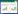中国科学院微生物研究所,中国微生物学会,中国菌物学会
文章信息
- 梅伶俐, 曾小波, 李友国, 陈大松. 2021
- Lingli Mei, Xiaobo Zeng, Youguo Li, Dasong Chen. 2021
- 华癸根瘤菌外膜孔蛋白编码基因MCHK_1326突变株的构建与共生固氮表型分析
- Construction and assignment of symbiotic phenotypes of outer membrane porin encoding gene MCHK_1326 in Mesorhizobium huakuii 7653R
- 微生物学报, 61(3): 631-643
- Acta Microbiologica Sinica, 61(3): 631-643
-
文章历史
- 收稿日期:2020-04-30
- 修回日期:2020-05-23
- 网络出版日期:2020-06-02
革兰氏阴性菌的细胞壁由外膜层和肽聚糖层组成,连同细胞膜一起,形成3个亚细胞间隔[1]:内膜(inner membrane,IM)、肽聚糖细胞壁和外膜(outer membrane,OM),其中外膜是革兰氏阴性细菌与外界环境接触的第一道屏障。外膜上镶嵌着多种蛋白质,称为外膜蛋白(outer membrane proteins,OMPs),执行着细胞内外物质运输、决定侵染性等多种功能。其中孔蛋白(porins)是一种充满水的开放通道,覆盖在细菌外膜上。细菌致病性在很大程度上取决于其表面结构[2-4]。
Henderson等[5]将孔蛋白定义为允许溶质通过或有助于包膜稳定性的外膜β-桶状蛋白,其包括OmpA家族的结构孔蛋白[6-7]、OmpW家族的小孔蛋白(8个β-折叠)[8-10]和带有18个β-折叠的扩散孔蛋白[11]。孔蛋白具有免疫调节特性,可作为PAMPs (病原体相关分子模式)被宿主细胞的模式识别受体(pattern recognition receptors,PRRs)如TLRs (Toll-like receptors)等分子识别[2, 12],诱导信号通路,进而影响免疫应答。
根瘤菌侵染宿主的方式与病原菌入侵植物高度相似[13],研究表明外膜蛋白在病原菌侵染植物的过程中起着重要作用,黄单胞菌(Xanthomonas axonopodis pv. glycines)中的OmpA突变后降低了大豆的病害严重程度,表明OmpA介导的侵染在大豆致病机理中的蛋白分泌中发挥功能[14];苜蓿根瘤菌(Sinorhizobium meliloti)中的外膜蛋白NsrA突变后导致植物的过度侵染表型,表明该基因在S. meliloti-Medicago相互作用过程中参与跨膜信号转导进而控制共生中的继发性侵染[15],因此我们推测根瘤菌的某些外膜蛋白在侵染过程中也可能起着重要作用。
根瘤菌在根瘤中与豆科植物共生,固定N2供宿主植物吸收利用[16],植物则为根瘤菌提供生长所必需的碳源[17]。华癸中慢生根瘤菌(Mesorhizobium huakuii 7653R)与紫云英有着专一共生关系[18],共生互作形成不定型根瘤。本研究室前期工作所做的M. huakuii 7653R全基因组测序[19]和共生全局转录组测序[20]为外膜蛋白基因的研究提供了便利,共生全局转录组测序的结果表明,外膜蛋白基因MCHK_1326在根瘤中的表达量相对于自生状态呈下调表达[20],表明其可能在共生固氮中发挥一定作用。本研究通过生物信息学分析MCHK_1326蛋白的结构特征和生物学功能、启动子原位表达技术检测该基因共生时空表达特征、利用Cre-loxp系统构建MCHK_1326缺失突变株,考察其早期侵染事件以及晚期共生表型,以期阐明MCHK_1326基因在共生固氮中的功能,为深入研究孔蛋白家族在共生固氮信号通路中的功能机制奠定一定基础。
1 材料和方法 1.1 材料1.1.1 植物材料: 紫云英(Astragalus sinicus):河南信阳农科院提供的“信紫一号”。
1.1.2 菌株和质粒: 本实验所用供试菌株和质粒见表 1。
| Strains and plasmids | Characteristics | Source |
| E. coli DH5α | Host of recombinant plasmids | This lab |
| E. coli S17-1 | The donor strain used for conjugation | This lab |
| Mesorhizobium huakuii 7653R | Wide type strain of Mesorhizobium huakuii, Strr | This lab |
| pMD19-T (simple) | TA cloning vector, Ampr | TaKaRa |
| pCM351 | Mutant strains construction vector, Gmr | This lab |
| pMP2463 | Green fluorescent label carrier, Gmr | This lab |
| pGD926 | Promoter expression vector, Tetr | China Agricultural University |
1.1.3 主要试剂及试剂盒: 限制性核酸内切酶为Fermentas公司产品;Taq DNA聚合酶、Ex Taq DNA聚合酶、dNTPs购自东胜科技公司;核酸电泳Marker购自诺唯赞公司;抗生素、培养基相关等分子生物学试剂均购自中国国药集团;PCR产物凝胶回收试剂盒购自上海华舜(Watson)生物公司,本研究所用PCR引物的合成和DNA的测序工作由武汉天一辉远有限公司进行。
1.1.4 引物: 本实验所用引物见表 2。
| ID of primers | Sequences of primers(5′→3′) | Characteristic |
| 1326-up-F | GGCCGCCGGACTGATTGATC | The forward primer of 1326 exchange fragment |
| 1326-up -R | CTAATTGGCTTGCATTGTTCTTTACTCCTT | The reverse primer of 1326 exchange fragment |
| 1326-down-F | TCGGCCTCGGTATGAAGTTCTAA | The forward primer of 1326 exchange fragment |
| 1326-down-R | CACCTCAACCGGATTTCGAAGA | The reverse primer of 1326 exchange fragment |
| 1326-CRmap-F | CAAGGCGCGGGCGAAATTCT | The verification forward primer of 1326 mutant |
| 1326-CRmap-R | TCGCAAGTGCCTGTCCAGTCAAC | The verification reverse primer of 1326 mutant |
| 1326-map-F | GCGAAGATGCAACGTCAAGGT | The verification forward primer of 1326 ORF |
| 1326-R | GCAGGATTTCCGGGGTCACG | The verification reverse primer of 1326 ORF |
| 1326-p-F | GGCCGCCGGACTGATTGATC | The forward promoter primer of 1326 |
| 1326-p-R | AGATTGGCTTGCATTGTTCTTTAC | The reverse promoter primer of 1326 |
1.2 培养基和培养条件
大肠杆菌(Escherichia coli)采用LB培养基,培养温度37 ℃。根瘤菌(Mesorhizobium huakuii 7653R)采用TY培养基,筛选突变株采用AMS培养基,培养温度均为28 ℃。
1.3 目标基因的生物信息学分析NCBI网站查找目的基因序列,然后通过NCBI Conserved Domain Search网页分析靶蛋白的保守结构域并预测目的蛋白的功能;通过BLASTp比对查找同源蛋白,挑选不同种属的同源蛋白序列,利用MEGA 6.0软件的邻位相接法(bootstrap N-J Tree)构建系统发育树。
1.4 启动子组织表达定位分析1.4.1 MCHK_1326启动子-LacZ融合表达载体的构建: 从NCBI数据库中获取MCHK_1326启动子序列,以7653R菌株基因组DNA为模板、1326-p-F & 1326-p-R为引物PCR扩增目的片段并连接到载体pGD926,构建融合表达载体pGD926-P1326-LacZ,经两亲本接合转移至宿主菌7653R中。
1.4.2 LacZ染色观察: pGD926中LacZ编码区5′端没有启动子,因此将目的基因启动子片段定向克隆后,经含X-gal的染色液处理后进行显色观察,进而检测启动子的表达活性,而未连入目的基因的载体因不能表达LacZ,经含X-gal的染色液处理后不会有显色反应。挑选紫云英种子,经表面消毒灭菌后,平铺于素琼脂平板中,于22 ℃光照培养2 d,将幼苗移植于无菌沙中,接菌7653R (pGD926-P1326-LacZ),分别收取接菌后5、7、15、20 dpi的植物根部组织或根瘤组织,经含X-gal的染色液处理后,在显微镜下观察并拍照。
1.5 Δ1326突变株的构建与筛选利用Cre-loxp系统构建MCHK_1326缺失突变株Δ1326[21]。以7653R菌株基因组DNA为模板,分别用1326-up-F & 1326-up-R和1326-down- F & 1326-down-R两对引物扩增目的基因上下游同源交换臂,获取目的片段。经核酸内切酶酶切后与相同内切酶线性化的pCM351载体连接并转化至E. coli DH5α,测序,比对正确后,获得含目的基因MCHK_1326上下游同源交换臂的重组质粒pCM351-1326-up+down,转化至E. coli S17-1,通过两亲本接合转移,稀释涂布于AMS+Str+Gm平板上,以引物1326-CRmap-F & 1326-CRmap-R筛选转化子,同时以野生型7653R为对照,获得突变体后,以1326-map-F & 1326-R为引物验证所获得的突变体为不含目的基因ORF的完全缺失突变株。
1.6 植物盆栽实验及共生表型测定1.6.1 植物盆栽实验: 75%乙醇清洗紫云英种子表面5 min,2% (V/V) NaClO溶液表面消毒10 min,再以无菌水清洗8–10次,彻底洗去NaClO残留,种子吸水膨胀4–8 h后铺于素琼脂平板表面,于22 ℃培养箱(16 h光照,8 h黑暗)中培养2 d催芽。将幼苗移植到无菌沙盆,每组处理4株,待长出第一片真叶后,分别接种野生型7653R和突变体7653RΔ1326,以不接菌紫云英为阴性对照,光照培养箱培养28–30 d,收取植株测定各项共生表型。
1.6.2 共生表型测定: 分别统计野生型和突变体植株的地上部分鲜重、根瘤数量、根瘤重量及固氮酶活变化。
2 结果和分析 2.1 MCHK_1326生物信息学分析2.1.1 MCHK_1326基因编码蛋白的结构域和功能分析: 生物信息学分析表明,MCHK_1326基因全长657 bp,编码218个氨基酸。其编码蛋白属于孔蛋白家族(porin family protein),包含LomR和OMP-β_barrel两个结构域(图 1)。其中LomR是外膜蛋白中的常见结构域,能帮助蛋白形成跨膜的β-桶状结构,有利于外膜稳定;OMP-β_barrel为外膜蛋白β-桶状结构域,广泛存在于外膜蛋白中;对MCHK_1326所编码蛋白进行SWISS- MODEL同源建模分析,发现其与OmpA蛋白三维结构相似,具有典型的β-桶状结构。由此推测该基因可能与OmpA蛋白有着相似的生物学功能,在病原菌与宿主细胞相互作用中有利于菌体定殖[22]。
|
| 图 1 MCHK_1326蛋白保守结构域分析以及三维结构预测 Figure 1 Putative conserved domains of MCHK_1326 protein and three-dimensional structure prediction. A: Putative conserved domains of MCHK_1326 protein; B: Three-dimensional structure prediction of MCHK_1326. |
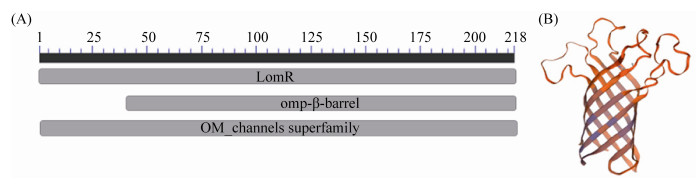
2.1.2 MCHK_1326同源性比对和系统发育分析: 经BLASTp和BLASTn检索发现,MCHK_1326的同源蛋白广泛存在于中慢生根瘤菌属中(Mesorhizobium),且相似度较高(> 75%),在其他根瘤菌属如慢生根瘤菌属(Bradyrhizobium)中相似度较低(< 45%),同时,该基因同源蛋白在Escherichia、Agrobacterium等非根瘤菌属中也存在,但相似度较低(< 42%),这些蛋白均属于孔蛋白家族或包含外膜蛋白β-桶状结构域。系统进化树结果显示(图 2),MCHK_1326在中慢生根瘤菌属中保守性较高,且与Mesorhizobium intechi (WP_143976456.1)亲缘关系最近。
|
| 图 2 MCHK_1326及其同源蛋白的系统发育分析 Figure 2 Phylogenetic analysis of the MCHK_1326 and homologous proteins. Numbers in bracket represent the sequences accession number in GenBank. The number at each branch points is the percentage supported by bootstrap. Bar, 0.2 sequence divergence. |
2.2 MCHK_1326启动子在共生条件下的时空表达特征
将启动子与报告基因LacZ融合,导入野生型M. huakuii 7653R,观察该基因在共生过程中的时空表达特征。结果显示(图 3),MCHK_1326在侵染早期(根毛卷曲)以及侵染线中正常表达(图 3-A、B),随着侵染线的延伸,直到根瘤原基中根瘤菌释放、增殖过程中都检测到持续表达,在15–20 dpi根瘤中,可见蓝色信号主要集中在侵染区,固氮区中则未见表达信号(图 3-C、D),这一结果表明MCHK_1326可能参与了根瘤菌早期侵染过程。
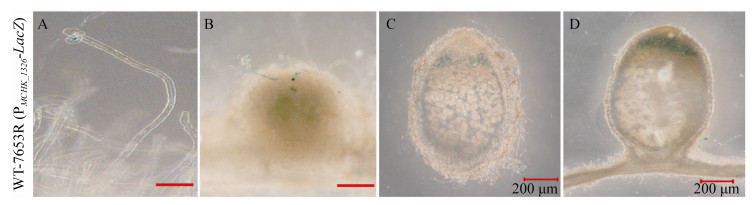
|
| 图 3 基于LacZ染色检测MCHK_1326启动子在共生过程中的时空表达 Figure 3 Detection of spatiotemporal expression of MCHK_1326 promoter during symbiosis based on LacZ staining. Roots or nodules were observed at 5 dpi (A), 7 dpi (B), 15 dpi (C), 20 dpi (D), respectively. Bars=200 μm. |
2.3 MCHK_1326突变株的构建和筛选鉴定
将构建好的同源重组载体pCM351-1326- up+down导入野生型M. huakuii 7653R,基因通过同源重组将质粒整合到染色体上,进而使目的基因缺失。由于Gm Cassette比MCHK_1326的ORF片段短123 bp,因此以引物1326-CRmap- F & 1326-CRmap-R筛选突变体时,对照组野生型7653R扩增出的产物片段大小为2167 bp,突变体的扩增产物片段为2471 bp (图 4-B),PCR扩增产物经测序比对分析表明突变体中的MCHK_1326ORF被Gm片段取代,将测序比对正确的突变体进一步划线分离,获得单菌落后,以1326-map- F & 1326-R为引物,其中1326-R位于MCHK_1326 ORF里面,因此以该对引物扩增突变体时无条带出现,进一步表明目的基因的ORF已消失(图 4-C),MCHK_1326缺失突变株(7653RΔ1326)构建成功。

|
| 图 4 基于Cre-loxp方法进行MCHK_1326基因的无痕敲除 Figure 4 The construction of MCHK_1326 deletion mutant based on Cre-loxp system. A: The diagram of strategy for MCHK_1326 knockout, DNA flanks upstream and downstream of the target gene are amplified by PCR with using primer pair up-F & up-R and down-F & down-R, and then cloned into pCM351; B: The products of PCR distinguish between mutant and wide-type by using primer CR-map-F and CR-map-R, due to the difference size of MCHK_1326 and the Gm Cassette. Lane marked "A1" to "A8" refer to 8 clones picked out a purification from a mutant identified in preliminary screening; C: The products of PCR distinguish between mutant and wide-type by using primer 1326-map-F & 1326-R. Lane marked "B1" to "B8" refer to 8 clones picked out a purification from a mutant identified in preliminary screening. |
2.4 突变体根瘤菌7653RΔ1326早期侵染事件观察
为了观察突变体在共生过程中的动态侵染特征,将含有报告基因GFP的质粒分别导入突变体和野生型中,获得GFP标记菌株,分别接种紫云英,先后选取3、6、9、13、18 dpi时间点观察和统计侵染事件。
结果如图 5所示,突变体的侵染线均能正常形成,形成根瘤原基的过程与野生型没有明显差异,表明该基因突变后,并不影响根瘤菌进入植物细胞,完成根瘤早期形成。从接菌后18 d可以看出,根瘤菌仅分布在突变体的侵染区,固氮区没有荧光,表明突变体根瘤菌在根瘤中演变为类菌体后,提前衰败,无法行使固氮功能,这与突变体在后期表现出明显的缺氮表型一致。分别选取10株长势一致的接菌后9 d的植株统计早期侵染事件(根毛弯曲、侵染线和根瘤原基)。结果表明(图 6),与野生型相比,突变体的侵染线和根瘤原基数量显著减少。根毛卷曲的数量也减少,但没有达到显著性水平,说明MCHK_1326突变后影响侵染线与根瘤原基的形成数量,但形成的侵染线表现正常。
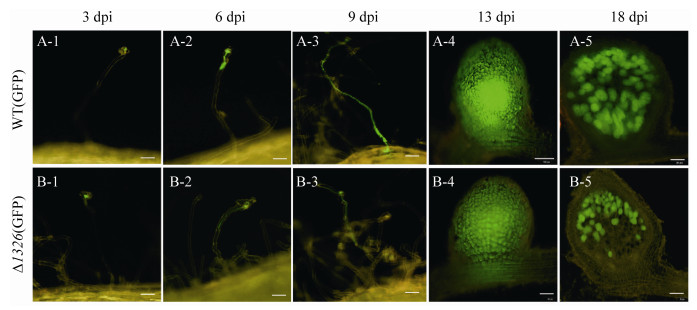
|
| 图 5 GFP标记的WT-7653R和7653RΔ1326突变体接种紫云英后的侵染观察 Figure 5 Observation of infection process of GFP-labeled 7653R-wild type and 7653RΔ1326 mutant after inoculation with Astragalus sinicus. The figure shows the different processes of infection of plants by rhizobia, including root hair curling, elongation of infection threads, entry into cortical cells, formation of nodule primordia and differentiation of bacteroids in mature nodules, bars=100 μm in A-4, bars=50 μm in the remaining figures. |

|
| 图 6 GFP标记的WT-7653R和7653RΔ1326突变体接种紫云英后的早期侵染统计分析 Figure 6 Statistical analysis of early infection processes after inoculation of GFP-labeled 7653R-wild type and 7653RΔ1326 mutant. A, B and C show the number of infected threads, the number of root hair curls, and the number of nodules about 7653R-wild type and 7653RΔ1326 mutant, respectively. *: compared with the wild-type control group, the mutant group showed significant difference at P < 0.05; **: compared with the wild-type control group, the mutant group showed significant difference at P < 0.01; the error bars represent the standard deviations of three independent experiments. |
2.5 共生固氮表型检测和分析
将筛选获得的突变株进行盆栽实验,以野生型7653R和7653RΔ1326为接种菌株,不接菌为对照,考察突变体与紫云英的共生表型。结果如图 7所示,接种突变体的植株同未接种根瘤菌的植株长势一样整体植株矮小、叶片发黄,表现出明显的缺氮状态,同时突变体植株所结根瘤较小,且为白色圆形根瘤,无固氮能力,并不同于野生型的红色长瘤。对植株地上部分鲜重、瘤数、瘤重及固氮酶活性进行生物学统计分析(图 8),结果发现相较于野生型,突变体Δ1326地上部分鲜重与固氮酶活性极显著降低,根瘤数量和根瘤重量显著降低,表明该基因突变失活后,显著影响了根瘤发育和固氮过程进而导致植株出现明显的缺陷表型(P < 0.01,n=9)。

|
| 图 7 7653RΔ1326突变体植株盆栽共生表型观察 Figure 7 Symbiotic phenotype of 7653RΔ1326 mutant tested on Astragalus sinicus. A, B, C: from left to right are plants inoculated with CK, 7653RΔ1326 and 7653R-wild type. A, B: appearances of plant growth; C: morphological observation of roots and nodules in the plant. Bars=1 mm. |
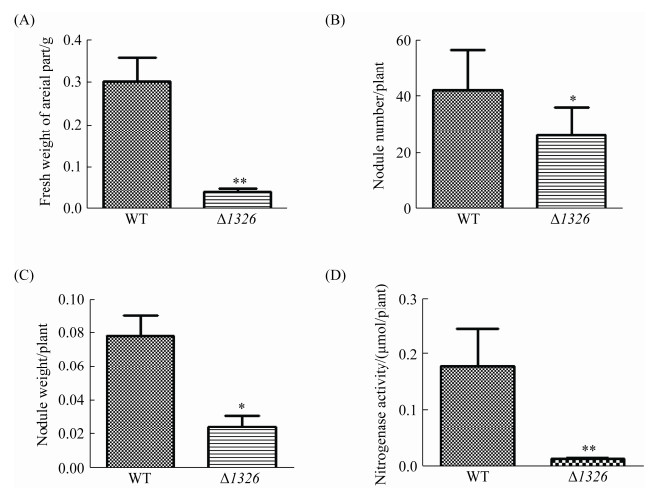
|
| 图 8 植株地上部分鲜重、瘤数、瘤重及固氮酶活的比较测定 Figure 8 Comparative quantification of the fresh weight of plant, number of nodules, fresh weight of nodules, and nitrogenase activity induced by 7653RΔ1326 mutant. A, B, C and D show the fresh weight of the aerial parts of the mutant plants, the number of nodules, fresh weight of the nodules, and the nitrogenase activity of each plant, respectively. **: compared with the wild-type control group, the mutant group showed significant difference at P < 0.01; the error bars represent the standard deviations of three independent experiments. |
2.6 额外添加无机氮源可恢复7653RΔ1326的共生固氮缺陷表型
在盆栽中额外补充无机氮源(5 mmol/L NH4NO3)以验证突变体植株所表现出的缺陷表型确实是由于目标基因的功能缺失所致,同时观察接种7653RΔ1326 28 dpi后植株的长势并拍照,以接种7653R的植株为阳性对照。结果如图 9所示,接种7653R植株的根瘤为正常红色根瘤,额外添加无机氮源后,突变株所结根瘤仍为白色无效瘤,但植物叶片恢复绿色;瘤数、瘤重及固氮酶活与野生型相比仍表现出显著性差异,但地上部分鲜重与野生型相比无显著性差异(表 3)。由此可见补充无机氮源能够弥补由于根瘤共生固氮缺陷造成的植物氮素养分不足,使得植物此时能够正常生长,进而地上部分叶片颜色以及长势恢复到野生型的状态。

|
| 图 9 补加无机氮源处理条件下突变体植株盆栽共生表型观察 Figure 9 Symbiotic phenotype changes of the mutant plants under the supplemental inorganic nitrogen source. A, B: Appearances of plant growth, 7653R-wild type (left, no nitrogen addition) and 7653RΔ1326 mutant (right, with nitrogen addition); C: Morphological observation of nodules in the plant, 7653R-wild type (left, no nitrogen addition) and 7653RΔ1326 mutant (right, with nitrogen addition). Bars=1 mm. |
| Treatments | Fresh weight of areial part/(g/plant) | Number of nodule/plant | Nodule weight/(g/plant) | Nitrogenase activity [μmol·g/(nodule·h)] |
| WT-7653R | 0.30±0.07 | 34.26±8.04 | 0.07±0.01 | 4.24±0.48 |
| Δ1326+N | 0.39a±0.12 | 9.16a±3.18 | 0.01 b±0.00 | 2.74a±0.43 |
| The data is average of three replicates. a: compared with the wild-type control group, the mutant group showed significant difference at P < 0.05; b: compared with the wild-type control group, the mutant group showed significant difference at P < 0.01. | ||||
3 讨论
革兰氏阴性细菌的外膜是细胞与外界环境进行物质交换、信号交流的界面,其外膜中的孔蛋白可诱导信号传递、核因子激活、基因启动子激活,并最终释放细胞因子。通过孔蛋白调节转录因子和亚基组成可能会影响适应性免疫机制,进而调节生物活性蛋白质或多肽的产生[12]。细菌外膜孔蛋白参与细菌侵染的致病过程与外膜内的成孔通道或各种裸露的结合位点活性有关[2]。其表面暴露区是噬菌体和大肠杆菌素的受体[23-24]。这暗示着外膜孔蛋白可能在病原菌侵染宿主初期发挥重要功能。在细菌与植物相互作用过程中,植物根的分泌物引起细菌转录组的广泛变化,如编码转运蛋白和酶相关基因的上调。同时伴随着细菌细胞壁性质的变化,细菌粘附在根表面,由菌毛、血凝素和卷曲介导完成侵染[25],如Rahnella aquatilis中的孔蛋白OmpC参与小麦根的粘附[26]。
本文以MCHK_1326为研究对象,对其进行生物信息学分析发现,MCHK_1326编码蛋白属于孔蛋白家族(porin family protein),包含有利于外膜稳定的LomR结构域。对该基因启动子在共生条件下的时空表达分析发现其在侵染早期表达;MCHK_1326基因的缺失导致紫云英的侵染线和根瘤原基数量显著减少,该基因编码蛋白与OmpA蛋白三维结构相似,有研究表明OmpA参与大肠杆菌中生物膜的形成,利于菌体附着[22];而在苯酚胁迫时,其表达量显著升高以增强E. coli的抵抗能力[27];牙龈卟啉单胞菌(Porphyromonas gingivalis)中的OmpA同源蛋白能协助宿主菌株抵抗外界对于菌株生长不利的环境,进而提高定殖效率[28]。由此推测MCHK_1326突变后降低了根瘤菌外膜稳定性,不能维持菌体结构,进而降低根瘤菌在紫云英上的定殖效率,亦或影响了根瘤菌与紫云英之间的部分信号通路,使紫云英对根瘤菌作出了错误的应答,导致紫云英的侵染线减少,进一步影响其后期固氮表型,导致植株矮小,出现提前衰老的现象。
菜豆根瘤菌Rhizobium etli CFN42中一编码与根瘤菌中铜转运有关的孔蛋白ropAe基因突变后增加了突变体的铜耐受性,导入野生型基因后,互补恢复了野生型对铜敏感性,其在铜含量充足时维持较低的基础转录水平,但在铜缺乏时上调,进一步证实了其可能参与铜的运输[29];MspA作为耻垢分枝杆菌(Mycobacterium smegmatis)中的主要孔蛋白,参与小麦的亲水性营养物质[30]的摄取,与其旁系同源物MspB、MspC和MspD等孔蛋白介导营养物质和β-内酰胺类抗生素的吸收[31-32]。其孔蛋白突变体在微量硫酸铜的条件下生长很差。本研究中当额外添加无机氮源后能恢复突变体植株因为缺氮引起的表型,地上部分长势与叶片颜色与野生型无差异,但根瘤发育仍然受阻,不能进行固氮作用。由此推测MCHK_1326在形成根瘤后的共生阶段,可能介导了根瘤菌营养物质的吸收。
本文首次研究了根瘤菌中外膜孔蛋白对M. huakuii-A. sinicus共生体系的影响。我们通过生物信息学及一系列实验结果分析,认为根瘤菌中外膜孔蛋白可能参与根瘤菌的早期侵染过程以及介导了共生过程中营养物质的吸收。但本研究中的表型变化是单一还是多种蛋白协同作用还有待深入探索。后续可利用RT-PCR的方法检测其他外膜孔蛋白在MCHK_1326突变后的表达情况或作为调控因子调控固氮相关基因,来探究孔蛋白在根瘤菌早期侵染以及结瘤过程中的作用机制,以深入了解孔蛋白在生物固氮体系中如何进行信号传导以及介导营养物质的运输。
| [1] | Silhavy TJ, Kahne D, Walker S. The bacterial cell envelope. Cold Spring Harbor Perspectives in Biology, 2010, 2(5): a000414. |
| [2] | Achouak W, Heulin T, Pagès JM. Multiple facets of bacterial porins. FEMS Microbiology Letters, 2001, 199(1): 1-7. DOI:10.1111/j.1574-6968.2001.tb10642.x |
| [3] | Galdiero M, Vitiello M, Galdiero S. Eukaryotic cell signaling and transcriptional activation induced by bacterial porins. FEMS Microbiology Letters, 2003, 226(1): 57-64. DOI:10.1016/S0378-1097(03)00562-7 |
| [4] | Naumann M, Rudel T, Meyer TF. Host cell interactions and signalling with Neisseria gonorrhoeae. Current Opinion in Microbiology, 1999, 2(1): 62-70. DOI:10.1016/S1369-5274(99)80011-3 |
| [5] | Henderson JC, Zimmerman SM, Crofts AA, Boll JM, Kuhns LG, Herrera CM, Trent MS. The power of asymmetry: architecture and assembly of the gram-negative outer membrane lipid bilayer. Annual Review of Microbiology, 2016, 70: 255-278. DOI:10.1146/annurev-micro-102215-095308 |
| [6] | Confer AW, Ayalew S. The OmpA family of proteins: roles in bacterial pathogenesis and immunity. Veterinary Microbiology, 2013, 163(3/4): 207-222. |
| [7] | Smith SG, Mahon V, Lambert MA, Fagan RP. A molecular Swiss army knife: OmpA structure, function and expression. FEMS Microbiology Letters, 2007, 273(1): 1-11. DOI:10.1111/j.1574-6968.2007.00778.x |
| [8] | Benz R, Jones MD, Younas F, Maier E, Modi N, Mentele R, Lottspeich F, Kleinekathöfer U, Smit J. OmpW of Caulobacter crescentus functions as an outer membrane channel for cations. PLoS One, 2015, 10(11): e0143557. DOI:10.1371/journal.pone.0143557 |
| [9] | Catel-Ferreira M, Marti S, Guillon L, Jara L, Coadou G, Molle V, Bouffartigues E, Bou G, Shalk I, Jouenne T, Vila-Farrés X, Dé E. The outer membrane porin OmpW of Acinetobacter baumannii is involved in iron uptake and colistin binding. FEBS Letters, 2016, 590(2): 224-231. DOI:10.1002/1873-3468.12050 |
| [10] | Hong H, Patel DR, Tamm LK, van den Berg B. The outer membrane protein OmpW forms an eight-stranded β-barrel with a hydrophobic channel. The Journal of Biological Chemistry, 2006, 281(11): 7568-7577. DOI:10.1074/jbc.M512365200 |
| [11] | Hancock REW, Brinkman FSL. Function of pseudomonas porins in uptake and efflux. Annual Review of Microbiology, 2002, 56: 17-38. DOI:10.1146/annurev.micro.56.012302.160310 |
| [12] | Galdiero S, Falanga A, Cantisani M, Tarallo R, Della Pepa ME, D'Oriano V, Galdiero M. Microbe-host interactions: structure and role of Gram-negative bacterial porins. Current Protein and Peptide Science, 2012, 13(8): 843-854. DOI:10.2174/138920312804871120 |
| [13] | Via VD, Zanetti ME, Blanco F. How legumes recognize rhizobia. Plant Signaling & Behavior, 2016, 11(2): e1120396. |
| [14] | Athinuwat D, Brooks S. The OmpA gene of Xanthomonas axonopodis pv. glycines is involved in pathogenesis of pustule disease on soybean. Current Microbiology, 2019, 76(7): 879-887. DOI:10.1007/s00284-019-01702-y |
| [15] | Garnerone AM, Sorroche F, Zou L, Mathieu-Demazière C, Tian CF, Masson-Boivin C, Batut J. NsrA, a predicted β-barrel outer membrane protein involved in plant signal perception and the control of secondary infection in Sinorhizobium meliloti. Journal of Bacteriology, 2018, 200(11): e00019-18. |
| [16] | Kouchi H, Imaizumi-Anraku H, Hayashi M, Hakoyama T, Nakagawa T, Umehara Y, Suganuma N, Kawaguchi M. How many peas in a pod? Legume genes responsible for mutualistic symbioses underground. Plant and Cell Physiology, 2010, 51(9): 1381-1397. DOI:10.1093/pcp/pcq107 |
| [17] | Desbrosses GJ, Stougaard J. Root nodulation: a paradigm for how plant-microbe symbiosis influences host developmental pathways. Cell Host & Microbe, 2011, 10(4): 348-358. |
| [18] | Hu GY, Li WW, Zhou JC. Incompatibility behavior of a symbiotic plasmid pMH7653Rb in Mesorhizobium huakuii 7653R. Science China Life Sciences, 2010, 53(6): 738-742. DOI:10.1007/s11427-010-4005-5 |
| [19] | Wang SM, Hao BH, Li JR, Gu HL, Peng JL, Xie FL, Zhao XY, Frech C, Chen NS, Ma BG, Li YG. Whole-genome sequencing of Mesorhizobium huakuii 7653R provides molecular insights into host specificity and symbiosis island dynamics. BMC Genomics, 2014, 15(1): 440. DOI:10.1186/1471-2164-15-440 |
| [20] | Peng JL, Hao BH, Liu L, Wang SM, Ma BG, Yang Y, Xie FL, Li YG. RNA-Seq and microarrays analyses reveal global differential transcriptomes of Mesorhizobium huakuii 7653R between bacteroids and free-living cells. PLoS One, 2014, 9(4): e93626. DOI:10.1371/journal.pone.0093626 |
| [21] | Marx CJ, Lidstrom ME. Broad-host-range cre-lox system for antibiotic marker recycling in gram-negative bacteria. BioTechniques, 2002, 33(5): 1062-1067. DOI:10.2144/02335rr01 |
| [22] | Orme R, Douglas CWI, Rimmer S, Webb M. Proteomic analysis of Escherichia coli biofilms reveals the overexpression of the outer membrane protein OmpA. Proteomics, 2006, 6(15): 4269-4277. DOI:10.1002/pmic.200600193 |
| [23] | Kim YC, Tarr AW, Penfold CN. Colicin import into E. coli cells: a model system for insights into the import mechanisms of bacteriocins. Biochimica et Biophysica Acta (BBA)-Molecular Cell Research, 2014, 1843(8): 1717-1731. DOI:10.1016/j.bbamcr.2014.04.010 |
| [24] | Rakhuba DV, Kolomiets EI, Dey ES, Novik GI. Bacteriophage receptors, mechanisms of phage adsorption and penetration into host cell. Polish Journal of Microbiology, 2010, 59(3): 145-155. DOI:10.33073/pjm-2010-023 |
| [25] | Pinski A, Betekhtin A, Hupert-Kocurek K, Mur L A J, Hasterok R. Defining the genetic basis of plant-endophytic bacteria interactions. International Journal of Molecular Sciences, 2019, 20(8): 1947. DOI:10.3390/ijms20081947 |
| [26] | Achouak W, Pages JM, De Mot R, Molle G, Heulin T. A major outer membrane protein of Rahnella aquatilis functions as a porin and root adhesin. Journal of Bacteriology, 1998, 180(4): 909-913. DOI:10.1128/JB.180.4.909-913.1998 |
| [27] | Zhang DF, Li H, Lin XM, Wang SY, Peng XX. Characterization of outer membrane proteins of Escherichia coli in response to phenol stress. Current Microbiology, 2011, 62(3): 777-783. DOI:10.1007/s00284-010-9786-z |
| [28] | Masuda T, Murakami Y, Noguchi T, Yoshimura F. Effects of various growth conditions in a chemostat on expression of virulence factors in Porphyromonas gingivalis. Applied and Environmental Microbiology, 2006, 72(5): 3458-3467. DOI:10.1128/AEM.72.5.3458-3467.2006 |
| [29] | González-Sánchez A, Cubillas CA, Miranda F, Dávalos A, García-de Los Santos A. The ropAe gene encodes a porin-like protein involved in copper transit in Rhizobium etli CFN42. MicrobiologyOpen, 2018, 7(3): e00573. DOI:10.1002/mbo3.573 |
| [30] | Faller M, Niederweis M, Schulz GE. The structure of a mycobacterial outer-membrane channel. Science, 2004, 303(5661): 1189-1192. DOI:10.1126/science.1094114 |
| [31] | Danilchanka O, Pavlenok M, Niederweis M. Role of porins for uptake of antibiotics by Mycobacterium smegmatis. Antimicrobial Agents and Chemotherapy, 2008, 52(9): 3127-3134. DOI:10.1128/AAC.00239-08 |
| [32] | Stephan J, Bender J, Wolschendorf F, Hoffmann C, Roth E, Mailänder C, Engelhardt H, Niederweis M. The growth rate of Mycobacterium smegmatis depends on sufficient porin-mediated influx of nutrients. Molecular Microbiology, 2005, 58(3): 714-730. DOI:10.1111/j.1365-2958.2005.04878.x |
 2021, Vol. 61
2021, Vol. 61