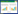中国科学院微生物研究所,中国微生物学会,中国菌物学会
文章信息
- 许昌超, 张俊涛. 2021
- Changchao Xu, Juntao Zhang. 2021
- 溶磷真菌ATMT转化条件优化及其对矮牵牛的促生效果评价
- Optimization of a phosphate-solubilizing fungus ATMT system and evaluation of its growth-promoting effect on Petunia hybrida
- 微生物学报, 61(2): 417-427
- Acta Microbiologica Sinica, 61(2): 417-427
-
文章历史
- 收稿日期:2020-03-26
- 修回日期:2020-05-21
- 网络出版日期:2020-05-29
土壤中有相当一部分的磷与Ca2+、Al3+、Fe3+等金属离子形成难溶物,短期内难以被植物利用,限制了土壤磷素的有效性以及水溶磷肥的肥效,尤其在我国南方地区,磷已经成为农林生产过程中的重要限制性因子[1]。研究表明,土壤中的部分微生物,可将植物难以利用的难溶性无机磷转化为植物可以吸收的可溶性磷,这类微生物被称作溶磷微生物,该发现为解决植物营养性缺磷问题指明了新方向[2-3]。
关于溶磷菌有较多研究和报道,但很少涉及到溶磷相关功能基因[4]。到目前为止,通过转座子随机插入突变的方式,已经克隆出了部分与细菌溶磷相关的基因[5-6],结果表明一些革兰氏阴性菌的溶磷能力与葡萄糖酸合成密切相关,葡萄糖向葡萄糖酸代谢过程中需要葡萄糖脱氢酶(glucose dehydrogenase,GDH)的参与,而GDH需要吡咯喹啉醌(pyrroloquinoline quinone,PQQ)作为辅基才能表现出酶活性。因此,与GDH和PQQ合成相关的基因在某些细菌的溶磷过程中发挥重要作用[6-7]。虽然从总体上来看真菌的溶磷效果要优于细菌,但关于溶磷真菌相关基因的研究很少[8, 9]。由于真菌具有细胞壁,存在转化效率低、操作难度大等问题的限制[10]。De Groot等成功将农杆菌介导的转化体系(Agrobacterium tumefaciens-mediated transformation,ATMT)应用于丝状真菌,使得利用基因随机插入突变的方式获得真菌突变体成为可能[11]。近年来,随着新根癌农杆菌株的使用、双元载体的改进以及转化方法的优化等,ATMT技术取得了快速发展,也成功地应用到真菌功能基因发掘中[12]。如Huang等[13]从A. fumigatus的ATMT文库中筛选出具有目的表型的突变体,再结合TAIL-PCR技术,发现tptA基因上发生的随机插入会导致菌株耐贫铁环境的能力下降。在Cercospora zeae-maydis、Fonsecaea pedrosoi和Kabatiella zeae等种中也实现了基因的随机插入[14-16]。前期从土壤中筛选出一株溶磷真菌,该菌具有高效溶磷能力并能够有效提高施用土壤环境中有效磷含量,依据形态学和分子数据,该株系被鉴定为咖啡果小蠹青霉菌Penicillium brocae。笔者针对该菌尝试通过条件优化建立合适的ATMT转化体系,对菌株进行分子标记,观察菌株在植物根部的定殖,并利用TAIL-PCR对一株转化子的插入位点进行了简单分析。此外,笔者还对该溶磷菌株的应用效果进行初试。
1 材料和方法 1.1 菌株和质粒转化对象P. brocae筛选自广州市南沙区甘蔗大田土壤(图 1-A),转化用农杆菌为AGL-1。选用质粒pCAMBIA1303-trpC-hyg-gpdA-gfp (图 1-B),其中trpC为真菌表达常用的启动子,与色氨酸合成相关;gpdA亦为真菌常用启动子,与甘油三磷酸脱氢酶合成相关,hyg为潮霉素抗性基因,gfp为绿色荧光蛋白基因。
|
| 图 1 菌株P. brocae菌落和转化用质粒图谱 Figure 1 Strain and plasmid used in this study. A: The phosphate solubilization zone produced by Penicillium brocae on a Ca3(PO4)2 containing medium; B: Map of pCAMBIA1303-trpC-hyg-gpdA-gfp. |
1.2 试剂和培养基
即用型GUS染色液(飞净科研试剂,国产),2.5×MM储存液(KH2PO4 3.625 g,K2HPO4 5.125 g,NaCl 0.375 g,MgSO4.7H2O 1.250 g,CaCl2 0.125 g,FeSO4.7H2O 0.0062 g,(NH4)2SO4 1.250 g。依次充分溶解在水中,终体积为1 L,现配现用),IM(诱导培养基,包含400 mL的2.5×MM储存液,1.8 g无水葡萄糖,5 mL甘油,终体积为1 L,调节pH至5.2。115 ℃、25 min灭菌,52 ℃水浴保温,添加AS至200 μmol/L),PVK培养基(glucose 10 g,(NH4)2SO4 0.5 g,Ca3(PO4)2 5.0 g,NaCl 0.2 g,KCl 0.2 g,MgSO4·7H2O 0.1 g,yeast extract 0.5 g,MnSO4·H2O 0.002 g,FeSO4·7H2O 0.002 g,加蒸馏水至1000 mL),利用PDA(马铃薯葡糖糖琼脂)平板来培养和收集P. brocae孢子。
1.3 P. brocae潮霉素敏感性检验接种环蘸取P. brocae孢子,在含不同浓度(0、25、50、100和200 mg/L)潮霉素的平板上划线,28 ℃连续培养7 d,观察并记录平板上菌株的生长情况。
1.4 ATMT转化将携带质粒的农杆菌AGL-1接种到LB平板上(含50 mg/L利福平和100 mg/L卡那霉素),28 ℃培养至产生菌落,挑取单菌落接种至LB液体培养基(含50 mg/L利福平和100 mg/L卡那霉素)中,28 ℃、200 r/min振荡培养(约24 h左右)。5000 r/min收集菌体,用新配的IM培养基稀释至OD600=0.3,继续振荡培养至OD600=0.6左右(该过程大约需要6 h左右)。与此同时,利用IM液体培养基将P. brocae孢子从PDA平板上冲洗下来,用IM培养基将孢子稀释至不同浓度(104、105、106或107个/mL),28 ℃预萌发6 h左右(此时农杆菌也达到预期浓度),将农杆菌和孢子等体积混匀,取200 μL涂布到表面铺有无菌玻璃纸的IM固体培养基上(AS浓度为50、100、150、200 μmol/L),待水分吸干后,将平板置于28 ℃的培养箱中,共培养侵染一段时间(24、36、48、60 h)后将玻璃纸转移到筛选培养基上(含头孢霉素300 mg/L,潮霉素200 mg/L),培养3–7 d,将可能的转化子在筛选培养基上连续传代3次来验证转化子的稳定性。
1.5 转化子PCR验证提取真菌基因组,通过扩增hyg和gfp基因片段对转化子进行验证,hyg基因片段扩增引物(hygF:5ʹ-TACACAGCCATCGGTCCAGACG-3ʹ,hyg R:5ʹ-CCGATTCCGGAAGTGCTTGACA-3ʹ),gfp基因片段引物(mgfp5 F:5ʹ-ACTGGAGTTGTC CCAATTCTTG-3ʹ,mgfp5 R:5ʹ-CATCCATGCCAT GTGTAATCCC-3ʹ)。PCR条件如下:95 ℃ 2 min;95 ℃ 30 s,57 ℃ 30 s,72 ℃ 1 min,30 cycles;72 ℃,3 min;4 ℃ forever。1.0%琼脂糖凝胶电泳检测。
1.6 转化子GUS染色和荧光信号检测将50×GUS染色液稀释到工作浓度,用刀片切取平板上菌落边缘含有菌丝的培养基小块,放入1.5 mL离心管,加入1 mL GUS染色液,用锡箔纸包裹,摇床振荡(往复140 r/min,37 ℃),染色时长约20–30 min即可观察着色。利用激光共聚焦显微镜观察菌丝GFP荧光(激发光波长为488 nm)。
1.7 P. brocae在矮牵牛根部定殖研究将平板上萌发的矮牵牛幼苗转移到灭菌栽培基质(陶粒和蛭石1︰3体积混匀,1/2B & D营养液浸透,装盆后表面覆盖珍珠岩),定殖1周后向根附近基质中注射2 mL(106孢子/mL)带GUS标记的P. brocae孢子悬液,2周后采集矮牵牛根部进行GUS染色和显微观察。自然土壤栽培试验则利用自然土壤代替灭菌基质即可。
1.8 溶磷能力测定将菌悬液接种到PVK液体培养基,培养7 d后,离心取上清测定有效磷含量(钼锑抗比色法)。
1.9 Southern blotting利用引物S1和S2(表 1)扩增出T-DNA中的gus-gfp片段作为探针。真菌基因组DNA用KpnⅠ酶切,0.7%(W/V)的琼脂糖凝胶电泳分离后转移到尼龙膜上(Millipore,Billerica,MA,USA)。按照试剂盒(Roche,Mannheim,Germany)说明进行探针地高辛标记、杂交以及显色。
| Primer name | Sequence (5′→3′) |
| S1 | GATCTGACTAGTTTACGTCC |
| S2 | CCCGATCTAGTAACATAG |
| RB1 | ACCAGCTCGAATTTCCCCGATCGTTC |
| RB2 | ATTGAATCCTGTTGCCGGTCTTGC |
| RB3 | GGGTTTTTATGATTAGAGTCCCGC |
| AD | GNANCANAGANNGC (N = G/A/T/C) |
1.10 TAIL-PCR
在质粒插入片段的right border上设计3条特异的嵌套引物(RB1、RB2和RB3),同时设计一条随机引物(AD),引物序列信息见表 1,TAIL-PCR条件设置参考Huang等[13]。
1.11 促生效果研究采集广州市郊红壤,与椰糠、绿化废弃物堆肥等体积混匀用于矮牵牛栽培,定殖1周后设置4组处理T1 (10 mL水)、T2 (10 g/L磷矿粉,10 mL)、T3 (106个/mL孢子,10 mL)和T4 (10 g/L磷矿粉+ 106个/mL孢子,10 mL),每周处理2次,连续处理4周后统计植株鲜重和干重。
2 结果和分析 2.1 ATMT转化及条件优化当潮霉素浓度达到25 mg/L时可以有效抑制P. brocae的生长,潮霉素浓度达到或超过50 mg/L时,平板上观察不到菌落(图 2-A),说明该菌株对潮霉素敏感,可用潮霉素筛选转化子。以获得尽可能多的转化子为目的,对转化条件进行优化。于设定的转化条件范围内,P. brocae的最适转化条件为孢子浓度106个/mL、200 μmol/L的AS以及48 h的共培养时间。在最优条件下,转化子的数目可以达到44个/106个孢子(图 2-B,C,D)。

|
| 图 2 转化P. brocae的条件优化 Figure 2 Optimization of the transformation parameters of P. brocae. A: Hygromycin sensitivity test of P. brocae; B: The effects of spore concentration on the transformant numbers of P. brocae; C: The effects of AS concentration on the transformant numbers of P. brocae; D: The effects of co-cultivation time on the transformant numbers of P. brocae. |
2.2 转化子的验证和根部定殖分析
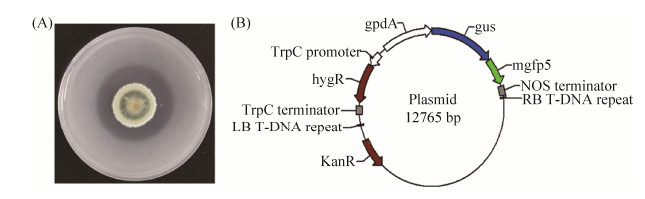
随机挑选5个转化子,提取基因组。对插入片段上的2个标记基因hyg和gfp片段进行扩增,结果如图 3,表明5个转化子均带有标记基因。利用GUS染色液对含有菌丝体的培养基团块进行染色,显色反应结果如图 4,含有转化子的培养基团块均可以被染成蓝色,野生型(W)则未显色,证明了转化子中外源gus基因的成功插入和表达。此外,笔者也通过显微镜对菌丝的GUS染色结果和GFP荧光进行了观察(图 5)。对在无菌条件下接种转化子孢子的矮牵牛根部进行染色,发现P. brocae主要定殖在根表(图 6)。在自然土壤环境下生长的矮牵牛根部进行同样的接种试验,却并未观察到矮牵牛根部的GUS染料着色和P. brocae的定殖(未列出)。

|
| 图 3 随机挑选的5个转化子中hyg(A)和gfp(B)片段扩增后电泳结果 Figure 3 The gel electrophoresis of hyg and gfp gene partial fragments cloned from randomly picked five transformants. The partial hyg gene fragment (A) and gfp gene fragment (B) was amplified with hygF/hygR primers and gfpF/gfpR primers, respectively. M is shorted for marker, W is the PCR product of wild type P. brocae, P is PCR product using plasmid as template. 1, 2, 3, 4, 5 were PCR products of the 5 transformants. |

|
| 图 4 5个转化子和野生型菌株的固体培养基团块GUS染色结果 Figure 4 GUS-staining results of the 5 transformants 1, 2, 3, 4 and 5. W is wild type P. brocae. The first line and second line showed the samples without staining and 2 hours after staining, respectively. |
|
| 图 5 P. brocae及其转化子菌丝的GUS染色和绿色荧光显微观察 Figure 5 The GUS staining and green fluorescence observation of P. brocae or transformant hyphae. The left and the right part is the hyphae of P. brocae and transformant, respectively. |

|
| 图 6 矮牵牛根部接种P. brocae转化子2周后的GUS染色结果 Figure 6 GUS staining of P. hybrida root that was harvested 2 weeks after P. brocae transformant inoculation. A: GUS staining result of P. hybrida root inoculated with wild type P. brocae (W) and transformant (M); B: The bright field of P. hybrida root inoculated with transformant. |
2.3 溶磷能力改变的转化子插入位点鉴定
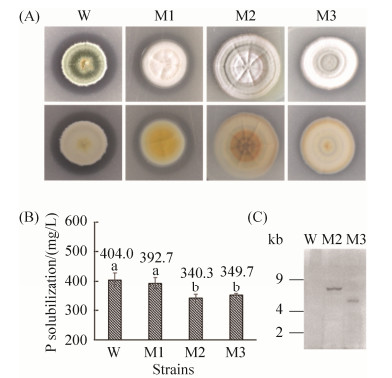
将转化子转移到含有Ca3(PO4)2的PVK平板上,从中随机挑选出3株与野生型对比在产生溶磷圈的能力或形态上具有肉眼可见的显著改变的转化子(图 7-A),第一列为野生型菌落(正、反面),其余三列为转化子。在液体培养条件下,转化子M1的溶磷能力较野生型W无显著差异;转化子M2和M3的溶磷能力较W有显著降低,且降幅分别达到15.8%和13.4%(P≤0.05)(图 7-B),说明T-DNA插入影响了M2和M3的溶磷能力。Southern blotting分析表明,M2和M3均为T-DNA单拷贝插入(图 7-C),可以通过TAIL-PCR获取插入位点信息,但最终只扩增出了M3的右翼序列(图 8)。图 8-A为3轮TAIL-PCR的扩增产物电泳结果,对第3轮PCR产物的电泳条带(图 8-A中的Target band)进行回收测序,通过序列分析找到了T-DNA在野生型基因组上的插入位点(图 8-C),右侧为野生型基因组上的部分序列信息(未完全列出)。为了验证测序结果的准确性,在测序所得的序列上设计了反向互补引物Cof (5′-GTTATTTGATGCGCA AAGTC-3′),并分别利用RB1、RB2和RB3三个嵌套引物作为上游引物,以转化子基因组和野生型为模板进行PCR,结果如图 8-B所示,验证了测序的准确性。另外,将获得的DNA序列或经翻译后获得肽段提交NCBI(national center for biotechnology information)数据库进行序列比对,均未获得有价值的信息。
|
| 图 7 野生型和转化子的溶磷能力和Southern blotting分析 Figure 7 Phosphate (P) solubilization capacity of P. brocae and transformants and Southern blotting analysis. A: P. brocae and transformants on Ca3(PO4)2 containing medium (5 g/L), the first line were the front views of strain colonies and the second line were the reverse views; B: Phosphate solubilization capacity of P. brocae and transformants in PVK liquid medium [Ca3(PO4)2 containing]; C: The Southern blotting result of P. brocae and transformants genome digested with KpnⅠ. |

|
| 图 8 利用TAIL-PCR获取转化子基因组上插入位点 Figure 8 TAIL-PCR was used to identify the sequence of insertion site and flanking sequence of right border in transformant genome. A: Gel electrophoresis of the three PCR reaction products of TAIL-PCR. M is marker, lane 1, 2, 3 were the PCR products with RB1/AD, RB2/AD and RB3/AD primer pairs, respectively. Transformant genome was used as template for lane 1. B: Gel electrophoresis of wild type or transformant genome PCR products with RB1/Cof, RB2/Cof and RB3/Cof. C: The sequence and location of primers RB1, RB2 and RB3 on right border of T-DNA insertion fragment. Tm is annealing temperature and the reversed solid triangle indicate the insertion site. |
2.4 P. brocae的促生效应
与对照(T1)相比,单独添加磷矿粉(T2)或P. brocae孢子悬液(T3)并不能显著增加矮牵牛的鲜重和干重,但磷矿粉和P. brocae孢子悬液共同施用(T4)对矮牵牛的鲜重和干重具有显著促进作用(P≤0.05)(图 9)。T4处理组鲜重较T1、T2和T3分别高出35.4%、35.6%和21.4%,T4处理组干重较T1、T2以及T3分别高出25.1%、37.8%和26.0%。

|
| 图 9 不同处理对矮牵牛鲜重和干重的影响 Figure 9 The effects of different treatments on the fresh and dry weight of P. hybrida. Quantification of fresh (A) and dry (B) weight per plant. Data indicate mean±S.D. (n=9). T4 treatment plant fresh weight was significantly higher than the other treatments and there were no significant difference among T1, T2 and T3. Different letters indicate significant differences, P≤0.05. |
3 讨论
自ATMT转化技术首次使用以来,目前至少已经实现了60多种丝状真菌的遗传转化[17]。虽然总体上转化步骤没有太大差异,但是对于不同真菌最佳转化条件的要求却有很大区别。目前转化条件优化的主要因素包括农杆菌和真菌孢子的浓度和比例,诱导和共培养阶段的时间长度、AS浓度、温度以及共培养介质(尼龙膜、玻璃纸、杂交膜、硝酸纤维素膜等)。绝大多数研究表明,在共培养阶段添加AS能够激活农杆菌的vir基因,促进农杆菌对丝状真菌的转化效率,如Zhang等[18]研究表明在诱导和共培养阶段,随着AS浓度(0– 800 μmol/L)的增加,农杆菌AGL-1对Lecanicillium lecanii的转化效率提升显著;也有研究表明,在共培养阶段添加AS对Cordyceps militaris的转化效率并无显著影响,可能与C. militaris自身代谢产生了某种能够诱导vir基因表达的代谢产物有关[19]。本研究表明,在50– 200 μmol/L,随着AS浓度升高,AGL-1对P. brocae的转化效率也呈现上升趋势,因此可以考虑继续提高AS浓度来提高转化效率。在针对Fusarium oxysporum的转化研究中发现,当AS浓度由200 μmol/L增加到300 μmol/L时,转化效率无显著提高,并且高浓度的AS会导致T-DNA插入拷贝数的增加,为后续研究增加了难度[20]。因此,也需要在平衡转化效率和插入拷贝数之间选择一个合适的AS浓度,不能单纯从提高转化效率角度出发。Mullins等[21]研究发现,在共培养之前用AS诱导农杆菌可以缩短产生转化子的时间,但是将单拷贝T-DNA插入的转化子数量由80%降低到53%。
共培养时间和孢子浓度也是影响转化效率的重要因素,在AGL-1转化P. digitatum的研究过程中发现,当孢子浓度低于104个/mL或共培养时间低于24 h会严重影响转化效率,甚至检测不到转化子[22]。笔者也发现当孢子浓度为104个/mL时可以检测到少量转化子,随着孢子浓度提升,转化子数量显著增加,但浓度从106个/mL提高到107个/mL时则变化不明显。在设置的时间梯度内,共培养时间在48 h最佳,延长至60 h反而有所降低。观察发现培养时间在60 h时,IM平板上农杆菌快速生长,孢子萌发量也显著增加,导致玻璃纸上菌落连接成片,给转化子筛选带来难度,这可能是导致挑选出来的转化子数量低的原因之一,在农杆菌介导的其他真菌转化体系中也有类似现象发生[23]。
本文通过TAIL-PCR从一株溶磷能力与野生型有显著差异的转化子中克隆出一段P. brocae基因组序列,但通过与NCBI数据库中的序列比对分析后,未获得该序列或其同源序列的描述,可能是由于插入位点位于非编码区或者数据库中尚未有相关序列的收录。真核生物中非编码区的外源基因插入也可能会影响某些基因的转录水平,进而影响转化子的表型,诸多研究表明一些非编码序列参与了启动子的活性调控[24-25]。目前,TAIL-PCR已经被广泛用于不同物种基因组中外源插入片段两侧未知序列的克隆,但关于这些序列进一步的功能分析尚受限于该物种基因组序列的测序情况和研究深度。
目前,溶磷菌在植物根部的定殖已经被报道,如王珍等研究了GFP标记的溶磷巨大芽孢杆菌(Bacillus megaterium)和蜡状芽孢杆菌(B. cereus)在小白菜根系不同组织上的定殖[26-27]。P. brocae属于某些植物的内生菌,并且在土壤环境中也有存在[28-29],这里通过ATMT成功实现了青霉菌的荧光和GUS标记,为研究该菌在植物根际的定殖提供了可行的技术依据。笔者发现在无菌栽培条件下,向矮牵牛根部接种带GUS标记的P. brocae,两周后对矮牵牛根系进行GUS染色,可在矮牵牛根表观察到染色的菌丝附着,但目前尚未在自然土壤栽培条件下观察到外源接种的P. brocae在矮牵牛根表附着的现象。此外,青霉菌属溶磷菌能够提升土壤有效磷素含量以及促生效果已经从不同方面得到了验证。史发超等通过玉米栽培田间试验证明了斜卧青霉菌(P. decumbens)菌剂具有非常显著的促生效果[30],类似的研究报道还包括草酸青霉菌、棘孢青霉菌等[31-32]。本文也通过栽培试验验证了P. brocae对矮牵牛的促生作用,但尚缺乏关于促生作用分子机制的探索,未来应加强这方面的研究。
| [1] |
Yang S, Yang T, Lin B, Liu XZ, Xiang MC. Isolation and evaluation of two phosphate-dissolving fungi. Acta Microbiologica Sinica, 2018, 58(2): 264-273.
(in Chinese) 杨顺, 杨婷, 林斌, 刘杏忠, 向梅春. 两株溶磷真菌的筛选、鉴定及溶磷效果的评价. 微生物学报, 2018, 58(2): 264-273. |
| [2] | Whitelaw MA, Harden TJ, Helyar KR. Phosphate solubilisation in solution culture by the soil fungus Penicillium radicum. Soil Biology and Biochemistry, 1999, 31(5): 655-665. DOI:10.1016/S0038-0717(98)00130-8 |
| [3] | Sane SA, Mehta SK. Isolation and evaluation of rock phosphate solubilizing fungi as potential biofertilizer. Journal of Fertilizers & Pesticides, 2015, 6(2): 1000156. |
| [4] | Liu ST, Lee LY, Tai CY, Hung CH, Chang YS, Wolfram JH, Rogers R, Goldstein AH. Cloning of an Erwinia herbicola gene necessary for gluconic acid production and enhanced mineral phosphate solubilization in Escherichia coli HB101:nucleotide sequence and probable involvement in biosynthesis of the coenzyme pyrroloquinoline quinone. Journal of Bacteriology, 1992, 174(18): 5814-5819. DOI:10.1128/JB.174.18.5814-5819.1992 |
| [5] | Goldstein AH, Liu ST. Molecular cloning and regulation of a mineral phosphate solubilizing gene from Erwinia herbicola. Bio/Technology, 1987, 5(1): 72-24. |
| [6] | Miller SH, Browne P, Prigent-Combaret C, Combes-Meynet E, Morrissey JP, O'gara F. Biochemical and genomic comparison of inorganic phosphate solubilization in Pseudomonas species. Environmental Microbiology Reports, 2010, 2(3): 403-411. |
| [7] |
Jiao ZW, Wu WL, Guo YB. Effect of glucose dehydrogenase on mineral phosphate solubilization with different carbon sources in Rahnella aquatilis HX2. Xinjiang Agricultural Sciences, 2015, 52(2): 268-274.
(in Chinese) 焦子伟, 吴文良, 郭岩彬. 不同碳源条件下GDH对植物促生菌HX2溶解无机磷影响的研究. 新疆农业科学, 2015, 52(2): 268-274. |
| [8] | 殷中伟.真菌溶磷相关基因的克隆与功能分析.中国农业大学博士学位论文, 2015. |
| [9] | 江红梅.真菌溶磷相关基因的克隆与功能验证.中国农业科学院博士学位论文, 2018. |
| [10] |
Yang CD, Liu G, Zheng YZ, Xing M. Application of Agrobacterium tumefaciens in transformation of filamentous fungi. Letters in Biotechnology, 2006, 17(5): 784-787.
(in Chinese) 杨长得, 刘刚, 郑易之, 邢苗. 根癌农杆菌在丝状真菌转化中的应用. 生物技术通讯, 2006, 17(5): 784-787. DOI:10.3969/j.issn.1009-0002.2006.05.032 |
| [11] | De Groot MJA, Bundock P, Hooykaas PJJ, Beijersbergen AGM. Agrobacterium tumefaciens-mediated transformation of filamentous fungi. Nature Biotechnology, 1998, 16(9): 839-842. DOI:10.1038/nbt0998-839 |
| [12] |
Chen YJ, Wei JF. Progress on the research of genetic transformation of filamentous fungi. Journal of Yunnan Agricultural University, 2009, 24(3): 448-454.
(in Chinese) 陈赟娟, 韦建福. 丝状真菌遗传转化的研究进展. 云南农业大学学报, 2009, 24(3): 448-454. DOI:10.3969/j.issn.1004-390X.2009.03.025 |
| [13] | Huang JJ, Ma ZH, Zhong GW, Sheppard DC, Zhang SZ. The mitochondrial thiamine pyrophosphate transporter tptA promotes adaptation to low iron conditions and virulence in fungal pathogen Aspergillus fumigatus. Virulence, 2019, 10(1): 234-247. DOI:10.1080/21505594.2019.1596505 |
| [14] | Lu YY, Xiao SQ, Wang F, Sun JY, Zhao LK, Yan LB, Xue CS. Agrobacterium tumefaciens-mediated transformation as an efficient tool for insertional mutagenesis of Cercospora zeae-maydis. Journal of Microbiological Methods, 2017, 133: 8-13. DOI:10.1016/j.mimet.2016.12.010 |
| [15] | Florencio CS, Brandão FAS, De Mello TM, Bocca AL, Felipe MSS, Vicente VA, Fernandes L. Genetic manipulation of Fonsecaea pedrosoi using particles bombardment and Agrobacterium mediated transformation. Microbiological Research, 2018, 207: 269-279. DOI:10.1016/j.micres.2018.01.001 |
| [16] | Sun JY, Xu RD, Xiao SQ, Lu YY, Zhang QF, Xue CS. Agrobacterium tumefaciens-mediated transformation as an efficient tool for insertional mutagenesis of Kabatiella zeae. Journal of Microbiological Methods, 2018, 149: 96-100. DOI:10.1016/j.mimet.2018.05.004 |
| [17] |
Jia PS, Ding LL, Zhou BJ, Guo HS, Gao F. Construction of a T-DNA insertional mutant library for Verticillium dahliae Kleb. and analysis of a mutant phenotype. Cotton Science, 2012, 24(1): 62-70.
(in Chinese) 贾培松, 丁丽丽, 周邦军, 郭惠珊, 高峰. 棉花黄萎病菌T-DNA插入突变体库的构建及其表型分析. 棉花学报, 2012, 24(1): 62-70. DOI:10.3969/j.issn.1002-7807.2012.01.008 |
| [18] | Zhang YJ, Zhao JJ, Xie M, Peng DL. Agrobacterium tumefaciens-mediated transformation in the entomopathogenic fungus Lecanicillium lecanii and development of benzimidazole fungicide resistant strains. Journal of Microbiological Methods, 2014, 105: 168-173. DOI:10.1016/j.mimet.2014.07.033 |
| [19] | Zheng ZL, Huang CH, Cao L, Xie CH, Han RC. Agrobacterium tumefaciens-mediated transformation as a tool for insertional mutagenesis in medicinal fungus Cordyceps militaris. Fungal Biology, 2011, 115(3): 265-274. DOI:10.1016/j.funbio.2010.12.011 |
| [20] |
Mao C, Dai QD, Wang J, Liu YX, Yang LY, Guo LJ, Huang JS. Establishment of ATMT Fusarium oxysporum f. sp. cubense race 4 effective transformation system and screening of the T-DNA insertional mutants. Journal of Southern Agriculture, 2013, 44(12): 1985-1991.
(in Chinese) 毛超, 戴青冬, 汪军, 刘一贤, 杨腊英, 郭立佳, 黄俊生. 香蕉枯萎病菌4号生理小种农杆菌介导遗传转化体系的建立及T-DNA插入突变体的筛选. 南方农业学报, 2013, 44(12): 1985-1991. DOI:10.3969/j:issn.2095-1191.2013.12.1985 |
| [21] | Mullins ED, Chen X, Romaine P, Raina R, Geiser DM, Kang S. Agrobacterium-mediated transformation of Fusarium oxysporum:an efficient tool for insertional mutagenesis and gene transfer. Phytopathology, 2001, 91(2): 173-180. DOI:10.1094/PHYTO.2001.91.2.173 |
| [22] | Vu TX, Ngo TT, Mai LTD, Bui TT, Le DH, Bui HTV, Nguyen HQ, Ngo BX, Tran VT. A highly efficient Agrobacterium tumefaciens-mediated transformation system for the postharvest pathogen Penicillium digitatum using DsRed and GFP to visualize citrus host colonization. Journal of Microbiological Methods, 2018, 144: 134-144. DOI:10.1016/j.mimet.2017.11.019 |
| [23] | Liu N, Chen GQ, Ning GA, Shi HB, Zhang CL, Lu JP, Mao LJ, Feng XX, Liu XH, Su ZZ, Lin FC. Agrobacterium tumefaciens-mediated transformation:an efficient tool for insertional mutagenesis and targeted gene disruption in Harpophora oryzae. Microbiological Research, 2016, 182: 40-48. DOI:10.1016/j.micres.2015.09.008 |
| [24] | Gius D, Grossman S, Bedell MA, Laimins LA. Inducible and constitutive enhancer domains in the noncoding region of human papillomavirus type 18. Journal of Virology, 1988, 62(3): 665-672. DOI:10.1128/JVI.62.3.665-672.1988 |
| [25] | Elkon R, Agami R. Characterization of noncoding regulatory DNA in the human genome. Nature Biotechnology, 2017, 35(8): 732-746. DOI:10.1038/nbt.3863 |
| [26] |
Wang Z, Cao CL, Ku YL, Xu GY, Lin YB, Yang XD. Bacillus megatherium WY4 labeled by GFP and its colonization in Chinese cabbage (Brassica chinensis). Journal of Agricultural Biotechnology, 2016, 24(12): 1925-1934.
(in Chinese) 王珍, 曹翠玲, 库永丽, 徐国益, 林雁冰, 柳晓东. 巨大芽胞杆菌WY4的GFP标记及其在小白菜上的定殖. 农业生物技术学报, 2016, 24(12): 1925-1934. |
| [27] | Wang Z, Xu GY, Ma PD, Lin YB, Yang XN, Cao CL. Isolation and characterization of a phosphorus-solubilizing bacterium from rhizosphere soils and its colonization of Chinese cabbage (Brassica campestris ssp. chinensis). Frontiers in Microbiology, 2017, 8: 1270. DOI:10.3389/fmicb.2017.01270 |
| [28] | Meng LH, Zhang P, Li XM, Wang BG. Penicibrocazines A-E, five new sulfide diketopiperazines from the marine-derived endophytic fungus Penicillium brocae. Marine Drugs, 2015, 13(1): 276-287. DOI:10.3390/md13010276 |
| [29] | Pradeep S, Faseela P, Josh MKS, Balachandran S, Devi RS, Benjamin S. Fungal biodegradation of phthalate plasticizer in situ. Biodegradation, 2013, 24(2): 257-267. DOI:10.1007/s10532-012-9584-3 |
| [30] |
Shi FC, Yin ZW, Jiang HM, Fan BQ. Screening, identification of P-dissolving fungus P83 strain and its effects on phosphate solubilization and plant growth promotion. Acta Microbiologica Sinica, 2014, 54(11): 1333-1343.
(in Chinese) 史发超, 殷中伟, 江红梅, 范丙全. 一株溶磷真菌筛选鉴定及其溶磷促生效果. 微生物学报, 2014, 54(11): 1333-1343. |
| [31] |
Zhang L, Fan BQ, Huang WY. Study on transformation of P-dissolving Penicillium oxalicum P8 with double-marker vector expressing green fluorescent protein and hygromycin B resistance. Acta Microbiologica Sinica, 2005, 45(6): 842-845.
(in Chinese) 张磊, 范丙全, 黄为一. 绿色荧光蛋白和潮霉素抗性双标记载体转化草酸青霉菌P8的研究. 微生物学报, 2005, 45(6): 842-845. DOI:10.3321/j.issn:0001-6209.2005.06.005 |
| [32] |
Gong MB, Fan BQ, Wang HY. Isolation and identification of a novel phosphate-dissolving strain Penicillium aculeatum Z32 and its colonization and phosphate-dissolving characteristics in soil. Acta Microbiologica Sinica, 2010, 50(5): 580-585.
(in Chinese) 龚明波, 范丙全, 王洪媛. 一株新的溶磷棘孢青霉菌Z32的分离、鉴定及其土壤定殖与溶磷特性. 微生物学报, 2010, 50(5): 580-585. |
 2021, Vol. 61
2021, Vol. 61