中国科学院微生物研究所,中国微生物学会,中国菌物学会
文章信息
- M. Zain Ul Arifeen, Xinyi Yang, Fangfang Li, Yarong Xue, Pixian Gong, Changhong Liu. 2020
- Zain Ul ArifeenM., 杨心怡, 李方方, 薛雅蓉, 公丕贤, 刘常宏. 2020
- Growth behaviors of deep subseafloor Schizophyllum commune in response to various environmental conditions
- 洋底深部裂褶菌在不同环境条件的生长行为
- Acta Microbiologica Sinica, 60(9): 1882-1892
- 微生物学报, 60(9): 1882-1892
-
文章历史
- 收稿日期:2020-03-17
- 修回日期:2020-05-12
- 网络出版日期:2020-07-02
Fungi are the most diverse and abundant eukaryotic organisms on the Earth and have been found in almost all ecosystems, from land to deep sea, and play essential ecological roles in biogeochemical cycles in these ecosystems[1-2].
Since the pilot study of fungi in marine sediment from the Chagos Trench in the Indian Ocean[3], fungi have been documented to be the dominant eukaryotes in a range of shallow ocean provinces including ridge flanks, continental margins, and abyssal plains[4]. Up to date, many fungi have been isolated from sediments ranging from a few centimetres to 2457 meters below seafloor (mbsf)[5-6]. Members of Basidiomycetes and Ascomycetes are the dominant fungal communities in the deep biosphere (> 1 mbsf) based on culture- dependent and culture-independent methods[4-7]. The deepest distribution of fungi was reported to be in coal-bearing subseafloor sediments for 20 million years off the Shimokita Peninsula, Japan[6]. In addition, it has been reported that fungi originated from subseafloor sediments are not significantly different from the fungi inhabiting in terrestrial environment[6, 8], indicating that fungi from the land and water environment might have the ability to adapt to the deep biosphere. A limited study also showed that fungi developed mechanisms such as a metabolic shift from oxidative phosphorylation to fermentation, to sustain development and growth in response to low concentration of oxygen[9-10]. This stunning ecological and morphological flexibility makes fungi the most versatile phylogenetic lineage, which helps them to survive the most extreme habitats, such as the deep biosphere[11]. Despite the increasing research on fungal diversity in the deep subseafloor biosphere, the biological, physiological, and metabolic characteristics of fungal survival in such extreme environments remain unknown.
The split gill fungus Schizophyllum commune (Agaricomycotina) is a mushroom forming fungus with world-wide occurrence due to its production of a large number of hydrolases, such as xylanase, pectinase, cellulase, endoglucanase, glycoside hydrolase, and oxidordeuctase[12]. Our previous study showed that S. commune was one of the dominant fungal species in the coal-bearing sediments and widely distributed in 1496, 1923, 1966, and 1993 mbsf[6], respectively. This study aims to investigate the growth characteristics of these strains and their adaptability to the extreme environment of submarine surface by comparing them with two strains isolated from terrestrial (S. commune CFCC7252) and marine (S. commune MCCC 3A00233) environments under different culture conditions.
1 Materials and methods 1.1 Fungal strainsFour strains 6R-2-F01, 15R-5-F01, 20R-7-F01, 24R-3-F01 of S. commune were isolated from deep coal-bearing sediments at different depths below sea floors during IODP Expedition 337 and maintained on mPDA (Potato Dextrose Agar in artificial seawater) at 4 ℃ in the laboratory[6]. Strain CFCC7252 isolated from terrestrial plant of Pterocarya stenoptera, was purchased from China Forestry Culture Collection Center. Strain MCCC 3A00233 collected from marine sediment of the Atlantic Ocean was purchased from Third Institute of Oceanography, State Oceanic Administration, People's Republic of China. The detailed inform about the in-situ condition and strains are shown in Table 1[13].
| Fungal strains | Depth/mbsf | Lithology | T/℃ | pH | Salinity/‰ | NH4+/(mmol/L) | TOC/wt% | TN/wt% | Fe/(µmol/L) |
| 6R-2-F01 | 1496 | Sandstone | 38.91 | 8.55 | 41.47 | 2.69 | 0.3 | 0.0 | 0.59 |
| 15R-5-F01 | 1923 | Coaly shale | 46.25 | 7.91 | 29.31 | 1.35 | 3.4 | 0.1 | 0.73 |
| 20R-7-F01 | 1966 | Sand | 46.99 | 8.17 | 28.82 | 1.34 | 0.1 | 0.0 | 3.11 |
| 24R-3-F01 | 1993 | Coal | 47.45 | ND | ND | ND | 50.0 | 1.2 | ND |
| CFCC7252 | – | Plants | |||||||
| MCCC 3A00233 | – | Marine sediment | |||||||
| TOC=Total Organic Carbon; TN=Total Nitrogen; ND=Not detected. The pH, salinity and Fe values of samples at depths of 1923 and 1966 mbsf were actually measured at adjacent depths of 1926 and 1962 mbsf, respectively. | |||||||||
1.2 Medium
mPAD medium was used in this study, which was prepared by dissolving 46 g of PDA powder (Ourchem, Sinnopharm Chemical Reagent Co., Ltd, China) in 1 L of the artificial seawater (ASW) or in-situ water (ISW)[6]. ASW contains (per liter) NaCl 33.43 g, NaSO4 0.64 g, KCl 5.05 g, NH4Cl 0.16 g, MgCl2 4.17 g, CaCl2 2.94 g, KBr 0.10 g, H3BO3 0.02 g, according to the salinity of seawater drilling sampling points (the ion concentration is 4.08%). ISW, according to the salinity of interstitial water of the cores (the ion concentration is 2.92%), consists (per liter) of NaCl 27.25 g, Na2SO4 0.16 g, KCl 0.11 g, NH4Cl 0.08 g, MgCl2 3.05 g, CaCl2 1.69 g, KBr 0.08 g, H3BO3 0.01 g, FeSO4 0.5 mg, ZnSO4 0.3 mg, MnCl2 2.0 mg, and CuSO4 0.5 mg.
1.3 Determination of optimum growth conditionsGrowth observation was set up on mPDA prepared with ASW, in 9-cm diameter Petri dishes[6]. The colony radius in the Petri dishes was measured daily and expressed as mm/day to reflect the growth rate at given conditions, including oxygen (aerobic, anaerobic), temperature (20, 30, 40, 45 ℃), pH (6, 8, 10), Fe2+ (0.27, 8.93, 89.28 µmol/L), salinity (freshwater, ASW, ISW), sodium lignin sulfonic acid (1, 5, 10 g/L), and NH4Cl (0.5, 1.0, 5.0 g/L), respectively. In addition to the oxygen and temperature experiments, the other experiments were carried out under anaerobic chamber (YQX-Ⅱ, Shanghai, China) at 30 ℃ for a total of five days. The morphology of each strain was photographed at the fifth day of incubation under aerobic and anaerobic conditions. Each experiment was repeated three times.
1.4 Statistic analysisThe results of the experiments were analyzed with GraphPad Prism (version 3.02) software to estimate the significance of the differences (P < 0.05) using an unpaired Student's t test, one-way ANOVA and the Dunnett's test. SPSS software (version 19) was used to perform hierarchical cluster analysis (default parameters) on the growth rates of fungal strains under different culture conditions.
2 Results and analysis 2.1 Effect of oxygen on S. commune growth and morphologyTo determine the impact of oxygen on fungal growth, all S. commune strains were grown under aerobic and anaerobic conditions. The strains 6R-2-F01, 20R-7-F01, and 24R-3-F01 showed almost similar morphology when grown on mPDA medium for five days of incubation at 30 ℃. The mycelia were relatively dense at aerobic conditions as compared to anaerobic conditions (Figure 1). In both conditions, the morphology of fungus looks like white cotton and has no concentric ring pattern. The growth behavior of strain 15R-5-F01 closely related to terrestrial strain CFCC7252. However, the strain MCCC 3A00233 showed significant morphological differences, the mycelium was found fluffy like white wool shape, as compared to other strains. The aerobic culture of all strains was less dense than that of anaerobic except for MCCC 3A00233, where the aerobic culture was more dense than anaerobic. These results indicated that the phenotype of S. commune isolated from different habitats (terrestrial plant, marine sediment, and deep subseafloor sediments), showed significant morphological differences, and also influenced by the availability of oxygen.

|
| Figure 1 Mycelium growth morphology of S. commune isolated from different sources under aerobic and anaerobic conditions. O–: anaerobic conditions; O+: aerobic conditions. |
In addition, the growth rates of the four subseafloor strains were all significantly higher than CFCC7252 and MCCC 3A00233 under anaerobic conditions. However, only strains 15R-5-F01 and 6R-2-F01 were significantly higher than CFCC7252 and MCCC 3A00233 under aerobic conditions. Moreover, the growth rates of strains 6R-2-F01 and 24R-3-F01 under anaerobic conditions were significantly higher than those under aerobic conditions. On the contrary, the growth rate of strain MCCC 3A00233 under aerobic conditions was significantly higher than that under anaerobic conditions. Overall, the growth rates of all the subseafloor strains and CFCC7252 were significantly higher than strain MCCC 3A00233 under both aerobic and anaerobic conditions (Figure 2). These results indicated that the deep subseafloor strains might have more extensive oxygen adaptability than the terrestrial and marine strains.

|
| Figure 2 Effects of oxygen on the growth rate of different S. commune strains. Capital letters indicate significant growth differences among the test strains under the same oxygen (One-way ANOVA test, P < 0.05). * indicates that there is a significant difference in the growth of the same strain under aerobic and anaerobic conditions (t-test, P < 0.05). |
2.2 Effect of temperature on S. commune growth
To determine the effect of temperature on growth, six strains of S. commune were cultured on mPDA under anaerobic condition with different temperatures. Except for strains 6R-2-F01 and MCCC 3A00233, the three subseafloor strains and the terrestrial strain CFCC7252 grew well at 30–40 ℃, but slowed down significantly at 20 ℃, and grew worst at 45 ℃. Moreover, the growth rates of subseafloor strains were significantly higher than that of the marine and terrestrial strains at given temperatures, except that of strain CFCC7252 at 40 ℃ (Figure 3). These results demonstrated that the subseafloor and terrestrial strains of S. commune showed similar temperature adaptability, and were significantly different from the marine strain.

|
| Figure 3 Effects of temperature on mycelium growth rate of different S. commune strains. Lowercase letters indicate significant differences in the growth rate of the same strain at different temperatures, while capital letters indicate significant differences in growth rates among strains at the same temperature (One-way ANOVA test, P < 0.05). The same indication of letters was used in the figures below. |
2.3 Effect of pH on S. commune growth
To determine the effect of pH on the growth of S. commune, all strains were cultured at pH 6, 8, 10 (Figure 4). Except for the growth of strains 24R-3-F01 and CFCC7252, which were significantly affected by pH (marked as a and b), the growth of other strains did not show significant difference under the given pH values. However, all strains collected from the deep subseafloor sediments grew faster than those isolated from terrestrial and marine environments at any given pH. In addition, the growth rate of strains 15R-5-F01 and 24R-3-F01 was significantly slower than that of strains 6R-2-F01 and 20R-7-F01 when they were close to the in-situ pH 8 (Table 1). These results suggest that the subseafloor strains of S. commune grow well at a wide range of pH values compared with strains from other habitats.

|
| Figure 4 Effects of pH on the growth rate of different S. commune strains. |
2.4 Growth capacity of S. commune at different salinities
To evaluate the adaptability and tolerance of S. commune to salinity, all the strains were grown in mPDA prepared with freshwater, ISW, and ASW respectively, and the growth rate was determined. With the exception of strain 24R-3-F01, the growth rates of all subseafloor strains and marine strain in the ISW (2.92% salinity) and ASW (4.02% salinity) media were significantly higher than that of freshwater medium (0% salinity) (Figure 5). The terrestrial and marine strains showed different salinity response growth behaviors. The growth rate of the terrestrial strain decreased significantly with the increase of salinity, while that of the marine strain increased significantly with the increase of salinity. All subseafloor strains showed good tolerance to salinity by exhibiting high growth rate in media prepared with ISW and ASW as compared to control strains CFCC7252 and MCCC 3A00233. The growth pattern of the subseafloor strains in the tested salinity is the same as that of marine strain, but its growth ability in freshwater is not different from that of terrestrial strain, which reflects that subseafloor strains have stronger salinity adaptability.

|
| Figure 5 Effects of salinity on the growth rate of different strains of S. commune. |
2.5 Effect of Fe2+ concentrations on S. commune growth
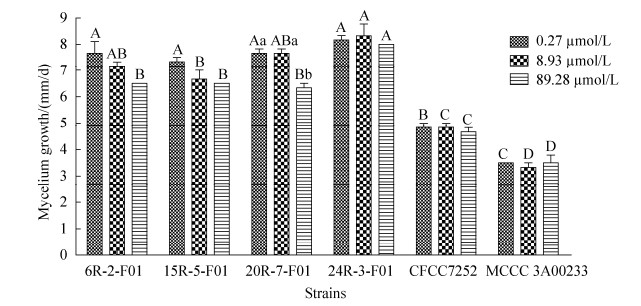
Iron is one of the mineral nutrients necessary for microorganisms and is relatively abundant in subseafloor sediments (Table 1). To evaluate its effect on the growth of S. commune, all strains were exposed to different Fe2+ concentrations, and their mycelia growth rates were measured. There was no significant difference in growth rate of all tested strains under the given ion concentration, except that the growth rate of strain 20R-7-F01 was significantly inhibited at 89.28 µmol/L. However, the subseafloor strains showed significantly higher growth rate at any level of iron ions, then marine and land strains (Figure 6).
|
| Figure 6 Effect of Fe2+ on the growth rate of different strains of S. commune. |
2.6 Effect of lignin on growth capacity
Since the subseafloor strains of S. commune were recovered from coal-bearing sediments with lignin as the main component, lignin was used as the carbon source to study the growth capacity of these strains. The results showed that the growth behavior of subseafloor strains 6R-2-F01 and 15R-5-F01 was similar to that of terrestrial strain CFCC7252, and the growth rate was the highest at 5 g/L lignin. Overall, the growth rates of all the subseafloor strains were not significantly different from that of the terrestrial strain, but significantly higher than that of the marine strain (Figure 7).

|
| Figure 7 Effects of lignin concentrations on the growth rate of different strains of S. commune. |
2.7 Effect of NH4+ on growth capacity of S. commune
Since NH4+ is the only nitrogen-containing inorganic compound detected in the deep subseafloor sediments, we studied the growth capacity of S. commune under different concentrations of NH4Cl. Except that the growth rates of strain 24R-3-F01 and CFCC7252 were not significantly different, the growth rates of other strains were significantly different at the given concentrations of NH4Cl. The highest growth rate of strains 6R-2-F01 and 15R-5-F01 was monitored at 1.0 g/L while that of strains 20R-07-F01 and 24R-3-F01 was at 0.5 g/L. The growth rate of all subseafloor strains was higher than that of strain MCCC 3A00233 when NH4Cl concentration was 0.5 g/L and 1.0 g/L (Figure 8). In addition, the growth rate of all subseafloor strains in ISW medium with in-situ concentration of NH4Cl (0.08 g/L) was significantly higher than that of terrestrial and marine strains (Figure 5), but there was no significant difference in the growth rate of different strains at high concentration of NH4Cl (5.0 g/L) (Figure 8). This result reflects that the growth of subseafloor fungal strains has a strong low ammonium adaptability.

|
| Figure 8 Effects of NH4Cl on the growth rate of different S. commune strains. |
2.8 Hierarchical cluster analysis
Growth characteristics and environmental conditions of the selected strains are summarized by using systematic cluster analysis model. The results showed that all the subseafloor strains were concentrated in one group, and the terrestrial and marine strains were two independent groups (Figure 9). In the subseafloor strains, 6R-2-F01 and 20R-7-F01 clustered on one branch, indicating that they had more similar growth characteristics than 15R-5-F01 and 24R-3-F01.

|
| Figure 9 The dendrogram of the hierarchical cluster analysis of the growth characteristics of S. commune strains isolated from different habitats. |
3 Discussion
The recent discovery of fungi in the deep biosphere beneath the seafloor pose seroius questions on the limit of life on earth. How fungi survived and adapted to the harsh and extreme conditions of the subseafloor is still unknown, and are these fungi are the actual resident of these environments or migrated from land or marine? It is also believed that these microboes are in the dormant form with physiological standby nature and are not sufficiently active in the deep biosphere ecosystems[2, 14]. However, many studies have cultured various fungi from the subseafloor sediment samples, showing that these fungi are in an active form and adapted well to survive these conditions[2, 6]. In this study, it was found for the first time that S. commune could grow well under both aerobic and anaerobic conditions; however, significant differences among strains from different habitats were monitored. Compared with terrestrial and marine strains, the deep subseafloor strains of S. commune, which were buried in about 2 kmbsf sediments at 20 million years ago, have stronger anaerobic growth capacity under different environmental factors such as lignin, NH4+, Fe2+, temperatures, and pH. These results suggest that the ancient strains of S. commune have a strong environmental adaptation capacity than the strains derived from other habitats.
Radial growth and mycelia dry weight measurements are two common methods for measuring fungal growth. Although the former is not well correlated with the biomass, it has the advantages of being fast, simple and easy to operate, so it is often used to detect the response of fungal growth to environmental factors[15]. The latter is relatively accurate, but requires a large amount of hyphae, and the operation is relatively complex and time-consuming[16]. Our preliminary study showed that S. commune strains grew well in both solid and liquid medium, but the growth rate of the fungal strains was faster in solid medium than that in liquid medium (data not shown). Therefore, all the experiments in this study were carried out on solid medium in order to quickly compare the growth differences of the test strains.
Oxygen is the most critical factor for life, and eukaryotic organisms usually do not survive anaerobic conditions; however, all the selected strains exhibited vegetative growth under anaerobic conditions with modification in their colony morphology (Figure 1). The subseafloor strains even showed a higher growth rate under the anaerobic conditions as compared to aerobic conditions (Figure 2). Although few studies have been reported on anaerobic fungi, there is evidence that fungi use various strategies to cope with the presence or absence of oxygen. For instance, Fusarium oxysporum, grow well under both aerobic and anaerobic conditions, but the nitrate reductase of the strain plays assimilation and dissimilation functions under aerobic and anaerobic conditions, respecticely[17]. Some anaerobic fungi have complex respiratory chains that contain alternative oxidases that allow electrons to bypass the electron transport chain if necessary[18]. It has also been reported that fungi can adapt to anoxic environments by metabolic changes from oxidative phosphorylation to fermentation and by reduced translation and cell growth[9-10]. The multiple mechanisms by which fungi cope with oxygen may partly explain why S. commune is one of the most widely distributed fungal species on Earth, even in the deep subseafloor environments up to approxmately 2 kmbsf for 20 million years were explored[6].
Temperature is one of the most critical environmental factors limiting the survival of microorganisms. Fungi have relatively weak high temperature resistance, with the upper limit of growth temperature of 62 ℃[19]. For S. commune, we found it could not grow at temperature ≥50 ℃ [6], but could grow well at temperature ≤45 ℃ (Figure 3). Moreover, strains of S. commune recovered from the deep subseafloor sediments grew better than those from the terrestrial and marine environments. All these data suggest that S. commune is not only alive but also may have grown in the deep subseafloor environment up to 1993 mbsf.
S. commune, a ubiquitous white rot fungus, is able to colonize at least 150 genera of woody plants with unique wood-degrading machinery[12]. Lignin is a major component of the cell wall of vascular plants and an important precursor of lignite. Since the four strains of S. commune in this study were isolated from coal-bearing sediments, their growth capacities were compared with those of two other strains isolated from land and marine environments in culture with lignin as a sole carbon source. The results showed that the growth rate of subseafloor strains was similar to that of terrestrial strain but significantly better than that of marine strain (Figure 7). The possible explanation to this astonishing finding is that fungi below the seafloor is not migrated from the marine environments but might be the actual resident to these sediments living there for more than 20 million years[6], where they developed new behaviors similar to terrestrial strain, to deal with the extreme conditions. Moreover, all subseafloor strains of S. commune showed similar growth characteristics under different culture conditions (Figure 9) and had more robust environmental adaptability against temperature, oxygen, Fe2+, NH4Cl, pH, lignin, and salinity. These data further confirmed our previous suggestion that fungi we isolated from the subseafloor sediments are likely to be deeply buried terrestrial fungi that may still reflect the original depositional conditions[6].
Compared with other habitat strains, the subseafloor strains of S. commune showed stronger adaptability to various environmental factors mimicking the in-situ conditions. These abilities help the fungus to survive in the harsh, dark ecosystems below seafloor, and play an important role in the biogeochemical cycles. Moreover, the subseafloor strains unexpectedly showed striking similarities with the terrestrial strain and obvious differences with marine strain, which further indicated that S. commune 6R-2-F01, 15R-5-F01, 20R-7-F01, and 24R-3-F01 were indigenous to deep subseafloor sediments. This study will provide a biological context for understanding fungal behavior in deep biosphere based cultural analysis.
| [1] | Hassett BT, Borrego EJ, Vonnahme TR, Rämä T, Kolomiets MV, Gradinger R. Arctic marine fungi:biomass, functional genes, and putative ecological roles. The ISME Journal, 2019, 13(6): 1484-1496. DOI:10.1038/s41396-019-0368-1 |
| [2] | Rédou V, Navarri M, Meslet-Cladière L, Barbier G, Burgaud G. Species richness and adaptation of marine fungi from deep-subseafloor sediments. Applied and Environmental Microbiology, 2015, 81(10): 3571-3583. DOI:10.1128/AEM.04064-14 |
| [3] | Raghukumar C, Raghukumar S, Sheelu G, Gupta SM, Nath BN, Rao BR. Buried in time:culturable fungi in a deep-sea sediment core from the Chagos Trench, Indian Ocean. Deep Sea Research Part Ⅰ:Oceanographic Research Papers, 2004, 51(11): 1759-1768. DOI:10.1016/j.dsr.2004.08.002 |
| [4] | Orsi W, Biddle JF, Edgcomb V. Deep sequencing of subseafloor eukaryotic rRNA reveals active fungi across marine subsurface provinces. PLoS One, 2013, 8(2): e56335. DOI:10.1371/journal.pone.0056335 |
| [5] | Nagano Y, Konishi M, Nagahama T, Kubota T, Abe F, Hatada Y. Retrieval of deeply buried culturable fungi in marine subsurface sediments, Suruga-Bay, Japan. Fungal Ecology, 2016, 20: 256-259. DOI:10.1016/j.funeco.2015.12.012 |
| [6] | Liu CH, Huang X, Xie TN, Duan N, Xue YR, Zhao TX, Lever MA, Hinrichs KU, Inagaki F. Exploration of cultivable fungal communities in deep coal-bearing sediments from~1.3 to 2.5 km below the ocean floor. Environmental Microbiology, 2017, 19(2): 803-818. DOI:10.1111/1462-2920.13653 |
| [7] | Edgcomb VP, Beaudoin D, Gast R, Biddle JF, Teske A. Marine subsurface eukaryotes:the fungal majority. Environmental Microbiology, 2011, 13(1): 172-183. DOI:10.1111/j.1462-2920.2010.02318.x |
| [8] | Orsi WD, Richards TA, Santoro AE. Cellular maintenance processes that potentially underpin the survival of subseafloor fungi over geological timescales. Estuarine, Coastal and Shelf Science, 2015, 164: A1-A9. DOI:10.1016/j.ecss.2015.04.009 |
| [9] | Songserm P, Karnchanatat A, Thitiprasert S, Tanasupawat S, Assabumrungrat ST, Yang ST, Thongchul N. Metabolic responses of Aspergillus terreus under low dissolved oxygen and pH levels. Annals of Microbiology, 2018, 68(4): 195-205. DOI:10.1007/s13213-018-1330-6 |
| [10] | Hillmann F, Shekhova E, Kniemeyer O. Insights into the cellular responses to hypoxia in filamentous fungi. Current Genetics, 2015, 61(3): 441-455. DOI:10.1007/s00294-015-0487-9 |
| [11] | Selbmann L, Egidi E, Isola D, Onofri S, Zucconi L, de Hoog GS, Chinaglia S, Testa L, Tosi S, Balestrazzi A, Lantieri A, Compagno R, Tigini V, Varese Giovanna Cristina. Biodiversity, evolution and adaptation of fungi in extreme environments. Plant Biosystems- An International Journal Dealing with all Aspects of Plant Biology, 2013, 147(1): 237-246. DOI:10.1080/11263504.2012.753134 |
| [12] | Ohm RA, de Jong JF, Lugones LG, Aerts A, Kothe E, Stajich JE, de Vries RP, Record E, Levasseur A, Baker SE, Bartholomew KA, Coutinho PM, Erdmann S, Fowler TJ, Gathman AC, Lombard V, Henrissat B, Knabe N, Kües U, Lilly WW, Lindquist E, Lucas S, Magnuson JK, Piumi F, Raudaskoski M, Salamov A, Schmutz J, Schwarze FWMR, vanKuyk PA, Horton JS, Grigoriev IV, Wösten HAB. Genome sequence of the model mushroom Schizophyllum commune. Nature Biotechnology, 2010, 28(9): 957-963. DOI:10.1038/nbt.1643 |
| [13] | Inagaki F, Hinrichs KU, Kubo Y, and the IODP Expedition 337 Scientists. Deep coalbed biosphere off Shimokita: microbial processes and hydrocarbon system associated with deeply buried coalbed in the ocean. IODP Expedition 337 Preliminary Report. publications. iodp.org/preliminary_report/337/index. html. Accessed 17, March, 2020. |
| [14] | Jørgensen BB. Deep subseafloor microbial cells on physiological standby. Proceedings of the National Academy of Sciences of the United States of America, 2011, 108(45): 18193-18194. DOI:10.1073/pnas.1115421108 |
| [15] | Kulmitra AK, Sahu N, Sahu MK, Kumar R, Kushram T, Kumar VBS. Growth of rice blast fungus Pyricularia oryzae (Cav.) on different solid and liquid media. International Journal of Current Microbiology and Applied Sciences, 2017, 6(6): 1154-1160. DOI:10.20546/ijcmas.2017.606.133 |
| [16] | Taniwaki MH, Pitt JI, Hocking AD, Fleet GH. Comparison of hyphal length, ergosterol, mycelium dry weight, and colony diameter for quantifying growth of fungi from foods//Hocking AD, Pitt JI, Samson RA, Thrane U. Advances in Food Mycology. Boston, MA: Springer, 2006: 49-67. |
| [17] | Kurakov AV, Nosikov AN, Skrynnikova EV, L'vov NP. Nitrate reductase and nitrous oxide production by Fusarium oxysporum 11dn1 under aerobic and anaerobic conditions. Current Microbiology, 2000, 41(2): 114-119. DOI:10.1007/s002840010104 |
| [18] | Stanić M, Zakrzewska J, Hadžibrahimović M, Žižić M, Marković Z, Vučinić Ž, Živić M. Oxygen regulation of alternative respiration in fungus Phycomyces blakesleeanus:connection with phosphate metabolism. Research in Microbiology, 2013, 164(7): 770-778. DOI:10.1016/j.resmic.2013.03.002 |
| [19] | Hoehler TM. Biological energy requirements as quantitative boundary conditions for life in the subsurface. Geobiology, 2004, 2(4): 205-215. DOI:10.1111/j.1472-4677.2004.00033.x |
 2020, Vol. 60
2020, Vol. 60