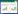中国科学院微生物研究所,中国微生物学会,中国菌物学会
文章信息
- 陈宏健, 周杨, 夏小洪, 赵欣怡, 乔恒, 谈家金, 郝德君. 2021
- Hongjian Chen, Yang Zhou, Xiaohong Xia, Xinyi Zhao, Heng Qiao, Jiajin Tan, Dejun Hao. 2021
- 松墨天牛成虫室内外种群肠道细菌的多样性及功能分析
- Diversity and function of intestinal bacteria in adult Monochamus alternatus Hope (Coleoptera: Cerambycidae) fed indoors and outdoors
- 微生物学报, 61(3): 683-694
- Acta Microbiologica Sinica, 61(3): 683-694
-
文章历史
- 收稿日期:2020-05-19
- 修回日期:2020-07-13
- 网络出版日期:2021-02-24
2. 南京林业大学林学院, 江苏 南京 210037
2. College of Forestry, Nanjing Forestry University, Nanjing 210037, Jiangsu Province, China
松墨天牛(Monochamus alternatus)属于鞘翅目(Coleoptera)天牛科(Cerambycidae),是我国松科植物重要蛀干害虫,也是松材线虫病扩散的主要媒介昆虫[1-3]。该害虫在野外以幼虫钻蛀取食生长衰弱木的韧皮部和木质部,成虫取食1–2年生枝条[4]。为了对松墨天牛进行深入的研究,国内外已经掌握了松墨天牛的人工饲养方法,同时配制了多种适宜松墨天牛生长发育的半人工饲料[5-7]。
昆虫的肠道大致分为前肠、中肠和后肠3个部分,其中又以中肠和后肠作为最主要的消化器官,肠道内部栖居着大量的微生物,能够影响宿主昆虫的生长发育、生理代谢、生殖和免疫等生命活动[8-11]。由于中、后肠的微生态环境存在差异,因而会影响不同肠段微生物的群落结构[12]。昆虫肠道微生物群落的形成高度依赖于饮食,取食不同食料或在不同生境中生长的昆虫,其肠道微生物的种类和数量会发生变化,优势菌群也可能随之改变[13-17]。
松墨天牛成虫在取食健康枝条的时候,也是从植物中获取生长发育所需营养物质的一个过程。然而,树木中的葡萄糖是以纤维素、半纤维素、葡聚糖和果胶等复杂的多糖聚合物存在,氮素含量也相对不足,植物细胞壁蛋白质与木质素和纤维素形成结合蛋白而难以被直接利用[18],因此,蛀干昆虫进化出利用肠道共生细菌产生纤维素或半纤维素酶[19],或者提高宿主昆虫体内相关消化酶活性[20]进而分解木质纤维素获得所需的葡萄糖。同时,萜类化合物是松属植物重要的组成性和诱导性化学防御物质[21],蛀干害虫可以利用肠道细菌降解针叶树萜类物质[22-23],以提高昆虫对寄主的适应性。
国外已有学者研究自然取食和人工饲养条件下松墨天牛成虫中肠、后肠细菌群落的组成[7],但是由于国内松墨天牛成虫在室外取食的松树种类不同,人工饲料的配方也有差异,可能会形成不同的肠道菌群。因此,国内松墨天牛成虫肠道菌群组成的研究仍很重要,并且松墨天牛成虫肠道细菌的功能尚未见报道。本研究选择从林间马尾松木段中羽化的松墨天牛成虫和室内人工饲料饲养的松墨天牛成虫为研究对象,研究2个种群成虫中、后肠不同肠段的细菌群落结构,进而分析这些菌群的潜在功能,为进一步揭示肠道细菌在不同食料、不同肠段中的组成变化以及帮助宿主昆虫获取营养、克服寄主植物化学防御的作用机制提供参考依据。
1 材料和方法 1.1 供试虫源松墨天牛成虫室外种群:将江苏省镇江市丹徒区感染松材线虫病死亡的马尾松砍伐后,锯成2 m的木段,放入人工自制的不锈钢网笼内(长×宽×高=2.5 m×2.5 m×2.0 m),每天上午8时检查木段中羽化的天牛成虫,一旦发现羽化的成虫,及时带回室内,体表消毒后置于–80 ℃冰箱中冷冻,备用。
松墨天牛成虫室内种群:将网笼内羽化的天牛,带回室内后除了进行上述直接处理冷冻后,一部分天牛成虫放入养虫笼(长×宽×高=2.5 m× 2.5 m×2.0 m),提供1–2年生马尾松枝条供其补充营养,2周后,将5对雌雄成虫置于相同的养虫笼内配对,提供长25 cm、直径7–10 cm的马尾松木段供其产卵,定期收集卵粒并转移至半人工饲料上,参考陈瑞旭等方法(2017)进行人工饲养。将羽化的松墨天牛成虫体表消毒后置于–80 ℃冰箱中冷冻,备用。每份半人工饲料的配方为马尾松木屑100 g、琼脂40 g、蔗糖20 g、酵母粉12.5 g、安息香酸钠2 g、山梨酸钾1 g、0.5 mol/L的硫酸10 mL、麦胚粉25g、胆固醇1.5 g、抗坏血酸4 g、酪蛋白20 g、氯化胆碱1 g、无菌水800 mL,饲料使用前需经121 ℃湿热灭菌20 min。
1.2 松墨天牛成虫肠道分离取健康的室内外种群松墨天牛成虫各15头,用75%乙醇体表消毒1 min,再用无菌水冲洗2次。无菌条件下进行解剖,取出完整肠道,分离中肠和后肠。不同种群成虫的中肠和后肠分别置于装有0.5 mL PBS缓冲液的1.5 mL离心管中,每管5头成虫,每个肠段3管,室内外种群共4个肠段,总计12管肠道样本用于提取肠道细菌DNA。
1.3 DNA提取和PCR扩增采用CTAB法[24-25]提取肠道细菌总DNA,利用Nanodrop 2000C (Thermo Scientific)评估DNA浓度,用1%琼脂糖凝胶电泳检测DNA纯度。以提取的细菌总DNA为模板,采用16S rDNA的V3–V4区引物进行PCR扩增,引物为341F (5′-CCTAYGGGRBGCASCAG-3′)和806R (5′-GG ACTACNNGGGTATCTAAT-3′)。PCR反应体系(30 μL):2×Phusion Master Mix 15 μL,2 μmol/L上下游引物各1.5 μL,DNA模板10 ng,ddH2O 2 μL。PCR扩增程序:98 ℃ 1 min;98 ℃ 10 s,50 ℃ 30 s,72 ℃ 30 s,30个循环;最后72 ℃ 5 min,PCR仪采用Bio-Rad T100。使用2%琼脂糖凝胶回收PCR产物,利用AxyPrep DNA Gel Extraction Kit (Axygen Biosciences,Union City,CA,USA) 进行纯化,Tris-HCl洗脱,2%琼脂糖电泳检测。
1.4 文库构建和高通量测序采用NEB NextⓇ UltraTM DNA Library Prep Kit for Illumina (NEB,USA)试剂盒完成文库的构建,具体步骤:(1) 连接“Y”字形接头;(2) 使用磁珠筛选去除接头自连片段;(3) 利用PCR扩增进行文库模板的富集;(4) 氢氧化钠变性,产生单链DNA片段。经过Qubit定量和文库检测合格后,利用Illumina HiSeq 2500平台进行双端测序。
1.5 序列数据分析原始序列数据使用Trimmomatic软件质控,FLASH软件[26]进行拼接。拼接后的序列用USEARCH软件(version 7.1 http://drive5.com/uparse/)进行OTU聚类[27]。再利用RDP classifier (http://rdp.cme.msu.edu/)对每条序列进行物种分类注释[28],最后利用R软件(version 3.5.1)分别在门和属分类水平上统计各样本的群落组成及物种丰度情况,同时绘制Venn图统计样本的OTU组成相似性及重叠情况。
采用Alpha多样性分析反映微生物群落的丰富度和多样性,用mothur (version v.1.30.1 http://www.mothur.org/wiki/Schloss_SOP#Alpha_diversity)指数分析[29]计算各样本的菌群丰富度(Ace和Chao指数)以及菌群多样性(Shannon和Simpson指数),并根据Shannon指数绘制稀释性曲线。数据采用SPSS 22.0软件进行单因素方差分析,使用Tukey HSD或Tamhane’s T2进行多重比较。采用Beta多样性分析研究不同样本菌群结构的相似性或差异关系,用Qiime计算Beta多样性距离矩阵,根据距离矩阵进行层级聚类分析,使用UPGMA算法和R软件(version 3.5.1)构建样本层级聚类树。
1.6 肠道菌群功能分析采用PICRUSt软件预测室内外松墨天牛成虫肠道菌群的功能[30]。首先对OTU丰度表进行标准化,去除16S rRNA marker gene在物种基因组中的拷贝数目的影响;然后根据每个OTU对应的greengene ID进行KEGG功能注释,获得OTU在各功能水平的注释信息及其在不同样本中的丰度信息。数据采用SPSS 22.0软件进行单因素方差分析,使用Tukey HSD或Tamhane’s T2进行多重比较。
2 结果和分析 2.1 室内外松墨天牛成虫肠道细菌16S rDNA序列拼接和组装从松墨天牛成虫室内外种群的12个肠段样本中共获得638141条细菌16S rDNA原始序列读数,经过质量过滤和去除嵌合体后,得到544180条高质量序列,每条序列平均长度为419.2 bp。按照最小样本序列数进行抽平,在97%相似度下将其聚类为用于物种分类的615个OTUs,总共注释到22个门、48个纲、112个目、172个科、285个属和408个种。
2.2 室内外松墨天牛成虫中、后肠细菌群落结构分析结果如图 1所示,松墨天牛成虫室外种群肠道的OTU数量远少于室内松墨天牛成虫。室外种群共有197个OTUs,其中中肠有170个,后肠有162个;室内种群共有519个OTUs,其中中肠有436个,后肠有438个。两个种群各自的中肠与后肠的OTU数量之间无显著差异。松墨天牛室内外两个种群的成虫共有相同的OTUs 101个,其中松墨天牛室外种群成虫中肠和室内种群成虫中肠共有相同的OTUs 58个,室外种群成虫后肠和室内成虫后肠共有相同的OTUs 72个;室外种群成虫中肠和后肠共有相同的OTUs 135个。室内种群成虫中肠和后肠共有相同的OTUs 355个。表明松墨天牛室内种群与室外种群成虫肠道的细菌群落组成存在较大差异,室内种群比室外种群的菌群丰富;无论室内种群还是室外种群,其成虫中肠和后肠的细菌群落组成具有相似性,并且丰度相当。
|
| 图 1 松墨天牛室内外种群成虫中、后肠细菌OTUs的韦恩图 Figure 1 Venn diagram of OTUs of bacteria in the midgut, hindgut of the adult of M. alternatus. FM: outdoor midgut; FH: outdoor hindgut; LM: indoor midgut; LH: indoor hindgut. |
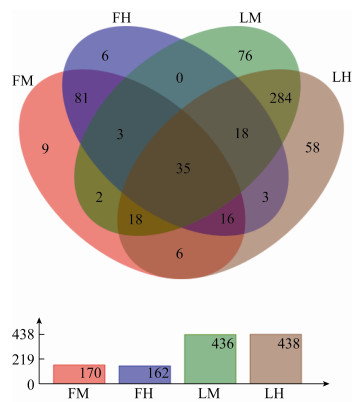
由图 2-A可以看出,在门水平上,松墨天牛室内外种群成虫肠道菌群的优势门为变形菌门(Proteobacteria)和厚壁菌门(Firmicutes),其中变形菌门在室内种群成虫肠道中表现出更大的优势。这2个优势门在不同样本中所占比例不同,变形菌门在室外种群成虫的中、后肠与室内种群成虫的中、后肠中分别为93.26%、76.85%、98.95%、99.21%,为绝对优势类群;厚壁菌门在这些样本中的占比分别为5.97%、22.35%、0.10%、0.06%。剩余占比较少的其他细菌分别为0.77%、0.80%、0.95%、0.73%。
|
| 图 2 松墨天牛室内外种群成虫中、后肠细菌优势门(A)和属(B)的相对丰度 Figure 2 Relative abundance of dominant phyla (A) and genera (B) of bacteria in the midgut, hindgut of the adult of M. alternatus. |
由图 2-B可以看出,在属水平上,丰度排序前10名的优势属在室外种群成虫的中、后肠与室内种群成虫的中、后肠中所占比例分别为肠杆菌属Enterobacter (68.60%: 70.21%: 2.34%: 67.08%)、沙雷氏菌属Serratia (0.46%: 0.45%: 65.93%: 5.32%)、拉乌尔菌属Raoultella (17.95%: 4.32%: 0.49%: 12.12%)、根瘤菌属Rhizobium (0.00%: 0.00%: 21.47%: 0.10%)、乳球菌属Lactococcus (3.04%: 16.99%: 0.00%: 0.00%)、埃希氏-志贺氏菌属Escherichia-Shigella (4.87%: 0.99%: 0.28%: 8.74%)、肠球菌属Enterococcus (2.90%: 5.36%: 0.00%: 0.00%)、气单胞菌属Aeromonas (0.02%: 0.00%: 2.79%: 0.46%)、醋杆菌属Acetobacter (0.00%: 0.00%: 3.19%: 0.03%)和肠杆菌科未分类属unclassified_f_Enterobacteriaceae (0.13%: 0.10%: 0.54%: 2.25%)。表明松墨天牛室外种群与室内种群成虫肠道的部分优势属种类不同,且同一优势属所占比例也不同;不同肠段之间的优势属比例存在差异。
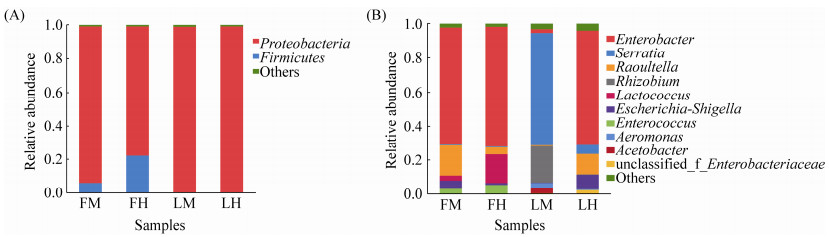
2.3 松墨天牛成虫中、后肠细菌多样性分析由图 3可知,全部样本基于Shannon指数的稀释性曲线趋于平缓,增加数据量产生的新OTU很少,说明测序数据量合理,测序达到足够深度。通过比较松墨天牛室内外种群成虫中、后肠细菌Alpha多样性的Ace指数、Chao指数、Shannon指数和Simpson指数(表 1),发现室外种群成虫肠道菌群的Ace与Chao指数均低于室内种群成虫肠道;室外种群与室内种群成虫肠道菌群的Shannon与Simpson指数之间无显著性差异;室外种群成虫的中肠与后肠,室内种群成虫的中肠与后肠之间在菌群丰度和多样性上均无显著差异。
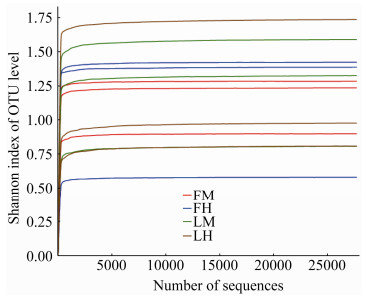
|
| 图 3 松墨天牛室内外种群成虫中、后肠细菌香农指数稀释曲线 Figure 3 Rarefaction curve of Shannon index of bacteria in the midgut, hindgut of the adult of M. alternatus. |
| Sample | Ace | Chao | Shannon | Simpson |
| Outdoor midgut | 159.16±21.77a | 161.04±19.09a | 1.14±0.21a | 0.49±0.13a |
| Outdoor hindgut | 156.90±10.11a | 156.79±14.79a | 1.13±0.48a | 0.49±0.27a |
| Indoor midgut | 480.79±102.99ab | 416.05±35.79b | 1.24±0.40a | 0.51±0.22a |
| Indoor hindgut | 457.73±25.20b | 424.58±16.15b | 1.17±0.35a | 0.57±0.28a |
| Data in the table are Mean±SD, different lowercase letters after each column of data indicate significant difference in the alpha diversity index of bacteria among different samples (P < 0.05, SPSS One-way ANOVA). | ||||
通过构建Beta多样性的样本层级聚类树,探索不同分组样本间菌群组成的相似性或差异性。本研究采用unweighted UniFrac的距离算法,同时考虑物种间进化关系和物种是否在样本中存在。结果如图 4所示,可知室外种群与室内种群成虫肠道样本各自聚成一簇,相互之间无交叉,说明2个种群成虫的肠道细菌群落组成存在差异。室外种群成虫的中肠与后肠样本之间相互交叉,室内种群成虫的中肠与后肠样本之间亦是如此,说明2个种群成虫的中肠与后肠在菌群组成上较相近。比较2个种群成虫肠道主要的OTU组成发现,室外种群成虫肠道样本的菌群均一性要优于室内种群,表明室外种群成虫肠道菌群的组成更稳定。

|
| 图 4 室内外松墨天牛成虫中、后肠细菌层级聚类树 Figure 4 Hierarchical clustering tree of bacteria in the midgut, hindgut of the adult of M. alternatus. |
2.4 松墨天牛成虫肠道菌群功能分析
为探究松墨天牛室内外种群成虫肠道菌群的潜在功能,通过PICRUSt预测不同样本菌群在KEGG第一、二级通路水平上的丰度。结果如表 2所示,可知松墨天牛2个种群成虫中、后肠的细菌群落中均注释到6种KEGG一级功能通路,分别为代谢(43.23%)、环境信息处理(21.52%)、遗传信息处理(14.06%)、细胞过程(3.56%)、人类疾病(1.03%)和组织系统(0.45%)。其中代谢功能通路的丰度占比接近所有一级通路丰度的一半,表明2个成虫的肠道菌群主要行使代谢各种物质的功能。除此以外,代谢功能的丰度在室外种群与室内种群成虫肠道样本之间未表现出显著差异,同时在中肠与后肠2个肠段样本之间的差异也不显著。在其他KEGG一级功能通路上,所有样本的丰度之间均无显著性差异。
| Pathway name | Outdoor midgut | Outdoor hindgut | Indoor midgut | Indoor hindgut |
| Metabolism | 12401305±1389759a | 17757599±9895476a | 12463332±6305946a | 10153094±839320a |
| Environmental information processing | 6120484±741717a | 8666210±4740440a | 6471449±3234407a | 4976935±367915a |
| Unclassified | 4799375±503720a | 6669713±3549445a | 4009493±1296600a | 4028980±329745a |
| Genetic information processing | 4187860±403752a | 5775337±2928125a | 3616539±1120312a | 3542418±273549a |
| Cellular processes | 1010093±44251a | 938872±74649a | 1146470±676850a | 1057070±82804a |
| Human diseases | 291732±27469a | 379807±172919a | 319782±222239a | 254382±22951a |
| Organismal systems | 116328±12393a | 165336±91382a | 162464±131240a | 98734±9243a |
| None | 44535±4092a | 55810±22755a | 45295±27155a | 40054±3853a |
| Data in the table are Mean±Sd, different lowercase letters after each row of data indicate significant difference in the KEGG level1 pathway abundance of bacteria among different samples (P < 0.05, SPSS One-way ANOVA). | ||||
对松墨天牛室内外种群成虫肠道菌群代谢通路里的二级通路进行映射注释,共发现12种二级代谢功能通路(表 3)。这些二级通路对应的功能名称及其在全部样本中的占比分别为糖类代谢(24.14%)、氨基酸代谢(19.01%)、能量代谢(10.79%)、辅助因子和维生素代谢(8.73%)、脂质代谢(6.61%)、核苷酸代谢(6.50%)、外源化学物质生物降解与代谢(5.88%)、聚糖生物合成与代谢(5.06%)、酶家族(4.51%)、其他氨基酸代谢(3.89%)、萜类和聚酮类化合物代谢(3.32%)及其他次生代谢产物生物合成(1.56%)。由此可知,2个种群成虫肠道菌群主要参与糖类和氨基酸的代谢,同时少部分参与降解外源化学物质、萜类和聚酮类化合物。这些功能的丰度在种群间和肠段间均无显著性差异。
| Pathway name | Outdoor midgut | Outdoor hindgut | Indoor midgut | Indoor hindgut |
| Carbohydrate metabolism | 3088861±383200a | 4547472±2648884a | 2749950±1163724a | 2434166±202324a |
| Amino acid metabolism | 2308328±257993a | 3332755±1898076a | 2483234±1362195a | 1901427±163224a |
| Energy metabolism | 1377037±149385a | 1894696±966210a | 1262033±505856a | 1143221±95101a |
| Metabolism of cofactors and vitamins | 1114979±118356a | 1542860±817751a | 1010957±367609a | 928575±71060a |
| Lipid metabolism | 782971±85960a | 1114780±619423a | 925025±614068a | 653966±58089a |
| Nucleotide metabolism | 815771±75551a | 1141440±589317a | 779911±325098a | 685448±50839a |
| Xenobiotics biodegradation and metabolism | 616019±82136a | 965095±629926a | 1045359±1019320a | 480643±55603a |
| Glycan biosynthesis and metabolism | 658779±61306a | 867047±405956a | 543181±103329a | 576634±40418a |
| Enzyme families | 580632±62357a | 800363±415286a | 501877±141647a | 490275±41244a |
| Metabolism of other amino acids | 474214±52589a | 684536±391393a | 502558±277841a | 389665±31817a |
| Metabolism of terpenoids and polyketides | 409926±39088a | 584943±322004a | 413739±248161a | 339324±22419a |
| Biosynthesis of other secondary metabolites | 173788±24052a | 281612±192528a | 245507±184063a | 129749±10127a |
| Data in the table are Mean±SD, different lowercase letters after each row of data indicate significant difference in the KEGG level2 pathway abundance of bacteria among different samples (P < 0.05, SPSS One-way ANOVA). | ||||
3 结论和讨论
本研究基于16S rDNA的高通量测序技术对来自寄主松树和室内人工饲料饲养的松墨天牛两个种群成虫的中、后肠细菌群落多样性和差异性进行了分析,共注释到22个门、48个纲、112个目、172个科、285个属和408个种。从马尾松木段中羽化的成虫肠道优势门为变形菌门和厚壁菌门,人工饲料饲养的成虫肠道优势门为变形菌门。室外种群中、后肠的优势属均为肠杆菌属,室内种群中、后肠的优势属分别为沙雷氏菌属和肠杆菌属。本研究的优势门类与已有松墨天牛肠道菌的研究结果相似,如Hu等[31]研究发现中国室外松墨天牛幼虫的肠道优势菌也为变形菌门;Kim等[7]对韩国松墨天牛成虫肠道菌进行研究,发现变形菌门和厚壁菌门为优势细菌;并且在天牛科其他多种昆虫的肠道中,也有类似优势门类的报道[20, 32-37]。对松墨天牛肠道细菌优势属的研究发现国内松墨天牛幼虫肠道以肠杆菌属为主[31],本研究的成虫肠道细菌优势属与幼虫结果类似;而国外研究显示松墨天牛室外种群成虫的中、后肠以克吕沃尔菌属(Kluyvera)为主,室内种群的中肠以沙雷氏菌属为主,后肠则以肠杆菌属、无色杆菌属(Achromobacter)和红球菌属(Rhodococcus)为主[7],结果与本研究存在差异。国内的松墨天牛均采自马尾松(Pinus massoniana),国外的松墨天牛采自赤松(Pinus densiflora),国内外松墨天牛由于取食不同的寄主植物,从而塑造了不同的肠道菌群结构;室外和室内成虫种群的肠道细菌组成也随着食料的变化而改变。在本研究中,室内饲养种群的中肠细菌以沙雷氏菌属为优势属,后肠细菌的优势属依然是肠杆菌属。针对鞘翅目和鳞翅目部分昆虫肠道酸碱性的研究发现,一般中肠碱性较高,而后肠接近中性[38-39]。东秀珠和蔡妙英[40]描述沙雷氏菌属细菌适宜生长的pH为5.0–9.0,因此推测沙雷氏菌属较肠杆菌属细菌更适应碱性环境,在中肠中的丰度更高。
从OTUs组成和Alpha多样性结果可以看出,室内饲养的松墨天牛成虫肠道细菌的丰度远高于室外种群的肠道细菌。Kim等[7]研究发现室内饲养种群的肠道细菌Alpha多样性结果也高于室外种群。本研究除了提供含有丰富碳源和氮源等营养成分的半人工饲料,同时也提供了恒定的饲养温度,为细菌的富集营造了良好的环境。Beta多样性的结果显示室外种群肠道各样本的菌群组成相近,室内种群肠道各样本的菌群组成差异明显。这种现象是因为室外种群肠道细菌长期定殖形成稳定群落的结果,室内种群的成虫种群由于刚羽化不久即被解剖提取肠道,菌群未定殖完成,形成样本间的差异。
昆虫完整的生活史离不开健康的生长发育,在此过程中肠道微生物的营养共生和解毒代谢作用显得尤为重要[10, 41]。本研究通过PICRUSt预测了松墨天牛室内外种群成虫中、后肠细菌的潜在功能,结果显示所有样本均表现出很高的代谢功能且各样本之间无差异。昆虫的中肠和后肠作为食物消化和吸收最主要的场所,肠道菌群发挥了重要的代谢作用[10, 42]。室内外松墨天牛成虫肠道细菌主要通过代谢糖类和氨基酸等物质为宿主提供充足的营养。本研究也预测了肠道细菌对外源化学物质、萜类和聚酮类化合物及其他次生代谢物质的降解功能,表明这些肠道细菌参与了代谢松树木质素和萜类物质的过程。研究表明肠杆菌属和沙雷氏菌属细菌在许多昆虫肠道中作为优势菌存在,并且能够帮助宿主昆虫高效的降解纤维素、单萜或双萜等物质[22-23, 43-45]。
本研究利用Illumina HiSeq测序技术完成了松墨天牛室内外种群成虫肠道中、后肠细菌组成和多样性的比较分析,预测了肠道细菌的潜在功能。研究结果表明取食不同食料的松墨天牛成虫的肠道菌群结构存在差异,说明食物可以塑造松墨天牛肠道的菌群结构。中、后肠作为营养消化吸收的主要器官,肠道细菌参与寄主植物木质素和纤维素分解的代谢,为宿主松墨天牛提供营养物质,也能降解次生代谢物质,帮助宿主昆虫解毒,后续研究将进行肠道中具有这些细菌的筛选和功能验证,进一步明确松墨天牛肠道共生菌的功能及其机制。
| [1] |
Hao DJ, Yang JX, Dai HG. Research prowess and prospect on chemical ecology of Monochamus alternatus. Chinese Journal of Ecology, 2008, 27(7): 1227-1233.
(in Chinese) 郝德君, 杨剑霞, 戴华国. 松墨天牛化学生态学. 生态学杂志, 2008, 27(7): 1227-1233. |
| [2] | Zhao BG, Futai K, Sutherland JR, Takeuchi Y. Pine wilt disease. Tokyo: Springer, 2008. |
| [3] |
Ye JR. Epidemic status of Pine Wilt Disease in China and its prevention and control techniques and counter measures. Scientia Silvae Sinicae, 2019, 55(9): 1-10.
(in Chinese) 叶建仁. 松材线虫病在中国的流行现状、防治技术与对策分析. 林业科学, 2019, 55(9): 1-10. |
| [4] | 萧刚柔. 中国森林昆虫. 北京: 中国林业出版社, 1992. |
| [5] | 任骥. 松褐天牛室内饲养、幼虫龄期及成虫产卵特性的研究. 山东农业大学硕士学位论文, 2014. |
| [6] |
Chen RX, Wang LJ, Lin T, Wei ZQ, Wang Y, Hao DJ. Rearing techniques of Monochamus alternatus Hope (Coleoptera: Cerambycidae) on artificial diets. Journal of Nanjing Forestry University (Natural Science Edition), 2017, 41(1): 199-202.
(in Chinese) 陈瑞旭, 王露洁, 林涛, 韦志强, 王焱, 郝德君. 松墨天牛的人工饲育技术研究. 南京林业大学学报(自然科学版), 2017, 41(1): 199-202. |
| [7] | Kim JM, Choi MY, Kim JW, Lee SA, Ahn JH, Song J, Kim SH, Weon HY. Effects of diet type, developmental stage, and gut compartment in the gut bacterial communities of two Cerambycidae species (Coleoptera). Journal of Microbiology, 2017, 55(1): 21-30. DOI:10.1007/s12275-017-6561-x |
| [8] | Dillon RJ, Dillon VM. The gut bacteria of insects: Nonpathogenic interactions. Annual Review of Entomology, 2004, 49: 71-92. DOI:10.1146/annurev.ento.49.061802.123416 |
| [9] | Colman DR, Toolson EC, Takacs-Vesbach CD. Do diet and taxonomy influence insect gut bacterial communities?. Molecular Ecology, 2012, 21(20): 5124-5137. DOI:10.1111/j.1365-294X.2012.05752.x |
| [10] | Engel P, Moran NA. The gut microbiota of insects-diversity in structure and function. Fems Microbiology Reviews, 2013, 37(5): 699-735. DOI:10.1111/1574-6976.12025 |
| [11] |
Tian XY, Song FP, Zhang J, Liu RM, Zhang XP, Duan JY, Shu CL. Diversity of gut bacteria in larval Protaetia brevitarsis (Coleoptera: Scarabaedia) fed on corn stalk. Acta Entomologica Sinica, 2017, 60(6): 632-641.
(in Chinese) 田小燕, 宋福平, 张杰, 刘荣梅, 张兴鹏, 段江燕, 束长龙. 饲喂玉米秸秆的白星花金龟幼虫肠道细菌多样性. 昆虫学报, 2017, 60(6): 632-641. |
| [12] |
Mei C, Fan S, Yang H. The strategies of isolation of insect gut microorganisms. Acta Microbiologica Sinica, 2018, 58(6): 985-994.
(in Chinese) 梅承, 范硕, 杨红. 昆虫肠道微生物分离培养策略及研究进展. 微生物学报, 2018, 58(6): 985-994. |
| [13] | Dillon R, Charnley K. Mutualism between the desert locust Schistocerca gregaria and its gut microbiota. Research in Microbiology, 2002, 153(8): 503-509. DOI:10.1016/S0923-2508(02)01361-X |
| [14] | Broderick NA, Raffa KF, Goodman RM, Handelsman J. Census of the bacterial community of the gypsy moth larval midgut by using culturing and culture-independent methods. Applied and Environmental Microbiology, 2004, 70(1): 293-300. DOI:10.1128/AEM.70.1.293-300.2004 |
| [15] | Hayashi A, Aoyagi H, Yoshimura T, Tanaka H. Development of novel method for screening microorganisms using symbiotic association between insect (Coptotermes formosanus Shiraki) and intestinal microorganisms. Journal of Bioscience and Bioengineering, 2007, 103(4): 358-367. DOI:10.1263/jbb.103.358 |
| [16] | Chandler JA, Lang JM, Bhatnagar S, Eisen JA, Kopp A. Bacterial communities of diverse Drosophila species: ecological context of a host-microbe model system. PLoS Genetics, 2011, 7(9): e1002272. DOI:10.1371/journal.pgen.1002272 |
| [17] |
Wei DF, Wang XJ, Yang J, Geng YX, Chen M. Analysis of the diversity and difference of intestinal bacteria in larvae Hyphantria cunea Drury (Lepidoptera: Arctiidae) on different diets. Journal of Environmental Entomology, 2017, 39(3): 515-524.
(in Chinese) 魏丹峰, 王秀吉, 杨锦, 耿涌鑫, 陈敏. 取食不同食料的美国白蛾幼虫肠道细菌多样性及差异性研究. 环境昆虫学报, 2017, 39(3): 515-524. |
| [18] | Calderón-Cortés N, Quesada M, Watanabe H, Cano-Camacho H, Oyama K. Endogenous plant cell wall digestion: a key mechanism in insect evolution. Annual Review of Ecology, Evolution, and Systematics, 2012, 43(1): 45-71. DOI:10.1146/annurev-ecolsys-110411-160312 |
| [19] | Zhou JP, Huang HQ, Meng K, Shi PJ, Wang YR, Luo HY, Yang PL, Bai YG, Zhou ZG, Yao B. Molecular and biochemical characterization of a novel xylanase from the symbiotic Sphingobacterium sp. TN19. Applied Microbiology and Biotechnology, 2009, 85(2): 323-333. DOI:10.1007/s00253-009-2081-x |
| [20] | Geib SM, Del Mar Jimenez-Gasco M, Carlson JE, Tien M, Hoover K. Effect of host tree species on cellulase activity and bacterial community composition in the gut of larval Asian Longhorned beetle. Environmental Entomology, 2009, 38(3): 686-699. DOI:10.1603/022.038.0320 |
| [21] | Raffa KF, Aukema BH, Erbilgin N, Klepzig KD, Wallin KF. Interactions among conifer terpenoids and bark beetles across multiple levels of scale: an attempt to understand links between population patterns and physiological processes. Recent Advances in Phytochemistry, 2005, 39: 79-118. |
| [22] | Adams AS, Aylward FO, Adams SM, Erbilgin N, Aukema BH, Currie CR, Suen G, Raffa KF. Mountain pine beetles colonizing historical and naÏve host trees are associated with a bacterial community highly enriched in genes contributing to terpene metabolism. Applied and Environmental Microbiology, 2013, 79(11): 3468-3475. DOI:10.1128/AEM.00068-13 |
| [23] | Berasategui A, Salem H, Paetz C, Santoro M, Gershenzon J, Kaltenpoth M, Schmidt A. Gut microbiota of the pine weevil degrades conifer diterpenes and increases insect fitness. Molecular Ecology, 2017, 26(15): 4099-4110. DOI:10.1111/mec.14186 |
| [24] | Stewart CN Jr, Via LE. A rapid CTAB DNA isolation technique useful for RAPD fingerprinting and other PCR applications. BioTechniques, 1993, 14(5): 748-750. |
| [25] | Wu XQ, Xue Q, Xiang Y, Ding XL, Xu XL, Ye JR. Community and functional diversity of bacteria associated with propagative and dispersal forms of Bursaphelenchus xylophilus. Nematology, 2016, 18(10): 1185-1198. DOI:10.1163/15685411-00003024 |
| [26] | Magoč T, Salzberg SL. Flash: fast length adjustment of short reads to improve genome assemblies. Bioinformatics, 2011, 27(21): 2957-2963. DOI:10.1093/bioinformatics/btr507 |
| [27] | Edgar RC. UPARSE: highly accurate OTU sequences from microbial amplicon reads. Nature Methods, 2013, 10(10): 996-998. DOI:10.1038/nmeth.2604 |
| [28] | Wang Q, Garrity GM, Tiedje JM, Cole JR. NaÏve bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Applied and Environmental Microbiology, 2007, 73(16): 5261-5267. DOI:10.1128/AEM.00062-07 |
| [29] | Schloss PD, Westcott SL, Ryabin T, Hall JR, Hartmann M, Hollister EB, Lesniewski RA, Oakley BB, Parks DH, Robinson CJ, Sahl JW, Stres B, Thallinger GG, van Horn DJ, Weber CF. Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Applied and Environmental Microbiology, 2009, 75(23): 7537-7541. DOI:10.1128/AEM.01541-09 |
| [30] | Langille MGI, Zaneveld J, Caporaso JG, McDonald D, Knights D, Reyes JA, Clemente JC, Burkepile DE, Vega Thurber RL, Knight R, Beiko RG, Huttenhower C. Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nature Biotechnology, 2013, 31(9): 814-821. DOI:10.1038/nbt.2676 |
| [31] | Hu X, Li M, Raffa KF, Luo QY, Fu HJ, Wu SQ, Liang GH, Wang R, Zhang FP. Bacterial communities associated with the pine wilt disease vector Monochamus alternatus (Coleoptera: Cerambycidae) during different larval instars. Journal of Insect Science, 2017, 17(6): 115. |
| [32] | Schloss PD, Delalibera I Jr, Handelsman J, Raffa KF. Bacteria associated with the guts of two wood-boring beetles: Anoplophora glabripennis and Saperda vestita (Cerambycidae). Environmental Entomology, 2006, 35(3): 625-629. DOI:10.1603/0046-225X-35.3.625 |
| [33] | Park DS, Oh HW, Jeong WJ, Kim H, Park HY, Bae KS. A culture-based study of the bacterial communities within the guts of nine longicorn beetle species and their exo-enzyme producing properties for degrading xylan and pectin. Journal of Microbiology, 2007, 45(5): 394-401. |
| [34] | Grünwald S, Pilhofer M, Höll W. Microbial associations in gut systems of woodand bark-inhabiting longhorned beetles[Coleoptera: Cerambycidae]. Systematic and Applied Microbiology, 2010, 33(1): 25-34. DOI:10.1016/j.syapm.2009.10.002 |
| [35] | Rizzi A, Crotti E, Borruso L, Jucker C, Lupi D, Colombo M, Daffonchio D. Characterization of the bacterial community associated with larvae and adults of Anoplophora chinensis collected in Italy by culture and culture-independent methods. BioMed Research International, 2013, 2013: 420287. |
| [36] | Vicente CSL, Nascimento FX, Espada M, Barbosa P, Hasegawa K, Mota M, Oliveira S. Characterization of bacterial communities associated with the pine sawyer beetle Monochamus galloprovincialis, the insect vector of the pinewood nematode Bursaphelenchus xylophilus. FEMS Microbiology Letters, 2013, 347(2): 130-139. |
| [37] | Alves M, Pereira A, Matos P, Henriques J, Vicente C, Aikawa T, Hasegawa K, Nascimento F, Mota M, Correia A, Henriques I. Bacterial community associated to the pine wilt disease insect vectors Monochamus galloprovincialis and Monochamus alternatus. Scientific Reports, 2016, 6: 23908. DOI:10.1038/srep23908 |
| [38] | Lemke T, Stingl U, Egert M, Friedrich MW, Brune A. Physicochemical conditions and microbial activities in the highly alkaline gut of the humus-feeding larva of Pachnoda ephippiata (Coleoptera: Scarabaeidae). Applied and Environmental Microbiology, 2003, 69(11): 6650-6658. DOI:10.1128/AEM.69.11.6650-6658.2003 |
| [39] | Chapman RF, Simpson SJ, Douglas AE. The Insects: structure and function. 5th ed. Cambridge: Cambridge University Press, 2013. |
| [40] | 东秀珠, 蔡妙英. 常见细菌系统鉴定手册. 北京: 科学出版社, 2001. |
| [41] |
Wei G, Bai L, Qu S, Wang SB. Insect microbiome and their potential application in the insect pest and vector-borne disease control. Acta Microbiologica Sinica, 2018, 58(6): 1090-1102.
(in Chinese) 魏舸, 白亮, 曲爽, 王四宝. 昆虫共生微生物在病虫害和疾病控制上的应用前景. 微生物学报, 2018, 58(6): 1090-1102. |
| [42] | Douglas AE. Multiorganismal insects: diversity and function of resident microorganisms. Annual Review of Entomology, 2015, 60(1): 17-34. DOI:10.1146/annurev-ento-010814-020822 |
| [43] | Adams L, Boopathy R. Isolation and characterization of enteric bacteria from the hindgut of Formosan termite. Bioresource Technology, 2005, 96(14): 1592-1598. DOI:10.1016/j.biortech.2004.12.020 |
| [44] | Howe M, Keefover-Ring K, Raffa KF. Pine engravers carry bacterial communities whose members reduce concentrations of host monoterpenes with variable degrees of redundancy, specificity, and capability. Environmental Entomology, 2018, 47(3): 638-645. DOI:10.1093/ee/nvy032 |
| [45] |
Hu X, Fu HJ, Li JN, Lin ZP, Zhang FP. Isolation and identification of cellulolytic bacteria associated with the gut of Monochamus alternatus larvae. Journal of Fujian Agriculture and Forestry University (Natural Science Edition), 2018, 47(3): 322-328.
(in Chinese) 胡霞, 傅慧静, 李俊楠, 林中平, 张飞萍. 松墨天牛幼虫肠道纤维素降解细菌的分离与鉴定. 福建农林大学学报(自然科学版), 2018, 47(3): 322-328. |
 2021, Vol. 61
2021, Vol. 61