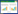中国科学院微生物研究所,中国微生物学会,中国菌物学会
文章信息
- 佘伟钰, 冯灿, 杨渐, 蒋宏忱. 2019
- Weiyu She, Can Feng, Jian Yang, Hongchen Jiang. 2019
- 三峡库区水体中固氮微生物多样性及其影响因素
- Nitrogen-fixing microbial diversity and its influencing environmental variables in waters Three Gorges Reservoir
- 微生物学报, 59(6): 1127-1142
- Acta Microbiologica Sinica, 59(6): 1127-1142
-
文章历史
- 收稿日期:2019-03-21
- 修回日期:2019-04-13
- 网络出版日期:2019-05-06
三峡工程是我国重要的水利水电工程,其在防洪、发电和航运等方面具有巨大的经济效益,但大坝建成后对库区的生态和水文环境问题造成了深远影响[1-2]。不同于世界上其他水库遵循的夏(或雨季)涨冬(或旱季)落蓄水方式,三峡水库采取夏落冬涨的蓄水方式[3-4]。即在每年汛期(6–9月)时降低水位至145 m,大量泥沙随排水流走;汛期过后将水位升至175 m,在冬季时(12–3月)保持高水位,拦蓄清水。由于大坝拦截作用,三峡库区河流的水文条件发生了巨大变化。例如,库区蓄水造成河面变宽,水流变缓,水体扩散能力减弱,和库区回水顶托等[5]。这些水文条件变化导致许多库湾和支流水体污染加重,同时造成局部水域的水体富营养化[6]。前人研究表明三峡库区水体富营养化程度存在时间和空间差异:春夏季发生水体富营养化的程度远高于秋冬季节;支流的水体富营养化程度明显高于干流[7-8]。以上研究表明高浓度的氮、磷营养盐是造成三峡库区水体富营养化问题的主要原因;且这些营养盐主要来自外源输入,包括上游来水、农田径流和城镇生活污水等[9-10]。尽管外源营养盐输入对水体富营养化具有重要贡献,但内源生物作用产生的营养盐(如生物固氮产生的氨态氮)对水体富营养化的贡献也不可忽视[11-13]。
生物固氮作用是氮循环中重要的生物地球化学过程之一。它指微生物在固氮酶作用下,将大气中的气态氮转化为生物可利用氨的过程[14]。因此,微生物固氮作用产生的氨可能是水体氮营养盐的另一个重要来源[15]。作为固氮作用的执行者,固氮微生物的群落多样性敏感地响应环境条件变化,并进而影响水体环境氮循环[7]。因此研究三峡库区水体固氮微生物的群落结构和多样性及其影响因素将有助于我们全面理解库区水体氮营养元素的来源。近年来,已有大量报道环境中固氮微生物多样性的文献,其研究手段主要采用功能基因分析方法。即通过分析固氮酶关键功能基因(nifH)研究固氮微生物多样性及其分布规律。该方法已被广泛应用于各类自然环境,包括湖泊[16]、土壤[17-20]、海洋[21-23]、深海热液[24]、陆地热泉[25-27]、河口[19, 28]和水库[29]等。
综上,本研究拟基于nifH功能基因分析,研究三峡库区不同时间(春季和夏季)和空间(干流与支流)水体样品固氮微生物多样性和种群构成,并结合三峡库区水体的地球化学参数,借助CCA、PCA等统计学手段来揭示固氮微生物的时空分布特征。该研究结果将有助于进一步加深认识三峡库区水体氮循环过程,并可为评价库区环境提供基础研究数据。
1 材料和方法 1.1 采样点分布本研究在湖北省三峡库区自下游向上游共选取9个采样点,如图 1。包括4个支流点(百岁溪、九畹溪、沙镇溪、神农溪)、5个干流点(所选支流与干流的河口交汇区),采样点选取与我们以前的研究一致[30]。分别于2017年三月(春)和六月(夏)在三峡库区共采集两批水体样品,三月水体样品标记为M1_M、M2_M、M3_M、M4_M、M5_M、B1_M、B2_M、B3_M、B4_M,六月水体样品标记为M1_J、M2_J、M3_J、M4_J、M5_J、B1_J、B2_J、B3_J、B4_J,编号前面的“M”代表干流(mainstream),“B”代表支流(branch);编号下划线后面的“M”代表三月(March),“J”代表六月(June)。

|
| 图 1 采样点位置 Figure 1 Sampling locations |
1.2 样品采集
在图 1每个取样点用孔径为0.22 μm的微孔滤膜(Whatman,英国)过滤500 mL表层水(–0.5 m处),滤液用于水样阴阳离子浓度检测,滤膜干冰存放运往实验室,用于后续提取DNA分析。根据《水质采样技术规程》(SL 187-96),分别采集用于测试溶解性有机碳(DOC)和溶解性无机碳(DIC)、总氮(TN)、氨态氮(NH4+)和总磷(TP)的水样。
1.3 水体理化参数测试参照我们以前的研究方法[30-32],在现场测试水体理化参数,包括水体pH和温度(上海三信仪表/SX711)、水体浊度(HANNA instruments/HI 98703)、水体DIC和DOC浓度(Analytic Jena/ multi N/C 2100)。返回实验室后,测试的水体理化参数包括:溶解无机碳浓度(双指示剂中和法测量)[33],总磷(磷钼蓝光度法)[34],总氮(碱性过硫酸钾消解紫外分光法),铵根浓度(纳氏试剂紫外分光法)[35]。
1.4 DNA提取和PCR扩增使用美国MP公司的Fast DNA Soil-Direct Kit试剂盒提取样品DNA。采用引物nifH3 (5′-ATRTT RTTNGCNGCRTA-3′)和nifH4 (5′-TTYTAYGGN AARGGNGG-3′)对样品总DNA进行第一轮扩增;用引物nifH1 (5′-TGYGAYCCNAARGCNGA-3′)和nifH2 (5′- ADNGCCATCATYTCNCC-3′)对上述扩增产物进行第二轮扩增,反应体系为2.5 μL 10×PCR缓冲液(Promega),2 μL dNTPs,17.2 μL无菌水,正反向引物各1 μL,0.3 μL rTaq DNA聚合酶以及1 μL DNA模板,共计25 μL。PCR扩增反应条件为:94 ℃ 5 min;94 ℃ 1 min,50 ℃ 1 min (引物nifH3/nifH4)或者55 ℃ 1 min (引物nifH1/nifH2),72 ℃ 1 min,反复进行30个循环,循环完成后再保持72 ℃ 10 min[32]。PCR产物采用大约1%琼脂糖凝胶电泳检测,凝胶内添加溴化乙锭(EB)用于染色,紫外灯照射下切取约为400 bp长度的目标条带,使用美国Axy gen公司的胶纯化试剂盒对PCR产物进行纯化回收。
1.5 克隆文库构建和系统发育分析参照文献[30, 36]方法构建克隆文库和系统发育分析,使用Mothur v1.34.1软件(Furthest neighbor方法)[37],以98%的序列相似性对测回序列进行划分分类操作单元(即Operational taxonomic unit,简称为OTU)。将所选取的代表性OTU序列翻译成氨基酸序列,并与NCBI蛋白数据库(http://www.ncbi.nlm.nih.gov)中的同源氨基酸序列进行比对,并选取出最高相似性的参考序列,使用BioEdit v7.0.9.0软件[38]中Clustal W将代表氨基酸序列和参考序列修剪整齐后,导入MEGA v7软件中迭代运算1000次构建系统发育树。该研究所测定的克隆序列的GenBank登录号为MF464113–MF464184。
1.6 数据分析参照我们之前的研究[36],使用公式C=1–n/N对nifH基因克隆文库的覆盖度进行计算(其中C为覆盖度,N为所构建文库的克隆总数,n为该文库中只出现1次的克隆数量)。使用PAST软件(http://folk.uio.no/ohammer/past/)进行稀释曲线(rarefaction curve)分析、聚类分析、Mantel检验、T检验、Kruskal-Wallis检验、群落组成相似性分析(Analysis of similarities,ANOSIM)和克隆文库多样性指数分析。使用R语言Vegan包进行环境变量的主坐标分析(principal component analysis,PCA)和固氮微生物群落组成典型对应分析(canonical correspondence analysis,CCA);使用SPSS 22.0分析固氮微生物群落多样性指数与三峡库区环境变量的Pearson相关性。
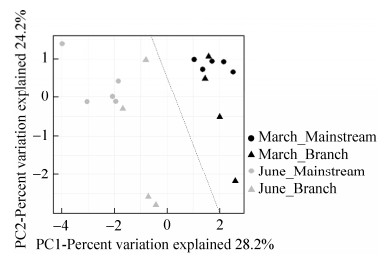
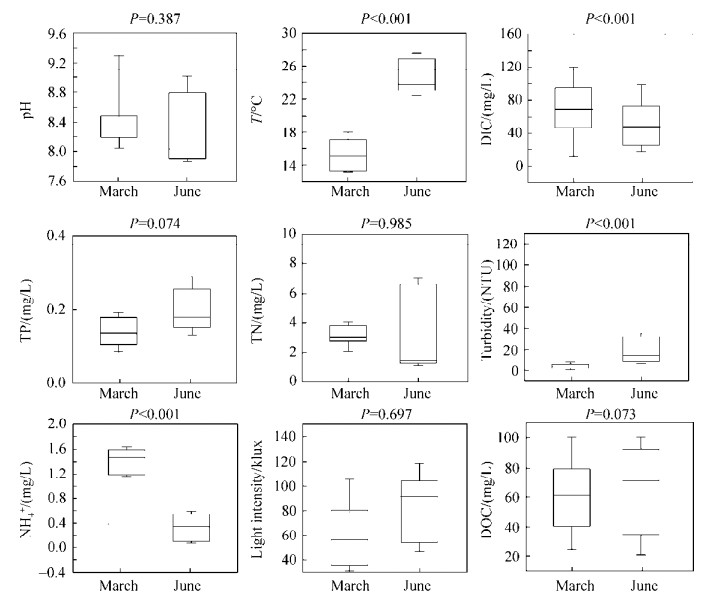
2 结果和分析 2.1 水体理化性质分析三峡库区三月和六月采样点(样点位置见图 1)表层水体的地球化学数据如表 1所示。PCA分析所有三峡库区水体样品的地球化学数据(图 2)显示:三月(春)和六月(夏)的地球化学参数差异显著(Kruskal-Wallis检验:P < 0.05),按照采样时间样点分别聚集在虚线两侧。同时可见三月干流样点聚集在一起,支流点较为分散;而六月的干流、支流样点之间均较为分散,无明显聚集规律。通过T检验分析三月与六月的各项地球化学参数发现,水体温度、浊度、DIC和氨氮浓度这4个环境参数季节性变化显著(P < 0.001)(图 3)。另外,干流和支流水体环境地球化学参数(包括温度、pH等)同样存在显著差异(Kruskal-Wallis检验,P < 0.05)(图 4)。
|
| 图 2 三峡库区三月份与六月份采样点水体地球化学参数的主成分分析二维散点图 Figure 2 Principal component analysis (PCA) on the geochemical parameters of the sampled waters in the TGR in March and June |
|
| 图 3 三峡库区三月和六月水体样品(包括干、支流所有样品)地球化学参数之间的差异 Figure 3 The variation of geochemical factors among the studied TGR water samples between March and June |
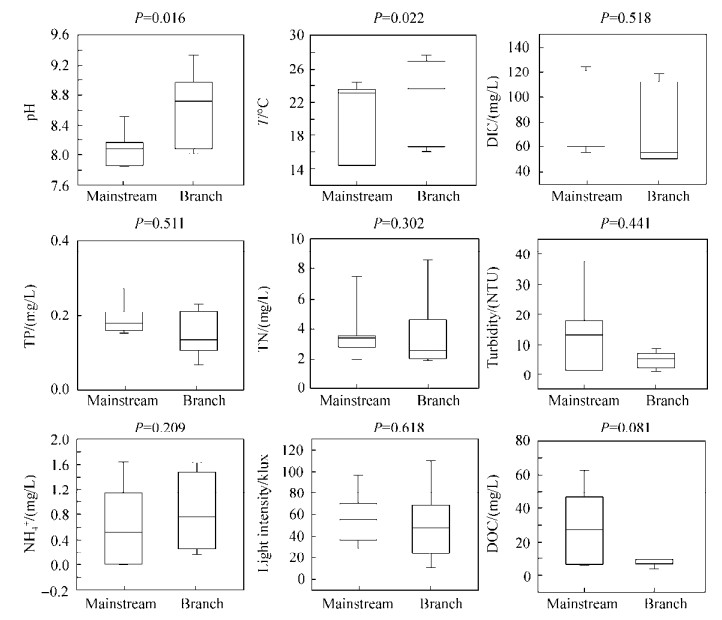
|
| 图 4 三峡库区干流和支流水体样品(包括三、六月所有样品)地球化学参数之间的差异 Figure 4 The variation of geochemical factors among the studied TGR water samples collected from the main stream and its corresponding tributary sites |
| Sample site (March/June) | M1 | M2 | M3 | M4 | M5 | B1 | B2 | B3 | B4 |
| T/℃ | 14.3/24.4 | 14.4/23.5 | 14.4/23.5 | 15.1/23.1 | 13.8/22.7 | 16.6/23.6 | 15.7/27.0 | 16.6/27.7 | 16.0/24.8 |
| Turbidity/(NTU) | 1.50/13.1 | 1.35/14.2 | 2.75/17.9 | 1.51/34.5 | 1.72/34.5 | 0.99/5.27 | 1.36/6.49 | 2.37/6.98 | 4.35/8.73 |
| pH | 8.08/8.52 | 8.14/7.86 | 8.16/7.85 | 8.14/7.97 | 8.17/7.88 | 8.56/8.01 | 8.30/8.79 | 8.72/8.87 | 9.34/9.08 |
| Intensity/klux | 69.8/29.0 | 76.9/36.7 | 63.9/53.3 | 55.4/97.1 | 3.5/12.5 | 78.4/25.0 | 23.1/57.9 | 42.3/110.0 | 1.4/53.6 |
| Water chemical parameters | |||||||||
| TN*/(mg/L) | 3.38/7.70 | 3.36/2.84 | 3.44/1.96 | 3.53/2.80 | 4.04/1.88 | 3.75/8.11 | 3.91/1.91 | 3.39/2.04 | 2.39/2.04 |
| TP*/(mg/L) | 0.21/0.27 | 0.13/0.16 | 0.18/0.18 | 0.16/0.15 | 0.11/0.36 | 0.14/0.23 | 0.18/0.14 | 0.13/0.11 | 0.07/0.21 |
| NH4+/(mg/L) | 1.08/0.53 | 1.33/0.00 | 1.64/0.28 | 1.130/0.005 | 1.56/0.00 | 1.49/0.76 | 1.50/0.15 | 1.16/0.26 | 1.62/0.26 |
| DOC*/(mg/L) | 6.31/62.60 | 6.09/27.30 | 6.04/20.90 | 30.40/46.20 | 4.85/28.60 | 3.57/8.62 | 5.51/6.91 | 6.65/6.54 | 25.4/9.15 |
| DIC*/(mg/L) | 96.0/60.0 | 125.0/60.0 | 125.0/55.2 | 122.0/60.0 | 128.0/55.0 | 112.0/50.4 | 122.0/55.2 | 118.0/50.4 | 96.0/52.8 |
| *TN: total nitrogen; TP: total phosphorus; DOC: dissolved organic carbon; DIC: dissolved inorganic carbon. | |||||||||
2.2 nifH基因克隆文库分析
三峡库区三月的9个nifH基因克隆文库共筛选出312个有效的克隆子,分属于61个OTU;统计分析克隆文库的覆盖度为82.6%–100% (表 2)。六月的9个克隆文库共筛选出279个有效克隆子,分属于74个OTU;覆盖度范围为70.8%–94.4% (表 2)。统计分析发现,六月水体样品的nifH基因OTU的平均丰度高于三月水体样品;支流水体样品的nifH基因OTU平均丰度高于干流水体样品(表 2)。相关性分析显示nifH基因文库多样性指数与环境变量没有显著相关性(数据未显示)。
| Libraries (March/June) | Clones | OTUs | Coverage/% | Simpson | Shannon | Chao-1 |
| M1 | 35/38 | 7/12 | 94.3/70.8 | 0.76/0.88 | 1.58/2.27 | 7.3/19.0 |
| M2 | 37/23 | 3/10 | 100.0/82.6 | 0.24/0.84 | 0.49/2.08 | 3.0/11.5 |
| M3 | 37/41 | 7/17 | 86.5/80.9 | 0.59/0.85 | 1.17/2.39 | 17.0/26.0 |
| M4 | 37/37 | 7/7 | 97.3/89.2 | 0.79/0.66 | 1.72/1.35 | 7.0/13.0 |
| M5 | 36/38 | 6/11 | 88.9/78.9 | 0.60/0.79 | 1.12/1.82 | 12.0/39.0 |
| B1 | 24/38 | 8/12 | 82.6/89.5 | 0.73/0.87 | 1.59/2.24 | 13.0/14.0 |
| B2 | 48/18 | 17/6 | 83.3/94.4 | 0.89/0.78 | 2.47/1.65 | 22.6/6.0 |
| B3 | 37/22 | 8/11 | 91.9/82.6 | 0.79/0.87 | 1.74/2.22 | 11.0/11.9 |
| B4 | 22/38 | 7/15 | 86.4/86.8 | 0.70/0.90 | 1.53/2.50 | 8.5/16.7 |
| “/” separated the data of March (left) and June (right). | ||||||
系统发育分析结果显示,本研究三月、六月三峡库区取样点表层水体样品的固氮微生物主要分属于Proteobacteria、Firmicutes和Cyanobacteria门(图 5)。其中,研究样品中固氮微生物群落的优势OTU (在所有样品中的平均相对丰度大于5%)分别与α, β, γ-Proteobacteria、Synechococcale和Clostridia纲的参考菌株相近,其nifH基因的氨基酸相似性均高于95% (表 3)。另外,三月和六月的优势OTU具有显著差异:OTU1、OTU3、OTU5、OTU6、OTU8、OTU9和OTU10主要出现在三月样品;然而OTU2、OTU4和OTU7主要出现在六月样品。干流与支流样品的优势OTU同样存在差异:OTU1、OTU2、OTU3、OTU6、OTU7、OTU8和OTU9主要分布于干流样品;然而OTU4、OTU5和OTU10主要分布于支流样品(表 3)。

|
| 图 5 nifH基因蛋白系统发育进化树 Figure 5 Neighbor-joining tree result showing the phylogenetic relationships of the deduced nifH protein sequences translated from the nifH gene clone sequences obtained in this study. The nifH gene OTUs obtained from Cyanobacteria/Proteobacteria (A) and Firmicutes/Euryarchaeota (B) with their closely related sequences from the GenBank database. The scale bar represents the expected changes at each homologous position. Bootstrap values of > 50% are displayed |
| Units | March/June | Main stream/tributary | Classification (Class) | Similar strains | Similarity/% |
| OTU 1 | 13.6%/0.6% | 12.7%/0.0% | α-Proteobacteria | Bradyrhizobium japonicum | 98 |
| OTU 2 | 0.0%/12.3% | 9.1%/2.0% | γ-Proteobacteria | Methylomonas sp. | 99 |
| OTU 3 | 9.6%/0.0% | 8.6%/0.0% | β-Proteobacteria | Sideroxydans sp. | 99 |
| OTU 4 | 0.0%/7.7% | 2.4%/4.6% | Synechococcales | Synechococcus sp. | 98 |
| OTU 5 | 6.1%/0.3% | 0.3%/5.5% | Clostridia | Clostridium drakei | 97 |
| OTU 6 | 6.3%/0.0% | 5.7%/0.0% | Clostridia | Clostridium sp. | 97 |
| OTU 7 | 0.0%/5.7% | 5.1%/0.0% | α-Proteobacteria | Bradyrhizobium japonicum | 99 |
| OTU 8 | 5.4%/0.0% | 4.9%/0.0% | α-Proteobacteria | Desulfobulbus elongatus | 98 |
| OTU 9 | 5.1%/0.0% | 3.5%/1.1% | Clostridia | Clostridium sp. | 97 |
| OTU 10 | 4.8%/0.0% | 0.0%/4.3% | Clostridia | Desulfosporosinus sp. | 98 |
此外,三月与六月样品的固氮微生物群落组成具有差异。三月样品的主要固氮微生物群落为Proteobacteria (50.3%)和Firmicutes (47.1%),还有少量的Cyanobacteria (2.6%)。在Proteobacteria中,主要包括Alphaproteobacteria (17.0%)、Betaproteobacteria (17.6%)、Deltaproteobacteria (12.2%)、Epsilonproteobacteria (1.3%)和Gammaproteobacteria (2.2%) 5个纲;在Firmicutes中,仅有Clostridia1个纲;Cyanobacteria中仅有Nostocales 1个纲(图 6)。
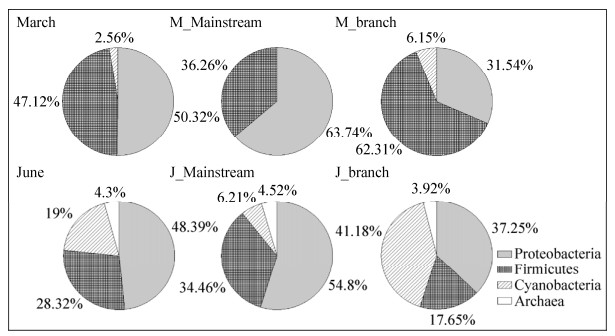
|
| 图 6 三月和六月的三峡库区干流和支流水体固氮微生物群落构成结构图 Figure 6 Community composition of nitrogen-fixing microbes in the TGR waters in March and June |
六月样品的主要固氮微生物群落为Proteobacteria (48.4%)、Firmicutes (28.3%)和Cyanobacteria (19.0%) 3个门。在Proteobacteria中,主要包括Alphaproteobacteria (11.8%)、Betaproteobacteria (5.0%)、Deltaproteobacteria (3.2%)、Epsilonproteobacteria (1.4%)和Gammaproteobacteria (26.9%) 5个纲;在Firmicutes中,以Clostridia (27.6%)为主和少量Negativicutes (0.7%);在Cyanobacteria中,有Synechococcales (8.6%)、Nostocales (6.5%)和Pleurocapsales (0.7%) 3个纲;此外,还有少量Euryarchaeota门的Methanobacteria (4.3%)(图 6)。
支流和干流水体样品的固氮微生物群落组成同样具有差异。在干流水体样品中,三月和六月均以Proteobacteria门固氮微生物种群为主;相较于三月干流样品,六月的干流样品中出现了属于Archaea和Cyanobacteria门的固氮微生物种群。在支流样品中,三月样品固氮微生物种群主要以Firmicutes为主,六月样品主要以Cyanobacteria为主(图 6)。
2.3 统计分析CCA分析(图 7)显示:本研究样品的固氮微生物群落结构差异与温度、pH、浊度、铵根和溶解无机碳等地球化学参数具有显著(P < 0.05)相关性;另外,三月和六月样点在CCA图上完全分开。上述这一结果与群落相似性检验(ANOSIM)结果一致,即三月和六月样品的固氮微生物群落组成具有明显差异(R=0.455,P=0.001)。CCA分析还显示:三月样品干流水体样点均聚在一起,但是支流水体样点较为分散;然而六月干流和支流水体样点均较为分散,分布无明显规律(图 7)。群落相似性检验(ANOSIM)也进一步证实三月干流和支流水体样品中的固氮微生物群落存在显著差异(R=0.622,P=0.007),而六月干流和支流水体样品中的固氮微生物群落不存在显著差异(R=0.194,P=0.157)。
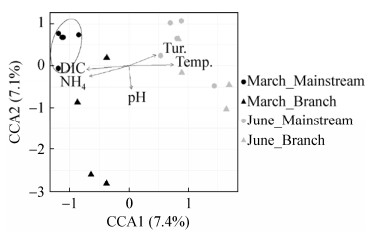
|
| 图 7 三峡库区所有水体样品nifH基因克隆文库与地球化学参数之间的CCA分析 Figure 7 CCA analysis showing correlation between the nifH gene clone libraries and geochemical parameters of the total TGR water samples. DIC: Dissolved inorganic carbon; Tur.: turbidity; Temp.: temperature; NH4: Ammonium |
Mantel检验显示,温度对研究样品固氮微生物群落组成的影响最强(R=0.576,P=0.001)(表 4)。同时,pH、溶解无机碳和铵根浓度对研究样品固氮微生物群落分布也具有显著影响(P < 0.05) (表 4);另外,针对三月和六月水体样品单独进行Mantel检验发现,这两个季节水体样品的固氮微生物群落组成均主要受到温度和pH的显著影响(表 4)。
| Environment factors | All samples | Samples in March | Samples in June | |||||
| R | P | R | P | R | P | |||
| T/℃ | 0.576 | 0.001 | 0.575 | 0.007 | 0.762 | 0.002 | ||
| pH | 0.329 | 0.001 | 0.587 | 0.002 | 0.763 | 0.001 | ||
| NH4+ | 0.394 | 0.001 | 0.145 | 0.265 | 0.050 | 0.313 | ||
| DIC | 0.461 | 0.001 | 0.177 | 0.231 | 0.127 | 0.182 | ||
| DOC | –0.024 | 0.595 | –0.063 | 0.640 | –0.130 | 0.791 | ||
| Turbitidy | 0.027 | 0.409 | 0.02 | 0.423 | –0.128 | 0.78 | ||
| TN | 0.081 | 0.226 | 0.031 | 0.309 | 0.021 | 0.388 | ||
| TP | –0.031 | 0.624 | –0.104 | 0.670 | –0.166 | 0.827 | ||
3 讨论
本文研究结果显示三月(春)和六月(夏)三峡库区支流和干流水体中的固氮微生物群落组成存在明显差异(图 5和6)。该结果意味着三峡库区水体固氮微生物群落分布具有时空差异性。这些固氮微生物群落分布的时空差异主要由三峡库区水体环境条件在时间和空间上变化导致(图 3和4)。本文样品分别来自蓄水期高水位的三月份与排水期低水位的六月份,这两个季度的温度、光照和水动力条件具有明显差异:夏季的温度和光照强度高于春季;同时,三峡库区六月份处于排水期,由于雨季到来,水流冲刷岸边土壤带走土壤颗粒造成库区水体浊度增加,进而土壤中大量营养物质(溶解性有机碳和磷元素等)输入到库区水体,并提高水体营养条件[39-42]。这种高温度和营养的环境条件会极大促使微生物(包括固氮微生物)的大量繁殖,故而会导致六月样品的固氮微生物种α-多样性高于三月样品,同时六月样品的固氮微生物群落组成也与三月份样品具有显著差异(图 7)。另外,三峡库区干流与支流的水体理化性质也有明显差异。这主要是由于受大坝的拦截,干流水体理化性质和水动力条件直接受到人为因素(如航运排污和水位调控)的影响,然而支流水体理化性质主要受周围植被类型、外源输入和土壤条件影响[43]。因此,上述空间环境因素的差异会导致了三峡库区支流和干流水体中的固氮微生物群落结构和α-多样性差异。此外,三月份是三峡库区的蓄水期,库区干流水动力学条件更类似湖泊,水体理化参数相对均一,因而三月干流固氮微生物群落会比较相似(图 7);六月份由于三峡库区处于排水期,此时干流水体运移速度要高于支流,支流更加容易形成营养物质的富集,也更利于藻类的繁盛[44]。因此,在三峡库区六月份支流样品中存有大量Cyanobacteria门固氮微生物种群(图 6)。
Mantel检验结果显示,温度的时空差异是影响三峡水库水体中固氮微生物群落结构最为重要的环境因素(表 4)。温度是限制微生物生长和功能代谢的重要环境因素之一;不同微生物种群具有不同的生长和代谢温度范围。因此,不同温度条件下会有不同的固氮微生物群落结构和多样性[21, 45-46]。前人在黄石公园热泉和南海北部沉积物的固氮微生物研究中发现,温度升高会促使藻类(如Cyanobacteria)繁盛[45-46]。三峡库区三月份与六月份的平均气温相差~10 ℃。同时,干流水体温度比支流水体温度平均低2 ℃左右。这主要是由于三峡库区干流流域面积较广,江上风速较大,使得表层与底层水体相互交换以致温度偏低[47]。这种三峡库区三月和六月、支流与干流的水体温度差异可能是影响三峡库区固氮微生物群落结构的空间差异的重要原因。
除温度外,pH、铵根和溶解无机碳等水体指标也影响着三峡库区水体固氮微生物群落组成和分布。三峡库区水体三月份(春)的pH和DIC含量均高于六月份(夏)。pH和DIC浓度均可以影响和调节水体酸碱度,并进而影响微生物胞外酶的分泌和活性。故pH和DIC会显著影响研究样品的固氮微生物群落分布[48-53]。另外,本研究三月份水体样品的总氮和氨氮含量均高于六月份,这与前人对三峡库区消落带土壤氮素吸附释放规律的研究结果相符,其研究表明三峡水库消落带土壤在低水位的干期大量吸纳来自农田和地表径流的氮素,在高水位的淹水期向相对稳定的水体中释放大量的氮素[54]。前人研究表明氨氮的增加会抑制固氮微生物的生长[55],故铵根离子会显著影响本研究样品固氮微生物的组成,同时它也可能是导致三月份样品固氮微生物α-多样性较六月份低的原因之一。
此外,值得我们注意的是本研究发现三峡库区水体存在大量(相对丰度 > 25%)的Firmiutes固氮微生物。前人研究表明,Firmicutes固氮微生物主要分布在沉积物和土壤中[57-58],也有少数分布在陆地热泉、海草和盐沼生态系统中[59]。在本研究中,Firmicutes在每个水体样品中均有较高的占比,且在三月的样品中比例最高,我们推测这与三峡库区蓄清排浑的水利调节方式导致的消落带有关。三月份水位由最低水位145 m上涨达到175 m的最高水位,高程差达到30 m,致使消落带土壤完全淹没在水下,大量土壤中的固氮微生物进入三峡库区水体中[60],从而导致三月研究样品的固氮微生物主要以土壤来源的Firmicutes固氮微生物为主。
4 结论本文以三峡水库不同时间(三月和六月)和不同空间(干流和支流)的水体样品中固氮微生物作为研究对象,通过对水体中固氮功能基因建立克隆文库并结合水体地球化学参数进行综合分析,对三峡库区固氮微生物多样性、群落构成及其环境影响因素进行了探究,结论如下:(1)三峡库区水体存在大量的固氮微生物群落,主要是Proteobacteria、Firmicutes和Cyanobacteria为主。该结果暗示内源固氮作用产生的氨态氮可能是库区水体氮营养元素的又一重要来源;(2)环境因素的时间(三月和六月)和空间(干流和支流)变化,导致了三峡库区水体固氮微生物多样性和群落结构的时空差异性。其中,温度和pH的时空差异是控制三峡库区固氮微生物群落组成差异的主要环境因素。另外,由于克隆文库分析方法本身的技术局限性,即该方法仅能探测到相对丰度 > 0.1%的微生物种群,而对稀有微生物种群(相对丰度 < 0.1%)检测能力有限[61],所以本研究工作结果仅能揭示三峡库区水体中优势固氮微生物群落的多样性及其分布规律。鉴于稀有微生物种群在环境生物地球化学循环中的重要作用[62],因此十分必要在后续工作中采用高通量测序手段深入研究三峡库区水体和沉积物固氮微生物群落构成。综上,本研究结果有助于进一步理解三峡库区水体氮元素的来源和及其循环过程,并可为全面评价库区水体环境变化提供基础研究数据。
| [1] | Chandra S, Zanden MJV, Heyvaert AC, Richards BC, Allen BC, Goldman CR. The effects of cultural eutrophication on the coupling between pelagic primary producers and benthic consumers. Limnology and Oceanography, 2005, 50(5): 1368-1376. DOI:10.4319/lo.2005.50.5.1368 |
| [2] |
Fei ZP, Xin XK, Hu S. Evaluation of nutrient status in the mainstream and tributaries of Three Gorges Reservoir. Water Power, 2018, 44(1): 1-4. (in Chinese) 裴中平, 辛小康, 胡圣.三峡库区干支流水体营养状态评价.水力发电, 2018, 44(1): 1-4, 58. http://www.cnki.com.cn/Article/CJFDTotal-SLFD201801001.htm |
| [3] | Nilsson C, Reidy CA, Dynesius M, Revenga C. Fragmentation and flow regulation of the world's large river systems. Science, 2005, 308(5720): 405-408. DOI:10.1126/science.1107887 |
| [4] | Wu JG, Huang JH, Han XG, Xie ZQ, Gao XM. Three-Gorges Dam--experiment in habitat fragmentation?. Science, 2003, 300(5623): 1239-1240. DOI:10.1126/science.1083312 |
| [5] | 长江水利委员会. 三峡工程生态环境影响研究. 武汉: 湖北科学技术出版社, 1997. |
| [6] |
Qiu GS, Tu M, Ye D, Chen SS, Guo WS, Zang XP. General investigation of eutrophication for tributaries in TGP reservoir area. Yangtze River, 2008, 39(13): 1-4.
(in Chinese) 邱光胜, 涂敏, 叶丹, 陈水松, 郭文思, 臧小平. 三峡库区支流富营养化状况普查. 人民长江, 2008, 39(13): 1-4. DOI:10.3969/j.issn.1001-4179.2008.13.001 |
| [7] | He JZ, Shen JP, Zhang LM, Zhu YG, Zheng YM, Xu MG, Di HJ. Quantitative analyses of the abundance and composition of ammonia-oxidizing bacteria and ammonia-oxidizing archaea of a Chinese upland red soil under long-term fertilization practices. Environmental Microbiology, 2007, 9(9): 2364-2374. DOI:10.1111/emi.2007.9.issue-9 |
| [8] |
Qin YW, Zhao YM, Ma YQ, Zheng BH, Wang X, Wang LJ, Li H. Prevention and control of nitrogen, phosphorus pollution in the Three Gorges Reservoir:ecological compensation, pollution control, quality assessment. Research of Environmental Sciences, 2018, 31(1): 1-8.
(in Chinese) 秦延文, 赵艳民, 马迎群, 郑丙辉, 汪星, 王丽婧, 李虹. 三峡水库氮磷污染防治政策建议:生态补偿·污染控制·质量考核. 环境科学研究, 2018, 31(1): 1-8. |
| [9] |
Qiu GS, Hu S, Ye D, Yuan L, Zang XP. Ivestigation on the present situation of eutrophication and water bloom in the branches of Three Gorges Reservoir. Resoures and Environment in the Yangtz Basin, 2011, 20(3): 311-316.
(in Chinese) 邱光胜, 胡圣, 叶丹, 袁琳, 臧小平. 三峡库区支流富营养化及水华现状研究. 长江流域资源与环境, 2011, 20(3): 311-316. |
| [10] |
Xu QJ, Zheng BH, Zhu YZ, Li H, Jiang LJ. Nutrient status evaluation for tributary of Three Gorges Reservoir. China Environmental Science, 2010, 30(4): 453-457.
(in Chinese) 许秋瑾, 郑丙辉, 朱延忠, 李涵, 蒋丽佳. 三峡水库支流营养状态评价方法. 中国环境科学, 2010, 30(4): 453-457. |
| [11] |
Liu J, Lin SZ, Hu ZP. Eutrophication of water source reservoir and its ecological management. Reservoir Fisheries, 2007, 27(5): 75-77.
(in Chinese) 刘军, 林叔忠, 胡和平. 水源水库富营养化问题及生态治理途径. 水利渔业, 2007, 27(5): 75-77. DOI:10.3969/j.issn.1003-1278.2007.05.032 |
| [12] |
Lan HX, Chen S, Zhang JS. Application of gray clustering method in the eutrophication evaluation for reservoir. Jounal of Fujian Normal University (Nature Science Edition), 2012, 28(1): 55-59, 65.
(in Chinese) 蓝华秀, 陈盛, 张江山. 灰色聚类法在水库富营养化评价中的应用. 福建师范大学学报(自然科学版), 2012, 28(1): 55-59, 65. DOI:10.3969/j.issn.1000-5277.2012.01.013 |
| [13] |
Zhu QF, Liao XL, Chen XG, Zhang SJ. The application of the grey clustering method to assess the eutrophication of lake of Liwan. Environmental Monitoring in China, 2004, 20(2): 47-50.
(in Chinese) 朱庆峰, 廖秀丽, 陈新庚, 张淑娟. 用灰色聚类法对荔湾湖水质富营养化程度的评价. 中国环境监测, 2004, 20(2): 47-50. DOI:10.3969/j.issn.1002-6002.2004.02.018 |
| [14] | Gruber N, Galloway JN. An Earth-system perspective of the global nitrogen cycle. Nature, 2008, 451(7176): 293-296. DOI:10.1038/nature06592 |
| [15] | Howard JB, Rees DC. Structural basis of biological nitrogen fixation. Chemical Reviews, 1996, 96(7): 2965-2982. DOI:10.1021/cr9500545 |
| [16] | Yan QY, Yu YH, Feng WS. Diversity of nifH gene amplified from plankton-community DNA in a shallow eutrophic lake (Lake Donghu, Wuhan, China). Limnology, 2011, 12(3): 245-251. DOI:10.1007/s10201-010-0340-2 |
| [17] | Izquierdo JA, Nüsslein K. Distribution of extensive nifH gene diversity across physical soil microenvironments. Microbial Ecology, 2006, 51(4): 441-452. DOI:10.1007/s00248-006-9044-x |
| [18] | Roesch LFW, Fulthorpe RR, Jaccques RJS, Bento FM, de Oliveira Camargo FA. Biogeography of diazotrophic bacteria in soils. World Journal of Microbiology and Biotechnology, 2010, 26(8): 1503-1508. DOI:10.1007/s11274-010-0326-6 |
| [19] | Thajudeen J, Yousuf J, Veetil VP, Varghese S, Singh A, Abdulla MH. Nitrogen fixing bacterial diversity in a tropical estuarine sediments. World Journal of Microbiology and Biotechnology, 2017, 33(2): 41. DOI:10.1007/s11274-017-2205-x |
| [20] | Rosado AS, Duarte GF, Seldin L, van Elsas JD. Genetic diversity of nifH gene sequences in Paenibacillus azotofixans Strains and soil samples analyzed by denaturing gradient gel electrophoresis of PCR-amplified gene fragments. Applied and Environmental Microbiology, 1998, 64(8): 2770-2779. |
| [21] | Dang HY, Luan XW, Zhao JY, Li J. Diverse and novel nifH and nifH-like gene sequences in the deep-sea methane seep sediments of the Okhotsk Sea. Applied and Environmental Microbiology, 2009, 75(7): 2238-2245. DOI:10.1128/AEM.02556-08 |
| [22] | Dang HY, Yang JY, Li J, Luan XW, Zhang YB, Gu GZ, Xue RR, Zong MY, Klotz MG. Environment-dependent distribution of the sediment nifH-harboring microbiota in the Northern South China Sea. Applied and Environmental Microbiology, 2013, 79(1): 121-132. DOI:10.1128/AEM.01889-12 |
| [23] | Stal LJ. Is the distribution of nitrogen-fixing Cyanobacteria in the oceans related to temperature?. Environmental Microbiology, 2009, 11(7): 1632-1645. DOI:10.1111/emi.2009.11.issue-7 |
| [24] | Mehta MP, Butterfield DA, Baross JA. Phylogenetic diversity of nitrogenase (nifH) genes in deep-sea and hydrothermal vent environments of the Juan de Fuca Ridge. Applied and Environmental Microbiology, 2003, 69(2): 960-970. DOI:10.1128/AEM.69.2.960-970.2003 |
| [25] | Hall JR, Mitchell KR, Jackson-Weaver O, Kooser AS, Cron BR, Crossey LJ, Takacs-Vesbach CD. Molecular characterization of the diversity and distribution of a thermal spring microbial community by using rRNA and metabolic genes. Applied and Environmental Microbiology, 2008, 74(15): 4910-4922. DOI:10.1128/AEM.00233-08 |
| [26] | Loiacono ST, Meyer-Dombard DR, Havig JR, Poret-Peterson AT, Hartnett HE, Shock EL. Evidence for high-temperature in situ nifH transcription in an alkaline hot spring of Lower Geyser Basin, Yellowstone National Park. Environmental Microbiology, 2012, 14(5): 1272-1283. DOI:10.1111/emi.2012.14.issue-5 |
| [27] | Hamilton TL, Boyd ES, Peters JW. Environmental constraints underpin the distribution and phylogenetic diversity of nifH in the Yellowstone geothermal complex. Microbial Ecology, 2011, 61(4): 860-870. DOI:10.1007/s00248-011-9824-9 |
| [28] | Affourtit J, Zehr JP, Paerl HW. Distribution of nitrogen-fixing microorganisms along the Neuse River Estuary, North Carolina. Microbial Ecology, 2001, 41(2): 114-123. |
| [29] | Yu Z, Zhou J, Yang J, Yu XQ, Liu LM. Vertical distribution of diazotrophic bacterial community associated with temperature and oxygen gradients in a subtropical reservoir. Hydrobiologia, 2014, 741(1): 69-77. DOI:10.1007/s10750-014-1832-6 |
| [30] |
Chu QL, Yang J, Jiang HC. Diversity of sulfur-oxidizing bacteria in waters of the Three Gorges Reservoir, China. Acta Microbiologica Sinica, 2018, 58(4): 584-597.
(in Chinese) 储巧玲, 杨渐, 蒋宏忱. 三峡库区水体中硫氧化菌的群落多样性. 微生物学报, 2018, 58(4): 584-597. |
| [31] |
Feng C, Yang J, Jiang HC. Diversity and distribution of nitrogen-fixing bacteria in two geothermal channels in Tengchong geothermal zone, Yunnan province. Earth Science, 2018, 43(S1): 10-18.
(in Chinese) 冯灿, 杨渐, 蒋宏忱. 云南腾冲两条热泉溪流的固氮细菌群落多样性. 地球科学, 2018, 43(S1): 10-18. |
| [32] |
Tang Y, Liu YC, Yang J, Jiang HC. Gene diversity involved in kalvin pathway of carbon fixation and its response to environmental variables in surface sediments of the northern Qinghai-Tibetan plateau lakes. Earth Science, 2018, 43(S1): 19-30.
(in Chinese) 唐阳, 刘永超, 杨渐, 蒋宏忱. 青藏高原北部湖泊表层沉积物参与卡尔文循环的固碳基因多样性及其影响因素. 地球科学, 2018, 43(S1): 19-30. |
| [33] |
Liu ZQ. Determination of carbonate and bicarbonate in mixed base--mixed indicator method. Journal of Zhengzhou Institute of Technology, 1981(1): 120-126.
(in Chinese) 刘镇琨. 混合碱中碳酸根和碳酸氢根含量的测定——双混合指示剂法. 郑州工学院学报, 1981(1): 120-126. |
| [34] |
Chen W, Zeng GN, Zhou Y, Yu J, Long H, Huang C, Peng JP. Determination of phosphorus in seaweed by molybdenum blue spectrophotometry. Marine Environmental Science, 2009, 28(S1): 79-82.
(in Chinese) 陈委, 曾淦宁, 周燕, 余骏, 龙华, 黄城, 彭建平. 磷钼蓝光度法测定海藻中磷的含量. 海洋环境科学, 2009, 28(S1): 79-82. |
| [35] |
Xiao YD. Alkaline potassium persulfate digestion UV spectrophotometric method for determination of total nitrogen in water by the method of. Guangdong Chemical, 2012, 39(4): 165-166, 135.
(in Chinese) 萧玉端. 碱性过硫酸钾消解紫外分光光度法测定水中总氮的方法探讨. 广东化工, 2012, 39(4): 165-166, 135. DOI:10.3969/j.issn.1007-1865.2012.04.086 |
| [36] | Jiang HC, Dong HL, Yu BS, Lv G, Deng SC, Berzins N, Dai MH. Diversity and abundance of ammonia-oxidizing archaea and bacteria in Qinghai Lake, northwestern China. Geomicrobiology Journal, 2009, 26(3): 199-211. DOI:10.1080/01490450902744004 |
| [37] | Schloss PD, Westcott SL, Ryabin T, Hall JR, Hartmann M, Hollister EB, Lesniewski RA, Oakley BB, Parks DH, Robinson CJ, Sahl JW, Stres B, Thallinger GG, van Horn DJ, Weber CF. Introducing mothur:open-source, platform-independent, community-supported software for describing and comparing microbial communities. Applied and Environmental Microbiology, 2009, 75(23): 7537-7541. DOI:10.1128/AEM.01541-09 |
| [38] | Hall TA. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT//Proceedings of the Nucleic acids Symposium Series. London: Information Retrieval Ltd., c1979-c2000, 1999. |
| [39] |
Wang Q, Yuan XZ, Liu H, Zhang YW, Chen ZL, Li B. Effect of initial impoundment on the vegetation and species diversity in water-level fluctuation zone of the Three Gorges Reservoir. Jounal of Nature Resourse, 2011, 26(10): 1680-1693.
(in Chinese) 王强, 袁兴中, 刘红, 张跃伟, 陈忠礼, 李波. 三峡水库初期蓄水对消落带植被及物种多样性的影响. 自然资源学报, 2011, 26(10): 1680-1693. DOI:10.11849/zrzyxb.2011.10.005 |
| [40] |
Zhang AY, Xiong GM, Fan DY, Yang D, Xie ZQ. Effects of Three Gorges Dam on Riparian vascular plants of the main stream of Yangtze River. Resources and Environment in the Yangtze Basin, 2018, 27(1): 145-156.
(in Chinese) 张爱英, 熊高明, 樊大勇, 杨丹, 谢宗强. 三峡水库蓄水对长江干流河岸植物组成的影响. 长江流域资源与环境, 2018, 27(1): 145-156. |
| [41] |
Hu XQ, Jiang CL, Fei HF, Xie XQ. Influences of water environment variety on acipenser sinensis. Water Resources and Power, 2009, 27(3): 35-37.
(in Chinese) 胡小琴, 姜翠玲, 裴海峰, 谢向前. 水环境变化对中华鲟的影响. 水电能源科学, 2009, 27(3): 35-37. DOI:10.3969/j.issn.1000-7709.2009.03.011 |
| [42] | Xie P. Three-Gorges Dam:risk to ancient fish. Science, 2003, 302(5648): 1149-1151. |
| [43] |
Cai QH, Hu ZY. Studies on eutrophication problem and control strategy in the Three Gorges Reservoir. Acta Hydrobiologica Sinica, 2006, 30(1): 7-11.
(in Chinese) 蔡庆华, 胡征宇. 三峡水库富营养化问题与对策研究. 水生生物学报, 2006, 30(1): 7-11. DOI:10.3321/j.issn:1000-3207.2006.01.002 |
| [44] |
Hu ZF, Zhang L, Qiu Q, Han G, Li XQ. Study of eutrophication in main stream and tributaries of Yangtze River in the Three Gorges Reservoir area. Shandong Agricultural Science, 2009(12): 74-80.
(in Chinese) 胡正峰, 张磊, 邱勤, 韩光, 李兴琼. 三峡库区长江干流和支流富营养化研究. 山东农业科学, 2009(12): 74-80. DOI:10.3969/j.issn.1001-4942.2009.12.020 |
| [45] | Butterwick C, Heaney SI, Talling JF. Diversity in the influence of temperature on the growth rates of freshwater algae, and its ecological relevance. Freshwater Biology, 2005, 50(2): 291-300. |
| [46] | Sharp CE, Brady AL, Sharp GH, Grasby SE, Stott MB, Dunfield PF. Humboldt's spa:microbial diversity is controlled by temperature in geothermal environments. The ISME Journal, 2014, 8(6): 1166-1174. DOI:10.1038/ismej.2013.237 |
| [47] |
Fan SP, Feng MQ, Fan SF, Liu Z. Change of water temperature structure under different water depth in reservoir. Engineering journal of Wuhan University (Engineering Science Edition), 2011, 44(1): 69-73.
(in Chinese) 范世平, 冯民权, 范术芳, 刘钊. 水库淤积前后水温结构的变化. 武汉大学学报(工学版), 2011, 44(1): 69-73. |
| [48] | Gérikas Ribeiro C, Dos Santos AL, Marie D, Pereira Brandini F, Vaulot D. Small eukaryotic phytoplankton communities in tropical waters off Brazil are dominated by symbioses between Haptophyta and nitrogen-fixing Cyanobacteria. The ISME Journal, 2018, 12(5): 1360-1374. DOI:10.1038/s41396-018-0050-z |
| [49] | Takahashi T, Broecker WS, Langer S. Redfield ratio based on chemical data from isopycnal surfaces. Journal of Geophysical Research:Oceans, 1985, 90(C4): 6907-6924. DOI:10.1029/JC090iC04p06907 |
| [50] | 罗光富.支流河口水动力作用对三峡库区干支流营养盐交换的影响.华东师范大学硕士学位论文, 2014. http://cdmd.cnki.com.cn/Article/CDMD-10269-1014321955.htm |
| [51] | 王敏.长江主流碳的时空输运特征及三峡工程对其影响.中国海洋大学硕士学位论文, 2010. http://cdmd.cnki.com.cn/Article/CDMD-10423-1011027104.htm |
| [52] | Altabet MA, Francois R, Murray DW, Prell WL. Climate-related variations in denitrification in the Arabian Sea from sediment 15N/14N ratios. Nature, 1995, 373(6514): 506-509. DOI:10.1038/373506a0 |
| [53] | Voss M, Bombar D, Loick N, Dippner JW. Riverine influence on nitrogen fixation in the upwelling region off Vietnam, South China Sea. Geophysical Research Letters, 2006, 33(7): L07604. |
| [54] |
Zhan YH, Wang LA, Jiao YJ. Adsorption&release of nitrogen of soils in Three Gorges Reservoir. Journal of Chongqing University (Nature Science Edition), 2006, 29(8): 10-13.
(in Chinese) 詹艳慧, 王里奥, 焦艳静. 三峡库区消落带土壤氮素吸附释放规律. 重庆大学学报(自然科学版), 2006, 29(8): 10-13. |
| [55] | Coelho MRR, Marriel IE, Jenkins SN, Lanyon CV, Seldin L, O'Donnell AG. Molecular detection and quantification of nifH gene sequences in the rhizosphere of sorghum (Sorghum bicolor) sown with two levels of nitrogen fertilizer. Applied Soil Ecology, 2009, 42(1): 48-53. DOI:10.1016/j.apsoil.2009.01.010 |
| [56] | Teng QH, Sun B, Fu XR, Li SP, Cui ZL, Cao H. Analysis of nifH gene diversity in red soil amended with manure in Jiangxi, South China. Journal of Microbiology, 2009, 47(2): 135-141. DOI:10.1007/s12275-008-0184-1 |
| [57] | Jungblut AD, Neilan BA. nifH gene diversity and expression in a microbial mat community on the McMurdo Ice Shelf, Antarctica. Antarctic Science, 2010, 22(2): 117-122. DOI:10.1017/S0954102009990514 |
| [58] | Wu LX, Cui YH, Chen SF. Diversity of nifH gene sequences in the sediments of South China Sea. African Journal of Microbiology Research, 2011, 5(32): 5972-5977. |
| [59] | Hamilton TL, Koonce E, Howells A, Havig JR, Jewell T, de la Torre JR, Peters JW, Boyd ES. Competition for ammonia influences the structure of chemotrophic communities in geothermal springs. Applied and Environmental Microbiology, 2014, 80(2): 653-661. DOI:10.1128/AEM.02577-13 |
| [60] |
Ye F, Wu XB, Jiang Y, Wu SJ, Wang Y. Community composition and distribution characteristics of soil archaea at different elevations in the water-level-fluctuating zone of the Three Gorges Reservoir. Ecology and Environmental Monitoring of Three Gorges, 2018, 10(3): 86-92.
(in Chinese) 叶飞, 吴晓冰, 姜毅, 吴胜军, 王雨. 不同高程消落带古菌群落组成与分布特征. 三峡生态环境监测, 2018, 10(3): 86-92. |
| [61] | Lynch MDJ, Neufeld JD. Ecology and exploration of the rare biosphere. Nature Reviews Microbiology, 2015, 13(4): 217-229. DOI:10.1038/nrmicro3400 |
| [62] | Jousset A, Bienhold C, Chatzinotas A, Gallien L, Gobet A, Kurm V, Küsel K, Rillig MC, Rivett DW, Salles JF, van der Heijden MGA, Youssef NH, Zhang XW, Wei Z, Hol WHG. Where less may be more:how the rare biosphere pulls ecosystems strings. The ISME Journal, 2017, 11(4): 853-862. DOI:10.1038/ismej.2016.174 |
 2019, Vol. 59
2019, Vol. 59