中国科学院微生物研究所,中国微生物学会,中国菌物学会
文章信息
- 张郁敏, 马家乐, 赵焱, 刘广锦. 2021
- Yumin Zhang, Jiale Ma, Yan Zhao, Guangjin Liu. 2021
- 链球菌引发宿主中毒性休克综合症相关机制的研究进展
- Host toxic shock syndrome induced by Streptococcus
- 微生物学报, 61(3): 501-511
- Acta Microbiologica Sinica, 61(3): 501-511
-
文章历史
- 收稿日期:2020-04-13
- 修回日期:2020-05-20
- 网络出版日期:2020-08-10
2. 中国农业科学院兰州兽医研究所, 家畜疫病病原生物学国家重点实验室, 甘肃 兰州 730030
2. State Key Laboratory of Veterinary Etiological Biology, Lanzhou Veterinary Research Institute, Chinese Academy of Agricultural Sciences, Lanzhou 730030, Gansu Province, China
中毒性休克综合症(toxic shock syndrome,TSS)首先是在金黄色葡萄球菌上发现,在感染宿主时其释放的毒素可引起以发热、休克和多器官或系统病变为特征的急性炎症[1]。80年代,Willoughby等[2]证实链球菌上存在TSS的特征,并将其称为链球菌性中毒性休克综合症(streptococcal toxic shock syndrome,STSS)。近40多年来,关于链球菌感染引发STSS的病例报告中,多为A族链球菌(group A Streptococcus,GAS)感染引起,少数为B族链球菌(group A Streptococcus,GBS)和猪链球菌感染病例[3-5]。与金黄色葡萄球菌引起的TSS相似,STSS可由链球菌的超抗原引发[6],临床上通常使用静脉注射免疫球蛋白阻断或失活超抗原进行治疗[7]。近年来也发现了不依赖超抗原的STSS病例,部分发病机制已阐明,例如链球菌分泌的穿孔毒素可激活细胞内炎性小体,并导致细胞焦亡,引发细胞因子风暴(cytokine storm)[8];GAS链球菌M蛋白介导的中性粒细胞的活化[9]。这类STSS与超抗原引发的STSS相比,难以判断病因,治疗措施有限,致死率高。
无论是何种原因引起的STSS,其共同特征是系统性的细胞因子风暴,引发过度炎症[9]。细胞因子风暴中的大量的细胞因子是过度炎症的重要参与者。在链球菌入侵时,免疫细胞会产生多种的细胞因子,包括促炎细胞因子和抑炎细胞因子,两者分别在炎症早期和炎症后期发挥主导作用,决定着炎症的发展。但一旦突破机体两者自身的平衡,短时间内产生过量的促炎细胞因子,导致细胞因子风暴[10-11]。若不及时控制,可导致自身组织的损伤,脓毒症和休克甚至死亡,这是STSS的典型症状[6]。本文主要对不依赖超抗原引发STSS的机制研究进行介绍,对STSS过程中宿主细胞对链球菌的识别、促炎细胞因子的释放、炎性小体和细胞焦亡的发生、细胞因子风暴及炎症信号通路等方面的研究进展进行综述,为进一步研究和防治STSS提供思路。
1 宿主对链球菌的识别天然免疫系统能够借助病原模式识别受体(pattem recognition receptors,PRRs)对病原微生物进行快速识别。PRRs是宿主细胞表达的蛋白质受体,能够识别外源性的病原相关分子模式(pathogen associated molecular patterms,PAMPs)和感染病原后组织损伤产生的危险信号分子模式(danger associated molecular patterns,DAMPs)[12]。DAMP与PAMP交错共享着许多下游的信号通路,导向免疫或炎症反应[13]。在PRRs中,Toll样受体(TLR)研究最多,在识别微生物亚结构中必不可少,当前有13种已知的哺乳动物TLR,其中10种是在人类和小鼠中发现[12, 14]。
天然免疫的前线效应细胞主要是巨噬细胞、树突状细胞及中性粒细胞[14]。其中巨噬细胞和树突状细胞(DC)是天然免疫系统响应链球菌入侵的中心协调者。如图 1所示,在链球菌感染时,巨噬细胞或树突状细胞的PRRs,通过与链球菌的表面脂蛋白酸(LTA,由TLR2识别)、肽聚糖(PGN,由TLR2识别)、溶血素(SLO/PLY,由TLR4识别)等成分的识别,通过依赖性MyD88信号通路,活化NF-κB,从而合成并分泌细胞因子[15-16]。链球菌被巨噬细胞或DC细胞内吞后,细胞内部会对链球菌的核酸成分(DNA和RNA)进行识别并进行信号传递,包括:(1) IFN合成途径:DNA被cGAS识别并激活cGAS-STING通路产生IFN-β[17-18];(2) 依赖性MyD88信号通路途径:例如,未甲基化CpG DNA被TLR9识别[19],链球菌RNA被人巨噬细胞的TLR8或小鼠同源物质TLR13识别[20-22],被树突状细胞的TLR 7识别[23-24],依赖MyD88和内质网膜蛋白UNC-93B释放促炎细胞因子。
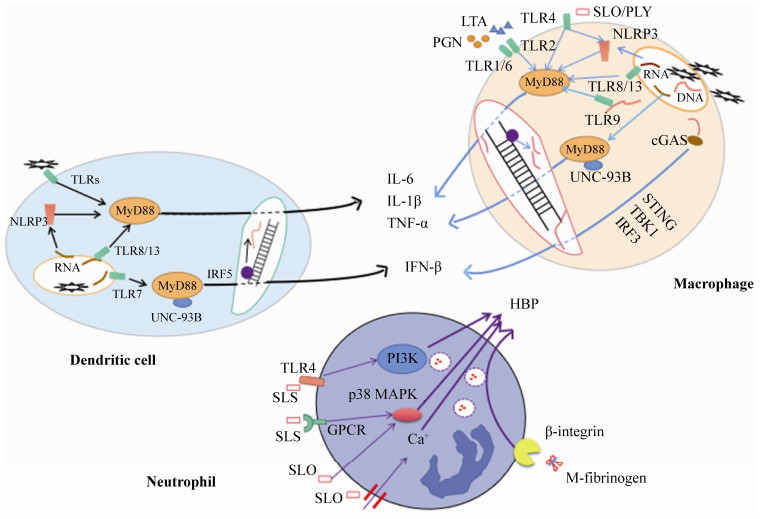
|
| 图 1 三种细胞对链球菌识别后胞内炎症信号通路 Figure 1 Inflammatory signaling pathways in three types cells activated by Streptococcus. |
中性粒细胞是募集到炎症中心的首批白细胞。在GAS链球菌感染时,M蛋白除了发挥超抗原的功能外,还可被蛋白酶剪切,和宿主纤维蛋白原形成复合物(M-fibrinogen),结合中性粒细胞表面β-整合素(β-integrin)来激活中性粒细胞,使其释放多种水解酶,尤其是肝素结合蛋白(heparin-binding protein,HBP),引起肺部病变的形成和血管渗漏、弥漫性血管内凝血和STSS特有的器官损伤[9, 25]。GAS溶血素O (streptolysin-O,SLO)直接穿透中性粒细胞,导致Ca2+内流和p38丝裂原活化蛋白激酶(p38 MAPK)信号通路的活化,促进中性粒细胞的脱颗粒作用[26]。猪链球菌溶血素(suilysin,SLS)通过与TLR4受体识别后触发磷脂酰肌醇3-激酶(PI3K)通路、p38 MAPK通路和G蛋白偶联受体(GPCR),共同作用下释放HBP (图 1)[27]。
2 炎性小体与细胞焦亡炎性小体是由凋亡相关斑点样蛋白(apoptosis-associated speck-like protein containing CARD,ASC)募集上游PRRs和下游胱冬肽酶-1前体(pro-caspase-1)形成的蛋白复合体。炎性小体的类型有NLRP1 (Nod-like receptor P1)、NLRP3 (Nod-like receptor P3)和NLRC4 (NLR-family CARD-containing protein 4)、AIM2 (absent in melanoma-2)和RIG-1 (retinoic acid-inducible gene I)[28]。如图 2所示,宿主细胞PRRs与某些PAMPs的识别结合,能够激活炎性小体,使pro-caspase-1剪切成活化的caspase-1。活化的caspase-1对pro-IL-1β和pro-IL-18进行切割,生成IL-1β/IL-18,作用于邻近免疫活性细胞,促进其他促炎细胞因子的产生,例如TNF-α、IL-6等[29]。同时,活化的caspase-1还可切割gasdermin-D (GSDMD),使GSDMD的N末端结构域释放,在细胞膜上形成10-15 nm的孔,细胞内外失衡并破裂,引起细胞死亡。这一独特的细胞程序性死亡的方式,被称为细胞焦亡(pyroptosis),于2001年首次提出[30],在2015年由我国科学家邵峰发现关键蛋白GSDMD并阐明其关键的分子机制[31]。细胞焦亡后,释放的大量细胞内容物又可引发强烈的炎症反应。
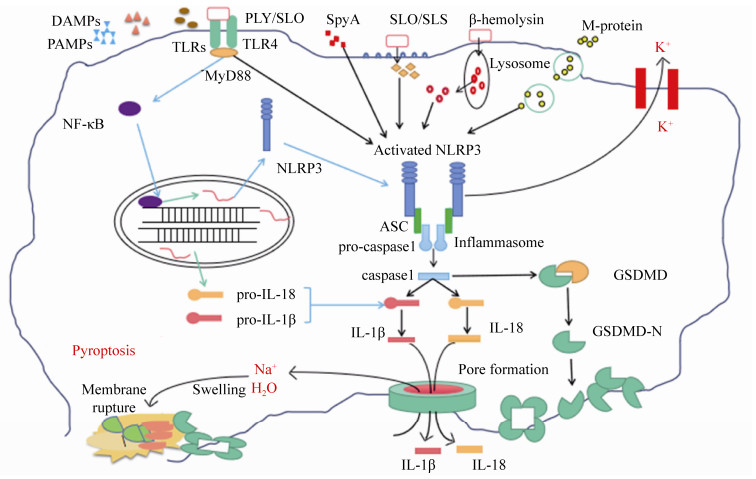
|
| 图 2 链球菌激活炎性小体的分子机制 Figure 2 Molecular mechanisms involved in inflammasome activation. |
目前链球菌上研究较多的炎性小体是NLRP3,已发现链球菌分泌的穿孔毒素(pore- forming toxin,PFT)是活化NLRP3的主要因素[32]。链球菌的穿孔毒素,属于胆固醇依赖性溶血素(cholesterol-dependent cytolysins,CDCs),已报道的有肺炎链球菌溶血素(pneumolysin,PLY)、GAS的溶血素SLO、GBS的β-溶血素(β-hemolysin)和猪链球菌溶血素SLY等[32-33]。例如,在肺炎链球菌中,PLY与TLR4受体结合,激活炎性小体NLRP3并产生高水平的IL-18和IL-1β,再进一步刺激免疫活性细胞分泌其他的细胞因子[34];GBS通过β-溶血素介导的溶酶体渗漏机制,即GBS被胞吞后β-溶血素介导细胞的溶酶体泄漏,增强RNA与NLRP3相互作用,从而诱导IL-1β的产生[35]。林岚等发现猪链球菌SLY的膜穿孔活性和导致的钾离子外流,能够激活炎性小体NLRP3,诱发细胞因子风暴和多器官的衰竭,从而证明了炎性小体NLRP3的激活是猪链球菌引发STSS的重要原因之一[8]。GAS的SLO能通过不依赖P2X7R和TLR信号传导的途径激活炎性小体NLRP3,并促进巨噬细胞caspase-1活化和IL-1β分泌[36]。此外,链球菌的M蛋白、链球菌ADP核糖基转移酶(streptococcal ADP-ribosyltransferase,SpyA)等也可激活炎性小体NLRP3,活化caspase 1,分泌IL-18和IL-1β,最终引起细胞焦亡,这属于链球菌导致的经典细胞焦亡途径(图 2)[37-38]。
3 链球菌感染与促炎细胞因子链球菌感染宿主后,机体的细胞因子的释放分为两个层次:首先是天然免疫系统的细胞因子释放和相互刺激,例如,链球菌感染后刺激NK细胞释放少量IFN-γ并激活巨噬细胞释放IFN-α/β、IL-1β、TNF-α、IL-12等促炎细胞因子,进一步促进炎症反应;其次是获得性免疫系统参战,主要是Th1细胞的影响,即DCs捕获链球菌并将抗原呈递给CD4+ T细胞,诱导CD4+ T细胞分化为Th1细胞并释放大量IFN-γ,IFN-γ又能刺激巨噬细胞活化并再次增强天然免疫应答,形成一个“正反馈循环”[39-40]。血清中IL-1、IL-6和TNF-α浓度升高与细菌性败血症的严重程度相关[41]。炎性小体激活能释放IL-1β,其分泌常常伴随细胞焦亡,细胞内容物的大量释放会触发其他的炎症信号通路,释放大量细胞因子[32]。TNF-α是最重要的促炎细胞因子之一,能激活血管内皮细胞,增加血管通透性,引起血管内容物漏出[42]。IL-6是细胞因子风暴中疾病严重程度和预后指标的生物标志物,因为高水平的IL-6可以激活凝血途径和血管内皮细胞,但会抑制心肌功能,导致高烧、低血压、多器官功能障碍[43-44]。IL-18可由激活的炎性小体介导释放,主要的功能是刺激NK细胞和诱导IFN-γ分泌[32]。
张松等[45]用表达的GAS的M蛋白和SpeB蛋白,刺激巨噬细胞RAW264.7,发现均能检测到高水平促炎细胞因子IL-1β、IL-6和TNF-α。Nikolai等[46]报道了一个免疫力健全的女性感染GBS菌株LUMC16后引发STSS,用LUMC16或提取该菌株的溶血素刺激人PBMC细胞,可检测到大量促炎细胞因子,包括TNF-α、IL-1β和IL-6等,推测有助于GBS引发STSS。Segura等[47]证明猪链球菌2型能够刺激人源性单核细胞,诱导大量促炎细胞因子TNF-α、IL-1、IL-6、IL-8和MCP-1的释放,参与炎症反应。
促炎细胞因子的水平,可以反映链球菌引起机体炎症反应的严重程度,推测STSS的发生,因此对促炎细胞因子的检测是开展研究必不可少的环节。目前商品化的细胞因子ELISA试剂盒很常用,可直接检测组织中的细胞因子水平。此外,另外一种常用的方法是提取血液或组织中的mRNA,反转录后通过荧光定量PCR的方法(RT-qPCR)对细胞因子mRNA水平进行定量测定。这两种方法各有优点,商品化的ELISA试剂盒检测,操作方便,直接反映细胞因子的蛋白水平;RT-qPCR可在实验室自行进行,反映的是细胞因子的转录水平。研究者通常两种方法并用,相互印证,增加实验结果的可信度。
4 细胞因子风暴如果免疫系统被过度激活时,免疫细胞会应激过度,促炎细胞因子水平超过阈值失去控制,会使“正反馈循环”无限放大,触发细胞因子风暴(cytokine storm)[11]。能激活细胞因子风暴的细菌或病毒感染,最后导致宿主死亡的原因都是过度炎症反应引起的机体多器官衰竭和休克[10]。研究表明细胞因子风暴是许多病毒感染致死的主要原因,比如西班牙流感大流行、SARS事件、H1N1流感爆发、埃博拉病毒疫情、登革热病毒疫情、新型冠状病毒COVID-19大流行等[48-53]。
细胞因子风暴也是链球菌引发STSS的重要特征,是临床上链球菌病死率高于一般感染的重要原因,其关键触发因子之一是SAg[54]。链球菌的超抗原与其他细菌的作用机制一致,可同时与MHC II类分子和T细胞受体的肽结合槽外侧发生结合,无需抗原的加工提呈,在极低浓度下就可非特异性地活化大量T细胞,造成众多细胞因子释放和细胞因子风暴,引发STSS[55-56]。研究发现,GAS存在12种超抗原,能够诱导宿主炎性因子大量表达[57]。本实验室前期研究未能在GBS中鉴定出超抗原样物质,对GBS基因组中分析也未发现编码类似的超抗原基因[58]。在能引发STSS的猪链球菌中,也存在类似的情况,至今未有关于猪链球超抗原的报道[59]。这些不产生超抗原的链球菌,引起STSS的机制更为复杂。所以对于这类非超抗原引发的STSS研究,需要从天然免疫系统对链球菌某些特定PAMPS的识别、是否有大量促炎细胞因子的释放、是否激活炎性小体和引发细胞焦亡等角度入手。
5 研究STSS的动物模型 5.1 小鼠小鼠是模拟链球菌体内感染最常用的模型。Valderrama等[37]在探究链球菌M蛋白对NLRP3的激活作用时,用M蛋白腹腔注射小鼠,收集腹膜液,通过ELISA分析细胞因子和腹膜液的细菌计数,显示可溶性M1蛋白对IL-1β激活是充分且特异的。Saito等[60]通过对感染GAS引起STSS的急性死亡和延迟死亡的小鼠模型中促炎细胞因子的分析,发现延迟死亡小鼠的血清能检测出IL-10和IL-12,同时TNF-α和IFN-γ水平是急性死亡小鼠的100倍以上。马可等[61]用无乳链球菌和cas 9基因缺失株分别注射小鼠后提取脾组织RNA,反转录后进行荧光定量PCR分析(RT-qPCR),发现cas 9基因缺失菌株在脾脏中触发了更高水平的IL-6表达,提示cas 9与无乳链球菌触发小鼠的促炎反应有关。Liu等[62]用猪链球菌05ZY菌株和HP1717基因缺失株注射小鼠后,分别于感染小鼠3、6、9、12 h后采血,进行RT-qPCR和直接ELISA检测促炎细胞因子IL-1β、MCP-1和TNF-α的产生水平,发现猪链球菌中HP1717的缺失降低了促炎细胞因子的产生,提示HP1717是一种促炎蛋白。
5.2 斑马鱼斑马鱼凭借高效的繁殖能力、完整的免疫系统和独特的形态特征逐渐发展成一个研究链球菌感染的成熟模型,其中主要选用成年斑马鱼以及斑马鱼胚胎作为感染对象开展研究[63]。Kim等[64]从感染链球菌48 h的斑马鱼胚胎提取RNA,通过实时qPCR发现IL-1β和CXCL8的转录水平显著上升。成年斑马鱼感染GBS,则可导致IL-1β和IL-6促炎细胞因子的表达增加[65]。
5.3 猪猪是猪链球菌的主要宿主。Segura等[66]通过使用猪全血模型,用PCR和ELISA的方法进行检测,结果表明猪链球菌可引起某些促炎症细胞因子释放。观察发现活菌比热灭活菌诱导更高水平的TNF-α、IL-1β和IL-6释放。相反热灭活菌刺激释放更多的IL-8和MCP-1。
5.4 奶牛奶牛乳腺炎是奶牛的重要疫病,引发的链球菌病原主要是无乳链球菌和乳房链球菌。Wedlock等[67]根据3种不同的乳房链球菌制备了含无细胞提取物的疫苗,皮下接种后收集血液,通过RT-qPCR分析,可以测到高水平的IFNγ,是该疫苗诱导牛的细胞和体液反应佐证之一。Weller等[68]屠宰时从有健康乳腺的奶牛身上取出4只乳房,灌注后在接种无乳链球菌0 h和3 h后取灌流液,进行RNA提取、测序和实时PCR分析,发现一组差异化表达的基因上调,其中CCL5、IL-8表达最高,涉及先天免疫反应和炎症反应。
6 展望链球菌与宿主细胞互作时,细胞存在不完全吞噬、自噬、炎症反应或焦亡等几种结果。一旦链球菌感染激发强烈的炎症反应,可迅速导致威胁生命的疾病表现,最常见的是以细胞因子风暴为特征的STSS。炎性小体在链球菌激发宿主炎症反应中起着重要作用,也是引发细胞焦亡的必要成分。信号转导的结果会促使宿主细胞释放不同的炎性介质,如果过量释放,就会导致细胞因子风暴,机体不分敌我地攻击入侵链球菌以及自身细胞和组织,引发STSS。当前,宿主细胞对链球菌及其特殊结构进行识别,激活炎症等一系列的机制研究多集中在构建链球菌缺失株,感染体外细胞系或者实验动物,检测细胞因子变化等。随着高通量单细胞测序技术、CRISPR技术、先导编辑技术等的发展,快速筛选与STSS发生相关的基因,对细胞或实验动物进行快速的基因剪辑,并为研究链球菌在体内触发STSS的关键因素提供便捷。对于链球菌引发宿主STSS相关机制的研究,可以为研究其他病原,尤其是新发传染病的致病机制提供支持,也为开发相关阻断药物、干预治疗奠定了理论基础。
| [1] | Chesney PJ. Toxic-shock syndrome: a commentary and review of the characteristics of Staphylococcus aureus strains. Infection, 1983, 11(4): 181-188. DOI:10.1007/BF01641192 |
| [2] | Willoughby R, Greenberg RN. The toxic shock syndrome and streptococcal pyrogenic exotoxins. Annals of Internal Medicine, 1983, 98(4): 559. |
| [3] | Ikebe T, Tominaga K, Shima T, Okuno R, Kubota H, Ogata K, Chiba K, Katsukawa C, Ohya H, Tada Y, Okabe N, Watanabe H, Ogawa M, Ohnishi M. Increased prevalence of group A streptococcus isolates in streptococcal toxic shock syndrome cases in Japan from 2010 to 2012. Epidemiology & Infection, 2015, 143(4): 864-872. |
| [4] | Ikebe T, Chiba K, Shima T, Masuda C, Okuno R, Ohya H, Ogata K, Katsukawa C, Kawahara R, Tominaga K, Yabata J, Tada Y, Okabe N, Watanabe H, Chang B, Ogawa M, Ohnishi M, Working Group for Beta-Hemolytic Streptococci in Japan. Evaluation of streptococcal toxic shock-like syndrome caused by group B streptococcus in adults in Japan between 2009 and 2013. Journal of Infection and Chemotherapy, 2015, 21(3): 207-211. DOI:10.1016/j.jiac.2014.12.002 |
| [5] | Szabó BG, Kiss R, Lénárt KS, Radka N, Kádár B. "Tempus fugit, venit mors"-about streptococcal toxic shock syndrome: a case report and mini-review. Orvosi Hetilap, 2019, 160(48): 1887-1893. DOI:10.1556/650.2019.31597 |
| [6] | Schmitz M, Roux X, Huttner B, Pugin J. Streptococcal toxic shock syndrome in the intensive care unit. Annals of Intensive Care, 2018, 8(1): 88. DOI:10.1186/s13613-018-0438-y |
| [7] | Parks T, Wilson C, Curtis N, Norrby-Teglund A, Sriskandan S. Polyspecific intravenous immunoglobulin in clindamycin-treated patients with streptococcal toxic shock syndrome: a systematic review and meta-analysis. Clinical Infectious Diseases, 2018, 67(9): 1434-1436. DOI:10.1093/cid/ciy401 |
| [8] | Lin L, Xu L, Lv WH, Han L, Xiang YZ, Fu L, Jin ML, Zhou R, Chen HC, Zhang AD. An NLRP3 inflammasome-triggered cytokine storm contributes to streptococcal toxic shock-like syndrome (STSLS). PLoS Pathogens, 2019, 15(6): e1007795. DOI:10.1371/journal.ppat.1007795 |
| [9] | Low DE. Toxic shock syndrome: major advances in pathogenesis, but not treatment. Critical Care Clinics, 2013, 29(3): 651-675. DOI:10.1016/j.ccc.2013.03.012 |
| [10] | Chousterman BG, Swirski FK, Weber GF. Cytokine storm and sepsis disease pathogenesis. Seminars in Immunopathology, 2017, 39(5): 517-528. DOI:10.1007/s00281-017-0639-8 |
| [11] | Tisoncik JR, Korth MJ, Simmons CP, Farrar J, Martin TR, Katze MG. Into the eye of the cytokine storm. Microbiology and Molecular Biology Reviews, 2012, 76(1): 16-32. DOI:10.1128/MMBR.05015-11 |
| [12] | Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell, 2006, 124(4): 783-801. DOI:10.1016/j.cell.2006.02.015 |
| [13] | Kumar H, Kawai T, Akira S. Toll-like receptors and innate immunity. Biochemical and Biophysical Research Communications, 2009, 388(4): 621-625. DOI:10.1016/j.bbrc.2009.08.062 |
| [14] | Medzhitov R. Recognition of microorganisms and activation of the immune response. Nature, 2007, 449(7164): 819-826. DOI:10.1038/nature06246 |
| [15] | Wennekamp J, Henneke P. Induction and termination of inflammatory signaling in group B streptococcal sepsis. Immunological Reviews, 2008, 225(1): 114-127. DOI:10.1111/j.1600-065X.2008.00673.x |
| [16] | Pancholi V. Group A Streptococcus-mediated host cell signaling. Microbiology Spectrum, 2019, 7(1): GPP3-0021-2018. |
| [17] | Feuerstein R, Gres V, Elias Perdigó N, Baasch S, Freudenhammer M, Elling R, Henneke P. Macrophages are a potent source of Streptococcus-induced IFN-β. The Journal of Immunology, 2019, 203(12): 3416-3426. DOI:10.4049/jimmunol.1900542 |
| [18] | Gratz N, Hartweger H, Matt U, Kratochvill F, Janos M, Sigel S, Drobits B, Li XD, Knapp S, Kovarik P. Type I interferon production induced by Streptococcus pyogenes-derived nucleic acids is required for host protection. PLoS Pathogens, 2011, 7(5): e1001345. DOI:10.1371/journal.ppat.1001345 |
| [19] | Charrel-Dennis M, Latz E, Halmen KA, Trieu-Cuot P, Fitzgerald KA, Kasper DL, Golenbock DT. TLR-independent type I interferon induction in response to an extracellular bacterial pathogen via intracellular recognition of its DNA. Cell Host & Microbe, 2008, 4(6): 543-554. |
| [20] | Signorino G, Mohammadi N, Patanè F, Buscetta M, Venza M, Venza I, Mancuso G, Midiri A, Alexopoulou L, Teti G, Biondo C, Beninati C. Role of Toll-like receptor 13 in innate immune recognition of group B streptococci. Infection and Immunity, 2014, 82(12): 5013-5022. DOI:10.1128/IAI.02282-14 |
| [21] | Fieber C, Janos M, Koestler T, Gratz N, Li XD, Castiglia V, Aberle M, Sauert M, Wegner M, Alexopoulou L, Kirschning CJ, Chen ZJ, von Haeseler A, Kovarik P. Innate immune response to Streptococcus pyogenes depends on the combined activation of TLR13 and TLR2. PLoS One, 2015, 10(3): e0119727. DOI:10.1371/journal.pone.0119727 |
| [22] | Eigenbrod T, Pelka K, Latz E, Kreikemeyer B, Dalpke AH. TLR8 senses bacterial RNA in human monocytes and plays a nonredundant role for recognition of Streptococcus pyogenes. The Journal of Immunology, 2015, 195(3): 1092-1099. DOI:10.4049/jimmunol.1403173 |
| [23] | Deshmukh SD, Müller S, Hese K, Rauch KS, Wennekamp J, Takeuchi O, Akira S, Golenbock DT, Henneke P. NO is a macrophage autonomous modifier of the cytokine response to streptococcal single-stranded RNA. The Journal of Immunology, 2012, 188(2): 774-780. DOI:10.4049/jimmunol.1101383 |
| [24] | Mancuso G, Gambuzza M, Midiri A, Biondo C, Papasergi S, Akira S, Teti G, Beninati C. Bacterial recognition by TLR7 in the lysosomes of conventional dendritic cells. Nature Immunology, 2009, 10(6): 587-594. DOI:10.1038/ni.1733 |
| [25] | Herwald H, Cramer H, Mörgelin M, Russell W, Sollenberg U, Norrby-Teglund A, Flodgaard H, Lindbom L, Björck L. M protein, a classical bacterial virulence determinant, forms complexes with fibrinogen that induce vascular leakage. Cell, 2004, 116(3): 367-379. DOI:10.1016/S0092-8674(04)00057-1 |
| [26] | Nilsson M, Sørensen OE, Mörgelin M, Weineisen M, Sjöbring U, Herwald H. Activation of human polymorphonuclear neutrophils by streptolysin O from Streptococcus pyogenes leads to the release of proinflammatory mediators. Thrombosis and Haemostasis, 2006, 95(6): 982-990. DOI:10.1160/TH05-08-0572 |
| [27] | Chen SL, Xie WL, Wu K, Li P, Ren ZQ, Li L, Yuan Y, Zhang CM, Zheng YL, Lv QY, Jiang H, Jiang YQ. Suilysin stimulates the release of heparin binding protein from neutrophils and increases vascular permeability in mice. Frontiers in Microbiology, 2016, 7: 1338. |
| [28] | Kelley N, Jeltema D, Duan YH, He Y. The NLRP3 Inflammasome: an overview of mechanisms of activation and regulation. International Journal of Molecular Sciences, 2019, 20(13): 3328. DOI:10.3390/ijms20133328 |
| [29] | Wang M, Jiang S, Zhang YF, Li PF, Wang K. The multifaceted roles of pyroptotic cell death pathways in cancer. Cancers, 2019, 11(9): 1313. DOI:10.3390/cancers11091313 |
| [30] | Cookson BT, Brennan MA. Pro-inflammatory programmed cell death. Trends in Microbiology, 2001, 9(3): 113-114. |
| [31] | Shi JJ, Zhao Y, Wang K, Shi XY, Wang Y, Huang HW, Zhuang YH, Cai T, Wang FC, Shao F. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature, 2015, 526(7575): 660-665. DOI:10.1038/nature15514 |
| [32] | LaRock CN, Nizet V. Inflammasome/IL-1β responses to streptococcal pathogens. Frontiers in Immunology, 2015, 6: 518. |
| [33] |
Chen X, Suo ZW, Xu JQ, Mu X. Review on classification and representative species of hemolysin. Chinese Agricultural Science Bulletin, 2008, 24(8): 16-22.
(in Chinese) 陈希, 索占伟, 许剑琴, 穆祥. 细菌溶血素的分类及代表性溶血素研究进展. 中国农学通报, 2008, 24(8): 16-22. |
| [34] | Witzenrath M, Pache F, Lorenz D, Koppe U, Gutbier B, Tabeling C, Reppe K, Meixenberger K, Dorhoi A, Ma JT, Holmes A, Trendelenburg G, Heimesaat MM, Bereswill S, van der Linden M, Tschopp J, Mitchell TJ, Suttorp N, Opitz B. The NLRP3 inflammasome is differentially activated by pneumolysin variants and contributes to host defense in pneumococcal pneumonia. The Journal of Immunology, 2011, 187(1): 434-440. DOI:10.4049/jimmunol.1003143 |
| [35] | Gupta R, Ghosh S, Monks B, DeOliveira RB, Tzeng TC, Kalantari P, Nandy A, Bhattacharjee B, Chan J, Ferreira F, Rathinam V, Sharma S, Lien E, Silverman N, Fitzgerald K, Firon A, Trieu-Cuot P, Henneke P, Golenbock DT. RNA and β-hemolysin of group B Streptococcus induce interleukin-1β (IL-1β) by activating NLRP3 inflammasomes in mouse macrophages. The Journal of Biological Chemistry, 2014, 289(20): 13701-13705. DOI:10.1074/jbc.C114.548982 |
| [36] | Harder J, Franchi L, Muñoz-Planillo R, Park JH, Reimer T, Núñez G. Activation of the NLRP3 inflammasome by Streptococcus pyogenes requires streptolysin O and NF-κB activation but proceeds independently of TLR signaling and P2X7 receptor. The Journal of Immunology, 2009, 183(9): 5823-5829. DOI:10.4049/jimmunol.0900444 |
| [37] | Valderrama JA, Riestra AM, Gao NJ, LaRock CN, Gupta N, Ali SR, Hoffman HM, Ghosh P, Nizet V. Group A streptococcal M protein activates the NLRP3 inflammasome. Nature Microbiology, 2017, 2(10): 1425-1434. DOI:10.1038/s41564-017-0005-6 |
| [38] | Lin AE, Beasley FC, Keller N, Hollands A, Urbano R, Troemel ER, Hoffman HM, Nizet V. A group A Streptococcus ADP-ribosyltransferase toxin stimulates a protective interleukin 1β-dependent macrophage immune response. mBio, 2015, 6(2). |
| [39] | Clarke D, Letendre C, Lecours MP, Lemire P, Galbas T, Thibodeau J, Segura M. Group B Streptococcus induces a robust IFN-γ response by CD4+ T cells in an in vitro and in vivo model. Journal of Immunology Research, 2016, 2016: 5290604. |
| [40] | Emgård J, Bergsten H, McCormick JK, Barrantes I, Skrede S, Sandberg JK, Norrby-Teglund A. MAIT cells are major contributors to the cytokine response in group A streptococcal toxic shock syndrome. Proceedings of the National Academy of Sciences of the United States of America, 2019, 116(51): 25923-25931. DOI:10.1073/pnas.1910883116 |
| [41] |
Shi YF, Wu BQ. The role of inflammatory cytokines in bacterial infection. International Journal of Internal Medicine, 2009, 36(2): 112-115.
(in Chinese) 石云峰, 吴本权. 炎性细胞因子在细菌感染中的作用. 国际内科学杂志, 2009, 36(2): 112-115. |
| [42] | Zelová H, Hošek J. TNF-α signalling and inflammation: interactions between old acquaintances. Inflammation Research, 2013, 62(7): 641-651. DOI:10.1007/s00011-013-0633-0 |
| [43] | Tanaka T, Narazaki M, Kishimoto T. Immunotherapeutic implications of IL-6 blockade for cytokine storm. Immunotherapy, 2016, 8(8): 959-970. DOI:10.2217/imt-2016-0020 |
| [44] | Hunter CA, Jones SA. IL-6 as a keystone cytokine in health and disease. Nature Immunology, 2015, 16(5): 448-457. DOI:10.1038/ni.3153 |
| [45] | 张松. A族链球菌M蛋白在诱导巨噬细胞活化及调节中的作用. 河北医科大学硕士学位论文, 2013. |
| [46] | Siemens N, Oehmcke-Hecht S, Hoßmann J, Skorka SB, Nijhuis RHT, Ruppen C, Skrede S, Rohde M, Schultz D, Lalk M, Itzek A, Pieper DH, van den Bout CJ, Claas ECJ, Kuijper EJ, Mauritz R, Sendi P, Wunderink HF, Norrby-Teglund A. Prothrombotic and Proinflammatory activities of the β-hemolytic group B streptococcal pigment. Journal of Innate Immunity, 2020, 12(4): 291-303. DOI:10.1159/000504002 |
| [47] | Segura M, Vadeboncoeur N, Gottschalk M. CD14-dependent and -independent cytokine and chemokine production by human THP-1 monocytes stimulated by Streptococcus suis capsular type 2. Clinical & Experimental Immunology, 2002, 127(2): 243-254. |
| [48] | Osterholm MT. Preparing for the next pandemic. The New England Journal of Medicine, 2005, 352(18): 1839-1842. DOI:10.1056/NEJMp058068 |
| [49] | Huang KJ, Su IJ, Theron M, Wu YC, Lai SK, Liu CC, Lei HY. An interferon-γ-related cytokine storm in SARS patients. Journal of Medical Virology, 2005, 75(2): 185-194. DOI:10.1002/jmv.20255 |
| [50] | Gu YN, Hsu AC, Pang ZQ, Pan H, Zuo X, Wang GQ, Zheng JT, Wang F. Role of the Innate Cytokine storm induced by the influenza a virus. Viral Immunology, 2019, 32(6): 244-251. DOI:10.1089/vim.2019.0032 |
| [51] | Younan P, Iampietro M, Nishida A, Ramanathan P, Santos RI, Dutta M, Lubaki NM, Koup RA, Katze MG, Bukreyev A. Ebola virus binding to Tim-1 on T lymphocytes induces a cytokine storm. mBio, 2017, 8(5): e00845-17. |
| [52] | Srikiatkhachorn A, Mathew A, Rothman AL. Immune-mediated cytokine storm and its role in severe dengue. Seminars in Immunopathology, 2017, 39(5): 563-574. DOI:10.1007/s00281-017-0625-1 |
| [53] | Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ, HLH Across Speciality Collaboration, UK. COVID-19:consider cytokine storm syndromes and immunosuppression. Lancet, 2020, 395(10229): 1033-1034. DOI:10.1016/S0140-6736(20)30628-0 |
| [54] | Barnett TC, Cole JN, Rivera-Hernandez T, Henningham A, Paton JC, Nizet V, Walker MJ. Streptococcal toxins: role in pathogenesis and disease. Cellular Microbiology, 2015, 17(12): 1721-1741. DOI:10.1111/cmi.12531 |
| [55] | Zeppa JJ, Kasper KJ, Mohorovic I, Mazzuca DM, Haeryfar SMM, McCormick JK. Nasopharyngeal infection by Streptococcus pyogenes requires superantigen-responsive Vβ-specific T cells. Proceedings of the National Academy of Sciences of the United States of America, 2017, 114(38): 10226-10231. DOI:10.1073/pnas.1700858114 |
| [56] | Shannon BA, McCormick JK, Schlievert PM. Toxins and superantigens of group A streptococci. Microbiology Spectrum, 2019, 7(1): GPP3-0054-2018. |
| [57] | Reglinski M, Sriskandan S. The contribution of group A streptococcal virulence determinants to the pathogenesis of sepsis. Virulence, 2014, 5(1): 127-136. DOI:10.4161/viru.26400 |
| [58] |
Gao TT, Liu GJ, Yao HC. Determination of no superantigen in piscine Streptococcus agalactiae GD201008-001. Animal Husbandry & Veterinary Medicine, 2018, 50(12): 68-72.
(in Chinese) 高婷婷, 刘广锦, 姚火春. 鱼源无乳链球菌GD201008-001中无超抗原的确定. 畜牧与兽医, 2018, 50(12): 68-72. |
| [59] | 许仲旻. 猪链球菌致STSLS相关转录因子的筛选及其调控机制的研究. 华中农业大学博士学位论文, 2018. |
| [60] | Saito M, Kajiwara H, Iida KI, Hoshina T, Kusuhara K, Hara T, Yoshida SI. Systemic cytokine response in moribund mice of streptococcal toxic shock syndrome model. Microbial Pathogenesis, 2011, 50(2): 109-113. DOI:10.1016/j.micpath.2010.12.001 |
| [61] | Ma K, Cao Q, Luo S, Wang ZF, Liu GJ, Lu CP, Liu YJ. cas9 enhances bacterial virulence by repressing the regR transcriptional regulator in Streptococcus agalactiae. Infection and Immunity, 2018, 86(3): e00552-17. |
| [62] | Liu L, Zhang Q, Xu ZM, Huang JJ, Zhu WF, Zhang AD, Sun XM, Jin ML. HP1717 contributes to Streptococcus suis virulence by inducing an excessive inflammatory response and influencing the biosynthesis of the capsule. Microorganisms, 2019, 7(11): 522. DOI:10.3390/microorganisms7110522 |
| [63] | Saralahti A, Rämet M. Zebrafish and streptococcal infections. Scandinavian Journal of Immunology, 2015, 82(3): 174-183. DOI:10.1111/sji.12320 |
| [64] | Kim BJ, Hancock BM, Del Cid N, Bermudez A, Traver D, Doran KS. Streptococcus agalactiae infection in zebrafish larvae. Microbial Pathogenesis, 2015, 79: 57-60. DOI:10.1016/j.micpath.2015.01.007 |
| [65] | Patterson H, Saralahti A, Parikka M, Dramsi S, Trieu-Cuot P, Poyart C, Rounioja S, Rämet M. Adult zebrafish model of bacterial meningitis in Streptococcus agalactiae infection. Developmental & Comparative Immunology, 2012, 38(3): 447-455. |
| [66] | Segura M, Vanier G, Al-Numani D, Lacouture S, Olivier M, Gottschalk M. Proinflammatory cytokine and chemokine modulation by Streptococcus suis in a whole-blood culture system. FEMS Immunology & Medical Microbiology, 2006, 47(1): 92-106. |
| [67] | Wedlock DN, Buddle BM, Williamson J, Lacy-Hulbert SJ, Turner SA, Subharat S, Heiser A. Dairy cows produce cytokine and cytotoxic T cell responses following vaccination with an antigenic fraction from Streptococcus uberis. Veterinary Immunology and Immunopathology, 2014, 160(1/2): 51-60. |
| [68] | Weller MMDCA, Fonseca I, Sbardella AP, Pinto ISB, Viccini LF, Brandão HM, Gern JC, Carvalho WA, Guimarães AS, Brito MAVP, Munari DP, Silva MVGB, Martins MF. Isolated perfused udder model for transcriptome analysis in response to Streptococcus agalactiae. Journal of Dairy Research, 2019, 86(3): 307-314. DOI:10.1017/S0022029919000451 |
 2021, Vol. 61
2021, Vol. 61