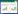中国科学院微生物研究所,中国微生物学会,中国菌物学会
文章信息
- 祝林, 向文良, 张楠笛, 许琴, 赵秋欢. 2021
- Lin Zhu, Wenliang Xiang, Nandi Zhang, Qin Xu, Qiuhuan Zhao. 2021
- 甲基营养型芽孢杆菌拮抗玉蜀黍尾孢菌、链格菌和灰葡萄孢菌及环脂肽分析
- Antagonism of Bacillus methylotrophicus against Cercospora zeae-maydis Tehon et Daniels, Alternaria alternate and Botrytis cinerea
- 微生物学报, 61(3): 644-654
- Acta Microbiologica Sinica, 61(3): 644-654
-
文章历史
- 收稿日期:2020-05-02
- 修回日期:2020-07-06
- 网络出版日期:2020-11-10
植物病害是威胁农业生产的主要自然灾害之一。对植物病害的防治,通常采用化学方法,其见效快、成本低,但长期大量使用会造成环境污染,导致农产品农药残留超标以及增加病原菌抗药性等不利因素[1-2]。随着生物技术发展,植物病害的生物防治因对人畜环境安全性高,具有改善环境[3]、促进植物生长发育等潜在价值,而受到越来越多的关注[4]。植物病害生物防治的机制主要有拮抗、诱导抗病性、竞争、重寄生、交叉保护以及捕食等,其中拮抗是植物真菌病害防治中最具应用潜力的生防机制。
玉蜀黍尾孢菌(Cercospora zeae-maydis Tehon et Daniels)是引发玉米灰斑病的病原真菌,在全球广泛传播,是导致玉米产量严重下降的主要病害菌之一[5]。链格菌(Alternaria alternate)是农作物的主要病原菌之一,能引起包括玉米叶枯病在内的多种植物疾病,给农业生产带来巨大损失[6]。灰葡萄孢菌(Botrytis cinerea)可以使200多种植物产生灰霉病,是世界范围内危害最为严重的真菌病害菌[7]。这些病原菌引发的植物病害严重影响了农业生产,阻遏了种植业发展。因此,寻求玉蜀黍尾孢菌、链格孢菌和灰葡萄孢菌病害的安全、有效的防治方法显得十分重要且紧迫。
甲基营养型芽孢杆菌(Bacillus methylotrophicus)是Madhaiyan等在2010年发现的芽孢杆菌属新成员[8],是一种植物根系促生菌,同时也是植物病害生物防治中重要的生防菌[9-10]。芽孢杆菌作为最有前途的生防菌,能产生重要的抗菌环脂肽(antifungal cyclic lipopeptides, ACLs)[11-12]。目前,有关产ACLs芽孢杆菌属的研究主要集中在枯草芽孢杆菌(Bacillus subtilis)[13]、解淀粉芽孢杆菌(Bacillus amyloliquefaciens)[11, 14-15]、短小芽孢杆菌(Bacillus brevis)[16]和苏云金芽孢杆菌(Bacillus thuringiensis)[17]等,对甲基营养型芽孢杆菌的研究鲜有报道。因此,本研究以玉蜀黍尾孢菌、链格菌和灰葡萄孢菌为指示菌,筛选甲基营养型芽孢杆菌拮抗菌,研究拮抗能力,分析拮抗物质,并通过田间试验评估生防效果,以期为甲基营养型芽孢杆菌防治植物真菌病害的深入研究和应用提供理论基础和科学依据。
1 材料和方法 1.1 材料1.1.1 菌株来源: 分离自污水中的20株芽孢杆菌,本实验室–20 ℃保藏。玉蜀黍尾孢菌T1 (Cercospora zeae-maydis Tehon et Daniels T1)、链格菌T2 (Alternaria alternata T2)和灰葡萄孢菌T3 (Botrytis cinerea T3)均为实验室保藏菌株。
1.1.2 培养基: 营养肉汤酵母膏培养基(NBY)[18]、马铃薯葡萄糖培养基(PDA/PDB)[18]和麦粒培养基[19]。
1.2 芽孢杆菌拮抗菌筛选1.2.1 平板对峙法筛菌: 芽孢杆菌接种于NBY液体培养基中,30 ℃、140 r/min活化48 h后,沿PDA平板上边长为65 mm的正方形边划线接种。同时,分别接种直径为5 mm的病原真菌菌饼于正方形对角线交点,30 ℃培养72 h。单独接种病原菌的平板作空白对照,当对照组病原菌爬满整个平板时计算抑制率(公式1)。

|
(1) |
1.2.2 杯碟法复筛: 挑选抑菌效果明显的菌株接种于70 mL NBY液体培养基中,30 ℃、140 r/min振荡培养48 h后,4 ℃、10000 r/min离心5 min,收集上清液备用。玉蜀黍尾孢菌T1和链格菌T2分别接种于50 mL PDB液体培养基中,30 ℃、140 r/min振荡培养48 h后,取100 µL菌液涂布于PDA平板。100 µL上述上清液加入PDA平板中心放置的牛津杯中,30 ℃、静置培养72 h,十字交叉法测量抑菌圈直径。
1.3 脂肽的分析鉴定1.3.1 脂肽粗提物制备: l00 mL NBY液体培养基中,接种1%的复筛种子液,30 ℃、140 r/min培养48 h后,4 ℃、10000 r/min离心15 min。上清液用6 mol/L HCl调至pH值2.0,4000 r/min离心5 min。取上清液,0.45 μm无菌滤膜过滤,滤液备用。同时,向离心的脂肽沉淀中加入甲醇,抽提4–8 h。抽提液经旋转蒸发仪真空浓缩后,0.02 mol/L、pH 7.4的磷酸盐缓冲液溶解,冷冻干燥得脂肽粗提物。
1.3.2 环脂肽鉴定: 薄层色谱(Thin-layer chromatography,TLC)分离纯化粗脂肽,原位酸水解茚三酮显色法鉴定环脂肽[11, 20]。取两块活化的TLC薄板,点样后在三氯甲烷: 甲醇: 水=65:25:4 (V/V/V)的溶液中展开40 min。待溶剂挥发后,其中一个TLC板以0.3%茚三酮丙酮溶液显色。另一板放入盛有2 mL浓盐酸的密封瓶,110 ℃原位酸水解2 h,冷却后吹去盐酸,茚三酮试剂显色。
1.3.3 脂肽编码基因鉴定: FastDNAⓇ Spin Kit for Soil (MP Biomedicals, 美国)提取菌株总DNA,–80 ℃保存。微量核酸蛋白分析仪(NanoDrop Technologies Inc,美国)测定DNA浓度和纯度。PCR扩增Surfactin、Fengycin和Iturin脂肽的编码基因片段[21-22],引物设计如表 1。
| Lipopeptides | Target gene | Sequences (5′→3′) |
| Surfactin | sfp | ATGAAGATTTACGGAATTTA |
| TTATAAAAGCTCTTCGTACG | ||
| Fengycia | fenB | CCTGGAGAAAGAATATACCGTACCY |
| GCTGGTTCAGTTKGATCACAT | ||
| Iturin A | ituD | TTGAAYGTCAGYGCSCCTTT |
| TGCCCTTCATAACCTTCT | ||
| Bacillomycin D | bamC | AGTAAATGAACGCGCCAATC |
| CCCTCTCCTGCCACATAGAG | ||
| bamD | TTGAAYGTCAGYGCSCCTTT | |
| TGCGMAAATAATGGSGTCGT |
1.4 菌株的鉴定
1.4.1 形态学观察: 拮抗性能良好的菌株划线于NBY固体平板上,观察菌落表面形态、边缘形态、隆起形状、透明度等,并革兰氏染色镜检。
1.4.2 16S rRNA分析: 菌株总DNA为模板,采用通用引物(Eu27F: 5′-AGAGTTTGATCCTGGCT CAG-3′;1492R: 5′-GGTTACCTTGTTACGACTT-3′),PCR扩增16S rRNA基因。扩增条件:95 ℃ 5 min;95 ℃ 1 min,50 ℃ 1 min,72 ℃ 2 min,30个循环;72 ℃ 10 min。电泳检验合格的PCR产物连接到pGEM-T载体上,克隆入感受态细胞E. coli DH5α中,提取重组质粒测序。16S rRNA基因序列提交至GenBank数据库中分析菌株的分类学地位,MEGA 5.0绘制16S rRNA基因的系统发育树。
1.5 田间生防试验1.5.1 玉米种子选择、拮抗菌菌液及病原菌接种体制备: 选择登海11号玉米种子,经精选、催芽,选择发芽一致的种子备用。将拮抗性能良好的菌株菌悬液稀释至107 CFU/mL左右备用。将3种直径为5 mm的病原真菌菌饼分别接种到麦粒培养基中,30 ℃培养4 d,待菌丝在麦粒上充分生长后,备用。
1.5.2 田间生防试验: 选择中等肥沃度的土壤作为试验地,面积为36 m2,窝距50 cm,行距50 cm。按每窝15 g分别将3种病原菌麦粒均匀加入窝内表层6 cm土壤后,每窝播种2粒在拮抗菌菌液中搅拌1 h后的种子,以不拌拮抗菌液的种子作为对照组。待玉米植株长到4叶期、拔节前期以及喇叭口期,分别用清水、拮抗菌菌液均匀喷施植株基部。
1.5.3 病情调查: 参照国标[23]对3种病原菌病害程度及防治效果进行调查统计。试验地随机取5点,每点取5株调查全部叶片,根据叶片病害程度计算病情指数和防治效果(公式2)。

|
(2) |
结果显示,20株芽孢杆菌中,菌株B-1841和B-1560对C. zeae-maydis T1、A. alternata T2和B. cinerea T3三种病原菌都具有明显拮抗作用(图 1)。总体而言,两株菌对A. alternata T2拮抗作用最好,抑制率达到70%左右;对C. zeae-maydis T1拮抗作用次之,为60%左右;对B. cinerea T3拮抗作用相对较弱,为40%左右(表 2,图 2)。芽孢杆菌类的生防菌通常产生可溶性环脂肽于发酵上清液中[11-12],为进一步研究拮抗菌是否产生环脂肽,选择A. alternata T2和C. zeae-maydis T1作为病原指示菌,对菌株B-1841和B-1560发酵上清液的抑菌能力进行分析。
|
| 图 1 菌株B-1841和B-1560对C. zeae-maydis T1 (A)、A. alternata T2 (B)和B. cinerea T3 (C)的拮抗作用 Figure 1 Antagonistic effects of B-1841 and B-1560 on C. zeae-maydis T1 (A), A. alternata T2 (B) and B. cinerea T3 (C). |
| Strains | C. zeae-maydis T1 | A. alternata T2 | B. cinerea T3 | |||||
| Colony diameter/mm | Inhibition rate/% | Colony diameter/mm | Inhibition rate/% | Colony diameter/mm | Inhibition rate/% | |||
| CK | 72.56±0.46 | – | 30.25±0.85 | – | 41.27±0.50 | – | ||
| B-1841 | 24.71±0.28 | 65.95aAB | 8.76±0.01 | 71.04aA | 22.00±1.35 | 46.69bB | ||
| B-1560 | 28.30±0.12 | 60.99abAB | 8.82±0.37 | 70.84aA | 25.10±1.02 | 39.18bB | ||
| Superscript lower case letters indicate extremely significant differences (P < 0.05); superscript upper case letters indicate extremely significant differences (P < 0.01). | ||||||||

|
| 图 2 菌株B-1841和B-1560对致病真菌的拮抗作用 Figure 2 Antagonistic effects of strain B-1841 and B-1560 on pathogenic fungi. ***: indicate extremely significant differences (P < 0.01). |
复筛结果表明,菌株B-1841和B-1560的发酵上清液对C. zeae-maydis T1和A. alternata T2均有拮抗作用(图 3),其中菌株B-1841的发酵上清液抑制作用更明显(表 3),表明菌株能产生可溶性的抑菌物质。

|
| 图 3 菌株B-1841和菌株B-1560发酵上清液对C. zeae-maydis T1 (A)和A. alternata T2 (B)的抑制作用 Figure 3 Inhibition effects of fermentation supernatants of B-1841 and B-1560 on C. zeae-maydis T1 (A) and A. alternata T2 (B). |
| Strains | Colony diameter/mm | |
| C. zeae-maydis T1 | A. alternata T2 | |
| B-1841 | 27.71±0.25aAB | 30.36±0.04aA |
| B-1560 | 23.45±0.03bB | 26.82±0.13aAB |
| Superscript lower case letters indicate extremely significant differences (P < 0.05); superscript upper case letters indicate extremely significant differences (P < 0.01). | ||
2.2 抑菌物质的分析鉴定
菌株B-1841和B-1560的发酵上清液经盐酸沉淀、甲醇抽提、真空干燥后得到抗菌粗提物产率分别为386 mg/mL和243 mg/mL。粗提物分别溶解于与上清液等体积的甲醇和水溶液,杯碟法探讨经盐酸沉淀后的上层清液、粗提物甲醇液和粗提物水溶液对A. alternata T2的抑菌活性。结果如图 4所示,两株菌的盐酸沉淀上清液、粗提物甲醇液和粗提物水溶液对A. alternata T2均表现出抑菌作用,其中盐酸沉淀上清液的抑菌效果弱于粗提物甲醇液和粗提物水溶液,表明大量抑菌物质能够被盐酸沉淀;粗提物甲醇液的抑菌效果优于水溶液。

|
| 图 4 菌株B-1841 (A)和B-1560 (B)芽孢杆菌脂肽粗提物对A. alternata T2抑制作用 Figure 4 Inhibition effect of crude extract of two strains of Bacillus lipopeptide on A. alternata T2. 1: methanol control solution; 2: supernatant after hydrochloric acid precipitation; 3: methanol extract from lower layer precipitation; 4: distilled water; 5: solid after vacuum drying dissolved in distilled water. A: Antagonistic effect of crude extract of strain B-1841 lipopeptide on A. alternata T2; B: Antagonistic effect of crude extract of strain B-1560 lipopeptide on A. alternata T2. |
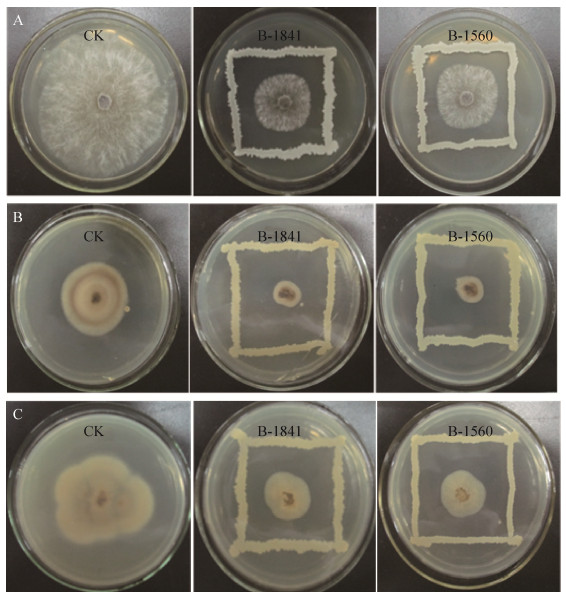
薄层色谱(TLC)是分离和定性鉴定混合物的重要手段之一,原位酸水解茚三酮显色法是鉴定环脂肽的方法之一[11, 20]。菌株B-1841抗菌粗提物经TLC分离后,色谱带中的分离物与茚三酮显色反应强,而菌株B-1560显色相对较弱(图 5),表明两株菌均能产生环脂肽类抗菌物质。脂肽类物质为环型结构,不含氨基末端,酸解后环型结构被打开,游离氨基会暴露出来与茚三酮发生显色反应,因而在TLC板中出现显色斑点,同时显色强度与含量呈正相关[3, 20, 24]。因此,菌株B-1841产生环状脂肽类抗菌物质能力高于菌株B-1560。
|
| 图 5 抗菌粗提物的薄层色谱(TLC)分离及酸水解茚三酮显色 Figure 5 Thin layer chromatography (TLC) separation of crude antibacterial extract and color development of acid hydrolyzed ninhydrin. A: plates are unacidified samples; B: plates are acid treated samples. 1: crude peptide crude extract of strains B-1841; 2: crude peptide crude extract of strains B-1560. |
选择环脂肽产生能力强的菌株B-1841,分析环脂肽种类。PCR扩增表 1中脂肽的编码基因,结果如图 6所示。Sfp基因编码Surfactin,FenB基因编码Fengycia,ItuD、BamC和BamD基因编码伊枯草菌素(Iturin A)和类似物杆菌霉素(Bacillomycin D)[21-22]。在琼脂糖凝胶电泳中仅检测到了ItuD、BamC和BamD基因,表明B-1841产生了伊枯草菌素和杆菌霉素。

|
| 图 6 脂肽编码基因的PCR检测 Figure 6 PCR detection of lipopeptide encoding gene. |
2.3 菌株B-1841的鉴定
在NBY平板上,菌株B-1841菌落表面干燥,无粘性,呈乳白色、圆形、扁平、边缘齿状(图 7-A)。细胞呈杆状[(0.63-0.64) µm×(1.8-2.7)µm],革兰氏阳性,芽孢椭圆形(图 7-B)。

|
| 图 7 菌株B-1841的形态学特征 Figure 7 Morphological characteristics of strain B-1841. A: colony morphology; B: gram staining. |
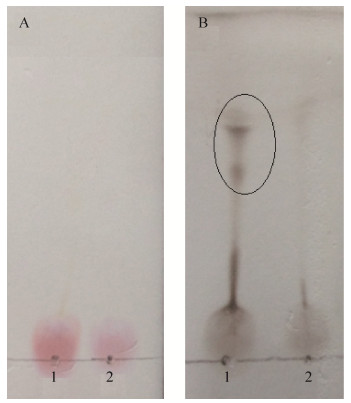
16S rRNA分析结果表明,菌株B-1841的16S rRNA基因(NCBI登录号为PCCU6E0001R)与甲基营养型芽孢杆菌(Bacillus methylotrophicus)的16S rRNA基因(NCBI登录号为KC790265.1)的序列相似度达到100%。Stackebrandt等[25]认为细菌16S rRNA序列同源性≥98%时可以认为是一个种。结合形态学观察,初步认定菌株B-1841为甲基营养型芽孢杆菌。基于Neighbor-joining算法绘制16S rRNA系统发育树进一步表明菌株B-1841属于芽胞杆菌属,甲基营养型芽孢杆菌种,与枯草芽孢杆菌亲缘关系较近(图 8)。
|
| 图 8 菌株B-1841的16S rRNA序列系统发育树 Figure 8 Phylogenetic tree of the 16S rRNA sequence of strain B-1841. Numbers in parentheses represent accession number in RPD. Number at each branch point represent the bootstrap values on Neighbor-joining analysis of 1000 replication data sets. Bar: 0.5% sequence divergence. |
2.4 田间生防效果
甲基营养型芽孢杆菌B-1841对C. zeae-maydis T1、A. alternata T2以及B. cinerea T3引起的玉米病害均有一定的防治效果(表 4)。其中,对A. alternata T2病害的防效最佳,相对防效为69.89%,对C. zeae-maydis T1的相对防效为60.25%,对B. cinerea T3防治效果最差,相对防效为45.21%。
| Strains | C. zeae-maydis T1 | A. alternata T2 | B. cinerea T3 | |||||
| Diseas index | Relative control effect/% | Diseas index | Relative control effect/% | Diseas index | Relative control effect/% | |||
| CK | 75.62 | – | 70.69 | – | 61.84 | – | ||
| B-1841 | 30.06 | 60.25aAB | 21.28 | 69.89aA | 33.88 | 45.21bB | ||
| Superscript lower case letters indicate extremely significant differences (P < 0.05); superscript upper case letters indicate extremely significant differences (P < 0.01). | ||||||||
3 讨论
植物真菌病害是导致农作物减产的主要原因之一。其中,玉蜀黍尾孢菌(C. zeae-maydis)、链格菌(A. alternata)和灰葡萄孢菌(B. cinerea)病原菌引发的农作物病害是全球农业生产广泛关注的问题[5-7]。玉蜀黍尾孢茵(Cercospora zeae-maydis Tehon Daniels)是玉米灰斑病的病原菌,感染玉米会导致减产,严重时甚至绝收,极大影响玉米产业发展。链格菌(Alternaria alternata)能产生多种非宿主特异性毒素,感染100多种农作物,如棉花叶片轮纹斑病[26]、烟草赤星病[27]。灰葡萄孢菌(Botrytis cinerea)是十大农作物病原真菌之一,感染农作物范围广,对作物生长及后期粮食储藏均会造成严重损害。因此,寻找最佳的防治方法,控制其危害性,十分紧迫。
农作物真菌病害的生物防治已成为研究热点。目前应用的生防菌主要包括荧光假单胞菌(Pseudomonas flurescens)、链霉菌属(Streptomyces)、木霉菌属(Trichoderma),但它们对病原菌的抑制率较低,普遍在30%–50%[28]。芽孢杆菌属是一类潜在的生防菌资源,能合成伊枯草菌素(iturin)、表面活性素(surfactin)和丰原素(fengycin)等环脂肽。环脂肽由非核糖体多肽合成酶(non-ribosomal peptide synthetases,NRPSs)识别特定的氨基酸直接连接形成,对植物病原菌具有很强的抑制作用,具有抗菌谱广、低残留和低毒的特点,在农作物真菌病害的生物防治领域具有巨大的研究价值和应用潜力[24, 29-32]。Cryptosporiopsis ericae Cc-HG-7对C. zeae-maydis抑菌率为36.11%[33];Bacillus subtilis 330-2对A. alternata和B. cinerea的抑菌率分别为47.96%和44.57%[34]。甲基营养型芽孢杆菌(B. methylotrophicus)是近年来报道的一种新型生防菌,能产生环脂肽,对植物病原菌具有较好的抑制作用,如B. methylotrophicus 39b能抑制根癌农杆菌(Agrobacterium tumefaciens)引起的番茄冠状胆瘤[35];B. methylotrophicus H8能抑制稻生黄单胞菌(Xanthomonas oryzae pv. oryzae)引起的水稻白叶枯病[36]。分离菌株B. methylotrophicus B-1841对C. zeae-maydis、A. alternata和B. cinerea的抑菌率分别为65.95%、71.04%和46.69%,优于Cryptosporiopsis ericae Cc-HG-7和Bacillus subtilis 330-2。
试验表明,B. methylotrophicus B-1841拮抗病原真菌的抑菌物质为伊枯草菌素和类似物杆菌霉素,属于Iturin类环脂肽,能在病原菌细胞膜上形成微孔,导致钾离子和其他离子渗漏,使细胞内外渗透压改变,从而导致病原菌死亡[30-32]。伊枯草菌素是Iturin家族中最具抑菌效果的成分之一,能抑制A. alternate[37],但对C. zeae-maydis和B. cinerea的抑制作用未见报道。杆菌霉素对病原真菌孢子和菌丝的细胞壁和细胞膜均会造成严重破坏,其纯化后对孢子形成和萌发的抑制能力明显提高[38]。B. methylotrophicus B-1841能产生伊枯草菌素和类似物杆菌霉素,不仅在平板试验中对A. alternata、C. zeae-maydis和B. cinerea表现出良好的抑制作用,在玉米田间生防试验中,也具有良好的防治效果。其研究结果奠定了B. methylotrophicus作为生防菌的应用基础,为防治农作物真菌病害的研究和应用提供了基础数据,同时丰富了农作物真菌病害的生防菌资源。
| [1] | Carvalho FP. Pesticides, environment, and food safety. Food and Energy Security, 2017, 6(2): 48-60. DOI:10.1002/fes3.108 |
| [2] | Gould F, Brown ZS, Kuzma J. Wicked evolution: can we address the sociobiological dilemma of pesticide resistance?. Science, 2018, 360(6390): 728-732. DOI:10.1126/science.aar3780 |
| [3] | Hanano A, Shaban M, Almousally I. Biochemical, molecular, and transcriptional highlights of the biosynthesis of an effective biosurfactant produced by Bacillus safensis PHA3, a petroleum-dwelling bacteria. Frontiers in Microbiology, 2017, 8: 77. |
| [4] | van Loon LC, Bakker PAHM, Pieterse CMJ. Systemic resistance induced by rhizosphere bacteria. Annual Review of Phytopathology, 1998, 36: 453-483. DOI:10.1146/annurev.phyto.36.1.453 |
| [5] | Lopez-Zuniga LO, Wolters P, Davis S, Weldekidan T, Kolkman JM, Nelson R, Hooda KS, Rucker E, Thomason W, Wisser R, Balint-Kurti P. Using maize chromosome segment substitution line populations for the identification of loci associated with multiple disease resistance. G3 Genes Genome Genetics, 2019, 9(1): 189-201. |
| [6] | Thomma BPHJ. Alternaria spp.: from general saprophyte to specific parasite. Molecular Plant Pathology, 2003, 4(4): 225-236. DOI:10.1046/j.1364-3703.2003.00173.x |
| [7] | Dean R, Van Kan JAL, Pretorius ZA, Hammond-Kosack KE, Pietro AD, Spanu PD, Rudd JJ, Dickman M, Kahmann R, Ellis J, Foster GD. The top 10 fungal pathogens in molecular plant pathology. Molecular Plant Pathology, 2012, 13(4): 414-430. DOI:10.1111/j.1364-3703.2011.00783.x |
| [8] | Madhaiyan M, Poonguzhali S, Kwon SW, Sa TM. Bacillus methylotrophicus sp. nov., a methanol-utilizing, plant-growth-promoting bacterium isolated from rice rhizosphere soil. International Journal of Systematic and Evolutionary Microbiology, 2010, 60(10): 2490-2495. DOI:10.1099/ijs.0.015487-0 |
| [9] | Wang CQ, Hu XN, Liu K, Hou QH, Yang QQ, Ding YQ, Du BH. Draft genome sequence of Bacillus methylotrophicus FKM10, a plant growth-promoting rhizobacterium isolated from apple rhizosphere. Genome Announcements, 2016, 4(1): e01739-15. |
| [10] | Jeukens J, Kukavica-Ibrulj I, Freschi L, Jabaji S, Levesquea RC. Draft genome sequences of two lipopeptide-producing strains of Bacillus methylotrophicus. Genome Announcements, 2015, 3(5): e01176-15. |
| [11] | Arrebola E, Jacobs R, Korsten L. Iturin A is the principal inhibitor in the biocontrol activity of Bacillus amyloliquefaciens PPCB004 against postharvest fungal pathogens. Journal of Applied Microbiology, 2010, 108(2): 386-395. DOI:10.1111/j.1365-2672.2009.04438.x |
| [12] | Pramudito TE, Agustina D, Nguyen TKN, Suwanto A. A novel variant of narrow-spectrum antifungal bacterial lipopeptides that strongly inhibit Ganoderma boninense. Probiotics and Antimicrobial Proteins, 2018, 10(1): 110-117. DOI:10.1007/s12602-017-9334-2 |
| [13] | García-Gutiérrez L, Zeriouh H, Romero D, Cubero J, de Vicente A, Pérez-García A. The antagonistic strain Bacillus subtilis UMAF6639 also confers protection to melon plants against cucurbit powdery mildew by activation of jasmonate-and salicylic acid-dependent defence responses. Microbial Biotechnology, 2013, 6(3): 264-274. DOI:10.1111/1751-7915.12028 |
| [14] | Yu GY, Sinclair JB, Hartman GL, Bertagnolli BL. Production of iturin A by Bacillus amyloliquefaciens suppressing Rhizoctonia solani. Soil Biology and Biochemistry, 2002, 34(7): 955-963. DOI:10.1016/S0038-0717(02)00027-5 |
| [15] | Chowdhury SP, Hartmann A, Gao XW, Borriss R. Biocontrol mechanism by root-associated Bacillus amyloliquefaciens FZB42-a review. Frontiers in Microbiology, 2015, 6: 780. |
| [16] | Che JM, Liu B, Liu GH, Chen QQ, Huang DD. Induced mutation breeding of Brevibacillus brevis FJAT-0809-GLX for improving ethylparaben production and its application in the biocontrol of Lasiodiplodia theobromae. Postharvest Biology and Technology, 2018, 146: 60-67. DOI:10.1016/j.postharvbio.2018.08.011 |
| [17] | Zghal RZ, Kharrat M, Rebai A, Khedher SB, Jallouli W, Elleuch J, Ginibre C, Chandre F, Tounsi S. Optimization of bio-insecticide production by Tunisian Bacillus thuringiensis israelensis and its application in the field. Biological Control, 2018, 124: 46-52. DOI:10.1016/j.biocontrol.2018.06.002 |
| [18] |
China Microbial Strains Deposit Management Committee. China Strain List. Light Industry Press, 1983. (in Chinese) 中国微生物菌种保藏管理委员会. 中国菌种目录. 北京: 轻工业出版社, 1983. |
| [19] |
Qian YF. How to make and use wheat spores. Edible Fungi of China, 1986(6): 32.
(in Chinese) 钱玉夫. 麦粒菌种的制作和使用方法. 中国食用菌, 1986(6): 32. |
| [20] | Razafindralambo H, Paquot M, Hbid C, Jacques P, Destain J, Thonart P. Purification of antifungal lipopeptides by reversed-phase high-performance liquid chromatography. Journal of Chromatography A, 1993, 639(1): 81-85. DOI:10.1016/0021-9673(93)83091-6 |
| [21] | Chung S, Kong H, Buyer JS, Lakshman DK, Lydon J, Kim SD, Roberts DP. Isolation and partial characterization of Bacillus subtilis ME488 for suppression of soilborne pathogens of cucumber and pepper. Applied Microbiology and Biotechnology, 2008, 80(1): 115-123. DOI:10.1007/s00253-008-1520-4 |
| [22] | 史翠娟. 防治脱氧雪腐镰刀烯醇毒素污染的脂肽类抗菌物质的研究. 哈尔滨工业大学博士学位论文, 2014. |
| [23] |
Institute of Pesticide Control, Ministry of Agriculture. GB/T 17980.107-2004 Pesticide-guidelines for the field efficacy trials(Ⅱ)-part 107:fungicides against northern leaf blight & southern leaf blight spot of corn. Beijing: China Standard Press, 2004: 319-323. (in Chinese) 农业部农药检定所. GB/T 17980.107-2004农药田间药效试验准则(二)第107部分: 杀菌剂防治玉米大小斑病药效试验. 北京: 中国标准出版社, 2004: 319-323. |
| [24] | Zheng Y, Chen F, Wang M. Use of Bacillus-based biocontrol agents for promoting plant growth and health//Maheshwari DK. Bacteria in Agrobiology: Disease Management. Berlin, Heidelberg: Springer, 2013: 243-258. |
| [25] | Stackebrandt E, Goebel BM. Taxonomic note: a place for DNA-DNA reassociation and 16S rRNA sequence analysis in the present species definition in bacteriology. International Journal of Systematic Bacteriology, 1994, 44(4): 846-849. |
| [26] | Zhao JQ, Li S, Jiang TF, Liu Z, Zhang WW, Jian GL, Qi FJ. Chilling stress-the key predisposing factor for causing Alternaria alternata infection and leading to cotton (Gossypium hirsutum L.) leaf senescence. PLoS One, 2012, 7(4): e36126. DOI:10.1371/journal.pone.0036126 |
| [27] | Sun HH, Wang L, Zhang BQ, Ma JH, Hettenhausen C, Cao GY, Sun GL, Wu JQ, Wu JS. Scopoletin is a phytoalexin against Alternaria alternata in wild tobacco dependent on jasmonate signalling. Journal of Experimental Botany, 2014, 65(15): 4305-4315. DOI:10.1093/jxb/eru203 |
| [28] | Shafi J, Tian H, Ji MS. Bacillus species as versatile weapons for plant pathogens: a review. Biotechnology & Biotechnological Equipment, 2017, 31(3): 446-459. |
| [29] | Meena KR, Kanwar SS. Lipopeptides as the antifungal and antibacterial agents: applications in food safety and therapeutics. BioMed Research International, 2015, 2015: 473050. |
| [30] | Lee DW, Kim BS. Antimicrobial cyclic peptides for plant disease control. The Plant Pathology Journal, 2015, 31(1): 1-11. DOI:10.5423/PPJ.RW.08.2014.0074 |
| [31] | Cochrane SA, Vederas JC. Lipopeptides from Bacillus and Paenibacillus spp.: a gold mine of antibiotic candidates. Medicinal Research Reviews, 2016, 36(1): 4-31. DOI:10.1002/med.21321 |
| [32] | Raaijmakers JM, Bruijn ID, Nybroe O, Ongena M. Natural functions of lipopeptides from Bacillus and Pseudomonas: more than surfactants and antibiotics. FEMS Microbiology Reviews, 2010, 34(6): 1037-1062. DOI:10.1111/j.1574-6976.2010.00221.x |
| [33] | Zhang XH, Zhang DJ, Liu JL, Pan HY, Qin JC, Zhang YH. Antifungal effects of volatile organic compounds from the endophytic fungus Cryptosporiopsis ericae Cc-HG-7 isolated from Coptis chinensis Franch. Biocontrol Science and Technology, 2018, 28(5): 496-508. DOI:10.1080/09583157.2018.1460744 |
| [34] | Ahmad Z, Wu J, Chen LL, Dong WB. Isolated Bacillus subtilis strain 330-2 and its antagonistic genes identified by the removing PCR. Scientific Reports, 2017, 7(1): 1777. DOI:10.1038/s41598-017-01940-9 |
| [35] | Frikha-Gargouri O, Abdallah DB, Ghorbel I, Charfeddine I, Jlaiel L, Triki MA, Tounsi S. Lipopeptides from a novel Bacillus methylotrophicus 39b strain suppress Agrobacterium crown gall tumours on tomato plants. Pest Management Science, 2017, 73(3): 568-574. DOI:10.1002/ps.4331 |
| [36] | Elshakh ASA, Anjum SI, Qiu W, Almoneafy AA, Li W, Yang Z, Cui ZQ, Li B, Sun GC, Xie GL. Controlling and defence-related mechanisms of Bacillus strains against bacterial leaf blight of rice. Journal of Phytopathology, 2016, 164(7/8): 534-546. |
| [37] | Xu YX, Cai DB, Zhang H, Gao L, Yang Y, Gao JM, Li YY, Yang CL, Ji ZX, Yu J, Chen SW. Enhanced production of iturin A in Bacillus amyloliquefaciens by genetic engineering and medium optimization. Process Biochemistry, 2020, 90: 55-57. |
| [38] | Bais HP, Fall R, Vivanco JM. Biocontrol of Bacillus subtilis against infection of arabidopsis roots by Pseudomonas syringae is facilitated by biofilm formation and surfactin production. Plant Physiology, 2004, 134(1): 307-319. DOI:10.1104/pp.103.028712 |
 2021, Vol. 61
2021, Vol. 61