| DE355 | duck | Hengxi, Jiangning, Jiangsu | + | – |
| DE354 | duck | Hengxi, Jiangning, Jiangsu | + | – |
| DE347 | duck | Hengxi, Jiangning, Jiangsu | + | – |
| DE344 | duck | Hengxi, Jiangning, Jiangsu | + | – |
| DE341 | duck | Hengxi, Jiangning, Jiangsu | + | – |
| DE336 | duck | Hengxi, Jiangning, Jiangsu | + | – |
| DE332 | duck | Hengxi, Jiangning, Jiangsu | + | – |
| DE331 | duck | Hengxi, Jiangning, Jiangsu | + | – |
| DE329 | duck | Hengxi, Jiangning, Jiangsu | + | – |
| DE328 | duck | Hengxi, Jiangning, Jiangsu | + | – |
| DE325 | duck | Hengxi, Jiangning, Jiangsu | + | – |
| DE322 | duck | Hengxi, Jiangning, Jiangsu | + | – |
| DE321 | duck | Hengxi, Jiangning, Jiangsu | + | – |
| DE319 | duck | Hengxi, Jiangning, Jiangsu | + | – |
| DE318 | duck | Hengxi, Jiangning, Jiangsu | + | – |
| DE317 | duck | Hengxi, Jiangning, Jiangsu | + | – |
| DE316 | duck | Hengxi, Jiangning, Jiangsu | + | – |
| DE314 | duck | Hengxi, Jiangning, Jiangsu | + | – |
| DE313 | duck | Hengxi, Jiangning, Jiangsu | + | – |
| DE312 | duck | Hengxi, Jiangning, Jiangsu | + | – |
| DE311 | duck | Hengxi, Jiangning, Jiangsu | + | – |
| DE310 | duck | Hengxi, Jiangning, Jiangsu | + | – |
| DE308 | duck | Hengxi, Jiangning, Jiangsu | + | – |
| DE306 | duck | Hengxi, Jiangning, Jiangsu | + | – |
| DE305 | duck | Hengxi, Jiangning, Jiangsu | + | – |
| DE304 | duck | Hengxi, Jiangning, Jiangsu | + | – |
| DE303 | duck | Hengxi, Jiangning, Jiangsu | + | – |
| DE301 | duck | Hengxi, Jiangning, Jiangsu | + | – |
| DE300 | duck | Hengxi, Jiangning, Jiangsu | + | – |
| DE298 | duck | Hengxi, Jiangning, Jiangsu | + | – |
| DE297 | duck | Hengxi, Jiangning, Jiangsu | + | – |
| DE294 | duck | Hengxi, Jiangning, Jiangsu | + | – |
| DE290 | duck | Hengxi, Jiangning, Jiangsu | + | – |
| DE288 | duck | Hengxi, Jiangning, Jiangsu | + | – |
| DE271 | duck | Hanhe Town, Lai'an County, Anhui | + | – |
| DE270 | duck | Hanhe Town, Lai'an County, Anhui | + | – |
| DE268 | duck | Hanhe Town, Lai'an County, Anhui | + | – |
| DE267 | duck | Hanhe Town, Lai'an County, Anhui | + | – |
| DE258 | duck | Hanhe Town, Lai'an County, Anhui | + | – |
| DE257 | duck | Hanhe Town, Lai'an County, Anhui | + | – |
| DE256 | duck | Hanhe Town, Lai'an County, Anhui | + | – |
| DE255 | duck | Hanhe Town, Lai'an County, Anhui | + | – |
| DE254 | duck | Hanhe Town, Lai'an County, Anhui | + | – |
| DE250 | duck | Hanhe Town, Lai'an County, Anhui | + | – |
| DE249 | duck | Hanhe Town, Lai'an County, Anhui | + | – |
| DE247 | duck | Hanhe Town, Lai'an County, Anhui | + | – |
| DE245 | duck | Hanhe Town, Lai'an County, Anhui | + | – |
| DE218 | duck | Tongjing, Jiangning, Jiangsu | + | – |
| DE192 | duck | Quanjiao County, Anhui | + | – |
| DE190 | duck | Quanjiao County, Anhui | + | – |
| DE189 | duck | Quanjiao County, Anhui | + | – |
| DE188 | duck | Quanjiao County, Anhui | + | + |
| DE186 | duck | Hengxi, Jiangning, Jiangsu | + | – |
| DE171 | duck | Dongshan, Jiangning, Jiangsu | + | – |
| DE169 | duck | Dongshan, Jiangning, Jiangsu | + | – |
| DE167 | duck | Dongshan, Jiangning, Jiangsu | + | – |
| DE166 | duck | Dongshan, Jiangning, Jiangsu | + | – |
| DE160 | duck | Guannan, Lianyungang, Jiangsu | + | – |
| DE152 | duck | Presented by Huazhong Agricultural University | + | – |
| DE151 | duck | Presented by Huazhong Agricultural University | + | – |
| DE150 | duck | Presented by Huazhong Agricultural University | + | – |
| DE144 | duck | Shandong | + | – |
| DE143 | duck | Shandong | + | – |
| DE139 | duck | Shandong | + | – |
| DE135 | duck | Shandong | + | + |
| DE134 | duck | Shandong | + | + |
| DE124 | duck | He County Anhui | + | – |
| DE099 | duck | Liuhe Nanjing | + | – |
| DE095 | duck | Liuhe Nanjing | + | – |
| DE089 | duck | Liuhe Nanjing | + | – |
| DE049 | duck | Quanjiao County, Anhui | + | – |
| DE040 | duck | He County Anhui | + | – |
| DE039 | duck | He County Anhui | + | – |
| DE037 | duck | He County Anhui | + | – |
| DE032 | duck | He County Anhui | + | – |
| DE029 | duck | He County Anhui | + | – |
| DE017 | duck | Lishui, Jiangsu | + | – |
| DE015 | duck | Lishui, Jiangsu | + | – |
| DE013 | duck | Lishui, Jiangsu | + | – |
| DE002 | duck | Lishui, Jiangsu | + | – |
| DE001 | duck | Lishui, Jiangsu | + | – |
| CVCC491 | piglets | Beijing | + | + |
| CVCC249 | cow | Beijing | + | – |
| CVCC247 | cow | Beijing | + | – |
| CVCC245 | cow | Beijing | + | – |
| CVCC244 | cow | Sichuan | + | – |
| CVCC241 | cow | Sichuan | + | + |
| CVCC240 | cow | Sichuan | + | – |
| CVCC221 | cow | France | + | – |
| CVCC215 | piglets | Beijing | + | – |
| CVCC204 | piglets | Beijing | + | – |
| CVCC198 | piglets | Beijing | + | – |
| CVCC196 | piglets | Beijing | + | – |
| CVCC195 | piglets | Beijing | + | – |
| CVCC1591 | cow | Lanzhou | + | – |
| CVCC1553 | chicken | Tianjin | + | – |
| CVCC1549 | cow | Sichuan | + | – |
| CVCC1548 | cow | Sichuan | + | – |
| CVCC1546 | milk calf | Sichuan | + | + |
| CVCC1539 | yak rectum | Lanzhou | + | + |
| CVCC1531 | lamb | Inner Mongolia | + | – |
| CVCC1530 | lamb | Inner Mongolia | + | – |
| CVCC1529 | lamb | Inner Mongolia | + | – |
| CVCC1526 | piglets | Gaochun, Zhejiang | + | + |
| CVCC1522 | piglet intestinal mucosa | Shanghai | – | + |
| CVCC1519 | piglets | Beijing | – | + |
| CVCC1511 | piglets | Beijing | – | + |
| CVCC1509 | piglets | Beijing | + | – |
| CVCC1502 | piglets | Shanghai | + | – |
| CVCC1498 | piglets | Guangxi | + | – |
| CVCC1495 | piglets | Guangxi | + | + |
| CVCC1494 | piglet intestinal contents | Beijing | + | – |
| CVCC1493 | piglets | Beijing | + | + |
| CVCC1491 | piglets | Beijing | + | + |
| CVCC1418 | piglets | Shanghai | – | + |
| CVCC1350 | piglets | Shanghai | – | + |
| CVCC1335 | piglets | Shanghai | – | + |
| +: plaque foremed; –: no plaque formed. | ||||
2.3 最佳感染复数(MOI)
当噬菌体与宿主菌浓度比为10︰1时,噬菌体PNJ1809-11和PNJ1809-13均释放出最多子代颗粒,效价分别为2.1×109 PFU/mL和1.8×109 PFU/mL,因此噬菌体PNJ1809-11和PNJ1809-13的最佳感染复数(MOI)均为10 (表 1)。
| MOI | Phage titer/(PFU/mL) | Bacteria concentration/(CFU/mL) | PNJ1809-11 titer/(PFU/mL) | PNJ1809-13 titer/(PFU/mL) |
| 100 | 1×109 | 1×107 | 2.6×108 | 1.3×109 |
| 10 | 1×109 | 1×108 | 2.1×109 | 1.8×109 |
| 1 | 1×108 | 1×108 | 6.4×108 | 9.2×108 |
| 0.1 | 1×107 | 1×108 | 1.2×109 | 7.6×108 |
| 0.01 | 1×106 | 1×108 | 1.5×109 | 1.7×108 |
| 0.001 | 1×105 | 1×108 | 4.4×108 | 1.3×108 |
2.4 一步生长曲线
一步生长曲线结果显示,噬菌体PNJ1809-11的潜伏期为15 min,15 min后进入爆发期,其效价在15-80 min迅速增加,并于80 min达到峰值,维持在7.0×108 PFU/mL不再明显改变,裂解量为52。噬菌体PNJ1809-13的潜伏期为15 min,15 min后进入爆发期,其效价在15-50 min迅速增加,并于50 min达到峰值,维持在2.8×108 PFU/mL不再明显改变,裂解量为21 (图 3)。

|
| 图 3 一步生长曲线 Figure 3 One-step growth curve. The error bar represents the standard deviation of three parallel samples. |
2.5 pH耐受性
pH耐受性检测显示,噬菌体PNJ1809-11、PNJ1809-13在pH 3-11范围内皆可存活,在pH≤2或pH≥12时,其活性完全丧失,最适pH为7 (图 4)。

|
| 图 4 QL01、QL02的pH耐受性 Figure 4 pH resistance of phage PNJ1809-11 and PNJ1809-13. The error bar represents the standard deviation of three parallel samples. |
2.6 热稳定性
热稳定性检测结果显示,噬菌体PNJ1809-11、PNJ1809-13在40 ℃时活性均保持稳定,60 ℃作用30 min后活性显著降低,作用1h则完全失活;噬菌体PNJ1809-11在70 ℃作用30 min仍有一定活性,PNJ1809-13在70 ℃条件下作用30 min则完全失活。80 ℃试验组2株噬菌体均不耐受,在30 min完全失活(图 5)。

|
| 图 5 噬菌体PNJ1809-11(A)和PNJ1809-13(B)的热稳定性 Figure 5 Thermal stability of PNJ1809-11 (A) and PNJ1809-13 (B). |
2.7 噬菌体体外抑菌效果
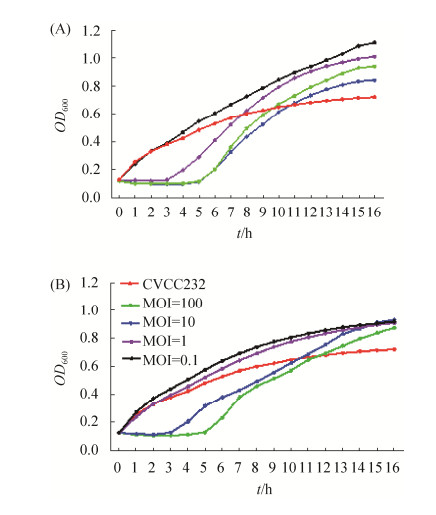
体外杀菌效果显示,当MOI=1时,噬菌体PNJ1809-11在前3 h显著抑制宿主菌的生长,当MOI=10和MOI=100时,PNJ1809-11抑制CVCC232生长的时间延长至5 h,OD600处的吸光值可低达0.09,由图可知,当MOI=10时,PNJ1809-11的体外抑菌效果最为显著(图 6-A)。当MOI=10时,噬菌体PNJ1809-13在前3 h显著抑制宿主菌的生长,当MOI=100时,PNJ1809-13抑制CVCC2323生长的时间延长至5 h,而当MOI=0.1和MOI=1时,噬菌体对CVCC232的生长则完全没有抑制作用(图 6-B)。
|
| 图 6 不同感染复数下噬菌体PNJ1809-11(A)和PNJ1809-13(B)对CVCC232的抑制作用 Figure 6 Inhibition of PNJ1809-11(A) and PNJ1809-13(B) against of CVCC232 with different MOI. |
2.8 模拟封闭装置中噬菌体制剂对细菌的杀灭效果 2.8.1 经雾化、喷雾处理后噬菌体制剂对宿主菌的杀灭效果:
经雾化处理后噬菌体PNJ1809-11、PNJ1809-13及噬菌体鸡尾酒对宿主菌CVCC232的杀菌效果均可达到99%以上,其中PNJ1809-11的杀菌效果稍强于PNJ1809-13,而噬菌体鸡尾酒的杀菌效果则介于二者之间;经喷雾处理后噬菌体PNJ1809-11、PNJ1809-13及其混合制剂的杀菌效果均可达99%以上,与雾化组杀菌效果无显著差异,但喷雾处理只需5 mL噬菌体悬液,因此后续试验选择喷雾处理方式进行杀菌效果评价(表 2,表 3)。
| t/h | PNJ1809-11/% | PNJ1809-13/% | Phage cocktail/% |
| 0.5 | 99.94 | 99.66 | 99.71 |
| 1.0 | 99.93 | 99.36 | 99.70 |
| 2.0 | 99.95 | 99.22 | 99.67 |
| t/h | PNJ1809-11/% | PNJ1809-13/% | Phage cocktail/% |
| 0.5 | 99.61 | 99.35 | 99.55 |
| 1.0 | 99.58 | 99.21 | 99.41 |
| 2.0 | 99.53 | 99.23 | 99.51 |
2.8.2 噬菌体喷雾对地面的杀菌效果:
喷雾处理的噬菌体对地面细菌的杀灭效果显示,与对照组相比,噬菌体PNJ1809-11对地面宿主菌处理后,残留菌落数下降了约2.5个数量级,杀灭效率为99.32%;噬菌体PNJ1809-13处理后,菌落数下降了约1个数量级,杀灭效率为91.23%;噬菌体鸡尾酒的杀菌效率为95.39%,杀菌效果介于两者之间(表 4)。
| Groups | Ground host bacteria/% | Host bacteria on fecal surface/% | Host bacteria in feces/% |
| PNJ1809-11 | 99.32 | 99.80 | 13.15 |
| PNJ1809-13 | 91.23 | 99.67 | 10.04 |
| Phage cocktail | 95.39 | 99.84 | 14.97 |
| SM buffer | 0.00 | 0.00 | 0.00 |
2.8.3 噬菌体喷雾对粪便的杀菌效果:
与空白对照相比,噬菌体单独处理组与鸡尾酒组对粪便表面细菌的杀灭效果均达到99.50%以上,杀菌效果依次为:鸡尾酒 > PNJ1809-11 > PNJ1809-13;而三组噬菌体制剂对粪便内部宿主菌的杀灭率均低于15.00%,杀灭效果依次为:鸡尾酒 > PNJ1809-11 > PNJ1809-13 (表 4),表明喷雾处理的噬菌体对粪便表面细菌的杀灭效果显著强于对内部细菌的杀灭效果。
2.9 噬菌体制剂对大肠杆菌CVCC232生物被膜的裂解CVCC232形成的生物被膜量在36 h时最高,此时OD/ODc=3.02,被膜形成能为中等阳性(表 5)。显微镜下观察CVCC232在细胞爬片上生长36 h后形成的被膜形态,可见有大量微菌落连成片状(图 7-A)。
| t/h | ODc | OD600 | OD/ODc |
| 24 | 0.141 | 0.372 | 2.64 |
| 36 | 0.182 | 0.550 | 3.02 |
| 48 | 0.181 | 0.322 | 1.77 |

|
| 图 7 显微镜下CVCC232的被膜形态(A)及噬菌体对CVCC232生物被膜的裂解效果(B) Figure 7 Morphology of biofilm formed by CVCC232 under microscope(A) and biofilm cleavage of CVCC232 by phage(B). A1-A3 were treated with SM buffer; B1-D1 were treated with PNJ1809-11; B2-D2 were treated with PNJ1809-13; B3-D3 were treated with phage cocktail. |
与对照组相比,噬菌体PNJ1809-11处理组和噬菌体鸡尾酒处理组孔内的液体明显变清,表明噬菌体PNJ1809-11和噬菌体鸡尾酒可有效裂解CVCC232形成的生物被膜(图 7-B)。经固定染色后测量各孔OD600处的吸光值,噬菌体PNJ1809-13试验组对CVCC232生物被膜的裂解效率为78%;PNJ1809-13的裂解效率为30%;噬菌体鸡尾酒的裂解效率为83%,为试验组中裂解效率最高的一组(表 6)。
| Groups | OD600 | Cleavage efficiency/% |
| PNJ1809-11 | 0.2197 | 78 |
| PNJ1809-13 | 0.6951 | 30 |
| Phage cocktail | 0.1649 | 83 |
| SM buffer | 0.9860 | 0.00 |
| Calculation formula of cleavage efficiency=[1-(OD test/OD negative)]×100%. | ||
2.10 噬菌体在养殖环境中的耐受性 2.10.1 噬菌体在养殖温度下的耐受性:
PNJ1809-11在26 ℃和37 ℃下活性变化较小,1周后效价均下降了约1个数量级,但37 ℃条件下活性变化稍大(图 8-A);而在26 ℃条件下,噬菌体PNJ1809-13在7 d内效价降低了约1个数量级,37 ℃条件下则降低了近2个数量级,表明噬菌体PNJ1809-11在养殖温度下耐受性更强(图 8-B)。

|
| 图 8 噬菌体在养殖环境中的耐受性 Figure 8 Tolerance of bacteriophage in culture environment. Titer variation of PNJ1809-11 (A) and PNJ1809-13 (B) at breeding temperature; titer variation of PNJ1809-11 (C) and PNJ1809-13 (D) at fecal pH; the effect of directe sunlight on phages PNJ1809-11 and PNJ1809-13 (E). The error bar represents the standard deviation of three parallel samples. |
2.10.2 噬菌体在粪便pH中的耐受性:
噬菌体PNJ1809-11的效价在0-4 d内在pH 6.0和pH 8.6的条件下均较稳定,5 d后效价开始缓慢下降,最终效价变化不超过1个数量级(图 8-C);噬菌体PNJ1809-13的效价在pH 6.0条件下于第2天和第4天明显降低,在pH 8.5条件下平缓下降,对pH 6.0弱酸条件下稍敏感(图 8-D)。
2.10.3 阳光直射对噬菌体活性的影响:经阳光照射6 h后,2株噬菌体的效价均有不同程度的下降,PNJ1809-11的滴度由初始的1.6×109 PFU/mL降至2.8×108 PFU/mL,约下降6倍;PNJ1809-13的滴度则由初始的4.8×108 PFU/mL降至4.5×107 PFU/mL,约下降1个数量级。噬菌体PNJ1809-11相对于PNJ1809-13在阳光照射下较稳定(图 8-E)。
2.11 宿主菌对噬菌体的抗性突变率经检测CVCC232对噬菌体PNJ1809-11产生抗性菌株的概率为2.5×10-3,对PNJ1809-13产生抗性菌株的概率为1.0×10-3(表 7)。
| Phages | Bacteria concentration after exposed to phage (CFU/mL) | Bacteria concentration without exposing to phage (CFU/mL) | Mutation frequency |
| PNJ1809-11 | 8.7×105 | 3.5×108 | 2.5×10-3 |
| PNJ1809-13 | 3.5×105 | 3.5×108 | 1.0×10-3 |
3 讨论
本研究评估了2株噬菌体PNJ1809-11、PNJ1809-13以单一形式和鸡尾酒形式分别经喷雾和雾化处理后对密闭箱中的细菌的杀灭效果。试验结果显示,不论是雾化组还是喷雾组,在噬菌体PNJ1809-11、PNJ1809-13和噬菌体鸡尾酒3组试验中,噬菌体PNJ1809-11的杀菌效果最好;与雾化处理相比,在相似杀菌率的条件下,喷雾处理组所需的噬菌体量是雾化处理组的1/6,若应用于养殖环境中,出于成本的考虑,喷雾处理的方式具有一定的优势。不同微生物对雾化的耐受力也不同,雾化器处理过程中,超声波也会降低噬菌体的存活量,雾化时间、雾化量等因素也会影响噬菌体的雾化效果,超声雾化后噬菌体的生物安全性也需要进一步考量;而喷雾处理操作简单方便,噬菌体用量少,因此后续使用喷雾化噬菌体作用于模拟养殖环境探讨噬菌体对地面、粪便的杀菌效果。喷雾处理的噬菌体对地面、粪便表面均有较好的杀灭效果,其中鸡尾酒的灭菌效果最佳,其次为噬菌体PNJ1809-11、效果最差的为PNJ1809-13,但喷雾处理的噬菌体对粪便内部细菌的杀灭效果并不理想。现实养殖场中粪便表面及内部中均有细菌定殖,无论是噬菌体喷雾还是其他化学喷雾剂,在对粪便进行消毒灭菌时主要对粪便表面的细菌起灭活作用,建议养殖场进行消毒前先清除粪便、饲料、垫料、有机物和污物等,做好消毒前准备。
生物被膜是细菌黏附的共同体,将细菌包裹在内使细菌比浮游状态具有更高的代谢能力,保护细菌免受免疫细胞的攻击,更是细菌产生抗性、难以被消毒剂杀灭的原因[14]。抗生素只能清除浮游态细菌却不能清除隐藏在生物被膜内的细菌[15]。细菌可在饲槽、笼架等表面形成生物被膜,这就要求养殖场的消毒剂具有清除生物被膜的能力。相关文献表明噬菌体、噬菌体裂解酶都可清除细菌表面的生物被膜[14]。本试验结果显示,宿主菌CVCC232形成的生物被膜在用噬菌体悬浮液作用24 h后可被显著破坏,但2株噬菌体对生物被膜的清除能力有显著差异:噬菌体PNJ1809-13单独作用于被膜时清除率很低,而PNJ1809-11对生物被膜的清除效果显著优于PNJ1809-13;然而,将两株噬菌体以鸡尾酒的形式作用于生物被膜后,对被膜的裂解效果要好于任意一株。该试验结果表明本实验室分离的两株噬菌体不仅能有效杀灭环境中的细菌,还可清除养殖环境中宿主菌形成的生物被膜,且噬菌体鸡尾酒的效果最佳。将来可考虑将噬菌体鸡尾酒运用于肉品、案板和医疗器械等生物被膜的清除,若与抗生素、钴离子和氯等物质联用效果可能会更好。
喷雾处理的噬菌体在模拟养殖环境的温度、粪便酸碱度、阳光直射下的活性变化试验中,PNJ1809-11对这些环境条件的耐受性更强,作为生物消毒剂具有更大优势,有望将其开发成新型环保、高效的生物消毒剂,这对养殖场的环境净化及大肠杆菌的防控具有重要意义。但由于试验条件限制,本试验仅使用密闭箱、水泥板和人工污染粪便等模拟养殖环境,未能在养殖场现场检测噬菌体的杀菌效果,且养殖场中的菌种更为复杂,若后续将噬菌体应用于临床,需分离更多的噬菌体,将具有不同裂解谱的噬菌体以鸡尾酒的形式喷洒于养殖环境,进一步探索噬菌体作为环境消毒剂的可能。
噬菌体与细菌共进化过程中会产生噬菌体抗性菌株。细菌可通过阻断噬菌体的吸附、抑制噬菌体DNA的注射、阻止噬菌体DNA的复制、CRISPR-Cas系统和流产感染等机制产生抗噬菌体突变株[16-17]。两种及以上具有不同裂解谱的噬菌体混合形成的制剂即为噬菌体鸡尾酒,使用噬菌体鸡尾酒不仅可以拓宽宿主谱,增强杀菌能力,还可以降低噬菌体抗性菌株的产生概率[17-18]。在国外,噬菌体鸡尾酒或噬菌体制剂已被应用于肉类、水果、蔬菜中食源性细菌的杀灭[19]。许多动物实验和临床实验也表明噬菌体鸡尾酒制剂具有非常广泛的裂解谱,可有效减少细菌的耐药性,且在动物模型上也有较好的保护力[20]。通过检测宿主菌CVCC232对2株噬菌体PNJ1809-11和PNJ1809-13抗性菌株的突变率,表明宿主菌产生抗噬菌体菌株的概率较大,还需分离更多的噬菌体,以噬菌体鸡尾酒的形式克服这一问题。
| [1] | Gomes TAT, Elias WP, Scaletsky ICA, Guth BEC, Rodrigues JF, Piazza RMF, Ferreira LCS, Martinez MB. Diarrheagenic Escherichia coli. Brazilian Journal of Microbiology, 2016, 47(S1): 3-30. |
| [2] | Nataro JP, Mai V, Johnson J, Blackwelder WC, Heimer R, Tirrell S, Edberg SC, Braden CR, Morris Jr JG, Hirshon JM. Diarrheagenic Escherichia coli infection in Baltimore, Maryland, and New Haven, Connecticut. Clinical Infectious Diseases, 2006, 43(4): 402-407. DOI:10.1086/505867 |
| [3] | Huang DB, Okhuysen PC, Jiang ZD, DuPont HL. Enteroaggregative Escherichia coli: an emerging enteric pathogen. American Journal of Gastroenterology, 2004, 99(2): 383-389. DOI:10.1111/j.1572-0241.2004.04041.x |
| [4] |
Wei YJ, Wang ZT, Xu YC, Xu WT. Current situation analysis and detection techniques of pathogenic Escherichia coli. Biotechnology Bulletin, 2016, 32(11): 80-92.
(in Chinese) 卫昱君, 王紫婷, 徐瑗聪, 许文涛. 致病性大肠杆菌现状分析及检测技术研究进展. 生物技术通报, 2016, 32(11): 80-92. |
| [5] |
Dong WW, Li F, Zhang SD, Wang CL, Ai HX, Li GM. Research progress of commonly used disinfectants and disinfection effect evaluation methods in poultry farms. Poultry Science, 2019(9): 53-56.
(in Chinese) 董雯雯, 李峰, 张世栋, 王春玲, 艾洪新, 李桂明. 家禽养殖场常用消毒剂及消毒效果评价方法研究进展. 家禽科学, 2019(9): 53-56. DOI:10.3969/j.issn.1673-1085.2019.09.025 |
| [6] |
Xu X, Zheng QL, Leng SZ, Liu L, Yin QP, Zhang C. Study on the disinfection effect of chlorine dioxide as a fumigant in farms. Guangdong Chemical Industry, 2017, 44(9): 98-99.
(in Chinese) 许详, 郑庆禄, 冷淑珍, 刘露, 尹曲平, 张超. 二氧化氯型熏蒸剂在养殖场所的现场杀菌效果研究. 广东化工, 2017, 44(9): 98-99. DOI:10.3969/j.issn.1007-1865.2017.09.041 |
| [7] | Bruynoghe R, Maisin J. Essais de thérapeutique au moyen du bactériophage du Staphylocoque. Comptes Rendus des Séances de la Société de Biologie et de ses Filiales, 1921(85): 1120-1121. |
| [8] | 欧阳凤菊. 禽致病大肠杆菌生物被膜检测及形成基因研究. 东北农业大学硕士学位论文, 2010. |
| [9] |
Yan YH, Han XX. Temperature difference of factors affecting livestock and poultry production. Animals Breeding and Feed, 2014(7): 20-21.
(in Chinese) 烟玉华, 韩秀欣. 畜禽生产影响因素之温差. 养殖与饲料, 2014(7): 20-21. DOI:10.3969/j.issn.1671-427X.2014.07.011 |
| [10] |
Huang YK, Liu J, Fan JY, Jiang DF. Correlation analysis between seasonal changes in the environment in chicken coops and mortality rate of chicken flocks. Chinese Journal of Animal Science, 2012, 48(6): 57-60, 64.
(in Chinese) 黄炎坤, 刘健, 范佳英, 姜东凤. 鸡舍内环境季节性变化与鸡群死淘率的关联分析. 中国畜牧杂志, 2012, 48(6): 57-60, 64. DOI:10.3969/j.issn.0258-7033.2012.06.012 |
| [11] |
Liang WF. Field measurement of porcine feces pH and its significance. Guangdong Journal of Animal and Veterinary Science, 2018, 43(6): 26-28.
(in Chinese) 梁雯霏. 猪粪便pH值现场测定及其意义. 广东畜牧兽医科技, 2018, 43(6): 26-28. DOI:10.3969/j.issn.1005-8567.2018.06.009 |
| [12] |
Wang Y, Liao XD, Wu YB. Effects of ambient temperature and humidity on water content, nitrogen and pH of layer manure. China Poultry, 2012, 34(4): 21-24.
(in Chinese) 王艳, 廖新俤, 吴银宝. 环境温度和湿度对蛋鸡粪便含水率、氮素和pH的影响. 中国家禽, 2012, 34(4): 21-24. DOI:10.3969/j.issn.1004-6364.2012.04.006 |
| [13] | O'Flynn G, Ross RP, Fitzgerald GF, Coffey A. Evaluation of a cocktail of three bacteriophages for biocontrol of Escherichia coli O157:H7. Applied and Environmental Microbiology, 2004, 70(6): 3417-3424. DOI:10.1128/AEM.70.6.3417-3424.2004 |
| [14] |
Lü YH, Quan XX, Shen MX, Yi QX, Cui ZL. Research progress in biofilm degradation by bacteriophage and its lysin. Microbiology China, 2015, 42(3): 568-573.
(in Chinese) 吕芸辉, 全心馨, 沈梦溪, 易秋雪, 崔泽林. 噬菌体及其裂解酶对细菌生物被膜作用的研究进展. 微生物学通报, 2015, 42(3): 568-573. |
| [15] |
Qu CL, Gao H, Zhao BH, Chen C. Progress on bacterial biofilm and the mechanism of antibiotic resistance. Progress in Veterinary Medicine, 2008, 29(3): 86-90.
(in Chinese) 屈常林, 高洪, 赵宝洪, 陈超. 细菌生物被膜与抗生素耐药机制研究进展. 动物医学进展, 2008, 29(3): 86-90. DOI:10.3969/j.issn.1007-5038.2008.03.022 |
| [16] |
Xu JL, Kan B. Bacterial resistance mechanisms to bacteriophage. Letters in Biotechnology, 2013, 24(3): 409-413.
(in Chinese) 徐嘉良, 阚飙. 细菌的噬菌体感染抗性机制. 生物技术通讯, 2013, 24(3): 409-413. DOI:10.3969/j.issn.1009-0002.2013.03.027 |
| [17] | Yu L, Wang S, Guo ZM, Liu HT, Sun DG, Yan GM, Hu DL, Du CT, Feng X, Han WY, Gu JM, Sun CJ, Lei LC. A guard-killer phage cocktail effectively lyses the host and inhibits the development of phage-resistant strains of Escherichia coli. Applied Microbiology and Biotechnology, 2018, 102(2): 971-983. DOI:10.1007/s00253-017-8591-z |
| [18] | Kelly D, McAuliffe O, Ross RP, O'Mahony J, Coffey A. Development of a broad-host-range phage cocktail for biocontrol. Bioengineered Bugs, 2011, 2(1): 31-37. DOI:10.4161/bbug.2.1.13657 |
| [19] | Spricigo DA, Bardina C, Cortés P, Llagostera M. Use of a bacteriophage cocktail to control Salmonella in food and the food industry. International Journal of Food Microbiology, 2013, 165(2): 169-174. DOI:10.1016/j.ijfoodmicro.2013.05.009 |
| [20] | Chan BK, Abedon ST, Loc-Carrillo C. Phage cocktails and the future of phage therapy. Future Microbiology, 2013, 8(6): 769-783. DOI:10.2217/fmb.13.47 |
 2021, Vol. 61
2021, Vol. 61




