中国科学院微生物研究所,中国微生物学会,中国菌物学会
文章信息
- 冯莹莹, 徐兴然, 邹祥. 2021
- Yingying Feng, Xingran Xu, Xiang Zou. 2021
- 钙调磷酸酶信号调控真菌生长代谢、毒力及抗逆性能
- Calcineurin signaling cascade regulates fungal growth, metabolism, virulence and stress resistance
- 微生物学报, 61(12): 3844-3855
- Acta Microbiologica Sinica, 61(12): 3844-3855
-
文章历史
- 收稿日期:2021-02-18
- 修回日期:2021-04-21
- 网络出版日期:2021-05-13
自然界真菌具有快速感知并适应各种环境的能力,其中钙调磷酸酶在该过程中发挥着非常重要的作用[1]。近年来,钙调磷酸酶信号级联反应已在真核生物中进行广泛研究,真菌通过钙泵或Ca2+通道来控制细胞质中的钙离子浓度[2],胞质Ca2+结合并激活包含Ca2+结合基序的蛋白质[3];Ca2+调节蛋白(钙调蛋白,calmodulin,CaM)是胞质钙离子的代表性传感器蛋白,可通过钙调蛋白结合蛋白(包括钙调磷酸酶)、钙调蛋白依赖性蛋白激酶和组蛋白脱乙酰基酶将钙离子信号转导为相应的响应信号,从而响应细胞的内部或外部信号[4]。
钙调磷酸酶作为Ca2+-钙调磷酸酶信号转导途径中的重要元件,在真菌中普遍保守[5]。钙调磷酸酶是一种丝氨酸/苏氨酸(Ser/Thr)蛋白磷酸酶,其通过使真核生物中的靶蛋白去磷酸而起作用[6]。在真菌中,Ca2+-钙调磷酸酶信号转导途径也具有保守性,并且参与许多生物学过程,例如细胞生长、细胞壁完整性的维持和应激反应等[1];在致病性真菌中,钙调磷酸酶信号通过多种机制参与其毒力,包括入侵和适应宿主或不同环境、形成感染性繁殖体以及与宿主相互作用等[7]。此外,钙调磷酸酶信号对调控真菌代谢产物的生成也有重要影响,同时与抗真菌药物耐药性有关,是治疗真菌感染的关键靶标[5]。因此,本文综述了钙调磷酸酶信号对真菌生长发育、毒力形成、抗逆性以及代谢产物合成的影响,并对钙调磷酸酶信号作为真菌合成生物学重要元件挖掘进行展望。
1 钙调磷酸酶相关信号转导途径及其组成元件钙调磷酸酶作为钙信号转导途径中的关键调控中心,在生命过程中发挥着重要作用[8]。另外,钙调磷酸酶是真菌中唯一受钙离子和钙调蛋白调节的磷酸酶,其下游调控网络靶向钙调磷酸酶依赖性转录因子,包括Crz1等,Crz1激活其靶基因的转录,从而调控细胞的生长、发育等生命活动[9]。
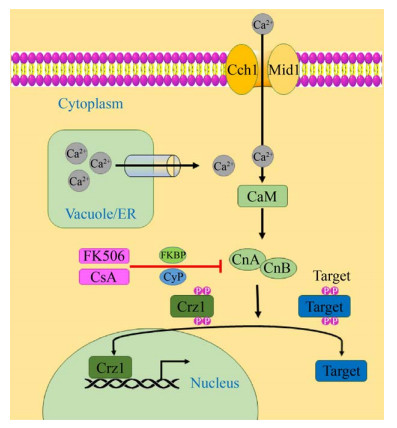
1.1 钙调磷酸酶及其上游信号转导途径钙调磷酸酶属于Ser/Thr蛋白磷酸酶家族成员,具有铁和锌离子双核金属中心的金属酶[10],以异源二聚体的形式存在,由催化亚基钙调磷酸酶A (CnA)和调节亚基钙调磷酸酶B (CnB)组成[6]。钙调磷酸酶上游信号传导途径由Ca2+通道(Cch1)、转运蛋白(Mid1)、钙离子感应蛋白(CaM)、钙调蛋白依赖性磷酸酶(钙调磷酸酶,CnA-钙调磷酸酶A亚基/CnB-钙调磷酸酶B亚基)以及和钙调磷酸酶相互作用的蛋白(即免疫亲和蛋白,FKBP、CyP)组成(图 1)。
在真核细胞中,Ca2+作为广泛存在的细胞内信使调控许多细胞过程,如细胞增殖、细胞程序性死亡等[9]。Ca2+主要通过高亲和力的Ca2+内流系统(HACS)进入细胞膜[12]。除此之外,真菌还通过Ca2+储存细胞器(如液泡、内质网和高尔基体)中的转运蛋白来维持Ca2+稳态[1]。在酵母中,液泡充当主要的Ca2+贮藏库,液泡膜包含多个Ca2+转运蛋白,包括Yvc1 (液泡电导蛋白)、Vcx1 (H+/Ca2+交换蛋白)和Pmc1 (Ca2+-ATP酶)。在高尔基体和内质网中,Ca2+泵Pmr1、Cod1和Eca1也起着恢复细胞质Ca2+基础水平的作用[13]。
CaM可以检测到胞质Ca2+浓度的变化[4],Ca2+-钙调蛋白复合物与钙调蛋白结合蛋白结合,包括钙调磷酸酶、钙调蛋白依赖性蛋白激酶和组蛋白脱乙酰基酶[6]。在低钙离子浓度下,CnB亚基与钙调蛋白结合域相互作用,而自抑制域则阻断CnA亚基的催化位点;当Ca2+浓度高时,钙离子与钙调蛋白和CnB亚基结合,导致CnB亚基的构象变化,钙调蛋白与CnA亚基结合,从底物结合位点释放自抑制域,从而激活钙调磷酸酶去磷酸化靶标[6]。
免疫亲和蛋白家族是一类广泛存在于真核生物体内并在结构上高度保守的多功能蛋白质,其中包括与环孢菌素(CsA)结合的CyP家族及与他克莫司(FK506)结合的FKBP家族,它们均具有催化含脯氨酸的寡肽底物顺反异构作用的肽脯氨酰顺反异构酶。免疫抑制剂CsA和FK506分别通过免疫亲和蛋白CyP和FKBP抑制钙调磷酸酶的A、B亚基,从而抑制钙信号级联反应。
钙调磷酸酶信号转导级联的几个组成部分在寄生原生生物和真菌之间是相似的。大多数真菌都含有钙调磷酸酶和钙调蛋白直系同源物,它们具有相似的结构域和功能。质膜Ca2+-ATPase (PMCA)是钙离子从细胞外环境流入的主要通道,在寄生生物中起着与真菌中的HACS通道相似的作用[12]。
1.2 钙调磷酸酶的下游调控途径在许多真菌中,反式激活因子Crz1是钙调磷酸酶下游的主要调控因子,钙调磷酸酶-Crz1途径也是钙离子诱导的细胞应答的主要信号传导模块[14]。Crz1包含一个或多个与C末端DNA结合的C2H2锌指结构域,是真菌中保守的钙调磷酸酶靶标。脱磷酸化的Crz1易位到细胞核中并激活Crz1依赖基因的表达,其中包括参与几丁质合成(Chs7)和膜转运(Pmc1)的基因等[15]。
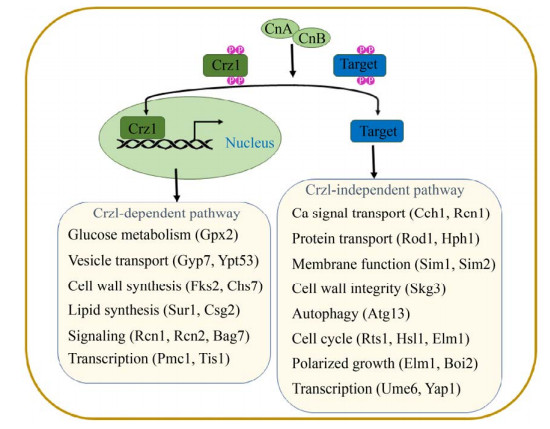
钙调磷酸酶的下游调控途径在模式真菌酿酒酵母中已有充分的研究,去磷酸化的Crz1进入细胞核,并与靶启动子中依赖钙调磷酸酶、依赖Crz1的反应元件(CDREs)结合,调节诸如Fks2、Pmc1、Gyp7、Sur1、Gpx2和Rcn1等基因(图 2)[16]。这些Crz1依赖性基因具有相关功能,例如细胞壁完整性、离子转运和葡萄糖代谢。虽然Crz1依赖性基因在不同的真菌物种中具有相似的作用,但一部分Crz1依赖性基因在真菌之间是保守的。包括酿酒酵母、烟曲霉和白色念珠菌等,具有相似的CDRE基序,但其他真菌中的Crz1直系同源物可识别不同的CDRE序列[15]。Crz1及其直系同源物的功能是保守的,但是Crz1调控网络具有物种特异性,并且对各种应力的反应不同。
尽管Crz1及其直系同源物是许多真菌中关键的钙调磷酸酶靶标,但有一些基因独立于Crz1之外,来控制许多细胞功能。如酿酒酵母中的Rts1、Rcn1和Atg13等基因,参与调控细胞周期、自噬蛋白质转运等过程(图 2)[9]。
也有研究表明转录因子Fg01350是独立于Crz1的钙调磷酸酶下游靶标,该基因缺失会导致禾谷镰刀菌毒力下降[17]。与钙调磷酸酶突变菌株相比,Crz1突变菌株导致生长或毒力缺陷的表型较少,这表明钙调磷酸酶还以不依赖Crz1的方式协调细胞功能[14–15]。因此,需要进一步的研究以阐明钙调磷酸酶在真菌中的作用,并全面了解该途径如何控制真菌的生长代谢。
2 钙调磷酸酶调控真菌生长及毒力形成钙调磷酸酶通过参与真菌胞内钙信号级联途径,从而控制与靶基因、靶蛋白相关的生命过程,最终调控真菌生长、发育和毒力形成来响应外界环境因素的变化,使真菌能够更好地适应环境,维持正常的生命活动。钙调磷酸酶信号通过多种机制参与毒力的形成,并且真菌毒力的形成与其生长发育过程密切相关。
2.1 钙调磷酸酶调控真菌生长发育钙调磷酸酶信号调节与细胞壁完整性相关基因的表达,参与传染性结构或繁殖体的形成,以及在许多致病真菌的双态转变过程中起关键作用。钙调磷酸酶缺陷型真菌菌株显示出无性孢子和有性结构的形成减少,并且无法完成形态发生转变[18]。钙调磷酸酶信号通路还控制病原性真菌与宿主的相互作用,例如控制真菌孢子的疏水性、真菌与宿主细胞受体的结合、宿主肌动蛋白的解聚等[19]。在裂殖酵母中,钙调磷酸酶的突变导致细胞极性缺陷以及胞质分裂延迟,钙调磷酸酶的过表达导致细胞形态异常[20]。而钙调磷酸酶的缺失导致细胞壁葡聚糖的合成模块Rho1-GTPase活性降低并伴有严重的细胞壁缺陷[21]。在酵母中,组成型钙调磷酸酶的缺失会导致胞质分裂缺陷[22]。在氮限制条件下,钙调磷酸酶信号在调控新生隐球菌有性繁殖、细胞融合和无性单倍体结实过程中的菌丝延伸中发挥重要作用[23]。钙调磷酸酶抑制剂磷脂结合蛋白Cts1与钙调磷酸酶协同可以控制细胞定位和细胞分离[24]。在新孢梭菌Cnb1基因突变体中,Fks1基因表达量增加,该基因负责细胞壁生物合成的β-1, 3-葡聚糖合酶复合物的成分[25]。
此外,钙调磷酸酶信号在构巢曲霉的细胞周期进程[26]、米曲霉的压力适应[27]、核盘菌菌核发育[28]以及稻瘟病菌感染结构“附着胞”的形成[29]中也发挥关键作用。在坏死真菌灰葡萄孢中,钙调磷酸酶的缺失会引起细胞壁和膜完整性缺陷,导致分生孢子和菌丝穿透叶片速度减慢,且对阳离子胁迫的响应也有所减弱[26]。在玉米黑穗菌中,钙调磷酸酶信号途径是其适应温度、pH、阳离子和氧化胁迫等多种环境胁迫所必需的[30]。钙调磷酸酶基因的缺失,除导致繁殖速度减慢及毒力降低外,由于细胞壁完整性缺陷而引起的细胞形态变化也很明显[30]。
2.2 钙调磷酸酶信号调控真菌毒力形成钙调磷酸酶信号对于菌体发育、形态转变、附着胞的形成、阳离子稳态和应激反应都是必需的[7],这些也是真菌病原体毒力形成和维持的前提。例如,钙调磷酸酶在维持真菌细胞壁完整性的过程中发挥重要作用,而细胞壁的完整性有助于真菌病原体在宿主环境中适应并存活。
对白色念珠菌[31]、假丝酵母[32]、隐球菌[33]和烟曲霉[34]等人类真菌病原体,核盘菌属[28]、灰葡萄孢[26]、稻瘟病菌[29]和玉米黑粉菌[35]等植物真菌病原体及白僵菌等昆虫病原体的毒力感染机制进行研究发现,尽管这些真菌具有不同的感染模式,但是其钙调磷酸酶在毒力或致病性状中具有保守作用。钙调磷酸酶对致病真菌毒力的调控机制如表 1所示。
| Pathogenic fungi | Virulence regulation mechanism | References |
| Cryptococcus gattii | Maintain cell wall integrity | [33] |
| Candida albicans | [36] | |
| Mucor circinelloides | [18] | |
| Sclerotinia sclerotiorum | [28] | |
| Beauveria bassiana | [37] | |
| Aspergillus fumigatus | Promote sporulation | [34] |
| Sclerotinia sclerotiorum | [28] | |
| Fusarium oxysporum | Promote hyphae extension | [38] |
| Aspergillus fumigatus | [34] | |
| Candida albicans | Regulate morphological changes | [36] |
| Mucor circinelloides | [18] | |
| Cryptococcus neoformans | Enhanced heat resistance | [39] |
| Cryptococcus gattii | [33] | |
| Candida glabrata | [40] | |
| Candida albicans | Maintain growth in serum | [31] |
| Candida dubliniensis | [32] | |
| Candida glabrata | [40] |
人类真菌病原体中,白色念珠菌是侵袭免疫力低下患者的主要病原体之一,钙调磷酸酶信号是白色念珠菌形态转变、唑耐受、膜应激、血清存活和毒力所必需的,其他种类的念珠菌中也有类似的作用,如光滑念球菌等[31, 36]。光滑念球菌在宿主体内温度(37 ℃)下生长的毒力属性也是受钙调磷酸酶信号传导途径控制[40]。此外,在碱性pH值下生长、繁殖和菌丝伸长也需要钙调磷酸酶信号的调控[41]。钙调磷酸酶信号在细胞壁完整性、应激反应、无性孢子产生和磷酸盐转移中起着至关重要的作用,所有这些都与烟曲霉的毒力有关[34]。研究表明,烟曲霉中单个钙调磷酸酶A或B亚基或两个亚基的缺失会导致菌丝生长、分生孢子萌发、压力适应和细胞壁形成缺陷进而导致毒力缺陷[42]。此外,与野生型菌株相比,钙调磷酸酶催化亚基A突变体在动物模型中无毒或毒力减弱,在肺组织中其真菌负荷降低[34]。钙调磷酸酶的突变会导致组织入侵期间抑制菌丝延伸,从而降低宿主死亡率。另外,在鼠类系统性感染模型和其他模型系统中,钙调磷酸酶催化亚基的缺失导致假丝酵母菌的毒力减弱,表明钙调磷酸酶信号是假丝酵母毒力所必需的[43]。在环毛霉菌中,钙调磷酸酶信号协调酵母细胞状-菌丝状双态转变和真菌孢子大小,这是导致细菌致病力的关键过程,并改变宿主与病原体的相互作用(吞噬体成熟和宿主细胞死亡)[18]。
丝状植物致病真菌的研究中,钙调磷酸酶信号对于菌核盘菌中的菌核发育和稻瘟病菌的传染性结构贴壁的形成很重要[28–29]。在性坏死真菌灰葡萄孢中,Crz1的缺失会引起细胞壁和膜完整性的缺陷,以及菌丝穿透植物组织的能力缺陷[26]。在玉米黑粉菌中,钙调磷酸酶信号是其适应各种环境压力、维持细胞壁完整性、繁殖和毒力所必需的,缺失钙调磷酸酶催化亚基导致多重出芽和有性繁殖[30]。
昆虫病原性真菌的研究中,白僵菌钙调磷酸酶催化亚基的缺失导致所产生的分生孢子数量减少,并且对各种胁迫条件的敏感性增加,也会导致细胞壁缺陷、对宿主的侵染力降低[37]。钙调磷酸酶信号与真菌毒力形成关系密切,但是对于该调控网络的研究较少,其具体的调控机制还需进一步明确。
3 钙调磷酸酶信号在调控真菌抗逆性中的作用真菌细胞会面临多种环境压力,主要包括酸碱、高渗、氧化胁迫以及抗真菌药物等,为了应对各种环境压力,真菌细胞必须快速感知这些压力信号,进而通过一系列应答反应,减少环境压力所造成的损伤。有研究表明,钙调磷酸酶信号在真菌应对环境压力并作出反应的过程中发挥重要作用[7]。钙调磷酸酶被因压力引起的Ca2+含量升高激活,并通过使蛋白质底物脱磷酸化而转导信号,诱导应激基因的表达。
酵母Cnb1基因突变后对碱性环境的敏感性增加,表明钙调磷酸酶信号对高pH耐受性具有一定的调控作用,研究证明钙调磷酸酶在介导Crz1转录因子的去磷酸化及其进入细胞核过程中诱导了几种高pH响应基因的表达,包括ENA1、PHO84、PHO89和PHO12等[44]。pH值升高时,钙通过Cch1-Mid1通道进入,可能会激活钙调磷酸酶[45]。然而,Crz1突变体对碱性pH无敏感性,表明了钙调磷酸酶信号也可以通过与其他碱性响应基因进行调控,这类基因包括Hph1和Hph2等[46]。
钙调磷酸酶使酿酒酵母对NaCl和LiCl具有一定的耐受性,并且由于Cnb1基因突变导致Ena1基因表达降低和钾离子转运系统缺陷,进而导致胞内高浓度锂离子的积累[47]。此外,有研究结果表明盐胁迫会引起细胞质中钙离子浓度的改变,并且钙调磷酸酶信号通过控制Na+外排和K+吸收系统来调控NaCl胁迫适应过程[48]。还有研究发现,与酿酒酵母Cnb1基因突变体相似,白色念珠菌Cna基因突变体对钙、锂或钠离子更敏感[49]。另外,Ypi1 (1型蛋白磷酸酶Glc7的调节亚基)调节酿酒酵母中的阳离子耐受性也与钙调磷酸酶信号紧密相关[50]。
粟酒裂殖酵母中编码P型Ca2+/Mn2+-ATPase的基因Pmr1的缺失导致细胞形态呈圆形,并且Pmr1基因的表达依赖钙调磷酸酶,表明钙调磷酸酶信号在Mn2+稳态中具有关键调控作用[51]。此外,烟曲霉中编码高尔基P型Ca2+/Mn2+-ATPase的基因pmrA的缺失导致基础生长缺陷,这是由于阳离子耐受性受钙调磷酸酶途径基因表达调控[52]。CsA能够调节3种钙转运蛋白pmcA、pmcB和pmcC突变菌株对钙和锰盐的敏感性,从而揭示了它们对钙调磷酸酶的依赖性[53]。钙和锰离子浓度增加会引起烟曲霉CrzA (Crz1同源物)缺失菌株生长严重缺陷,此外还有分生孢子表面形态异常、钙转运蛋白mRNA表达量改变和毒力缺陷[54]。同时构巢曲霉CrzA突变体对碱性pH、高Ca2+和Mn2+浓度表现出敏感性,并介导了P型Ca2+-ATPase同源基因的表达[55]。在后续的结果中,阐明了CrzA的核质穿梭及其通过响应钙离子和碱性pH的磷酸化/去磷酸化,以及酪蛋白激酶和3β-糖原合酶激酶参与其调节的机制[56]。真菌抗逆性不仅受钙调磷酸酶的影响,还可能受整个钙调磷酸酶信号级联途径的调控。
4 钙调磷酸酶信号对真菌代谢产物的影响钙调磷酸酶信号作为胞内重要的信号传导系统,感受并将变化了的环境信号因子级联传递至细胞内,引起特定的基因表达,参与调控真菌代谢产物的生物合成。工业上,许多真菌发酵过程需要添加碳酸钙为中和剂,以维持稳定酸碱环境[57];此外,Ca2+和CO32–可能在真菌发酵过程中起调控作用[57–58]。钙信号转导工程作为一种增加高附加值代谢产物的方法,得到了广泛应用。已有研究证明,出芽短梗霉在产聚苹果酸(polymalic acid,PMA)过程中,Ca2+-PMA的形成需要CaCO3的存在[59–60]。Chi等[61]认为聚苹果酸合成酶(PMAs)是苹果酸聚合成PMA的关键酶。Ca2+信号途径中的转录激活因子Crz1控制PMAs基因的表达和PMA的生物合成,本课题组也发现类似现象;此外在存在CaCO3的情况下,分泌的PMA可以与培养基中的CaCO3反应形成Ca2+-PMA,避免了培养基中pH的降低和低pH值对细胞生长的抑制,这也可能对PMA生产有帮助[57]。Lu等也发现添加Ca2+可以调节钙/钙调蛋白信号转导来调节炭壳菌聚酮的生物合成基因,从而诱导炭壳菌聚酮的生物合成[62]。Chung认为Ca2+/CaM信号转导可能在烟隐孢子菌的头孢菌素生物合成中起关键作用,且表明维持内源性Ca2+稳态是该途径所必需的[63]。Xu等[64]发现Na+能调控灵芝中的钙调磷酸酶信号转导途径,进而提高灵芝酸(GA)产量。Zhang等[65]发现Ca2+参与调控热应激(HS)条件下灵芝中GA的生物合成,随后Liu等[66]进一步研究发现HS条件下,一氧化氮(NO)促进Ca2+浓度的增加和CaM基因表达。NO减少了HS诱导条件下GA的积累,并且Ca2+正调控GA的生物合成。钙信号对真菌代谢的调控作用如表 2所示。
| Fungus species | Regulatory factors | Effect | References |
| Torulopsis glabrata | Ca2+ | Increase the activity of pyruvate carboxylase | [59] |
| Saccharomyces cerevisiae | Ca2+ | Increase in malic acid production by about 5% | [60] |
| Yarrowia lipolytica | CaCO3 | As a Ca2+ provider and pH buffer in the production of α-ketoglutarate | [67] |
| Curvularia sp. | Ca2+ | Indolizidine alkaloid production increased by 2.33 times | [68] |
| Clostridium acetobutylicum | CaCO3 | Up-regulate most enzymes involved in glycolysis, redox balance and amino acid metabolism | [69] |
| Aureobasidium melanogenum | Crz1 | Control the expression of polymalate synthase gene and polymalate biosynthesis | [61] |
| Daldinia eschscholzii | Ca2+-CaM | Regulates the biosynthesis of polyketones from Xylaria spp., increasing the output by 2.59 times | [62] |
| Cercospora nicotianae | Ca2+-CaM | Regulate cephalosporin biosynthesis | [63] |
| Ganoderma lucidum | CnA/CnB | Regulate the biosynthesis of GS | [64] |
可见,钙离子以及钙信号途径可能对真菌代谢物合成起调控作用,而钙调磷酸酶作为钙信号途径的调控中心,对代谢产物合成的调控机制缺乏深入解析。
5 总结和展望过去20年中,钙调磷酸酶作为真菌细胞钙稳态的调控中心,受到了广泛研究,主要体现在生长发育、应激反应、毒力发生、抗菌耐药等方面。钙调磷酸酶信号调控真菌代谢物合成的机制虽然不太清楚,但已经越来越受到关注。钙调磷酸酶作为重要的信号元件,响应于Ca2+浓度及外界环境条件(包括温度、酸碱、渗透压和调节剂等),参与真菌胞内重要的磷酸化级联反应,调节遗传环路的输出,从而调控代谢相关基因的表达。随着合成生物学的发展,通过人工构建的细胞工厂来生产高附加值化学品受到关注。生物元件挖掘及基因回路的设计、代谢网络重构及其与底盘的适配性等是目前合成生物学的主要研究方向。真菌信号途径研究为合成生物学提供了重要的生物合成元件、修饰元件和有效的调控元件,钙调磷酸酶信号级联途径对真菌代谢的重要调控作用使其有望成为新型的动态调控开关,以设计可控的基因表达及调控系统。钙调磷酸酶信号作为一种信号调节工程策略,未来可能会成为真菌生物学研究中的重要调控工具。
| [1] | Clapham DE. Calcium signaling. Cell, 2007, 131(6): 1047-1058. DOI:10.1016/j.cell.2007.11.028 |
| [2] | Carafoli E. Calcium signaling: a tale for all seasons. PNAS, 2002, 99(3): 1115-1122. DOI:10.1073/pnas.032427999 |
| [3] | Nowycky MC, Thomas AP. Intracellular calcium signaling. Journal of Cell Science, 2002, 115(19): 3715-3716. DOI:10.1242/jcs.00078 |
| [4] | Stull JT. Ca2+-dependent cell signaling through calmodulin-activated protein phosphatase and protein kinases minireview series. The Journal of Biological Chemistry, 2001, 276(4): 2311-2312. DOI:10.1074/jbc.R000030200 |
| [5] | Juvvadi PR, Lee SC, Heitman J, Steinbach WJ. Calcineurin in fungal virulence and drug resistance: Prospects for harnessing targeted inhibition of calcineurin for an antifungal therapeutic approach. Virulence, 2017, 8(2): 186-197. DOI:10.1080/21505594.2016.1201250 |
| [6] | Rusnak F, Mertz P. Calcineurin: form and function. Physiological Reviews, 2000, 80(4): 1483-1521. DOI:10.1152/physrev.2000.80.4.1483 |
| [7] | Park HS, Chow EWL, Fu C, Soderblom EJ, Moseley MA, Heitman J, Cardenas ME. Calcineurin targets involved in stress survival and fungal virulence. PLoS Pathogens, 2016, 12(9): e1005873. DOI:10.1371/journal.ppat.1005873 |
| [8] | Armstrong-James D, de Boer L, Bercusson A, Shah A. From phagocytosis to metaforosis: Calcineurin's deadly role in innate processing of fungi. PLoS Pathogens, 2018, 14(1): e1006627. DOI:10.1371/journal.ppat.1006627 |
| [9] | Goldman A, Roy J, Bodenmiller B, Wanka S, Landry CR, Aebersold R, Cyert MS. The calcineurin signaling network evolves via conserved kinase-phosphatase modules that transcend substrate identity. Molecular Cell, 2014, 55(3): 422-435. DOI:10.1016/j.molcel.2014.05.012 |
| [10] | Aramburu JO, Rao A, B.Kill C. Calcineurin: from structure to function. Current Topics in Cellular Regulation, 2001, 36(1): 237-295. |
| [11] | Park HS, Lee SC, Cardenas ME, Heitman J. Calcium-calmodulin-calcineurin signaling: a globally conserved virulence cascade in eukaryotic microbial pathogens. Cell Host Microbe, 2019, 26(4): 453-462. DOI:10.1016/j.chom.2019.08.004 |
| [12] | Alber J, Jiang LH, Geyer J. CaRch1p does not functionally interact with the high-affinity Ca2+ influx system (HACS) of Candida albicans. Yeast, 2013, 30(11): 449-457. DOI:10.1002/yea.2981 |
| [13] | Gao YY, Li W, Liu XB, Gao FS, Zhao XH. Reversing effect and mechanism of soluble resistance-related calcium-binding protein on multidrug resistance in human lung cancer A549/DDP cells. Molecular Medicine Reports, 2015, 11(3): 2118-2124. DOI:10.3892/mmr.2014.2936 |
| [14] | Thewes S. Calcineurin-Crz1 signaling in lower eukaryotes. Eukaryotic Cell, 2014, 13(6): 694-705. DOI:10.1128/EC.00038-14 |
| [15] | Chow EWL, Clancey SA, Billmyre RB, Averette AF, Granek JA, Mieczkowski P, Cardenas ME, Heitman J. Elucidation of the calcineurin-Crz1 stress response transcriptional network in the human fungal pathogen Cryptococcus neoformans. PLoS Genetics, 2017, 13(4): e1006667. DOI:10.1371/journal.pgen.1006667 |
| [16] | Yoshimoto H, Saltsman K, Gasch AP, Li HX, Ogawa N, Botstein D, Brown PO, Cyert MS. Genome-wide analysis of gene expression regulated by the calcineurin/Crz1p signaling pathway in Saccharomyces cerevisiae. The Journal of Biological Chemistry, 2002, 277(34): 31079-31088. DOI:10.1074/jbc.M202718200 |
| [17] | Zhang XX, Cao SL, Li W, Sun HY, Deng Y, Zhang AX, Chen HG. Functional characterization of calcineurin- responsive transcription factors Fg01341 and Fg01350 in Fusarium graminearum. Frontiers in Microbiology, 2020, 11: 597998. DOI:10.3389/fmicb.2020.597998 |
| [18] | Lee SC, Li A, Calo S, Heitman J. Calcineurin plays key roles in the dimorphic transition and virulence of the human pathogenic zygomycete Mucor circinelloides. PLoS Pathogens, 2013, 9(9): e1003625. DOI:10.1371/journal.ppat.1003625 |
| [19] | Paul AS, Saha S, Engelberg K, Jiang RHY, Coleman BI, Kosber AL, Chen CT, Ganter M, Espy N, Gilberger TW, Gubbels MJ, Duraisingh MT. Parasite calcineurin regulates host cell recognition and attachment by apicomplexans. Cell Host & Microbe, 2015, 18(1): 49-60. |
| [20] | Koyano T, Konishi M, Martin SG, Ohya Y, Hirata D, Toda T, Kume K. Casein kinase 1γ gamma ensures monopolar growth polarity under incomplete DNA replication downstream of Cds1 and calcineurin in fission yeast. Molecular and Cellular Biology, 2015, 35(9): 1533-1542. DOI:10.1128/MCB.01465-14 |
| [21] | Viana RA, Pinar M, Soto T, Coll PM, Cansado J, Pérez P. Negative functional interaction between cell integrity MAPK pathway and Rho1 GTPase in fission yeast. Genetics, 2013, 195(2): 421-432. DOI:10.1534/genetics.113.154807 |
| [22] | Martín-García R, Arribas V, Coll PM, Pinar M, Viana RA, Rincón SA, Correa-Bordes J, Ribas JC, Pérez P. Paxillin-mediated recruitment of calcineurin to the contractile ring is required for the correct progression of cytokinesis in fission yeast. Cell Reports, 2018, 25(3): 772-783. DOI:10.1016/j.celrep.2018.09.062 |
| [23] | Kalem MC, Subbiah H, Leipheimer J, Glazier VE, Panepinto JC. Puf4 mediates post-transcriptional regulation of cell wall biosynthesis and caspofungin resistance in Cryptococcus neoformans. mBio, 2021, 12(1): e03225-20. |
| [24] | Fox DS, Cox GM, Heitman J. Phospholipid-binding protein Cts1 controls septation and functions coordinately with calcineurin in Cryptococcus neoformans. Eukaryotic Cell, 2003, 2(5): 1025-1035. DOI:10.1128/EC.2.5.1025-1035.2003 |
| [25] | Kraus PR, Fox DS, Cox GM, Heitman J. The Cryptococcus neoformans MAP kinase Mpk1 regulates cell integrity in response to antifungal drugs and loss of calcineurin function. Molecular Microbiology, 2003, 48(5): 1377-1387. DOI:10.1046/j.1365-2958.2003.03508.x |
| [26] | Schumacher J, de Larrinoa IF, Tudzynski B. Calcineurin-responsive zinc finger transcription factor CRZ1 of Botrytis cinerea is required for growth, development, and full virulence on bean plants. Eukaryotic Cell, 2008, 7(4): 584-601. DOI:10.1128/EC.00426-07 |
| [27] | Juvvadi PR, Kuroki Y, Arioka M, Nakajima H, Kitamoto K. Functional analysis of the calcineurin-encoding gene cnaA from Aspergillus oryzae: evidence for its putative role in stress adaptation. Archives of Microbiology, 2003, 179(6): 416-422. DOI:10.1007/s00203-003-0546-3 |
| [28] | Harel A, Bercovich S, Yarden O. Calcineurin is required for sclerotial development and pathogenicity of Sclerotinia sclerotiorum in an oxalic acid-independent manner. Molecular Plant-Microbe Interactions, 2006, 19(6): 682-693. DOI:10.1094/MPMI-19-0682 |
| [29] | Choi J, Kim Y, Kim S, Park J, Lee YH. MoCRZ1, a gene encoding a calcineurin-responsive transcription factor, regulates fungal growth and pathogenicity of Magnaporthe oryzae. Fungal Genetics and Biology, 2009, 46(3): 243-254. DOI:10.1016/j.fgb.2008.11.010 |
| [30] | Cervantes-Chávez JA, Ali S, Bakkeren G. Response to environmental stresses, cell-wall integrity, and virulence are orchestrated through the calcineurin pathway in Ustilago hordei. Molecular Plant-Microbe Interactions, 2011, 24(2): 219-232. DOI:10.1094/MPMI-09-10-0202 |
| [31] | Blankenship JR, Wormley FL, Boyce MK, Schell WA, Filler SG, Perfect JR, Heitman J. Calcineurin is essential for Candida albicans survival in serum and virulence. Eukaryotic Cell, 2003, 2(3): 422-430. DOI:10.1128/EC.2.3.422-430.2003 |
| [32] | Chen YL, Brand A, Morrison EL, Silao FGS, Bigol UG, Malbas FF, Nett JE, Andes DR, Solis NV, Filler SG, Averette A, Heitman J. Calcineurin controls drug tolerance, hyphal growth, and virulence in Candida dubliniensis. Eukaryotic Cell, 2011, 10(6): 803-819. DOI:10.1128/EC.00310-10 |
| [33] | Chen YL, Lehman VN, Lewit Y, Averette AF, Heitman J. Calcineurin governs thermotolerance and virulence of Cryptococcus gattii. G3: Bethesda, Md, 2013, 3(3): 527-539. DOI:10.1534/g3.112.004242 |
| [34] | Steinbach WJ, Cramer RA, Perfect BZ, Asfaw YG, Sauer TC, Najvar LK, Kirkpatrick WR, Patterson TF, Benjamin DK, Heitman J, Perfect JR. Calcineurin controls growth, morphology, and pathogenicity in Aspergillus fumigatus. Eukaryotic Cell, 2006, 5(7): 1091-1103. DOI:10.1128/EC.00139-06 |
| [35] | Egan JD, García-Pedrajas MD, Andrews DL, Gold SE. Calcineurin is an antagonist to PKA protein phosphorylation required for postmating filamentation and virulence, while PP2A is required for viability in Ustilago maydis. Molecular Plant-Microbe Interactions, 2009, 22(10): 1293-1301. DOI:10.1094/MPMI-22-10-1293 |
| [36] | Bader T, Bodendorfer B, Schröppel K, Morschhäuser J. Calcineurin is essential for virulence in Candida albicans. Infection and Immunity, 2003, 71(9): 5344-5354. DOI:10.1128/IAI.71.9.5344-5354.2003 |
| [37] | Li F, Wang ZL, Zhang LB, Ying SH, Feng MG. The role of three calcineurin subunits and a related transcription factor (Crz1) in conidiation, multistress tolerance and virulence in Beauveria bassiana. Applied Microbiology and Biotechnology, 2015, 99(2): 827-840. DOI:10.1007/s00253-014-6124-6 |
| [38] | Hou YH, Hsu LH, Wang HF, Lai YH, Chen YL. Calcineurin Regulates Conidiation, Chlamydospore Formation and Virulence in Fusarium oxysporum f. sp. lycopersici. Frontiers in Microbiology, 2020, 11: 539702. DOI:10.3389/fmicb.2020.539702 |
| [39] | Kozubowski L, Aboobakar EF, Cardenas ME, Heitman J. Calcineurin colocalizes with P-bodies and stress granules during thermal stress in Cryptococcus neoformans. Eukaryotic Cell, 2011, 10(11): 1396-1402. DOI:10.1128/EC.05087-11 |
| [40] | Chen YL, Konieczka JH, Springer DJ, Bowen SE, Zhang J, Silao FGS, Bungay AAC, Bigol UG, Nicolas MG, Abraham SN, Thompson DA, Regev A, Heitman J. Convergent evolution of calcineurin pathway roles in thermotolerance and virulence in Candida glabrata. G3 : Genes/Genomes/Genetics, 2012, 2(6): 675-691. |
| [41] | Cruz MC. Calcineurin is required for hyphal elongation during mating and haploid fruiting in Cryptococcus neoformans. The EMBO Journal, 2001, 20(5): 1020-1032. DOI:10.1093/emboj/20.5.1020 |
| [42] | Juvvadi P, Steinbach W. Calcineurin orchestrates hyphal growth, septation, drug resistance and pathogenesis of Aspergillus fumigatus: where do we go from here?. Pathogens, 2015, 4(4): 883-893. DOI:10.3390/pathogens4040883 |
| [43] | Yu SJ, Chang YL, Chen YL. Calcineurin signaling: lessons from Candida species. FEMS Yeast Research, 2015, 15(4): fov016. |
| [44] | Serrano R, Ruiz A, Bernal D, Chambers JR, Ariño J. The transcriptional response to alkaline pH in Saccharomyces cerevisiae: evidence for calcium-mediated signalling. Molecular Microbiology, 2002, 46(5): 1319-1333. DOI:10.1046/j.1365-2958.2002.03246.x |
| [45] | Viladevall L, Serrano R, Ruiz A, Domenech G, Giraldo J, Barceló A, Ariño J. Characterization of the calcium-mediated response to alkaline stress in Saccharomyces cerevisiae. The Journal of Biological Chemistry, 2004, 279(42): 43614-43624. DOI:10.1074/jbc.M403606200 |
| [46] | Heath VL, Shaw SL, Roy S, Cyert MS. Hph1p and Hph2p, novel components of calcineurin-mediated stress responses in Saccharomyces cerevisiae. Eukaryotic Cell, 2004, 3(3): 695-704. DOI:10.1128/EC.3.3.695-704.2004 |
| [47] | Serra-Cardona A, Canadell D, Ariño J. Coordinate responses to alkaline pH stress in budding yeast. Microbial Cell: Graz, Austria, 2015, 2(6): 182-196. DOI:10.15698/mic2015.06.205 |
| [48] | Matsumoto TK, Ellsmore AJ, Cessna SG, Low PS, Pardo JM, Bressan RA, Hasegawa PM. An osmotically induced cytosolic Ca2+ transient activates calcineurin signaling to mediate ion homeostasis and salt tolerance of Saccharomyces cerevisiae. Journal of Biological Chemistry, 2002, 277(36): 33075-33080. DOI:10.1074/jbc.M205037200 |
| [49] | Sanglard D, Ischer F, Marchetti O, Entenza J, Bille J. Calcineurin A of Candida albicans: involvement in antifungal tolerance, cell morphogenesis and virulence. Molecular Microbiology, 2003, 48(4): 959-976. DOI:10.1046/j.1365-2958.2003.03495.x |
| [50] | Marquina M, González A, Barreto L, Gelis S, Muñoz I, Ruiz A, Álvarez MC, Ramos J, Ariño J. Modulation of yeast alkaline cation tolerance by Ypi1 requires calcineurin. Genetics, 2012, 190(4): 1355-1364. DOI:10.1534/genetics.112.138370 |
| [51] | Maeda T, Sugiura R, Kita A, Saito M, Lu D, Yi H, Lu YB, Fujita Y, Takegawa K, Shuntoh H, Kuno T. Pmr1, a P-type ATPase, and Pdt1, an Nramp homologue, cooperatively regulate cell morphogenesis in fission yeast: The importance of Mn2+ homeostasis. Genes to Cells, 2004, 9(1): 71-82. DOI:10.1111/j.1356-9597.2004.00699.x |
| [52] | Pinchai N, Juvvadi PR, Fortwendel JR, Perfect BZ, Rogg LE, Asfaw YG, Steinbach WJ. The Aspergillus fumigatus P-type Golgi apparatus Ca2+/Mn2+ ATPase PmrA is involved in cation homeostasis and cell wall integrity but is not essential for pathogenesis. Eukaryotic Cell, 2010, 9(3): 472-476. DOI:10.1128/EC.00378-09 |
| [53] | Dinamarco TM, Freitas FZ, Almeida RS, Brown NA, dos Reis TF, Ramalho LNZ, Savoldi M, Goldman MHS, Bertolini MC, Goldman GH. Functional characterization of an Aspergillus fumigatus calcium transporter (PmcA) that is essential for fungal infection. PLoS One, 2012, 7(5): e37591. DOI:10.1371/journal.pone.0037591 |
| [54] | Cramer RA, Perfect BZ, Pinchai N, Park S, Perlin DS, Asfaw YG, Heitman J, Perfect JR, Steinbach WJ. Calcineurin target CrzA regulates conidial germination, hyphal growth, and pathogenesis of Aspergillus fumigatus. Eukaryotic Cell, 2008, 7(7): 1085-1097. DOI:10.1128/EC.00086-08 |
| [55] | Hagiwara D, Kondo A, Fujioka T, Abe K. Functional analysis of C2H2 zinc finger transcription factor CrzA involved in calcium signaling in Aspergillus nidulans. Current Genetics, 2008, 54(6): 325-338. DOI:10.1007/s00294-008-0220-z |
| [56] | Hernández-Ortiz P, Espeso EA. Phospho-regulation and nucleocytoplasmic trafficking of CrzA in response to calcium and alkaline-pH stress in Aspergillus nidulans. Molecular Microbiology, 2013, 89(3): 532-551. DOI:10.1111/mmi.12294 |
| [57] | Zou X, Tu GW, Zan ZQ. Cofactor and CO2 donor regulation involved in reductive routes for polymalic acid production by Aureobasidium pullulans CCTCC M2012223. Bioprocess and Biosystems Engineering, 2014, 37(10): 2131-2136. DOI:10.1007/s00449-014-1182-9 |
| [58] | Zou X, Zhou YP, Yang ST. Production of polymalic acid and malic acid by Aureobasidium pullulans fermentation and acid hydrolysis. Biotechnology and Bioengineering, 2013, 110(8): 2105-2113. DOI:10.1002/bit.24876 |
| [59] | Chen XL, Xu GQ, Xu N, Zou W, Zhu P, Liu LM, Chen J. Metabolic engineering of Torulopsis glabrata for malate production. Metabolic Engineering, 2013, 19: 10-16. DOI:10.1016/j.ymben.2013.05.002 |
| [60] | Zelle RM, Hulster ED, Kloezen W, Pronk JT, Van Maris AJA. Key process conditions for production of C4 dicarboxylic acids in bioreactor batch cultures of an engineered Saccharomyces cerevisiae strain. Applied and Environmental Microbiology, 2010, 76(3): 744-750. DOI:10.1128/AEM.02396-09 |
| [61] | Wang K, Chi Z, Liu GL, Qi CY, Jiang H, Hu Z, Chi ZM. A novel PMA synthetase is the key enzyme for polymalate biosynthesis and its gene is regulated by a calcium signaling pathway in Aureobasidium melanogenum ATCC62921. International Journal of Biological Macromolecules, 2020, 156(1): 1053-1063. |
| [62] | Lu YH, Pan ZH, Tao J, An FL. Induced effect of Ca2+ on dalesconols A and B biosynthesis in the culture of Daldinia eschscholzii via calcium/calmodulin signaling. Journal of Bioscience and Bioengineering, 2018, 125(2): 205-210. DOI:10.1016/j.jbiosc.2017.08.018 |
| [63] | Chung KR. Involvement of calcium/calmodulin signaling in cercosporin toxin biosynthesis by Cercospora nicotianae. Applied and Environmental Microbiology, 2003, 69(2): 1187-1196. DOI:10.1128/AEM.69.2.1187-1196.2003 |
| [64] | Xu YN, Xia XX, Zhong JJ. Induced effect of Na+ on ganoderic acid biosynthesis in static liquid culture of Ganoderma lucidum via calcineurin signal transduction. Biotechnology and Bioengineering, 2013, 110(7): 1913-1923. DOI:10.1002/bit.24852 |
| [65] | Zhang X, Ren A, Li MJ, Cao PF, Chen TX, Zhang G, Shi L, Jiang AL, Zhao MW. Heat stress modulates mycelium growth, heat shock protein expression, ganoderic acid biosynthesis, and hyphal branching of Ganoderma lucidum via cytosolic Ca2+. Applied and Environmental Microbiology, 2016, 82(14): 4112-4125. DOI:10.1128/AEM.01036-16 |
| [66] | Liu R, Shi L, Zhu T, Yang T, Ren A, Zhu J, Zhao MW. Cross talk between nitric oxide and calcium-calmodulin regulates ganoderic acid biosynthesis in Ganoderma lucidum under heat stress. Applied and Environmental Microbiology, 2018, 84(10): e00043-18. |
| [67] | Zhou JW, Zhou HY, Du GC, Liu LM, Chen J. Screening of a thiamine-auxotrophic yeast for α-ketoglutaric acid overproduction. Letters in Applied Microbiology, 2010, 51(3): 264-271. DOI:10.1111/j.1472-765X.2010.02889.x |
| [68] | Wei XC, Liu CQ, An FL, Lu YH. Induced effect of Ca2+ on curvulamine synthesis by marine-derived fungus Curvularia sp. IFB-Z10 under submerged fermentation. Process Biochemistry, 2019, 83: 18-26. DOI:10.1016/j.procbio.2019.03.011 |
| [69] | Su ZP, Wang FQ, Xie YH, Xie H, Mao GT, Zhang HS, Song AD, Zhang ZY. Reassessment of the role of CaCO3 in n-butanol production from pretreated lignocellulosic biomass by Clostridium acetobutylicum. Scientific Reports, 2020, 10: 17956. DOI:10.1038/s41598-020-74899-9 |
 2021, Vol. 61
2021, Vol. 61