中国科学院微生物研究所,中国微生物学会,中国菌物学会
文章信息
- 虞德夫, 朱晓峰, 冯江银, 康翠翠, 张明钰, 朱伟云, 杭苏琴. 2019
- Defu Yu, Xiaofeng Zhu, Jiangyin Feng, Cuicui Kang, Mingyu Zhang, Weiyun Zhu, Suqin Hang. 2019
- 低蛋白质日粮对断奶仔猪生长相关激素和肠道微生物区系的影响
- Effect of low-protein diet on hormones associated with growth and on gut microbiota in weaned piglets
- 微生物学报, 58(9): 1695-1704
- Acta Microbiologica Sinica, 58(9): 1695-1704
-
文章历史
- 收稿日期:2019-03-15
- 修回日期:2019-05-01
- 网络出版日期:2019-07-11
传统的玉米-豆粕型日粮中赖氨酸等必需氨基酸的含量相对缺乏,不能满足仔猪断奶后的生长需要。通过提高日粮中粗蛋白比例能够满足机体对必需氨基酸的生长需要,但同时会导致非必需氨基酸的相对过剩,从而降低氮的利用率[1]。近年来,降低日粮蛋白质同时添加必需氨基酸的日粮配制方案受到广泛关注。我们前期研究发现,断奶仔猪日粮蛋白质水平从20%降低到17%并补充赖氨酸、蛋氨酸、苏氨酸和色氨酸能够维持断奶后仔猪的生长性能,进一步降低至14%,虽然补充了必需氨基酸但仔猪的生长明显受到抑制[2]。这说明,日粮蛋白质降低过多不利于仔猪断奶后的生长发育。家畜的生长主要受到下丘脑-垂体-生长轴和下丘脑-垂体-甲状腺轴的共同调控,参与调控的激素有生长激素和甲状腺激素T3、T4等,当机体蛋白质营养摄入不足时,促生长的调控轴暂时性被抑制以便减少体内营养物质的消耗[3-4]。本实验室前期研究表明,生长猪日粮蛋白质水平降低4个百分点时,生长激素水平显著下降,这很可能是导致生长性能下降的直接原因[5]。然而此类研究在猪上仍然较少,因而低蛋白质日粮是否通过降低生长相关激素的分泌从而抑制仔猪的生长性能仍需提供更多的试验证据。
哺乳动物肠道中含有超过1000种微生物[6-7]。Palmer认为[8],肠道微生物区系随着宿主的成长而不断演化,相比于成年宿主肠道内微生物区系,仔猪断奶阶段的肠道微生物区系不稳定,易受多种因素影响,不利于宿主健康。其中,日粮的转换(由母乳转变为固体饲料)是引起仔猪断奶前后肠道微生物区系变化的主要因素[9-10],研究表明,低蛋白质日粮能够通过抑制有害菌增殖从而缓解仔猪腹泻[11-12],然而前期研究显示当日粮蛋白质水平降低6个百分点时,大肠杆菌比例反而显著上升[13]。这说明日粮蛋白质降低过多可能不利于肠道有益菌的建立。因而,低蛋白质日粮对断奶后仔猪肠道微生物区系的影响仍需进一步研究。
因此,本研究以断奶后仔猪为研究对象,饲喂不同水平的低蛋白质日粮,检测生长相关激素,同时进行高通量测定肠道微生物16S rRNA基因的V3–V4可变区,分析肠道菌群组成和多样性,以期为低蛋白质日粮在断奶仔猪上的合理利用提供参考。
1 材料和方法 1.1 试验动物、试验日粮和试验设计选择健康的、日龄基本一致的杜×长×大三元杂交断奶仔猪54头,初始体重为9.58±0.62 kg,随机分为3组,每组18个重复。试验前适应3 d,正式试验45 d,对照组饲喂NRC (2012)推荐的标准日粮(表 1),其粗蛋白含量为20% (NP组);在对照组基础之上,处理组仔猪饲喂低蛋白质日粮,其粗蛋白含量分别为17% (MP组)和14% (LP组),在处理组中补充赖氨酸、蛋氨酸、苏氨酸和色氨酸4种必需氨基酸以满足仔猪断奶后的生长需要,同时补充大豆油使得三组之间消化能一致。试验期间仔猪自由采食及饮水,环境温度控制在24 ℃。
| Composition | Dietary protein level /% | ||
| NP (20% CP) |
MP (17% CP) |
LP (14% CP) |
|
| Ingredients /% | |||
| Corn | 63.70 | 66.50 | 71.80 |
| Soya bean meal | 19.80 | 18.80 | 13.40 |
| Whey powder | 4.30 | 4.30 | 4.40 |
| Fish meal | 9.00 | 4.00 | 1.50 |
| Soya bean oil | 0.80 | 2.60 | 4.10 |
| L-Lysine | 0.38 | 0.62 | 0.88 |
| D/L-Methionine | 0.10 | 0.19 | 0.27 |
| L-Threonine | 0.09 | 0.21 | 0.33 |
| L-Tryptophan | 0.01 | 0.04 | 0.08 |
| Monocalcium phosphate | 0.00 | 0.74 | 1.15 |
| Limestone | 0.52 | 0.70 | 0.79 |
| NaCl | 0.30 | 0.30 | 0.30 |
| Mineral premix* | 1.00 | 1.00 | 1.00 |
| Nutrient composition# [% in dry matter] | |||
| Digestible energy/(MJ/kg) | 14.60 | 14.60 | 14.60 |
| Crude protein | 20.27 | 17.32 | 14.14 |
| Lysine | 1.26 | 1.25 | 1.26 |
| Methionine + cysteine | 0.63 | 0.65 | 0.63 |
| Threonine | 0.76 | 0.75 | 0.76 |
| Tryptophan | 0.20 | 0.20 | 0.20 |
| Arginine | 1.09 | 0.93 | 0.71 |
| Histidine | 0.44 | 0.37 | 0.30 |
| Isoleucine | 0.71 | 0.60 | 0.46 |
| Leucine | 1.52 | 1.32 | 1.11 |
| Phenylalanine | 0.81 | 0.70 | 0.56 |
| Valine | 0.72 | 0.64 | 0.54 |
| Calcium | 0.70 | 0.71 | 0.70 |
| Phosphorus | 0.57 | 0.55 | 0.53 |
| Starch | 40.22 | 41.95 | 45.16 |
| Neutral detergent fiber | 8.54 | 8.66 | 8.40 |
| Acid detergent fiber | 3.29 | 3.30 | 3.05 |
| *Premix provided per kg of complete diet: 3800 IU vitamin A, 800 IU vitamin D3, 9 mg vitamin E, 1 mg vitamin K3, 1 mg vitamin B1, 2 mg vitamin B2, 1.2 mg vitamin B6, 10 µg vitamin B12, 10 mg nicotinic acid, 50 µg biotin, 0.4 mg folic acid, 80 mg iron (as FeSO4·H2O), 5 mg copper as (CuSO4·5H2O), 80 mg zinc (as ZnSO4·H2O and ZnO), 0.14 mg iodine (as KI), 0.25 mg Se (as Na2SeO3), 3 mg Mn (as MnSO4·H2O); #Analyzed values except for digestible energy. | |||
1.2 样品采集
分别在试验的第10、25和45天屠宰采样,每个时间点每组屠宰6头,静脉注射50 mg/kg戊巴比妥钠,颈静脉采集血液样品,静置1 h后,在4 ℃下3000×g离心15 min后,取上清液置于–20 ℃保存。采集血样后颈动脉放血处死,剖开腹腔后迅速将各个肠段结扎分离,分别采集回肠和盲肠食糜于2 mL冻存管中,立即置于液氮中保存。
1.3 血液相关指标的测定采用全自动生化分析仪(BECKMAN CONLTER AU5800)检测血常规;胰岛素(货号:H091)、胰高血糖素(货号:H183)、生长激素(货号:H091)、甲状腺激素(T3和T4)(货号:H222和H223)检测试剂盒购自南京建成生物公司,严格按照试剂盒说明书进行测定。
1.4 肠道微生物测序及其群落分析用酚氯仿抽提法提取肠道食糜中微生物总DNA,用微量核酸蛋白分析仪(NanoDrop Technologies Inc,美国)测定DNA浓度和纯度,浓度 > 100 ng/μL,OD260/OD280为1.6–1.8的样品于–80 ℃保存。
高通量测序检测猪肠道微生物群落结构并分析多样性。引物341F (5′-AGAGTTTGATCCTGGC TCAG-3′)和806R (5′-TTACCGCGGCTGCTGGC AC-3′)扩增16S rRNA基因V3–V4可变区,Illuminate Hiseq 2500 (上海美吉生物医药有限公司)平台完成测序。使用QIIME软件对测序数据进行过滤,通过Flash软件将有overlap的一对reads进行拼接。用UClust软件对序列进行聚类,将97%相似性的序列聚类成为OTUs (Operational taxonomic units),RDP classifier对序列进行物种注释。统计每个样品在各分类水平上的构成,利用OTUs的数值及比对注释种类到属水平上计算各个采样点的Ace、Chao、Shannon和Simpson指数。
1.5 微生物代谢产物的测定 1.5.1 短链脂肪酸的测定: 取0.3 g肠道食糜于2 mL灭菌后的离心管中,加入1.5 mL去离子水,用vortex仪混匀后,3000×g离心10 min,取1 mL上清液于新的2 mL离心管中,加入25% (W/V)偏磷酸,保存于–20 ℃。使用气相色谱(Agilent GC–2014,Japan)检测分析各样品中短链脂肪酸(SCFA)的浓度,气相色谱配备氢火焰离子检测器和毛细管柱(30 m×0.32 mm×0.25 μm,Agilent Technologies Inc.,USA)。 1.5.2 生物胺的测定: 取0.5 g食糜置于2 mL离心管中,加入1 mL 5% (5 g/100 mL)三氯乙酸,振荡1 h,充分混匀后3000 g离心10 min,取900 μL上清并加入等量的正己烷,混匀5 min,弃上清有机相。取下层溶液,加入丹磺酰氯,充分反应后,使用高效液相色谱测定样品中各种生物胺的含量。 1.5.3 氨氮的测定: 取1 g食糜置于1.5 mL离心管中,加入1 mL 0.2 mol/L HCl,充分混匀后,4000 r/min离心10 min,取40 µL上清加入0.96 mL 0.2 mol/L HCl,保存于–20 ℃。氨氮浓度的测定参照靛酚法[14]。 1.6 统计分析试验数据经Excel(2016)初步处理后,采用SPSS 25.0软件中的单因素方差(one-way ANOVA)进行分析,多重比较采用Student-Newman-Keuls进行检验,P < 0.05表示差异显著。数据呈现用GraphPad Prism 5软件作图。
2 结果和分析 2.1 血液生化参数由表 2可知,总蛋白、白蛋白、球蛋白和血糖水平在不同日粮蛋白质水平下差异不显著;与对照组相比,在第25和45天时,低蛋白质日粮显著降低了尿素氮水平(P < 0.05);在第45天时,14% CP显著提高了胆固醇的含量(P < 0.05);在第25天时,甘油三脂在14% CP粗蛋白组中显著提高(P < 0.05)。
| Item | day | NP | MP | LP | SEM | P-value |
| Total protein/(g/L) | 10 | 51.98 | 55.17 | 59.15 | 1.430 | 0.127 |
| 25 | 50.37 | 49.00 | 48.28 | 1.195 | 0.791 | |
| 45 | 58.90 | 55.77 | 57.80 | 1.071 | 0.490 | |
| Albumin/(g/L) | 10 | 31.46 | 35.55 | 37.00 | 1.020 | 0.073 |
| 25 | 32.02 | 32.70 | 31.63 | 0.794 | 0.872 | |
| 45 | 39.40 | 37.02 | 37.84 | 0.635 | 0.296 | |
| Globulin/(g/L) | 10 | 20.52 | 19.62 | 22.15 | 0.787 | 0.419 |
| 25 | 18.35 | 16.30 | 16.65 | 0.593 | 0.339 | |
| 45 | 19.50 | 18.75 | 19.96 | 0.783 | 0.838 | |
| Blood glucose/(mmol/L) | 10 | 5.14 | 5.90 | 5.28 | 0.249 | 0.441 |
| 25 | 5.48 | 6.05 | 5.85 | 0.336 | 0.804 | |
| 45 | 6.32 | 5.48 | 5.12 | 0.411 | 0.509 | |
| Blood urea nitrogen/(mmol/L) | 10 | 4.78 | 3.32 | 7.47 | 1.333 | 0.444 |
| 25 | 4.07a | 2.28b | 2.20b | 0.255 | < 0.001 | |
| 45 | 4.65a | 3.03b | 2.24b | 0.320 | 0.001 | |
| Cholesterol/ (mmol/L) | 10 | 1.76 | 1.80 | 2.02 | 0.109 | 0.615 |
| 25 | 1.85 | 2.1 | 2.05 | 0.071 | 0.338 | |
| 45 | 1.95b | 2.15b | 2.44a | 0.066 | 0.003 | |
| Triglyceride/ (mmol/L) | 10 | 0.74 | 0.64 | 0.60 | 0.047 | 0.496 |
| 25 | 0.62b | 0.53b | 0.86a | 0.054 | 0.019 | |
| 45 | 0.44 | 0.54 | 0.68 | 0.056 | 0.235 | |
| SEM: standard error of the means. The different lowercase letters in the same column indicate significant difference at the 0.05 level. | ||||||
2.2 血液生长相关激素
由表 3可知,仔猪采食不同蛋白质水平的日粮后,血液胰高血糖素、胰岛素、生长激素、T3和T4浓度差异均不显著。
| Item | Time/d | NP | MP | LP | SEM | P-value |
| Glucagon/(pg/mL) | 10 | 631.22 | 658.33 | 553.78 | 35.271 | 0.465 |
| 25 | 326.63 | 362.02 | 494.34 | 32.649 | 0.963 | |
| 45 | 224.88 | 224.75 | 221.00 | 19.397 | 0.119 | |
| Growth hormone/(ng/mL) | 10 | 0.66 | 0.64 | 0.55 | 0.088 | 0.079 |
| 25 | 0.40 | 0.92 | 0.63 | 0.138 | 0.320 | |
| 45 | 0.47 | 0.51 | 0.48 | 0.070 | 0.529 | |
| Insulin/(μIU/mL) | 10 | 8.43 | 7.06 | 7.67 | 0.356 | 0.996 |
| 25 | 7.98 | 9.30 | 7.18 | 0.504 | 0.233 | |
| 45 | 6.81 | 7.02 | 7.54 | 0.367 | 0.753 | |
| Triiodothyronine(T3)/(ng/mL) | 10 | 0.59 | 0.62 | 0.56 | 0.035 | 0.875 |
| 25 | 0.57 | 0.72 | 0.60 | 0.032 | 0.730 | |
| 45 | 0.51 | 0.55 | 0.48 | 0.023 | 0.262 | |
| Tetraiodothyronine(T4)/(ng/mL) | 10 | 58.10 | 65.64 | 66.50 | 4.573 | 0.330 |
| 25 | 46.52 | 53.51 | 55.92 | 2.390 | 0.832 | |
| 45 | 57.80 | 54.97 | 45.46 | 2.679 | 0.143 |
2.3 回肠和盲肠微生物丰富度和多样性
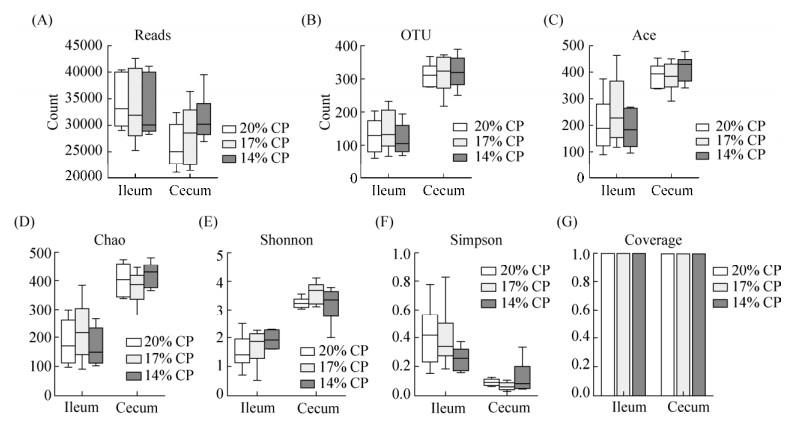
从图 1可知,回肠和盲肠测得序列总数均在2万条以上(图 1-A),结肠中微生物OTU数高于回肠(图 1-B)。Ace和Chao指数可反映微生物丰富度,Shannon和Simpson指数可反映微生物的多样性。在不同日粮蛋白质水平下Ace、Chao、Shannon和Simpson指数差异均不显著(图 1-CDEF);相比于回肠,盲肠中Ace、Chao和Shannon指数较高,Simpson指数较低,反映了盲肠微生物的丰富度和多样性高于回肠。Coverage是样本的覆盖率,其数值越高则样本中序列被测出的概率越高,测序结果反映了不同日粮蛋白水平下样本中微生物的真实情况(图 1-G)。
|
| 图 1 第45天回肠和盲肠微生物丰富度和多样性 Figure 1 Richness and diversity of microbiota in ileum and cecum in pigs fed different dietary protein level. A: reads of 16S rRNA; B: OTUs with 97% similarity; C, D, E and F: index of Ace, Chao, Shannon, and Simpson; G: coverage of OTUs. |
2.4 回肠和盲肠微生物组成
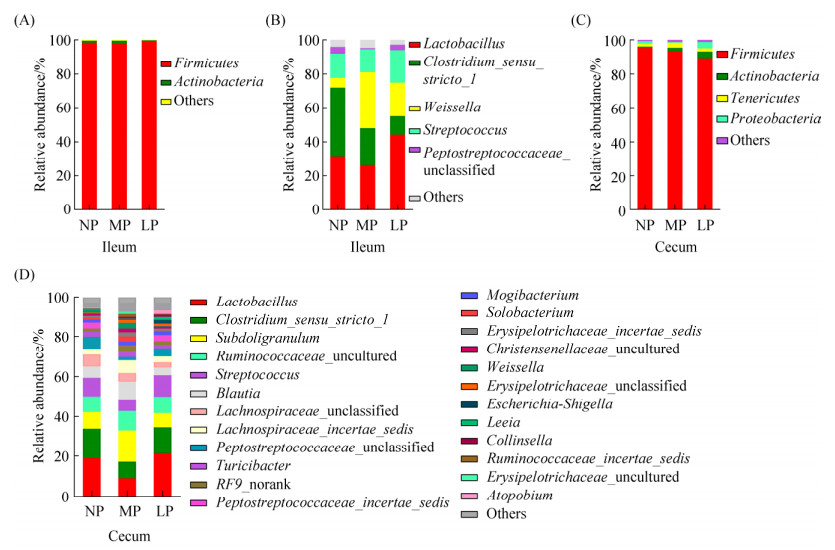
在门水平上,回肠和盲肠中的微生物均以厚壁菌门(Firmicutes)为主,但在各组之间差异不显著(图 2-A、C)。在属水平上,乳酸杆菌属(Lactobacillus)和严格梭菌属(Clostridium_sensu_ stricto_1)为优势菌属,随着日粮蛋白质含量的降低,回肠中乳酸杆菌属(Lactobacillus)和魏斯菌属(Weissella)呈上升趋势,严格梭菌属(Clostridium_sensu_stricto_1)呈下降趋势,但差异均不显著;回肠和盲肠中其他属水平菌群差异不显著(图 2-B、D)。
|
| 图 2 第45天回肠和盲肠微生物组成 Figure 2 Microbiota in ileum and cecum in pigs fed different dietary protein level. A and C: Phyla microbiota; B and D: Microbiota in genus; NP: 20% CP; MP: 17% CP; LP: 14% CP. |
2.5 回肠和盲肠中微生物的代谢产物
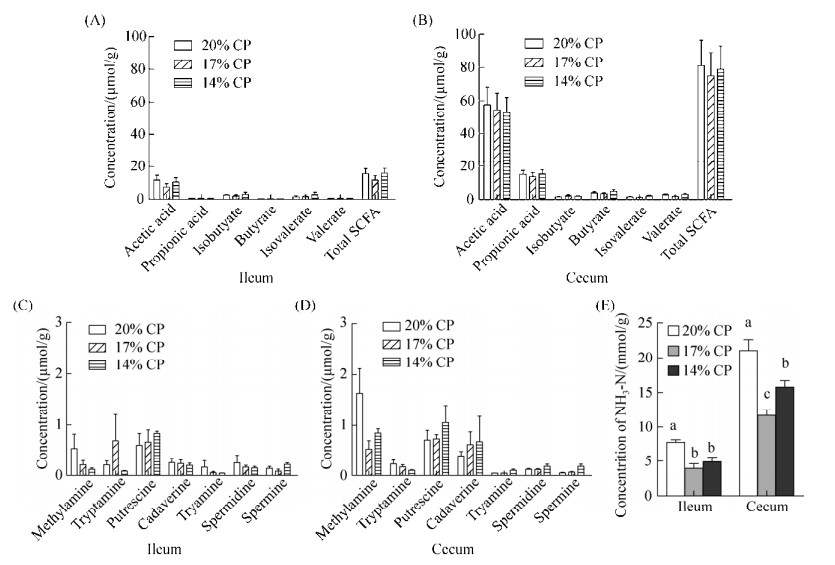
由图 3可知,回肠和盲肠中各SCFA的浓度在3组之间差异不显著(图 3-A、B);与对照组相比,降低日粮蛋白质含量使回肠和盲肠中甲胺浓度呈下降趋势,但差异不显著(图 3-C、D);降低日粮蛋白质含量显著减少了回肠和盲肠中氨氮的产量(P < 0.05,图 3-E)。
|
| 图 3 回肠和盲肠中微生物的代谢产物 Figure 3 Microbial metabolites in ileum and cecum among three groups with different dietary protein level. A and B: Concentration of short chain fatty acids (SCFAs) in ileum and cecum; C and D: Concentration of biogenic amines in ileum and cecum; E: Concentration of ammonia nitrogen in ileum and cecum. abc: The different lowercase letters above the bars indicate significant difference at the 0.05 level. |
3 讨论
本课题组前期研究结果表明,与对照组相比,17% CP对生长性能无显著影响,而14% CP显著降低了仔猪采食量和平均日增重[2]。本文进一步研究发现,14% CP组血液尿素氮浓度显著下降,表明低蛋白质日粮提高了仔猪对氮的利用率;同时,血液甘油三脂和胆固醇浓度上升,暗示机体对脂肪的吸收增强。很多研究报道,低蛋白质日粮能显著增加脂肪在肌肉和背膘中的沉积[15-17]。此外,尽管低蛋白质日粮导致仔猪采食量下降,然而未引起生长激素、甲状腺激素T3和T4、胰岛素和胰高血糖素的变化,这与前人报道不一致,即给大鼠饲喂不含蛋白质的日粮,抑制了生长轴并降低了生长相关激素的分泌,从而减少机体能量消耗,维持能量平衡[3-4]。以上结果说明,不含蛋白质的日粮对生长相关激素有显著抑制作用,而一定程度上降低日粮蛋白质水平可能并不会影响生长相关激素的分泌。因此,在低蛋白质日粮条件下,生长相关激素的变化及其对机体生长的调控作用还有待进一步探索。
断奶容易引起仔猪肠道一系列生理功能紊乱,稳定的微生物组成对于降低仔猪腹泻促进免疫功能的完善十分重要[18]。研究表明,适当降低日粮中粗蛋白含量并补充必需氨基酸,能够有效缓解分别由断奶和大肠杆菌攻毒导致的仔猪腹泻[11-12],然而在饲喂低蛋白质日粮条件下,有关断奶仔猪肠道内微生物区系的丰富度和多样性报道不一致。Peng等[13]发现,生长猪日粮蛋白质水平降低5个百分点能够提高结肠微生物多样性(Shannon指数);Zhou等研究表明[19],日粮蛋白质水平降低3个百分点不影响盲肠和结肠的微生物的丰富度和多样性(Ace、Chao、Shannon和Simpson指数),但在属水平上,多种微生物菌群相对丰度差异显著。而我们的研究发现,日粮蛋白质水平降低3或6个百分点均不影响微生物的丰富度和多样性,在门和属水平上各主要微生物菌群也不受日粮蛋白质水平的显著影响。肠道微生物倾向于优先发酵利用未被宿主消化吸收的碳水化合物,其次是未被消化吸收的日粮蛋白质[20-23],这意味着碳水化合物比蛋白质更容易影响微生物的多样性和组成。根据本试验日粮配方可知,3组之间淀粉、中性洗涤纤维和酸性洗涤纤维含量相当,这可能是微生物区系无组间差异的主要原因。
未被消化吸收的蛋白质进入大肠后被微生物发酵并产生多种潜在的有害物质,如生物胺和氨氮,进而促使腹泻的发生[24-25]。当降低日粮蛋白质含量后,微生物发酵蛋白质的产物下降,且腹泻减少[10-11]。本文结果显示,低蛋白质日粮显著降低了回肠和盲肠中氨氮的含量,而对于生物胺和SCFA的浓度并无显著影响。相似的研究也表明,低蛋白质日粮能够降低回肠、盲肠和结肠中氨氮含量,对盲肠中生物胺浓度无显著影响[13]。这说明低蛋白质日粮对回肠和盲肠微生物的代谢产物生物胺和SCFA影响较小,但显著降低了氨氮的浓度,有利于减少氮排放。He等也发现[26],在猪生长和肥育阶段低蛋白质日粮均提高了小肠对氨基酸的吸收利用,从而减少了氮排放。
综上所述,本研究较为全面地揭示了饲喂不同日粮蛋白质水平对仔猪回肠和盲肠中微生物的多样性和丰富度以及在门和属水平上主要优势菌群相对丰度的影响,粗蛋白水平降低3或6个百分点对生长相关激素和肠道菌群无明显影响,但仍需进一步试验验证并在实验中对不同日粮营养素和环境等因素进行严格控制,以期为确定合理的日粮低蛋白水平提供依据。
| [1] | Houmard NM, Mainville JL, Bonin CP, Huang S, Luethy MH, Malvar TM. High-lysine corn generated by endosperm-specific suppression of lysine catabolism using RNAi. Plant Biotechnology Journal, 2007, 5(5): 605-614. DOI:10.1111/j.1467-7652.2007.00265.x |
| [2] | Wu L, Liao P, He QH, Tan B, Guo FF, Tang M, Li TJ. Chronic feeding with protein-restricted diets affect ileal amino acid digestibility and the expression of nutrient-sensing, hormone secretion, gastrointestinal digestive enzyme, and nutrient transporter genes in young weaned pigs. Oncotarget, 2018. DOI:10.18632/oncotarget.24093 |
| [3] | Boelen A, Wiersinga WM, Fliers E. Fasting-induced changes in the hypothalamus-pituitary-thyroid axis. Thyroid, 2008, 18(2): 123-129. |
| [4] | Shi ZX, Levy A, Lightman SL. The effect of dietary protein on thyrotropin-releasing hormone and thyrotropin gene expression. Brain Research, 1993, 606(1): 1-4. DOI:10.1016/0006-8993(93)91561-6 |
| [5] | Yu M, Zhang CJ, Yang YX, Mu CL, Su Y, Yu KF, Zhu WY. Long-term effects of early antibiotic intervention on blood parameters, apparent nutrient digestibility, and fecal microbial fermentation profile in pigs with different dietary protein levels. Journal of Animal Science and Biotechnology, 2017, 8: 60. DOI:10.1186/s40104-017-0192-2 |
| [6] | Savage CD. Microbial ecology of the gastrointestinal tract. Annual Review of Microbiology, 1977, 31: 107-133. DOI:10.1146/annurev.mi.31.100177.000543 |
| [7] | Xu J, Gordon JI. Honor thy symbionts. Proceedings of the National Academy of Sciences of the United States of America, 2003, 100(18): 10452-10459. DOI:10.1073/pnas.1734063100 |
| [8] | Palmer C, Bik EM, Digiulio DB, Relman DA, Brown PO. Development of the human infant intestinal microbiota. PLoS Biology, 2007, 5(7): e177. DOI:10.1371/journal.pbio.0050177 |
| [9] | Hayashi H, Shibata K, Sakamoto M, Tomita S, Benno Y. Prevotella copri sp6 nov. and Prevotella stercorea sp. nov., isolated from human faeces. International Journal of Systematic and Evolutionary Microbiology, 2007, 57: 941-946. DOI:10.1099/ijs.0.64778-0 |
| [10] | Lamendella R, Domingo JWS, Ghosh S, Martinson J, Oerther DB. Comparative fecal metagenomics unveils unique functional capacity of the swine gut. BMC Microbiology, 2011, 11: 103. DOI:10.1186/1471-2180-11-103 |
| [11] | Heo JM, Kim JC, Hansen CF, Mullan BP, Hampson DJ, Pluske JR. Effects of feeding low protein diets to piglets on plasma urea nitrogen, faecal ammonia nitrogen, the incidence of diarrhoea and performance after weaning. Archives of Animal Nutrition, 2008, 62(5): 343-358. DOI:10.1080/17450390802327811 |
| [12] | Heo JM, Kim JC, Hansen CF, Mullan BP, Hampson DJ, Pluske JR. Feeding a diet with decreased protein content reduces indices of protein fermentation and the incidence of postweaning diarrhea in weaned pigs challenged with an enterotoxigenic strain of Escherichia coli. Journal of Animal Science, 2009, 87(9): 2833-2843. DOI:10.2527/jas.2008-1274 |
| [13] | Peng Y, Yu KF, Mu CL, Hang S, Che LQ, Zhu WY. Progressive response of large intestinal bacterial community and fermentation to the stepwise decrease of dietary crude protein level in growing pigs. Applied Microbiology and Biotechnology, 2017, 101(13): 5415-5426. DOI:10.1007/s00253-017-8285-6 |
| [14] | Weatherburn MW. Phenol-hypochlorite reaction for determination of ammonia. Analytical Chemistry, 1967, 39(8): 971-974. DOI:10.1021/ac60252a045 |
| [15] | Kerr BJ, Southern LL, Bidner TD, Friesen KG, Easter RA. Influence of dietary protein level, amino acid supplementation, and dietary energy levels on growing-finishing pig performance and carcass composition. Journal of Animal Science, 2003, 81(12): 3075-3087. DOI:10.2527/2003.81123075x |
| [16] | Ruusunen M, Partanen K, Pösö R, Puolanne E. The effect of dietary protein supply on carcass composition, size of organs, muscle properties and meat quality of pigs. Livestock Science, 2007, 107(2/3): 170-181. |
| [17] | Morazán H, Alvarez-Rodriguez J, Seradj AR, Balcells J, Babot D. Trade-offs among growth performance, nutrient digestion and carcass traits when feeding low protein and/or high neutral-detergent fiber diets to growing-finishing pigs. Animal Feed Science and Technology, 2015, 207: 168-180. DOI:10.1016/j.anifeedsci.2015.06.003 |
| [18] | Pluske JR, Turpin DL, Kim JC. Gastrointestinal tract (gut) health in the young pig. Animal Nutrition, 2018, 4(2): 187-196. DOI:10.1016/j.aninu.2017.12.004 |
| [19] | Zhou LP, Fang LD, Sun Y, Su Y, Zhu WY. Effects of the dietary protein level on the microbial composition and metabolomic profile in the hindgut of the pig. Anaerobe, 2016, 38: 61-69. DOI:10.1016/j.anaerobe.2015.12.009 |
| [20] | Bauer E, Williams BA, Voigt C, Mosenthin R, Verstegen MWA. Microbial activities of faeces from unweaned and adult pigs, in relation to selected fermentable carbohydrates. Animal Science, 2001, 73(2): 313-322. DOI:10.1017/S135772980005829X |
| [21] | Högberg A, Lindberg JE, Leser T, Wallgren P. Influence of cereal non-starch polysaccharides on ileo-caecal and rectal microbial populations in growing pigs. Acta Veterinaria Scandinavica, 2004, 45(2): 87-98. DOI:10.1186/1751-0147-45-87 |
| [22] | Metzler BU, Vahjen W, Baumgärtel T, Rodehutscord M, Mosenthin R. Changes in bacterial populations in the ileum of pigs fed low-phosphorus diets supplemented with different sources of fermentable carbohydrates. Animal Feed Science and Technology, 2009, 148(1): 68-89. DOI:10.1016/j.anifeedsci.2008.03.009 |
| [23] | Ball RO, Aherne FX. Influence of dietary nutrient density, level of feed intake and weaning age on young pigs. Ⅱ. Apparent nutrient digestibility and incidence and severity of diarrhea. Canadian Journal of Animal Science, 1987, 67(4): 1105-1115. DOI:10.4141/cjas87-116 |
| [24] | Buddle JR, Bolton JR. The pathophysiology of diarrhoea in pigs. Pig News and Information, 1992, 13: 41N-45N. |
| [25] | Nyachoti CM, Omogbenigun FO, Rademacher M, Blank G. Performance responses and indicators of gastrointestinal health in early-weaned pigs fed low-protein amino acid-supplemented diets. Journal of Animal Science, 2006, 84(1): 125-134. DOI:10.2527/2006.841125x |
| [26] | He LQ, Wu L, Xu ZQ, Li TJ, Yao K, Cui ZJ, Yin YL, Wu GY. Low-protein diets affect ileal amino acid digestibility and gene expression of digestive enzymes in growing and finishing pigs. Amino Acids, 2016, 48(1): 21-30. |
 2019, Vol. 58
2019, Vol. 58