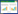中国科学院微生物研究所,中国微生物学会,中国菌物学会
文章信息
- Peipei Chen, Xiaoming Liu, Huipeng Lu, Xiaohui Zhou, Xinyu Zhang, Xiaoli Xia, Huaichang Sun. 2021
- 陈裴裴, 刘晓明, 卢会鹏, 周晓慧, 张鑫宇, 夏晓莉, 孙怀昌. 2021
- Quick purification of recombinant adeno-associated viruses with the receptor-binding capture
- 快速纯化重组腺联病毒的受体结合捕捉方法
- Acta Microbiologica Sinica, 61(3): 621-630
- 微生物学报, 61(3): 621-630
-
文章历史
- 收稿日期:2020-04-27
- 修回日期:2020-05-20
- 网络出版日期:2020-05-27
2. Jiangsu Key Laboratory for High-Tech Research and Development of Veterinary Biopharmaceuticals, Jiangsu Agri-animal Husbandry Vocational College, Taizhou 225300, Jiangsu Province, China
2. 江苏农牧科技职业技术学院, 江苏省兽用生物制品高技术研发重点实验室, 江苏 泰州 225300
Recombinant adeno-associated viruses (rAAVs) have broad applications in gene therapy, vaccine development and biomedical research[1-2]. However, the application of rAAV technology is hindered by the scale production and downstream processing[3]. The traditional method for rAAV purification is cesium chloride gradient centrifugation[4]. Although both yield and purity are generally high, this technique has the drawbacks of limited cell lysate loading capacity and prolonged exposure to harmful cesium chloride[3]. Iodixanol is an alternative gradient medium for rAAV purification with the advantage of low toxicity[5]. However, iodixanol gradient centrifugation has the same drawback of limited cell lysate loading capacity as cesium chloride gradient centrifugation. Ion-exchange and affinity chromatography have been developed for scale rAAV purification with enhanced yield and purity[6]. However, chromatographic techniques can not separate the vector-related impurities from fully functional vector particles[3]. Therefore, there is the necessity to develop cost-effective methods for scale purification of rAAV vectors.
Receptor-binding capture and magnetic sequestration has been developed to concentrate noroviruses from large volume samples[7-9]. Although this technique is simple and highly sensitive, it is not suitable for virus purification at scales. Elastin-like polypeptides (ELPs) are synthetic polymers of pentapeptide repeats (e.g., Val-Pro-Gly-Xaa-Gly), where Xaa can be any amino acid except proline. ELPs have an unequal property, reversible phase transition, allowing the change from soluble monomers to insoluble aggregates at specific transition temperatures[10]. Therefore, ELP fusion proteins can be purified simply by inverse transition cycling (ITC) with broad applications such as recombinant protein purification, drug delivery and vaccine development[11-12]. More recently, a simple method for quick purification of adenoviruses has been developed by combining the receptor-binding capture with ELP-mediated precipitation[13].
AAV receptor (AAVR) or KIAA0319L is a type-Ⅰ membrane protein with five Ig-like polycystic kidney disease (PKD) domains[14]. Although AAVR is an N-linked glycoprotein, the glycosylation is not a strict requirement for AAV binding. AAV2 or AAV5 interacts predominantly with the PKD2 or PKD1 domain of AAVR. Other AAV serotypes require a combination of PKD1 and PKD2 domains for optimal binding[15]. In this study, a novel receptor-binding capture method was established for quick purification of rAAV from different cell types.
1 Materials and methods 1.1 Bacterial strains, cell lines and vectorsE. coli strains DH5α and BL21(DE3) (Stratagene, USA) were used as the host strains for gene cloning or recombinant protein expression. E. coli strain HH10Bac (Invitrogen, USA) was used for Bacmid preparation in insect cells. Sf9 insect cells (Invitrogen, USA) were cultured in serum-free SF-900 Ⅱ medium (Gibco, USA) at 27 ℃ and agitated at 110 r/min. Human kidney AAV-293 cells (ATCC, USA) were cultured in Dulbecco's modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum (FBS). Baculovirus vectors pFastBacTM 1 and pFastBacTM Dual (Invitrogen, USA) were used for generation of recombinant baculoviruses in insect cells. AAV vectors pAAV-RC, pAAV-CMV and pHelper (Stratagene, USA) were used for generation of rAAV in mammalian cells.
1.2 Construction of prokaryotic expression vectorELP fusion expression vector pEI/GFP was kindly provided by Professor Wood at Princeton University, USA. The modified vector pET-ELP was constructed as previously described[16]. Briefly, the coding sequence for ELP was excised from pEI/GFP vector with Nde I and Sac I digestion, and cloned into pET-30a (+) vector (Invitrogen, USA) after digestion with the same restriction enzymes. The coding sequences for PKD1 and PKD2 domains of AAVR (GenBank accession: NM_024874) were adapted to E. coli codon usage using JAVA Codon Adaption Tool[17]. The synthetic sequence was cloned into pET-ELP vector with Hind Ⅲ and Xho I digestion. The recombinant vector pELP-PKD was identified by restriction digestion and sequence analysis.
1.3 ELP-PKD fusion protein expressionThe pELP-PKD vector was transformed into BL21(DE3) E. coli. After growing for 5 h at 37 ℃ in 2× YT medium (10 g yeast extract, 16 g tryptone, 5 g NaCl/L) containing 50 µg/mL kanamycin, the expression of ELP-PKD fusion protein was induced for 18 h at 20 ℃ with 0.2 mmol/L IPTG (Isopropyl β-D-thiogalactoside). After centrifugation for 10 min at 500 g, cells were washed and disrupted in PBS (pH 7.2) by sonication treatment. Following centrifugation for 20 min at 12000×g at 4 ℃, the cell lysate was used for ELP-PKD protein purification.
1.4 ELP-PKD fusion protein purificationELP-PKD fusion protein was purified by ITC as previously described[18]. Briefly, the centrifuged cell lysate was mixed with an equal volume of 6 mol/L NaCl and incubated for 10 min at different temperatures. After centrifugation for 5 min at room temperature, the protein pellet was dissolved in cold PBS, centrifuged for 10 min at 4 ℃, and analyzed by 12% SDS-PAGE for protein purity. Then, the cell lysate was incubated for 10 min with different concentrations of NaCl at the determined temperature, and centrifuged for 5 min at room temperature. The protein pellet was dissolved in cold PBS and analyzed as described. Finally, 200 mL of the cell lysate was incubated for 10 min at the determined temperature with the determined concentration of NaCl. After centrifugation for 5 min at room temperature, the protein pellet was washed, dissolved in cold PBS and centrifuged for 10 min at 12000×g before SDS-PAGE analysis.
1.5 Generation of rAAV-GFP in Sf9 insect cellsRecombinant baculovirus (rBac) rBac-RC and rBac-GFP were prepared as previously described[19]. Briefly, the rep and cap genes of AAV2 were codon-optimized for optimal expression in insect cells. The synthetic genes were cloned into pFasBactTM Dual vector with Bgl Ⅱ and Xba I or Sma I and Sph I digestion. The recombinant vector was called pBac-RC. The inverse terminal repeats (ITRs) of AAV2 were excised from pAAV-CMV vector and cloned into pFastBacTM 1 vector with BamH I and Hind Ⅲ digestion. The resultant vector was called pBac-ITR. Green fluorescent protein (GFP) gene was amplified from pEGFP-N1 vector (Clontech, USA) using the sense primer (5′-ATCTCGAGATGGTGAGCAAGGGCGAG-3′) and the antisense primer (5′-CGAAGCTTTTACT TGTACAGCTCGTCC-3′). The PCR product was cloned into pBac-ITR vector with EcoR I and BamH I digestion. The recombinant vector was called pBac-GFP. Both pBac-RC and pBac-GFP vectors were transformed individually into HH10Bac E. coli and the purified recombinant bacmids were transfected individually into Sf9 insect cells using LipofectamineTM 3000 transfecting agent (Invitrogen, USA) by following the manufacturer's instruction. The rBac-RC and rBac-GFP were harvested at 72 h post transfection. Sf9 cells were co-infected with rBac-RC and rBac-GFP (MOI of 1). The resultant rAAV-GFP was harvested at 72 h post infection (hpi), titrated on AAV-293 cells and expressed as fluorescence formation units (FFU)/mL as previously described[20].
1.6 Generation of AAV-GFP in AAV-293 cellsGFP gene was amplified from pEGFP-N1 vector using the sense primer (5′-GCCACCATGGT GAGAGAGAG-3′) and the antisense primer (5′-TC ACTACTTATACAGCTCGTCCA-3′). The PCR product was cloned into pAAV-CMV vector with EcoR I and BamH I digestion. The recombinant pAAV-GFP vector, together with commercially available pAAV-RC and pHelper vectors, was transfected into AAV-293 cells using LipofectamineTM 3000 transfecting agent as described. The resultant rAAV-GFP was harvested 72 h post transfection and titrated on AAV-293 cells as described.
1.7 Virus binding assayThe insect cell-derived rAAV-GFP (107 FFU/mL) was mixed with an equal volume (100 µL) of ELP-PKD fusion protein or ELP control protein (200 µg/mL). After incubation for 60 min at 4 ℃, 200 µL of 4 mol/L NaCl was added and incubated for 10 min at 26 ℃. After centrifugation for 5 min at 12000×g at room temperature, the protein-bound rAAV-GFP was washed three times with 2 mol/L NaCl and suspended in 100 µL of PBS. The viral DNA was extracted using Viral DNA Extraction Kit (OMEGA, China). PCR (50 µL) was performed using GFP-specific primer pair as described.
1.8 rAAV-GFP purificationFirstly, ELP-PKD fusion protein was diluted to 50, 100, 150, 200, 250 µg/mL with PBS (pH 7) and mixed with an equal volume (100 µL) of the vector-transfected insect cell lysate. After incubation for 60 min at 4 ℃, the protein-bound rAAV-GFP was collected by ITC and titrated on AAV-293 cells as described. Secondly, ELP-PKD protein was diluted to the determined concentration with PBS (pH 7) and incubated with the insect cell lysate for 5, 15, 3, 45 or 60 min at 4 ℃ before rAAV-GFP recovery and titration. Thirdly, ELP-PKD protein was diluted to the determined concentration with PBS (pH 7) and incubated with the insect cell lysate for the determined time at 4, 20, 25, 30 or 37 ℃ before rAAV-GFP recovery and titration. Fourthly, ELP-PKD protein was diluted to the determined concentration with PBS (pH 4, 5, 6, 7 and 8) and incubated with the insect cell lysate for the determined time at the determined temperature before rAAV-GFP recovery and titration. Finally, rAAV-GFP was purified from 20 mL of the vector-transfected insect or AAV-293 cell lysate under the optimized conditions.
1.9 AAV-GFP elutionFirstly, ELP-PKD-bound rAAV-GFP was eluted from ELP-PKD protein for 30 min at 4 ℃ with PBS of pH 3, 4, 5, 8, 9 and 10. After immediate neutralization to pH 7, ELP-PKD protein was removed by ITC and the recovered rAAV-GFP was titrated on AAV-293 cells as described. Secondly, the protein-bound rAAV-GFP was eluted for 5, 15, 30, 45 or 60 min at the determined pH at 4 ℃ before rAAV-GFP recovery and titration. Thirdly, the protein-bound rAAV-GFP was eluted for the determined time at the determined pH at 4, 20, 25, 30 or 37 ℃ before rAAV-GFP recovery and titration. Finally, rAAV-GFP was eluted from ELP-PKD protein under the optimized conditions.
1.10 AAV-GFP characterizationTo analyze the morphology of rAAV-GFP, the purified virus was absorbed onto copper grids (400-mesh) for 2.5 min at room temperature, stained with 3% phosphotungstic acid for 2.5 min, and observed under transmission electron microscope (Philips, Tecnai 12, Germany) at an acceleration voltage of 75 kV. To analyze the infectivity of rAAV-GFP, PK-15 cells were infected with the purified rAAV-GFP (MOI=1) and observed for GFP-positive cells under fluorescent microscope 48 h post transduction. To analyze the structural proteins of rAAV-GFP, the purified virus was separated by 12% SDS-PAGE and transferred onto nitrocellulose membrane. After blocking for 1 h at 37 ℃ with 5% defatted milk powder in PBS, Western blotting was performed using mouse anti-AAV Cap serum (1:2000) as the first antibody and DylightTM 800-labeled goat anti-mouse IgG (1:10000; KPL, USA) as the second antibody. The hybridization signal was scanned on Odyssey Infrared Imaging System (LI-COR, USA) at 800 nm.
2 Results 2.1 ELP-PKD protein expressionThe recombinant vector pELP-PKD (Figure 1-A) was transformed into BL21(DE3) E. coli for ELP-PKD fusion protein expression. For ITC to be useful, a large enough fraction of ELP-PKD fusion protein must be expressed as a soluble protein. Therefore, the expression of ELP-PKD fusion protein was slowly induced at 20 ℃ with 0.2 mmol/L IPTG. SDS-PAGE analysis showed that, compared to the non-induced cell control, an expected 67-kDa extra protein was present in the cell extract of pELP-PKD recombinant E. coli, more than 90% of which was present in the soluble fraction of centrifuged cell extract (Figure 1-B).
|
| Figure 1 SDS-PGE analysis of ELP-PKD fusion protein expression and purification. A: The schematic structure of pELP-PKD vector. The PKD1 and PKD2 gene segments of AAVR were fused in frame with ELP coding sequence and the expression of ELP-PKD fusion gene is under the control of T7 promoter (PT7). B: SDS-PAGE analysis of ELP-PKD fusion protein expression. The un-induced cell extract (1), IPTG-induced cell extract (2), supernatant (3) and pellet (4) of centrifuged cell extract are shown. C: SDS-PAGE analysis of purified ELP-PKD fusion protein. D: Western blotting of ELP-PKD fusion protein. |
2.2 ELP-PKD protein purification
To optimize the ITC conditions for ELP-PKD protein purification, the bacterial extract was incubated first at different temperatures in 3 mol/L NaCl solution, and then at the determined temperature in different concentrations of NaCl solution. SDS-PAGE analysis showed that the optimal ITC conditions for ELP-PKD protein purification was incubation for 10 min at 26 ℃ in 2 mol/L NaCl solution. After once cycle of ITC, ELP-PKD fusion protein was purified to 92% purity (Figure 1-C), which reacted positively with the mAb specific for His-tag at its C-terminus (Figure 1-D).
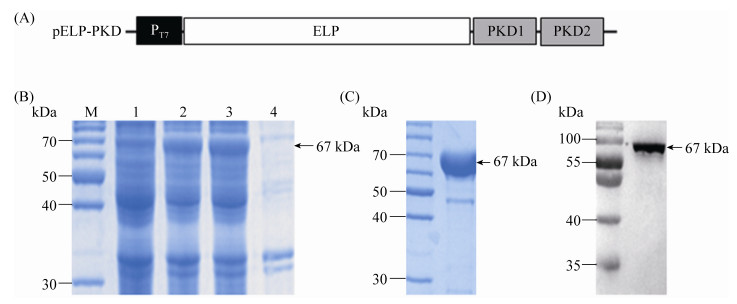
2.3 Binding of ELP-PKD protein to rAAV-GFPThe rAAV-GFP was incubated with ELP-PKD protein or ELP control protein and then precipitated by one cycle of ITC. The protein-bound rAAV-GFP was collected for DNA extraction and PCR analysis using GFP-specific primers. An expected 720-bp product was amplified from ELP-PKD-incubated rAAV-GFP, but not from the control protein-incubated rAAV-GFP (Figure 2).
|
| Figure 2 The viral DNA was extracted from rAAV- GFP (1), ELP-PKD-bound rAAV-GFP (2) or ELP control protein-incubated rAAV-GFP (3), and amplified by PCR using gfp-specific primers with negative control (4). |
2.4 Purification of rAAV-GFP
To optimize the conditions for rAAV-GFP purification, rBac-infected insect cell extract was incubated for different times at different temperatures at different pH values with different concentrations of ELP-PKD protein. The protein-bound rAAV-GFP was precipitated by one cycle of ITC. Virus titration showed that the binding of ELP-PKD protein to rAAV-GFP was protein concentration-, pH-, temperature- and time-dependent (Figure 3). Finally, rAAV-GFP was purified from 20 mL of cell extracts under the optimized conditions (pH 7.0/4 ℃/15 min/100 µg of ELP-PKD protein/mL) with 58% recovery from insect cells or 56% recovery from AAV-293 cells.

|
| Figure 3 Optimization of the conditions for rAAV-GFP purification by ELP-PKD-binding capture. The rAAV was incubated with different concentrations ELP-PKD protein (A) for different times (B) at different temperatures (C) and at different pH 7 values (D). The protein-bound rAAV was precipitated by ITC and titrated directly on AAV-293 cells. |
The rAAV was incubated with ELP-PKD fusion protein or ELP control protein and the protein-bound rAAV was precipitated by ITC. The viral DNA was extracted from rAAV (1), ELP-PKD-bound rAAV (2) or ELP-bound rAAV (3) and detected by PCR using gfp-specific primers with PBS as the negative control (4).
2.5 Elution of rAAV-GFPTo optimize the conditions for rAAV-GFP elution, ELP-PKD protein-bound rAAV-GFP was incubated for different times at different temperatures at different pH values, and the eluted rAAV-GFP was recovered after an additional round of ITC. Virus titration showed that elution of rAAV-GFP was pH-, temperature-dependent and slightly time-dependent (Figure 4). Finally, rAAV-GFP was eluted under the optimized conditions (pH 3/25 ℃/30 min) with final recovery of 47% from insect cells or 44% from AAV-293 cells.

|
| Figure 4 Optimization of the conditions for rAAV-GFP elution. The protein-bound rAAV was eluted at different pH values (A) for different times (B) at different temperatures (C). ELP-PKD protein was removed by ITC and the recovered rAAV-GFP was titrated on AAV-293 cells. |
2.6 rAAV-GFP characterization
Electron microscopy showed that the purified rAAV-GFP had the typical AAV morphology with a diameter of about 22 nm (Figure 5-A). Cell infection assay showed that the purified rAAV-GFP was highly infectious for AAV-293 cells (Figure 5-B). Western blotting analysis showed that rAAV-GFP before and after purification was recognized by anti-AAV antibody (Figure 5-C).
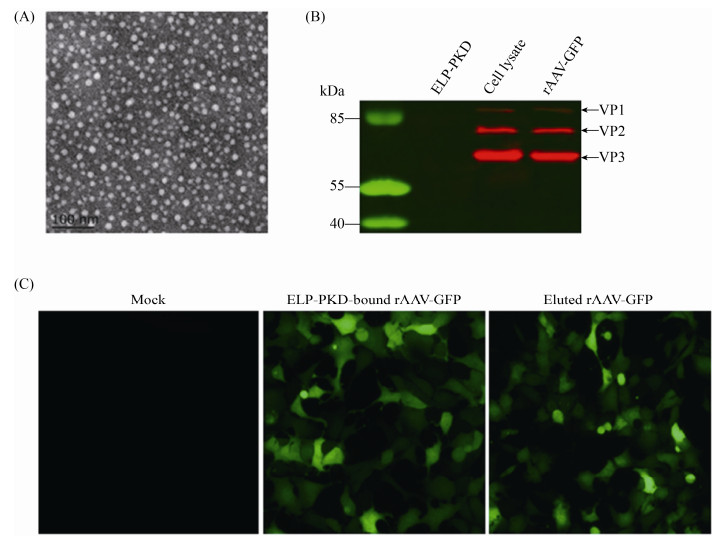
|
| Figure 5 Identification of rAAV-GFP purified with ELP-PKD-binding capture. A: Electron microscopy of virus particles; B: Western blotting of viral structural proteins; C: Fluorescent analysis of virus infectivity in AAV-293 cells. |
3 Discussion
The current methods for rAAV purification are time-consuming, expensive and/or scale-limited. Although AAVR is a 150-kDa glycoprotein, the glycosylation is not strictly required for AAV binding or functional transduction[15]. PKD domains of AAVR with different affinities for AAV binding have been expressed in E. coli as maltose or glutathione S-transferase (GST) fusion proteins and purified by affinity chromatography[14-15]. This warranted us to develop a novel method for rAAV purification by combining PKD-binding capture with ELP-mediated precipitation. To this end, both PKD1 and PKD2 domains of AAVR were expressed as an ELP fusion protein and purified simply by ITC. Under the optimized conditions, a sufficient amount of ELP-PKD fusion protein was expressed as a soluble protein and purified to high purity (> 90%) after one cycle of ITC. Although the ELP used in this study is a 55-kDa large protein, the ELP-PKD fusion protein remained the specific affinity for rAAV binding, confirming that ELPs do not interfere with recombinant protein folding[21].
The optimization experiments showed that the binding of ELP-PKD protein to rAAV-GFP was pH-, temperature-, time- and protein concentration-dependent. Under the optimized conditions, the rAAV was efficiently captured from the vector-transfected insect cell extract after one cycle of ITC. The purification process could be accomplished within 30 min, confirming the simplicity and speediness of ELP-PKD-binding capture for rAAV purification.
Although the main principle of ELP- PKD-binding capture for rAAV purification is similar to that of ELP-CAR-binding capture for rAd purification[13], the two purification methods have the following differences. Although the recovery of ELP-PKD-bound rAAV-GFP was lower than that of ELP-CAR-bound rAd-GFP, the final recovery of rAAV-GFP was higher than that of rAd-GFP after elution. This indicates that elution of rAAV from ELP-PKD protein was more easier than rAd from ELP-CAR protein, which could be explained by different binding affinities of the two virus receptors[13]. In addition, the cell transduction efficiency of ELP-CAR-bound rAd-GFP is significantly lower than that of eluted rAd-GFP. In contrast, the cell transduction efficiency of ELP-PKD-bound rAAV-GFP was similar to or even higher than the eluted rAAV-GFP, which could be explained by the finding that ELPs can enhance AAV-mediated gene delivery into mammalian cells[22]. These data suggest that ELP-PKD-bound rAAV can be used directly for gene therapy and vaccine development.
Recombinant AAVs can be prepared in mammalian and insect cells with significant difference in yield. To evaluate the feasibility of ELP-PKD-binding capture for rAAV purification from different cell types, two versions of rAAV-GFP were generated in insect and AAV-293 cells. After ELP-PKD-binding capture, the recovery rate (58%) of ELP-PKD-bound rAAV-GFP from insect cells was similar to that (56%) from AAV-293 cells. After elution, the final recovery rates of rAAV-GFP from the two cell types were also similar (47% vs. 44%). Such rAAV recovery rates were comparable to that with iodixanol gradient centrifugation[3] or with the combination of affinity and ion exchange chromatography[23].
In conclusion, the PKD1 and PKD2 domains of AAVR were expressed as an ELP fusion protein and purified to high purity simply by ITC. The ELP-PKD-binding capture method developed is feasible for quick purification of rAAVs from different cell types.
| [1] | Berns KI, Muzyczka N. AAV: an overview of unanswered questions. Human Gene Therapy, 2017, 28(4): 308-313. DOI:10.1089/hum.2017.048 |
| [2] | Nieto K, Salvetti A. AAV vectors vaccines against infectious diseases. Frontiers in Immunology, 2014, 5: 5. |
| [3] | Lock M, Alvira M, Vandenberghe LH, Samanta A, Toelen J, Debyser Z, Wilson JM. Rapid, simple, and versatile manufacturing of recombinant adeno-associated viral vectors at scale. Human Gene Therapy, 2010, 21(10): 1259-1271. DOI:10.1089/hum.2010.055 |
| [4] | Zolotukhin S, Byrne BJ, Mason E, Zolotukhin I, Potter M, Chesnut K, Summerford C, Samulski RJ, Muzyczka N. Recombinant adeno-associated virus purification using novel methods improves infectious titer and yield. Gene Therapy, 1999, 6(6): 973-985. DOI:10.1038/sj.gt.3300938 |
| [5] | Hermens WT, Brake O, Dijkhuizen PA, Sonnemans MA, Grimm D, Kleinschmidt JA, Verhaagen J. Purification of recombinant adeno-associated virus by iodixanol gradient ultracentrifugation allows rapid and reproducible preparation of vector stocks for gene transfer in the nervous system. Human Gene Therapy, 1999, 10(11): 1885-1891. DOI:10.1089/10430349950017563 |
| [6] | Kaludov N, Handelman B, Chiorini JA. Scalable purification of adeno-associated virus type 2, 4, or 5 using ion-exchange chromatography. Human Gene Therapy, 2002, 13(10): 1235-1243. DOI:10.1089/104303402320139014 |
| [7] | Pan LW, Zhang QG, Li X, Tian P. Detection of human norovirus in cherry tomatoes, blueberries and vegetable salad by using a receptor-binding capture and magnetic sequestration (RBCMS) method. Food Microbiology, 2012, 30(2): 420-426. DOI:10.1016/j.fm.2011.12.026 |
| [8] | Tian P, Yang D, Pan LW, Mandrell R. Application of a receptor-binding capture quantitative reverse transcription-PCR assay to concentrate human norovirus from sewage and to study the distribution and stability of the virus. Applied and Environmental Microbiology, 2012, 78(2): 429-436. DOI:10.1128/AEM.06875-11 |
| [9] | Tong HI, Connell C, Boehm AB, Lu YN. Effective detection of human noroviruses in Hawaiian waters using enhanced RT-PCR methods. Water Research, 2011, 45(18): 5837-5848. DOI:10.1016/j.watres.2011.08.030 |
| [10] | Meyer DE, Chilkoti A. Purification of recombinant proteins by fusion with thermally-responsive polypeptides. Nature Biotechnology, 1999, 17(11): 1112-1115. DOI:10.1038/15100 |
| [11] | Hassouneh W, Christensen T, Chilkoti A. Elastin-like polypeptides as a purification tag for recombinant proteins. Current Protocols in Protein Science, 2010, 61(1): Unit-6.11. |
| [12] | Yeboah A, Cohen RI, Rabolli C, Yarmush ML, Berthiaume F. Elastin-like polypeptides: a strategic fusion partner for biologics. Biotechnology and Bioengineering, 2016, 113(8): 1617-1627. DOI:10.1002/bit.25998 |
| [13] | Wu Q, Liu WJ, Xu B, Zhang XY, Xia XL, Sun HC. Single-step concentration and purification of adenoviruses by coxsackievirus-adenovirus receptor-binding capture and elastin-like polypeptide-mediated precipitation. Archives of Virology, 2016, 161(2): 279-287. DOI:10.1007/s00705-015-2664-z |
| [14] | Pillay S, Meyer NL, Puschnik AS, Davulcu O, Diep J, Ishikawa Y, Jae LT, Wosen JE, Nagamine CM, Chapman MS, Carette JE. An essential receptor for adeno-associated virus infection. Nature, 2016, 530(7588): 108-112. DOI:10.1038/nature16465 |
| [15] | Pillay S, Zou W, Cheng F, Puschnik AS, Meyer NL, Ganaie SS, Deng XF, Wosen JE, Davulcu O, Yan ZY, Engelhardt JF, Brown KE, Chapman MS, Qiu JM, Carette JE. Adeno-associated Virus (AAV) serotypes have distinctive interactions with domains of the cellular AAV Receptor. Journal of Virology, 2017, 91(18): e00391-17. |
| [16] | Zong Y, Tan X, Xiao JJ, Zhang XY, Xia XL, Sun HC. Half-life extension of porcine interferon-α by fusion to the IgG-binding domain of streptococcal G protein. Protein Expression and Purification, 2019, 153: 53-58. DOI:10.1016/j.pep.2018.08.012 |
| [17] | Grote A, Hiller K, Scheer M, Münch R, Nörtemann B, Hempel DC, Jahn D. JCat: a novel tool to adapt codon usage of a target gene to its potential expression host. Nucleic Acids Research, 2005, 33(S2): W526-W531. |
| [18] | Liu WJ, Wu Q, Xu B, Zhang XY, Xia XL, Sun HC. Single-step purification of recombinant proteins using elastin-like peptide-mediated inverse transition cycling and self-processing module from Neisseria meningitides FrpC. Protein Expression and Purification, 2014, 98: 18-24. DOI:10.1016/j.pep.2014.02.016 |
| [19] | Urabe M, Ding CT, Kotin RM. Insect cells as a factory to produce adeno-associated virus type 2 vectors. Human Gene Therapy, 2002, 13(16): 1935-1943. DOI:10.1089/10430340260355347 |
| [20] | Koeberl DD, Alexander IE, Halbert CL, Russell DW, Miller AD. Persistent expression of human clotting factor IX from mouse liver after intravenous injection of adeno-associated virus vectors. Proceedings of the National Academy of Sciences of the United States of America, 1997, 94(4): 1426-1431. DOI:10.1073/pnas.94.4.1426 |
| [21] | Floss DM, Sack M, Stadlmann J, Rademacher T, Scheller J, Stöger E, Fischer R, Conrad U. Biochemical and functional characterization of anti-HIV antibody-ELP fusion proteins from transgenic plants. Plant Biotechnology Journal, 2008, 6(4): 379-391. DOI:10.1111/j.1467-7652.2008.00326.x |
| [22] | Kim JS, Chu HS, Park KI, Won JI, Jang JH. Elastin-like polypeptide matrices for enhancing adeno-associated virus-mediated gene delivery to human neural stem cells. Gene Therapy, 2012, 19(3): 329-337. DOI:10.1038/gt.2011.84 |
| [23] | Nass SA, Mattingly MA, Woodcock DA, Burnham BL, Ardinger JA, Osmond SE, Frederick AM, Scaria A, Cheng SH, O'Riordan CR. Universal method for the purification of recombinant AAV vectors of differing serotypes. Molecular Therapy-Methods & Clinical Development, 2018, 9: 33-46. |
 2021, Vol. 61
2021, Vol. 61