中国科学院微生物研究所,中国微生物学会,中国菌物学会
文章信息
- 李娜, 温继龙, 宋福平, 张杰, 段江燕, 彭琦. 2020
- Na Li, Jilong Wen, Fuping Song, Jie Zhang, Jiangyan Duan, Qi Peng. 2020
- 苏云金芽胞杆菌dxr1基因的转录受SigH控制
- Transcription of dxr1 is controlled by SigH in Bacillus thuringiensis
- 微生物学报, 60(1): 200-210
- Acta Microbiologica Sinica, 60(1): 200-210
-
文章历史
- 收稿日期:2019-03-26
- 修回日期:2019-04-24
- 网络出版日期:2019-05-21
2. 中国农业科学院植物保护研究所, 植物病虫害生物学国家重点实验室, 北京 100193
2. State Key Laboratory for Biology of Plant Diseases and Insect Pests, Institute of Plant Protection, Chinese Academy of Agricultural Sciences, Beijing 100193, China
萜类化合物是小分子天然化合物,广泛分布在生物界,是重要的生命物质[1],在细胞壁的合成、蛋白翻译后修饰和信号传导等过程中具有重要的作用[2]。目前发现有2条萜类化合物的生物合成途径,即甲羟戊酸(MVA)途径和2-甲基-D-赤藓糖醇-4-磷酸(MEP)途径。MVA途径主要存在于哺乳动物、高等植物胞液、真菌和少数细菌中;MEP途径主要存在于高等植物质体、大多数原核生物和病原体中[2]。在MEP途径中,以丙酮酸和3-磷酸甘油醛为反应底物,经过6个中间产物,最终形成二甲烯丙基焦磷酸(DMAPP)及其异构物、异戊二烯焦磷酸(IPP)[3],这两种化合物是类异戊二烯的共同前体。MEP代谢途径在不同的生物体中具有不同的功能,例如在玉米中发现SCD酶(种子类胡萝卜素缺乏酶,SEED CAROTENOID DEFICIENT)催化MEP途径中的倒数第二步:由2-C-甲基-D-赤藓糖醇-2, 4-环二磷酸(MEcPP)转化成4-羟基-3-甲基-2-丁烯基-焦磷酸(HMBPP)。scd基因的缺失导致植物的生长发育受损,在叶片和种子中MEP代谢的衍生物(如类胡萝卜素、叶绿素、维生素E、脱落酸和赤霉素等)的水平下降[4]。在疟原虫(Plasmodium falciparum)的顶质体[5]和一些致病菌中,如流感嗜血杆菌(Haemophilus influenzae)[6]、结核分枝杆菌(Mycobacterium tuberculosis)[7]和大肠杆菌(Escherichia coli)[8]等,MEP代谢途径是抑制病原微生物活性物质的药物分子靶标,通过研究和改造MEP代谢途径相关酶的特性,对于筛选新型稳定廉价的抑菌药物具有潜在的应用价值。枯草芽胞杆菌(Bacillus subtilis, Bs)中[9],过表达MEP代谢途径中的相关酶,使类胡萝卜素的产量显著增加,可以将Bs发展成为用于生产类异戊二烯的细胞工厂[1]。
MEP代谢途径的起始反应是在1-脱氧-D-木酮糖-5-磷酸合成酶(DXS)的催化下将3-磷酸甘油醛和丙酮酸生成1-脱氧-D-木酮糖-5-磷酸(DXP),DXP在1-脱氧-D-木酮糖-5-磷酸-还原异构酶(DXR)的催化下,生成MEP,这两步反应是MEP代谢途径的限速步骤和关键步骤[10–12]。在一些药用植物中,如雷公藤(Tripterygium wilfordii)、香茅(Cymbopogon winterianus)、薄荷(Mentha)等,克隆和表达了编码DXR的基因,并通过过表达DXR来提高类异戊二烯的产量,使植株积累和产生更多有利于生长和药用价值的物质[13–15]。在一些病源微生物中,如结核分枝杆菌、大肠杆菌和创伤弧菌(Vibrio vulnificus)中,克隆表达dxr基因,并解析了DXR的晶体结构,发现了与金属离子结合的活性位点,这些发现为用于抗菌化合物的DXR抑制剂的设计提供基础[16–17]。目前已经发现膦胺霉素及其类似物是DXR的抑制剂[18–20]。
苏云金芽胞杆菌(Bacillus thuringiensis,Bt)是革兰氏阳性细菌,属于蜡样芽胞杆菌族,其特点是在产生芽胞的同时还能生成对多种农林害虫具有杀虫活性的晶体蛋白[21]。在芽胞形成的过程中,与枯草芽胞杆菌相似,不同Sigma因子,包括SigA、SigH、SigE、SigK、SigF和SigG,通过与启动子的结合调控芽胞形成的相关基因在时间和空间上有序且准确无误地表达[22]。其中,SigH主要在芽胞杆菌从指数生长期到平稳期时发挥重要功能,除了芽胞形成相关的基因,SigH还在其他生理过程中指导相关基因的表达,包括细胞色素生物合成、营养物质的利用和运输、细胞壁代谢等[23]。
通过基因组序列比对分析发现,Bt HD73菌株中也存在MEP代谢途径,从丙酮酸和3-磷酸甘油醛,生成DMAPP和IPP需要7种酶的催化,由8个基因编码。其中DXR由两个基因编码,即dxr1(HD73_3605)和dxr2(HD73_4104),它们与枯草芽胞杆菌中的同源基因dxr[1]的相似性分别为56.6%和70.1%。目前细菌中对于dxr基因的克隆和抑制剂的筛选方面的研究报道较多,而关于dxr基因的转录调控未见报道。本文拟对Bt中的dxr1基因的转录调控机制开展研究,为更深入了解细菌MEP代谢途径的调控机制提供基础。
1 材料和方法 1.1 材料 1.1.1 菌株、质粒和培养基: 所用菌株和质粒见表 1。| Strains and plasmids | Characterization | Resource |
| Strains | ||
| HD73 | B. thuringiensis subsp. kurstaki carrying the cry1Ac gene | This lab |
| HD(ΔsigH) | B. thuringiensis HD73 sigH gene insertion mutant | [25] |
| HD(Pdxr1) | HD73 strain containing plasmid pHTPdxr1 | This study |
| ΔsigH(Pdxr1) | HD(ΔsigH) strain containing plasmid pHTPdxr1 | This study |
| HD(Δdxr1) | B. thuringiensis HD73 dxr1 gene insertion mutant | This study |
| HD(Δdxr2) | B. thuringiensis HD73 dxr2 gene insertion mutant | This study |
| E. coli TG1 | ∆(lac-proAB) supE thi hsd-5 (F' traD36 proA+ proB+ lacIq lacZ∆M15), general purpose cloning host | This lab |
| E. coli ET | F- dam-13::Tn9 dcm-6 hsdM hsdR recF143 zjj-202: :Tn10 galK2 galT22 ara14 pacY1 xyl-5 leuB6 thi-1, for generation of unmethylated DNA | This lab |
| Plasmids | ||
| pHT304-18Z | Promoterless lacZ vector, Eryr, Ampr | This lab |
| pHTPdxr1 | pHT304-18Z carrying promoter upstream from dxr1 | This study |
| pMAD | ApR, EmR shuttle vector, thermosensitive origin of replication | Institute Pasteur |
| pMAD∆dxr1 | pMAD with dxr1 insertion fragment | This study |
| pMAD∆dxr2 | pMAD with dxr2 insertion fragment | This study |
大肠杆菌(Escherichia coli,E. coli)使用LB培养基,培养条件:37 ℃、220 r/min;Bt的培养分别使用LB培养基和SSM培养基[24],培养条件:30 ℃、220 r/min。抗生素的使用终浓度分别为:氨苄青霉素100 g/mL,红霉素5 μg/mL,卡那霉素100 μg/mL
1.1.2 主要生化试剂: 限制性内切酶、DNA聚合酶、DNA连接酶均购自TaKaRa公司;质粒提取、DNA回收和PCR产物纯化试剂盒购自Axygen公司。细菌RNA提取试剂盒RNAprep Pure Cell/Bacteria kit购自天根生化科技(北京)有限公司。SMARTerTM RACE cDNA Amplification试剂盒购自Clontech公司。无缝克隆试剂盒购自中美泰和生物技术(北京)有限公司。 1.1.3 引物合成及序列测定: 根据Bt HD73基因组(GenBank登录号:CP004069)序列[26]设计引物,引物合成由生工生物工程(上海)股份有限公司北京合成部完成,序列测定由北京诺赛基因组研究中心有限公司完成,引物名称及序列见表 2。| Primer name | Sequence (5'→3')a |
| UPM | AAGCAGTGGTATCAACGCAGAGTACATGGG |
| dxr1-RACE | GGCATTCTCATATCAGGAGCACCGAGC |
| Pdxr1-F | CCAAGCTTACTAACTCCTCCTTTCGC |
| Pdxr1-R | CGGGATCCGAAAAAACCTCCAAAGTTG |
| dxr1-A1 | GGCGATATCGGATCCTCTGAATCATGCACA |
| dxr1-A2 | TCAAATGGTTCGCTGGGATCCTGTGGAACC |
| dxr1-B1 | GCCTACGAGGAATTTGAAGCGGATCAATGG |
| dxr1-B2 | CGGGAGCTCGAATTCAGCAATTGCCGAATC |
| d1Kan-1 | GGTTCCACAGGATCCCAGCGAACCATTTGA |
| d1Kan-2 | CCATTGATCCGCTTCAAATTCCTCGTAGGC |
| dxr2-A1 | CCATATGACGTCGACCATGGATTGGATTAG |
| dxr2-A2 | TCAAATGGTTCGCTGACCAATTGATCCGCT |
| dxr2-B1 | GCCTACGAGGAATTTAGACGGTTTGTGATG |
| dxr2-B2 | CGGGAGCTCGAATTCCAAATCCAAGAATCAC |
| d2Kan-1 | AGCGGATCAATTGGTCAGCGAACCATTTGA |
| d2Kan-2 | CATCACAAACCGTCTAAATTCCTCGTAGGC |
| a: Restriction enzyme sites are underscored. | |
1.2 5'RACE分析
HD73菌株在SSM中培养至T5时期(T0为对数期结束的时期,Tn为T0后的n小时),取菌液低温离心,沉淀用预冷的TRIzol重悬,RNA提取参照RNAprep Pure Cell/Bacteria kit说明。以纯化后的RNA为模板,以dxr1RACE和UPM为引物合成cDNA,cDNA的合成参考SMARTerTM RACE cDNA Amplification试剂盒说明。得到的PCR产物经纯化后连接pMD19-T载体并测序,对测序结果进行比对分析。
1.3 dxr1基因启动子融合lacZ基因表达载体构建以Bt HD73基因组为模板,用Pdxr1-F和Pdxr1-R引物扩增dxr1(HD73_3605)基因启动子Pdxr1片段(389 bp),将含有lacZ报告基因的载体pHT304-18Z与PCR产物经过BamH I和Hind Ⅲ双酶切,连接、转化至E. coli TG1菌株获得重组质粒pHTPdxr1,将重组质粒转入E. coli ET 12567菌株中去甲基化后通过电击转入HD73出发菌株和ΔsigH突变菌株,转化方法见文献[27],获得菌株HD(Pdxr1)和ΔsigH(Pdxr1)。
1.4 dxr基因突变菌株构建及筛选Bt HD73菌株中,有两个基因编码DXR,分别为HD73_3605基因(记为dxr1)和HD73_4104基因(记为dxr2),利用同源重组的原理[28],分别构建dxr1和dxr2突变体。以dxr1为例简述如下:以Bt HD73基因组为模板,用引物dxr1-A1/dxr1-A2扩增dxr1基因上游片段(dxr1-A,595 bp);用引物dxr1-B1/dxr1-B2扩增dxr1基因下游片段(dxr1-B,604 bp)。以cwlC突变体[29]为模板(含有卡那霉素抗性基因),用引物d1Kan-1/d1Kan-2扩增卡那霉素抗性基因(kan,1473 bp)。温敏穿梭载体pMAD经限制性内切酶BamH I和EcoR I双酶切后,应用无缝克隆试剂盒,与dxr1-A、dxr1-B和kan三个片段连接,转化至大肠杆菌TG1菌株中,获得重组质粒pMADΔdxr1。将重组质粒转入ET去甲基化,再电击转入HD73菌株,获得具有红霉素和卡那霉素抗性的HD (pMADΔdxr1)菌株。该菌株在LB培养基中进行38 ℃高温突变,传代3次,筛选出有卡那霉素抗性并且没有红霉素抗性的菌株。经过PCR鉴定,获得的突变菌株命名为HD (Δdxr1)。dxr2基因的突变菌株HD (Δdxr2)的获得方法与HD(Δdxr1)菌株相同,所用引物分别为:dxr2-A1/dxr2-A2扩增dxr2基因上游片段(dxr2-A,634 bp);dxr2-B1/dxr2- B2扩增dxr2基因下游片段(dxr2-B,621 bp);d2Kan-1/ d2Kan-2扩增卡那霉素抗性基因(kan,1473 bp)。
1.5 β-半乳糖苷酶活性测定Bt菌株活化8 h,1%转接至SSM培养基,30 ℃、220 r/min振荡培养,从T0至T10,每小时取样2 mL,低温离心,沉淀用于测定β-半乳糖苷酶活性,方法见参考文献[29],每组数据3次独立重复取平均值并计算标准差。
1.6 生长曲线测定方法见参考文献[30],简述如下:将Bt菌株分别接种于LB和SSM培养基中,30 ℃、220 r/min培养。每1 h取样,测定600 nm波长下的吸光值OD600,每组数据至少独立重复3次。
1.7 芽胞形成率方法见参考文献[30],Bt菌株在芽胞形成培养基SSM中,30 ℃、220 r/min培养至90%左右菌体释放芽胞时,取适量菌液梯度稀释涂于LB固体培养基,30 ℃培养10 h,对LB固体培养基上的菌落进行计数,数值记为A。取10 mL菌液65 ℃热处理25 min后,梯度稀释涂于LB固体培养基,30 ℃培养10 h,对LB固体培养基上的菌落进行计数,数值记为B,芽胞形成率=(B/A)×100%。每组数据至少独立重复3次,取平均值并计算标准差。
1.8 Cry1Ac蛋白产量测定Bt菌株在SSM培养基中培养至T24,取样离心,沉淀中加入50 mmol/L Tris-HCl,机械振荡破碎100 s后,加上样缓冲液,沸水浴10 min,离心取上清液,利用PierceⓇ 660 nm protein Assay Kit试剂盒测定蛋白的浓度,取相同总蛋白量的样品进行SDS-PAGE,方法见参考文献[30]。
1.9 1-脱氧-D-木酮糖-5-磷酸还原异构酶酶活测定Bt菌株在SSM培养基中培养至T3、T5和T7,分别取50 mL菌液,离心弃上清,沉淀中加入500 μL 1×PBS缓冲液和石英砂,机械振荡破碎180 s,离心取240 μL上清,应用1-脱氧-D-木酮糖-5-磷酸还原异构酶(DXR)酶联免疫分析试剂盒(江莱生物)检测DXR活性,方法见说明书。
2 结果和分析 2.1 dxr1基因转录起始位点和启动子序列分析用SMARTerTM RACE cDNA Amplification试剂盒将HD73菌株T5时期的总RNA反转录为cDNA,用引物UPM和dxr1RACE进行PCR扩增,将纯化后的DNA与pMD19-T载体进行连接并转化E. coli TG1菌株,挑取20个阳性克隆测序,将测序结果与dxr1基因起始密码子上游序列和接头引物序列(UPM)比对,结果表明(图 1),有18个克隆的测序结果与dxr1基因的上游序列一致,接头序列之后的核苷酸即为dxr1的转录起始位点,位于起始密码子上游39 bp处的G碱基(图 2,标星号的核苷酸)。通过在DBTBS数据库(http://dbtbs.hgc.jp/)中检索dxr1基因启动子序列的转录调控因子结合位点,发现了SigH的结合位点,具有保守的–10区和–35区序列(图 2,加粗的核苷酸序列,双下划线表示–35区和–10区),说明dxr1基因的转录可能受SigH的控制。

|
| 图 1 序列比对结果 Figure 1 Sequence alignment. |

|
| 图 2 dxr基因启动子序列分析 Figure 2 Sequence analysis of the promoter of dxr gene. |
2.2 dxr1基因转录调控分析
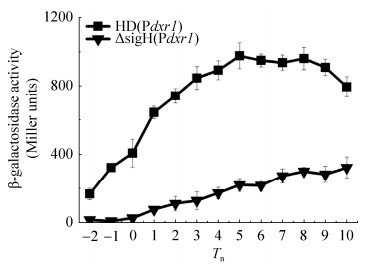
为了研究dxr1基因的转录活性,构建了dxr1的启动子Pdxr1融合lacZ基因的表达载体pHTPdxr1,分别电击转入Bt HD73菌株和sigH突变体,获得菌株HD (Pdxr1)和ΔsigH (Pdxr1)。β-半乳糖苷酶活性测定表明,在SSM培养基中,HD (Pdxr1)菌株的转录活性在T-2与T5之间持续增加,从T5之后开始略有下降(图 3)。与出发菌株相比,从T-2至T10,Pdxr1在sigH突变体中的转录活性显著下降(图 3),说明dxr1基因的转录受SigH的控制。
|
| 图 3 Pdxr的转录活性 Figure 3 Transcriptional activity of Pdxr. The standard deviation reflects the degree of dispersion of the value relative to the mean. |
2.3 dxr突变体的表型分析
为了进一步明确dxr基因在Bt中的功能,利用同源重组的方法,分别构建了dxr1和dxr2的突变体,并进行了表型分析。生长曲线测定实验表明(图 4-A):无论是在营养丰富的LB培养基,还是在营养贫瘠的SSM培养基,2个dxr基因的缺失对菌体生长均无明显影响。芽胞形成率实验表明(图 4-B),HD(Δdxr1)和HD(Δdxr2)菌株芽胞形成率与野生型HD73菌株相比无明显差异,说明dxr基因的缺失不影响芽胞形成率。将HD73菌株、HD(Δdxr1)和HD(Δdxr2)菌株在LB培养基中培养至T24,收集菌体,分析蛋白产量,结果表明在总蛋白量相同的条件下,dxr突变体和HD73野生菌株的Cry1Ac蛋白产量无明显差异(图 4-C),说明dxr基因的缺失对Cry蛋白产量无显著影响。1-脱氧-D-木酮糖-5-磷酸还原异构酶活性测定实验表明(图 4-D),HD73出发菌株中,T5时期的DXR活性最高,与启动子的转录活性趋势一致(图 3);从T3到T7,与HD73出发菌株比较,HD(Δdxr1)和HD(Δdxr2)突变菌株的DXR活性显著下降,说明dxr基因的缺失影响了DXR的活性。

|
| 图 4 dxr突变体的表型分析 Figure 4 Phenotype of dxr mutants. A: growth curve; B: sporulation efficiency; C: Cry1Ac protein production; D: DXR activity. The standard deviation reflects the degree of dispersion of the value relative to the mean. |
3 讨论和结论
本研究发现,dxr1基因上游启动子序列存在SigH结合位点,通过β-半乳糖苷酶活性测定明确了dxr1基因启动子的转录受SigH的控制。但是在sigH突变体中(图 3),dxr1基因启动子的转录活性明显降低,没有完全丧失,可能还存在其他的Sigma因子控制dxr1的转录。通过BPROM预测(http://www.softberry.com/berry.phtml?topic=bprom&group=programs&subgroup=gfindb),dxr1基因的上游可能还存在另外一个启动子(TTCATTGCTTT TTCGTTATTTTTTACAGTTTATCAC)。前期通过生物信息学分析和RT-PCR实验发现(未发表),dxr2基因与其上游的6个基因及下游的3个基因共转录,形成一个转录单元,该转录单元的上游启动子区域无SigH的结合位点,因此推测dxr2基因的转录可能不受SigH的直接控制。
枯草芽胞杆菌中,DXR由一个dxr基因编码,而在Bt HD73中,有2个dxr基因(dxr1和dxr2)编码DXR,通过序列比对分析发现,Bt中的dxr2基因及其上下游基因在基因组中的位置排列与Bs相似(图 5),而在Bs中没有发现与Bt的dxr1基因及其上下游基因相似的组织结构。本研究发现,单独突变dxr1或dxr2,对菌体的生长、芽胞形成和Cry蛋白的产量没有影响,但使DXR活性下降,可能是dxr1和dxr2基因存在互补功能,缺失其中一个基因,不会使DXR的活性完全丧失,也不会使菌株的重要表型受到影响。

|
| 图 5 Bt和Bs中dxr基因组成结构 Figure 5 The organization of the dxr gene in Bt and Bs. |
在Bt中,通过MEP代谢途径产生的DMAPP和IPP可以进一步生成法尼基二磷酸(FPP)和香叶基香叶基二磷酸(GGPP),FPP和GGPP是(E)-4, 8-二甲基-1, 3, 7-壬三烯(DMNT)和(E, E)-4, 8, 12-三甲基-1, 3, 7, 11-十三碳四烯(TMTT)合成的前体。当大部分裸子植物和被子植物受到食草动物和病原菌胁迫时会大量释放DMNT和TMTT这些挥发性萜烯类化合物,它们作为化学信号,可以被食草性昆虫识别,驱使其他的食草昆虫远离该植株,避免再次被取食[31–32]。最近的研究揭示了植物DMNT和TMTT合成途径上的关键2个萜烯类合成酶基因,并将这2个基因转入水稻中,使水稻挥发出DMNT和TMTT,显著吸引了水稻害虫天敌二化螟盘绒茧蜂,起到了对害虫的间接防御作用,行为学检测的结果也证明DMNT和TMTT对小菜蛾绒茧蜂也具有明显的吸引作用[33]。因此,通过将植物来源的萜烯类合成酶基因转入Bt菌株中,使其利用自身代谢产生的FPP和GGPP,产生对天敌有吸引作用的DMNT和TMTT,可以成为一种既对害虫有毒杀作用,又吸引天敌的双重绿色防控策略。通过对Bt MEP代谢途径的转录调控机制的研究,加深对该途径的认识,为更好地利用该代谢途径生产高效广谱的工程菌提供理论基础。
| [1] | Guan Z, Xue D, Abdallah Ⅱ, Dijkshoorn L, Setroikromo R, Lv GY, Quax WJ. Metabolic engineering of Bacillus subtilis for terpenoid production. Applied Microbiology and Biotechnology, 2015, 99(22): 9395-9406. DOI:10.1007/s00253-015-6950-1 |
| [2] | Frank A, Groll M. The methylerythritol phosphate pathway to isoprenoids. Chemical Reviews, 2017, 117(8): 5675-5703. DOI:10.1021/acs.chemrev.6b00537 |
| [3] | Rohmer M. The discovery of a mevalonate-independent pathway for isoprenoid biosynthesis in bacteria, algae and higher plants. Natural Product Reports, 1999, 16(5): 565-574. DOI:10.1039/a709175c |
| [4] | Zhang LL, Zhang X, Wang XJ, Xu J, Wang M, Li L, Bai GH, Fang H, Hu ST, Li JG, Yan JB, Li JS, Yang XH. Seed carotenoid deficient functions in isoprenoid biosynthesis via the plastid MEP pathway. Plant Physiology, 2019. DOI:10.1104/pp.18.01148 |
| [5] | He L, He P, Luo XY, Li MX, Yu L, Guo JY, Zhan XY, Zhu G, Zhao JL. The MEP pathway in Babesia orientalis apicoplast, a potential target for anti-babesiosis drug development. Parasites & Vectors, 2018, 11(1): 452. DOI:10.1186/s13071-018-3038-7 |
| [6] | Matsue Y, Mizuno H, Tomita T, Asami T, Nishiyama M, Kuzuyama T. The herbicide ketoclomazone inhibits 1-deoxy-D-xylulose 5-phosphate synthase in the 2-C-methyl-D-erythritol 4-phosphate pathway and shows antibacterial activity against Haemophilus influenzae. Journal of Antibiotics, 2010, 63(10): 583-588. DOI:10.1038/ja.2010.100 |
| [7] | San Jose G, Jackson ER, Uh E, Johny C, Haymond A, Lundberg L, Pinkham C, Kehn-Hall K, Boshoff HI, Couch RD, Dowd CS. Design of potential bisubstrate inhibitors against Mycobacterium tuberculosis (Mtb) 1-Deoxy-D-Xylulose 5-phosphate reductoisomerase (Dxr)-evidence of a novel binding mode. MedChemComm, 2013, 4(7): 1099-1104. DOI:10.1039/c3md00085k |
| [8] | Borel F, Barbier E, Krasutsky S, Janthawornpong K, Chaignon P, Poulter CD, Ferrer JL, Seemann M. Further insight into crystal structures of Escherichia coli Isph/Lytb in complex with two potent inhibitors of the MEP pathway:a starting point for rational design of new antimicrobials. ChemBioChem, 2017, 18(21): 2137-2144. DOI:10.1002/cbic.201700363 |
| [9] | Xue D, Abdallah Ⅱ, de Haan IEM, Sibbald MJJB, Quax WJ. Enhanced C30 carotenoid production in Bacillus subtilis by systematic overexpression of MEP pathway genes. Applied Microbiology and Biotechnology, 2015, 99(14): 5907-5915. DOI:10.1007/s00253-015-6531-3 |
| [10] | Xue JF, Ahring BK. Enhancing isoprene production by genetic modification of the 1-deoxy-d-xylulose-5-phosphate pathway in Bacillus subtilis. Applied and Environmental Microbiology, 2011, 77(7): 2399-2405. DOI:10.1128/AEM.02341-10 |
| [11] | Hui X, Yue Q, Zhang DD, Li H, Yang SQ, Gao WY. Antimicrobial mechanism of theaflavins:they target 1-deoxy-D-xylulose 5-phosphate reductoisomerase, the key enzyme of the MEP terpenoid biosynthetic pathway. Scientific Reports, 2016, 6: 38945. DOI:10.1038/srep38945 |
| [12] | Kuzuyama T, Seto H. Two distinct pathways for essential metabolic precursors for isoprenoid biosynthesis. Proceedings of the Japan Academy. Series B, Physical and Biological Sciences, 2012, 88(3): 41-52. DOI:10.2183/pjab.88.41 |
| [13] | Tong YR, Su P, Zhao YJ, Zhang M, Wang XJ, Liu YJ, Zhang XN, Gao W, Huang LQ. Molecular cloning and characterization of DXS and DXR genes in the terpenoid biosynthetic pathway of tripterygium wilfordii. International Journal of Molecular Sciences, 2015, 16(10): 25516-25535. DOI:10.3390/ijms161025516 |
| [14] | Devi K, Dehury B, Phukon M, Modi MK, Sen P. Novel insights into structure-function mechanism and tissue-specific expression profiling of full-length dxr gene from Cymbopogon winterianus. FEBS Open Bio, 2015, 5: 325-334. DOI:10.1016/j.fob.2015.04.005 |
| [15] | Mahmoud SS, Croteau RB. Metabolic engineering of essential oil yield and composition in mint by altering expression of deoxyxylulose phosphate reductoisomerase and menthofuran synthase. Proceedings of the National Academy of Sciences of the United States of America, 2001, 98(15): 8915-8920. DOI:10.1073/pnas.141237298 |
| [16] | Ussin NK, Bagnell AM, Offermann LR, Abdulsalam R, Perdue ML, Magee P, Chruszcz M. Structural characterization of 1-deoxy-D-xylulose 5-phosphate reductoisomerase from Vibrio vulnificus. Biochimica et Biophysica Acta (BBA)-Proteins and Proteomics, 2018, 1866(12): 1209-1215. DOI:10.1016/j.bbapap.2018.09.008 |
| [17] | Armstrong CM, Meyers DJ, Imlay LS, Freel Meyers C, Odom AR. Resistance to the antimicrobial agent fosmidomycin and an FR900098 prodrug through mutations in the deoxyxylulose phosphate reductoisomerase gene (dxr). Antimicrobial Agents and Chemotherapy, 2015, 59(9): 5511-5519. DOI:10.1128/AAC.00602-15 |
| [18] | Jackson ER, Dowd CS. Inhibition of 1-deoxy-D-xylulose-5-phosphate reductoisomerase (Dxr):a review of the synthesis and biological evaluation of recent inhibitors. Current Topics in Medicinal Chemistry, 2012, 12(7): 706-728. DOI:10.2174/156802612799984599 |
| [19] | Sooriyaarachchi S, Chofor R, Risseeuw MDP, Bergfors T, Pouyez J, Dowd CS, Maes L, Wouters J, Jones TA, Van Calenbergh S, Mowbray SL. Targeting an aromatic hotspot in Plasmodium falciparum 1-Deoxy-D-xylulose-5-phosphate reductoisomerase with β-arylpropyl analogues of fosmidomycin. ChemMedChem, 2016, 11(18): 2024-2036. DOI:10.1002/cmdc.201600249 |
| [20] | Cobb RE, Bae B, Li Z, DeSieno MA, Nair SK, Zhao HM. Structure-guided design and biosynthesis of a novel FR-900098 analogue as a potent Plasmodium falciparum 1-deoxy-D-xylulose-5-phosphate reductoisomerase (Dxr) inhibitor. Chemical Communications, 2015, 51(13): 2526-2528. DOI:10.1039/C4CC09181G |
| [21] | van Frankenhuyzen K. Insecticidal activity of Bacillus thuringiensis crystal proteins. Journal of Invertebrate Pathology, 2009, 101(1): 1-16. DOI:10.1016/j.jip.2009.02.009 |
| [22] | Errington J. Bacillus subtilis sporulation:regulation of gene expression and control of morphogenesis. Microbiological Reviews, 1993, 57(1): 1-33. DOI:10.1128/MMBR.57.1.1-33.1993 |
| [23] | Britton RA, Eichenberger P, Gonzalez-Pastor JE, Fawcett P, Monson R, Losick R, Grossman AD. Genome-wide analysis of the stationary-phase sigma factor (sigma-H) regulon of Bacillus subtilis. Journal of Bacteriology, 2002, 184(17): 4881-4890. DOI:10.1128/JB.184.17.4881-4890.2002 |
| [24] | Schaeffer P, Millet J, Aubert JP. Catabolic repression of bacterial sporulation. Proceedings of the National Academy of Sciences of the United States of America, 1965, 54(3): 704-711. DOI:10.1073/pnas.54.3.704 |
| [25] | Du LX, Qiu LL, Peng Q, Lereclus D, Zhang J, Song FP, Huang DF. Identification of the promoter in the intergenic region between orf1 and cry8Ea1 controlled by sigma H factor. Applied and Environmental Microbiology, 2012, 78(12): 4164-4168. DOI:10.1128/AEM.00622-12 |
| [26] | Liu GM, Song L, Shu CL, Wang PS, Deng C, Peng Q, Lereclus D, Wang XM, Huang DF, Zhang J, Song FP. Complete genome sequence of Bacillus thuringiensis subsp. kurstaki strain HD73. Genome Announcements, 2013, 1(2): e0008013. DOI:10.1128/genomeA.00080-13 |
| [27] | Lereclus D, Arantès O, Chaufaux J, Lecadet MM. Transformation and expression of a cloned δ-endotoxin gene in Bacillus thuringiensis. FEMS Microbiology Letters, 1989, 60(2): 211-217. |
| [28] | Arnaud M, Chastanet A, Débarbouillé M. New vector for efficient allelic replacement in naturally nontransformable, low-GC-content, gram-positive bacteria. Applied and Environmental Microbiology, 2004, 70(11): 6887-6891. DOI:10.1128/AEM.70.11.6887-6891.2004 |
| [29] | Chen XM, Gao TT, Peng Q, Zhang J, Chai YR, Song FP. Novel cell wall hydrolase CwlC from Bacillus thuringiensis is essential for mother cell lysis. Applied and Environmental Microbiology, 2018, 84(7): e02640-17. |
| [30] |
Huang MZ, Peng Q, Zheng J, Gao JG, Song FP. Transcriptional regulation of aco gene cluster in Bacillus thuringiensis. Acta Microbiologica Sinica, 2015, 55(9): 1144-1153.
(in Chinese) 黄闽忠, 彭琦, 张杰, 高继国, 宋福平. 苏云金芽胞杆菌3-羟基丁酮代谢基因簇的转录调控. 微生物学报, 2015, 55(9): 1144-1153. |
| [31] | Tholl D, Lee S. Terpene specialized metabolism in Arabidopsis thaliana. Arabidopsis Book, 2011, 9: e0143. DOI:10.1199/tab.0143 |
| [32] | Boggia L, Sgorbini B, Bertea CM, Cagliero C, Bicchi C, Maffei ME, Rubiolo P. Direct contact-sorptive tape extraction coupled with gas chromatography-mass spectrometry to reveal volatile topographical dynamics of lima bean (Phaseolus lunatus L.) upon herbivory by Spodoptera littoralis Boisd. BMC Plant Biology, 2015, 15: 102. DOI:10.1186/s12870-015-0487-4 |
| [33] | Li FQ, Li W, Lin YJ, Pickett JA, Birkett MA, Wu KM, Wang GR, Zhou JJ. Expression of lima bean terpene synthases in rice enhances recruitment of a beneficial enemy of a major rice pest. Plant, Cell & Environment, 2018, 41(1): 111-120. |
 2020, Vol. 60
2020, Vol. 60


 ,
,