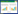中国科学院微生物研究所,中国微生物学会,中国菌物学会
文章信息
- 张萍华, 靳丽萍, 尹彩萍, 陈佳琪, 孙飞飞, 李宗平, 张应烙. 2021
- Pinghua Zhang, Liping Jin, Caiping Yin, Jiaqi Chen, Feifei Sun, Zongping Li, Yinglao Zhang. 2021
- 白蚁巢来源链霉菌T12的分离鉴定及其抗菌活性代谢产物
- Isolation, identification and antimicrobial metabolites of Streptomyces sp. T12 from termite nest
- 微生物学报, 61(2): 444-451
- Acta Microbiologica Sinica, 61(2): 444-451
-
文章历史
- 收稿日期:2020-04-16
- 修回日期:2020-08-13
- 网络出版日期:2020-11-10
2. 湖北省烟草科学研究院, 湖北 武汉 430030;
3. 安徽农业大学生命科学学院, 安徽 合肥 230036
2. Tobacco Research Institute of Hubei Province, Wuhan 430030, Hubei Province, China;
3. College of Life Sciences, Anhui Agricultural University, Hefei 230036, Anhui Province, China
由于抗生素的不规范使用和致病微生物不断增强的耐药性,导致搜寻更新颖、更安全和副作用少的抗菌化合物的需求与日俱增[1-2]。土壤微生物代谢产物曾经是抗生素的丰富来源,如氯霉素、链霉素和青霉素等抗生素都是从土壤微生物中分离得到。然而,由于长期重复从相同或相似的土壤微生物中分离天然产物往往是已知化合物,获取新颖骨架抗菌天然产物的可能性大大降低[3]。因昆虫的特殊生活环境及与周围生物的相互作用关系,从其共生微生物中能够发现结构独特、具有抗菌活性的天然产物[4]。
白蚁巢具有食药用性功能[5],是等翅目昆虫土白蚁的主要生活与繁殖场所,富含巨大的放线菌物种多样性[6];对泰国3种白蚁巢放线菌的抗菌活性研究表明其中超过20%的放线菌至少能抑制1种供试致病菌的生长[7]。在对1株黑翅土白蚁巢放线菌的抗菌活性研究的基础上[8],我们进一步从黑翅土白蚁巢中筛选出1株具有显著抗菌活性的菌株并对其活性成分进行解析,以期为新型抗生素的开发奠定研究基础。
1 材料和方法 1.1 材料1.1.1 样品来源: 供试白蚁巢:黑翅土白蚁巢采自浙江师范大学校园内(29°00'17.37" N,119°29'54.84" E)。
1.1.2 供试致病菌: 枯草芽孢杆菌(Bacillus subtilis,ATCC6633)、金黄色葡萄球菌(Staphylococcus aureus,ATCC6538)、大肠杆菌(Escherichia coli,ATCC8739)、白色念珠菌(Canidiaalbicans,ATCC10231)。
1.1.3 供试培养基: 马铃薯葡萄糖(PDA)培养基、胰蛋白胨酵母(LB)培养基、高氏一号(GS)培养基,按常规方法配制。
1.1.4 仪器与试剂: 超净工作台(苏净集团苏州安泰空气技术有限公司);ZWZR-2102摇床(上海智诚分析仪器制造有限公司);PCR仪(德国Eppendorfs公司);BUCHI旋转蒸发仪R-210 (瑞士步琦有限公司);核磁共振仪(瑞士Bruker公司);Mariner质谱仪(美国ABI公司)。
Ezup柱式细菌基因组DNA提取试剂盒[生工生物工程(上海)有限公司];柱层层析硅胶(青岛海洋化工有限公司);氘代试剂(宁波旋光医药)。
1.2 菌株的分离根据文献中的方法[9],取约0.1 cm3的黑翅土白蚁巢,在无菌条件下,添加适量无菌生理盐水研磨至均匀,并制成10-1、10-2和10-3等3个浓度梯度。分别取上述稀释液200 μL涂布于高氏一号固体培养基上,28 ℃恒温培养。待长出菌落后,挑取边缘菌体多次划线获得纯培养物并保存于GS试管斜面上备用。
1.3 菌株的发酵及活性测试将分离得到的放线菌菌株接种于含GS液体培养基的250 mL锥形瓶中,180 r/min、28 ℃下摇床培养7 d,用纱布过滤发酵物得到滤液。参考文献的牛津杯法测定滤液的抗菌活性[10]:将大肠杆菌(E. coli)、枯草芽孢杆菌(B. subtilis)和金黄色葡萄球菌(S. aureus)置于LB液体培养基中37 ℃培养24 h,白色念珠菌(C. albicans)置于PDA液体培养基中28 ℃培养24 h,随后用无菌水将菌液浓度稀释到(0.8-1.2)×108 CFU/mL并取200 μL均匀涂布于相应培养基上。用镊子挑取牛津杯置于培养基中,每杯加入100 μL待测发酵液,每处理设3次重复,分别置于适宜温度下培养,观察是否有抑菌圈产生。
1.4 菌株T12的分子生物学鉴定菌株活化后,提取基因组DNA,参照文献进行PCR扩增、测序,并构建系统发育树[11]。
1.5 菌株T12发酵液不同极性部分的抗菌活性参照1.3的方法,对菌株T12进行液体发酵,用石油醚、乙酸乙酯和正丁醇分别对1 L发酵液进行萃取,获得3个有机相(石油醚相、乙酸乙酯相、正丁醇相)和水相的不同极性的萃取物。分别用丙酮将4种提取物配制为6 mg/mL的母液。用滤纸片法测试提取物对病原菌的拮抗活性,用“十字交叉法”记录抑菌圈,具体方法参见文献[11]。
1.6 活性次级代谢产物的分离与鉴定及抗菌活性菌株T12发酵液共25 L,用乙酸乙酯等体积萃取3次,减压浓缩获得2.8 g粗浸膏,粗浸膏通过多种色谱方法(硅胶柱层析、凝胶柱层析、薄层层析等)分离与纯化得到单体化合物。用质谱仪测定分子量,用核磁共振仪进行1H-NMR谱和13C-NMR谱分析。参考1.5的滤纸片法,对分离得到的单体化合物的抗菌活性进行测试。
2 结果和分析 2.1 菌株的筛选与鉴定从白蚁巢中共筛选到32株放线菌,对菌株发酵液进行活性测试,其中菌株T12抑菌能力最强,对金黄色葡萄球菌和白色念珠菌的抑菌圈直径分别为18.6 mm和19.5 mm。经过PCR扩增所得的T12菌株16S rRNA基因片段长度为1431 bp。测序结果经BLAST比对,发现T12的序列与链霉菌属Streptomyces malaysiensis的相关序列(GenBank登录号:HQ607429)相似性达99%,两者在系统发育树上处于同一分支(图 1),将菌株T12初步鉴定为链霉菌属菌株(Streptomyces sp.)。

|
| 图 1 基于16S rRNA序列构建的白蚁巢相关链霉菌T12系统发育树 Figure 1 Phylogenetic tree based on the 16S rRNA sequences of termite nest associated T12. |
2.2 菌株粗提物的抗菌活性
链霉菌T12提取物对4种致病菌的生长抑制作用结果见表 1,在供试浓度为30 μg/滤纸片时,乙酸乙酯提取物对金黄色葡萄球菌、大肠杆菌和白色念珠菌的抑菌效果明显,抑菌圈直径分别达20.1 mm、16.1 mm和17.4 mm,与阳性对照相当;而石油醚提取物对供试菌的抑制作用较弱,其抑菌圈直径范围为12.0-13.6 mm;正丁醇提取物和水相均无活性。
| Pathogenic microorganism | Petroleum ether extract | Ethyl acetate extract | Butanol extract | Water extract | Gentamicin sulfate | Amphotericin |
| E. coli | NI | 16.1±0.7 | NI | NI | 13.2±0.8 | NI |
| S. aureus | 12.0±0.7 | 20.1±1.3 | NI | NI | 18.2±1.1 | NI |
| B. subtilis | NI | NI | NI | NI | 16.6±1.3 | NI |
| C. albicans | 13.6±0.4 | 17.4±0.6 | NI | NI | NI | 13.2±0.8 |
| Amphotericin and gentamycin sulfate were the positive control of C. albicans and pathogenic bacteria respectively; NI=not inhibitory effect. | ||||||
2.3 活性代谢产物的结构鉴定及其抗菌活性
在活性跟踪指导下,经凝胶柱层析等多种色谱方法,从链霉菌T12乙酸乙酯粗提物中分离获得两个单体化合物1和2,经ESI-MS和核磁共振谱分析,获得相应波谱数据并对其结构进行鉴定。
化合物1:高分辨质谱(图 2)显示:最强的m/z出现在583.2640,与C29H40N2O9Na [M+Na]+的583.2640相符合,说明其分子式为C29H40N2O9,分子量为560.2748,与理论值560.2734误差仅2.5,分子式准确;1H NMR图(图 S1,http://journals.im.ac.cn/html/actamicrocn/2021/2/202102018.htm,氘代试剂为CDCl3) δ=8.71 (1H, s, -NH), 7.28 (1H, s, H-19), 6.94 (1H, d, J = 11.6 Hz, H-3), 6.57 (1H, t, J = 11.6 Hz, H-4), 5.87 (1H, t, J = 10.3 Hz,H-5), 5.81 (1H, d, J = 9.4 Hz, H-9), 5.19 (1H, s, H-7), 4.80 (2H, br s, -OCONH2), 4.31 (1H, d, J = 9.3 Hz, H-6), 4.12 (3H, s, 17-OCH3), 3.53 (1H, m, H-11), 3.39 (1H, m, H-12), 3.36 (3H, s, 12-OCH3), 3.29 (3H, s, 6-OCH3), 3.02 (1H, br s, 11-OH), 2.78 (1H, m, H-10), 2.47 (2H, m, H-15), 2.02 (3H, s, 2-CH3), 1.82 (1H, m, H-13A), 1.79 (3H, br s, 8-CH3), 1.76 (1H, m, H-13B), 1.66 (1H, m, H-14), 0.97 (6H, overlap, 10-CH3, 14-CH3),以上氢谱说明其含有5个不饱和氢(δ=6.94, 6.57, 5.87, 5.81, 5.19)、4个甲基(δ=2.02, 1.79, 0.97)和3个甲氧基(δ=4.12, 3.36, 3.29);13C NMR图(图 S2,http://journals.im.ac.cn/html/actamicrocn/2021/2/202102018.htm,氘代试剂为CDCl3) δ 185.1 (C-21), 184.2 (C-18), 168.2 (C-1), 157.0 (C-17), 156.2 (-OCONH2), 138.1 (C-20), 136.5 (C-5), 134.9 (C-2), 133.4 (C-8), 133.1 (C-9), 127.7 (C-16), 127.3 (C-3), 126.3 (C-4), 111.8 (C-19), 81.8 (C-7), 81.4 (C-6), 81.1 (C-12), 72.8 (C-11), 61.7 (17-OCH3), 57.4 (6-OCH3), 56.9 (12-OCH3), 34.7 (C-13), 32.9 (C-15), 32.3 (C-10), 28.1 (14-CH3), 23.0 (C-14), 13.0 (2-CH3), 12.6 (8-CH3), 12.5 (10-CH3),上述碳谱说明其含有2个羰基(δ=185.1, 184.2)和化学位移位于111.8-168.2间的12个不饱和碳。以上波谱数据与文献[12-13]报道的geldanamycin数据基本一致,因此,确定该化合物为geldanamycin。

|
| 图 2 化合物1的质谱 Figure 2 Mass spectrogram of compound 1. |
|
| 图 S1 化合物1的核磁共振氢谱图 Figure S1 1H NMR of compound 1. |

|
| 图 S2 化合物1的核磁共振碳谱图 Figure S2 13C NMR of compound 1. |
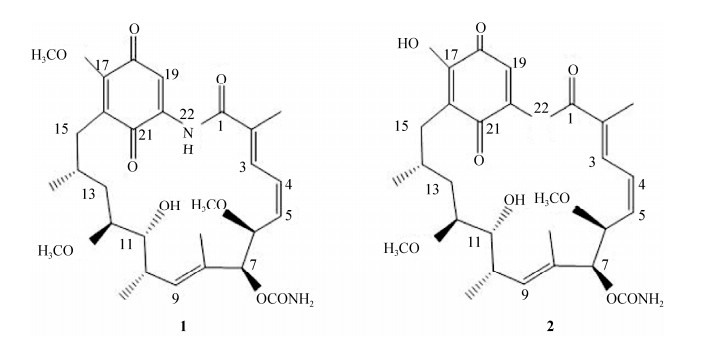
化合物2:高分辨质谱(图 3)显示:最强的m/z出现在569.2471,与C28H38N2O9Na [M+Na]+的569.2471相符合,说明其分子式为C28H38N2O9,分子量为546.2579,与理论值546.2577误差仅0.4,分子式准确;1H NMR图(图 S3,氘代试剂为CDCl3) δ=8.95 (1H, s, -NH), 7.51 (1H, s, 17-OH), 7.42 (1H, s, H-19), 6.96 (1H, d, J=11.6 Hz, H-3), 6.58 (1H, t, J=11.3 Hz, H-4), 5.91 (1H, t, J=15.5 Hz, H-5), 5.81 (1H, d, J=9.9 Hz, H-9), 5.18 (1H, s, H-7), 4.72 (2H, br s, -OCONH2), 4.32 (1H, d, J=9.3 Hz, H-6), 3.54 (1H, m, H-11), 3.39 (1H, m, H-12), 3.36 (3H, s, 12-OCH3), 3.31 (3H, s, 6-OCH3), 2.80 (1H, m, H-10), 2.45 (2H, m, H-15), 2.03 (3H, s, 2-CH3), 1.80 (3H, d, J=0.9 Hz, 8-CH3), 1.77 (2H, m, H-13A), 1.73 (1H, m, H-13B), 1.01 (3H, d, J=6.2 Hz, 14-CH3), 0.97 (3H, d, J=7.0 Hz, 10-CH3),上述氢谱说明其含有5个不饱和氢(δ=6.96, 6.58, 5.91, 5.81, 5.18)、4个甲基(δ=2.03, 1.80, 1.01, 0.97)和2个甲氧基(δ=3.36, 3.31)。以上波谱数据与文献[13]报道的17-O-demethylgeldanamycin数据基本一致,因此,确定该化合物为17-O-demethylgeldanamycin。这2个化合物具体的分子结构如图 4所示。

|
|
图 3 化合物2的质谱 Figure 3 Mass spectrogram of compound 2. |

|
| 图 S3 化合物2的氢谱图 Figure S3 1H NMR of compound 2. |
|
| 图 4 化合物1和2结构图 Figure 4 Molecular structure of compounds 1 and 2. |
两个单体化合物的抑菌作用如表 2和图 5所示,化合物1和2在浓度为30 μg/滤纸片时,对金黄色葡萄球菌有较强的的拮抗作用,抑菌圈直径分别达14.6 mm和14.5 mm,与硫酸庆大霉素的抑菌效果相当;与阳性对照两性霉素相比,两种单体化合物对白色念珠菌具较好的抑制作用,抑菌圈直径分别为10.9 mm和13.9 mm。
| Compounds | E. coli | C. albicans | S. aureus | B. subtilis |
| 1 | 8.9±1.2 | 10.9±1.9 | 14.6±1.9 | NI |
| 2 | NI | 13.9±1.5 | 14.5 ±0.6 | NI |
| Gentamicin sulfate | 20.3±2.8 | NI | 18.2±1.1 | 13.2±0.8 |
| Amphotericin | NI | 16.6±1.3 | NI | NI |
| Amphotericin and gantamycin sulfate were the positive control of C. albicans and pathogenic bacteria respectively; results were presented as the mean ± standard error; NI=no inhibitory effect. | ||||

|
| 图 5 化合物1和2的抗菌活性 Figure 5 Antimicrobial activities of compounds 1 and 2. Antibacterial activities of compounds 1 (A) and 2 (B) against S. aureus; antimicrobial activities of compound 2 against C. albicans (C). |
3 讨论
本文从黑翅白蚁巢筛选到的链霉菌T12具有较好的抗菌活性,其发酵液对金黄色葡萄球菌和白色念珠菌的抑菌圈直径均大于18.6 mm,因此,黑翅土白蚁巢链霉菌T12作为微生物源杀菌剂值得进一步研究。经分子生物学鉴定将菌株T12确定为链霉菌(Streptomyces sp.)。进一步从目标菌株链霉菌T12乙酸乙酯粗浸膏中分离出2个单体化合物geldanamycin和17-O-demethylgeldanamycin,这两个化合物均属于安莎霉素类化合物中的萘安莎霉素亚族,目前已有报道这两种化合物均具有抗肿瘤活性[14-15],也有报道geldanamycin对多种病原微生物具有抑制作用[16-17],而17-O-demethylgeldanamycin的抗菌活性尚未见报道。本文研究首次发现:当供试浓度为30 μg/滤纸片时,化合物17-O-demethylgeldanamycin对白色念珠菌和金黄色葡萄球菌具有较强的抑制活性,抑菌圈直径分别为13.9 mm和14.5 mm,与阳性对照抑菌作用相当;geldanamycin对白色念珠菌与金黄色葡萄球菌也表现出一定的抑制作用,抑菌圈直径分别为10.9 mm和14.6 mm。因此单体化合物1与2作为抗菌先导化合物具有进一步开发的潜力。关于这两种单体化合物的安全性及活性作用机理等,还有待于进一步的研究探讨。
| [1] | Fisher MC, Hawkins NJ, Sanglard D, Gurr SJ. Worldwide emergence of resistance to antifungal drugs challenges human health and food security. Science, 2018, 360(6390): 739-742. DOI:10.1126/science.aap7999 |
| [2] | Hossain MN, Rahman MM. Antagonistic activity of antibiotic producing Streptomyces sp. against fish and human pathogenic bacteria. Brazilian Archives of Biology and Technology, 2014, 57(2): 233-237. DOI:10.1590/S1516-89132014000200011 |
| [3] | Kumar PS, Duraipandiyan V, Ignacimuthu S. Isolation, screening and partial purification of antimicrobial antibiotics from soil Streptomyces sp. SCA 7. The Kaohsiung Journal of Medical Sciences, 2014, 30(9): 435-446. DOI:10.1016/j.kjms.2014.05.006 |
| [4] | DeCorte BL. Underexplored opportunities for natural products in drug discovery. Journal of Medicinal Chemistry, 2016, 59(20): 9295-9304. DOI:10.1021/acs.jmedchem.6b00473 |
| [5] |
Yan SH, Peng XF, Zhang YQ, Sun X. Utilization of termite resources in China. Chinese Bulletin of Entomology, 2008, 45(2): 336-339.
(in Chinese) 严少辉, 彭心赋, 张永强, 孙叙. 我国白蚁资源利用的现状. 昆虫知识, 2008, 45(2): 336-339. |
| [6] | Manjula A, Sathyavathi S, Pushpanathan M, Gunasekaran P, Rajendhran J. Microbial diversity in termite nest. Current Science, 2014, 106(10): 1430-1434. |
| [7] | Sujada N, Sungthong R, Lumyong S. Termite nests as an abundant source of cultivable actinobacteria for biotechnological purposes. Microbes and Environments, 2014, 29(2): 211-219. DOI:10.1264/jsme2.ME13183 |
| [8] |
Lu YH, Li S, Zhou DX, Zhang YL. Isolation and identification of termitarium antagonistic actinomycetes BYC 01 and its active metabolites. Acta Microbiologica Sinica, 2014, 54(7): 754-759.
(in Chinese) 卢贻会, 李帅, 周端顼, 张应烙. 白蚁巢拮抗放线菌BYC01代谢产物的分离和鉴定. 微生物学报, 2014, 54(7): 754-759. |
| [9] | Poulsen M, Oh DC, Clardy J, Currie CR. Chemical analyses of wasp-associated Streptomyces bacteria reveal a prolific potential for natural products discovery. PLoS One, 2011, 6(2): e16763. DOI:10.1371/journal.pone.0016763 |
| [10] |
Zhao SL, Ren FE, Liu JL, Qin JC, Pan HY. Screening, identification and optimization of fermentation conditions of an antagonistic actinomycetes strain to Setosphaeria turcica. Acta Microbiologica Sinica, 2012, 52(10): 1228-1236.
(in Chinese) 赵淑莉, 任飞娥, 刘金亮, 秦建春, 潘洪玉. 玉米大斑病生防放线菌的筛选鉴定及发酵条件优化. 微生物学报, 2012, 52(10): 1228-1236. |
| [11] | 靳丽萍.蜻蜓和白蚁共生菌的活性次生代谢产物的研究.浙江师范大学硕士学位论文, 2017. |
| [12] | Qin HL, Panek JS. Total synthesis of the Hsp90 inhibitor geldanamycin. Organic Letters, 2008, 10(12): 2477-2479. DOI:10.1021/ol800749w |
| [13] |
Shi NN, Wang HX, Lu CH, Liu Z, Shen YM. Ansamycins produced by Streptomyces sp. LZ35. Chinese Pharmaceutical Journal, 2011, 46(17): 1317-1320.
(in Chinese) 石妞妞, 王浩鑫, 鲁春华, 刘最, 沈月毛. 海洋放线菌Streptomyces sp. LZ35中的安莎霉素类化合物. 中国药学杂志, 2011, 46(17): 1317-1320. |
| [14] | Li S, Shao MW, Lu YH, Kong LC, Jiang DH, Zhang YL. Phytotoxic and antibacterial metabolites from Fusarium proliferatum ZS07 isolated from the gut of long-horned grasshoppers. Journal of Agricultural and Food Chemistry, 2014, 62(36): 8997-9001. DOI:10.1021/jf502484n |
| [15] | Ikeda H, Hideshima T, Fulciniti M, Lutz RJ, Yasui H, Okawa Y, Kiziltepe T, Vallet S, Pozzi S, Santo L, Perrone G, Tai YT, Cirstea D, Raje NS, Uherek C, Dälken B, Aigner S, Osterroth F, Munshi N, Richardson P, Anderson KC. The monoclonal antibody nBT062 conjugated to cytotoxic maytansinoids has selective cytotoxicity against CD138-positive multiple myeloma cells in vitro and in vivo. Clinical Cancer Research, 2009, 15(12): 4028-4037. DOI:10.1158/1078-0432.CCR-08-2867 |
| [16] | Deboer C, Meulman PA, Wnuk RJ, Peterson DH. Geldanamycin, a new antibiotic. The Journal of Antibiotics, 1970, 23(9): 442-447. DOI:10.7164/antibiotics.23.442 |
| [17] | Zhang JQ, Liu W, Tan JW, Sun Y, Wan Z, Li RY. Antifungal activity of geldanamycin alone or in combination with fluconazole against Candida species. Mycopathologia, 2013, 175(3/4): 273-279. |
 2021, Vol. 61
2021, Vol. 61