中国科学院微生物研究所,中国微生物学会,中国菌物学会
文章信息
- 元超, 李刚, 张影波, 庞玉新. 2019
- Chao Yuan, Gang Li, Yingbo Zhang, Yuxin Pang. 2019
- 艾纳香内生真菌Diaporthe sp.次生代谢产物
- Secondary metabolites of an endophytic fungus Diaporthe sp. derived from Blumea balsamifera (L.) DC.
- 微生物学报, 59(4): 753-761
- Acta Microbiologica Sinica, 59(4): 753-761
-
文章历史
- 收稿日期:2018-07-24
- 修回日期:2018-09-14
- 网络出版日期:2019-03-04
2. 青岛大学药学院, 山东 青岛 266021;
3. 海南省艾纳香工程技术研究中心, 海南 儋州 571737
2. School of Pharmacy, Qingdao University, Qingdao 266021, Shandong Province, China;
3. Hainan Engineering Research Center for Blumea balsamifera, Danzhou 571737, Hainan Province, China
中国传统药用植物用于治疗和保障人们身体健康通常具有上千年的历史,它们不仅在中国,在亚洲甚至很多西方国家中也具有广泛的应用[1]。植物内生菌是指长期存在于健康植物体内并且不引起植物产生明显症状的微生物总称[2]。大量研究表明传统药用植物内生菌可以产生结构多样、生物活性广泛的次生代谢产物,进而成为抑菌药物先导化合物发现的重要宝库[3-4]。尽管目前有很多关于植物内生菌次生代谢产物的研究报道,然而已经研究报道的内生菌的数量仅占内生菌总数的1/10左右[5]。通常认为植物内生菌能够产生结构丰富多样的次生代谢产物是其对寄主植物体内微环境的一种适应[6],同时对寄主植物亦具有一定生态功能。如Cook等[7-8]研究发现北美草原上很多牧草植物中均含有一种吲哚里西啶类生物碱成分swainsonine,畜牧动物食用该牧草后能够引起体重下降、精神萎靡等神经中毒症状,进一步研究显示这种真菌毒素是由牧草中的内生真菌Undifilum oxytropis产生,表明此内生菌可通过分泌毒性次生代谢产物帮助寄主抵御牲畜的掠食。李露莹等[9]从沙漠植物沙蒿内生真菌Embellisia chlamydospora中分离的酚酸类化合物barceloneic lactone和2′-O-methyl-barceloneate可以明显抑制拟南芥根系伸长,推测该内生菌可通过分泌此类次生代谢产物协助寄主抑制其他植物生长。
艾纳香作为传统黎药具有悠久用药历史,海南黎族民间将其用于刀枪伤等外伤伤口,具防治伤口感染和加速伤口愈合作用,煮水后用于妇女产后沐浴,可防治妇科疾病发生等[10]。现代药理学研究亦显示其具有很好的抑菌活性[11]。在我们从海南传统黎药植物中寻找新型抑菌先导化合物的过程中,获得一株艾纳香植物内生真菌Diaporthe sp.,对其次生代谢产物研究进一步获得7个单体化合物:Dicerandrol A (1),Dicerandrol B (2),4, 6-dihydroxy-1H-isoindole1, 3(2H)-dione (3),Cytochalasin H (4),Cytochalasin J (5),4, 6-dihydroxy-2, 3-dihydro-1H-iso indol-1-one(6),Cerebroside C (7)。
1 材料和方法 1.1 材料 1.1.1 样品采集: 艾纳香采自农业农村部儋州药用植物种质资源圃,由中国热带农业科学院热带作物品种资源研究所庞玉新研究员鉴定,标本存放于中国热带农业科学院热带作物品种资源研究所南药室,采集后1 h内运送到实验室进行内生真菌分离。 1.1.2 仪器与试剂: 核磁共振波谱仪(Bruker Avance 500 MHz);高分辨质谱仪(Waters Synapt G2,USA),Waters 2489半制备液相色谱系统(Waters Corporation,Milford,MA,USA);SW-CJ-1D洁净工作台(江苏苏洁净化设备厂),葡聚糖凝胶Sephadex LH-20 (GE Healthcare Bio-Sciences AB,Sweden);ODS-A反相键和硅胶(北京慧德易科技有限责任公司);高效液相色谱柱YMC-Pack ODS-A (5 μm,250 mm×10 mm),GF254薄层硅胶板(青岛海洋化工有限公司);石油醚、乙酸乙酯、氯仿、甲醇等均为分析纯(天津市富宇精细化工有限公司);色谱甲醇(TEDIA)。 1.2 内生菌菌株分离分离方法主要参考文献[12]的方法,艾纳香茎秆剪段,自来水冲洗1 min,超净台上用70%乙醇消毒1 min,无菌水洗净,1.3 mol/L的次氯酸钠溶液浸泡3 min,无菌水冲洗,70%乙醇再次消毒0.5 min,无菌水冲洗干净,无菌枝剪成1 cm左右的组织段,接种到加有100 mg/L链霉素的水琼脂培养基中,避光28 ℃培养4-6周,从茎段断面长出内生真菌菌丝,接种环挑出少量菌丝接种于PDA培养基,经过继代形成单一菌落,取出少量菌块用30%甘油水保存到-80 ℃冰箱。
1.3 菌株鉴定以PDA培养基上生长3-5 d的菌株为材料,经液氮研磨后采用CTAB法提取基因组DNA[13],参照White等[14]报道真菌的nrDNA ITS序列(ITS1:5′-TCCGTAGGTGAACCTGCGG-3′;ITS4:5′-TCCTCCGCTTATTGATATGC-3′)为引物,经PCR扩增与电泳检测后,选取阳性产物委托华大基因科技有限公司进行测序。其中PCR扩增反应在50 μL体系中完成,DNA模板5.0 μL (50-100 ng/μL),2× PCR Mix (北京Tiangen公司) 25.0 μL,双向引物各2.5 μL (10 μmol),ddH2O 15.0 μL,以ddH2O代替模板DNA作空白对照。PCR反应程序为:94 ℃ 4 min;94 ℃ 1 min,50 ℃ 45 s,72 ℃ 2 min,共30个循环后;72 ℃ 10 min补齐。
测序峰图使用MEGA-X[15]首先进行拼接,并去除低质量序列及引物区。然后参照文献[13]等文献报道的真菌系统分类地位鉴定方法,将序列提交NCBI数据库中进行BLAST比对分析,并以E-value < 10-10为筛选条件,选取前11条无重复序列保存为*.aln格式,然后使用MEGA-X中NJ法[16]构建系统发育树,其中进化模型参数设置为Kimura2- parameter[17],并采用bootstrap[18]法进行1000次抽检后验,以确定目标菌株的系统分类地位。
1.4 菌株发酵500 mL三角瓶中装有60 g大米,加80 mL水,121 ℃高压蒸汽灭菌20 min,获得大米培养基,共20瓶,目标菌株接种到PDA平板培养基中培养1周左右,无菌条件下将菌块接种到大米培养基中,24 ℃静置培养1个月左右,加入乙酸乙酯终止发酵。
1.5 次生代谢产物提取分离将发酵后的20瓶大米培养基用乙酸乙酯超声提取3次,每次30 min,过滤,合并滤液,减压浓缩至干获得粗提物(3.2 g)。采用硅胶柱色谱分离,二氯甲烷/甲醇梯度洗脱(100/0,99/1,…,0/100),根据薄层色谱检测情况,共获得8个组分(Fr.1-8),Fr.4组分经过Sephadex LH-20凝胶色谱,甲醇洗脱,结合半制备HPLC,获得化合物1 (2 mg,65%甲醇/水保持2 min,65%-100%甲醇用20 min,流速=2 mL/min,tR=23 min)和2 (4 mg,65%甲醇/水保持2 min,65%-100%甲醇用20 min,流速=2 mL/min,tR=24.5min),Fr.7组分经过Sephadex LH-20结合半制备HPLC,获得化合物3 (5 mg,55%甲醇/水,流速=2 mL/min,tR=8.2 min)和5 (3 mg,65%甲醇/水保持2 min,65%-100%甲醇用20 min,流速=2 mL/min,tR=14.5 min)。Fr.5组分经过Sephadex LH-20凝胶色谱,甲醇洗脱,结合甲醇中重结晶,获得化合物4 (33 mg)。Fr.8组分的甲醇溶液经过静置一段时间后瓶底出现白色结晶性粉末,滤出得化合物7 (4 mg),滤液经过半制备HPLC,获得化合物6 (3 mg,60%甲醇/水,流速=2 mL/min,tR=7.5 min)。
2 结果和分析 2.1 菌株鉴定结果通过对菌株的nrDNA的ITS序列分析,结合系统发育树,该菌株与Diaporthe phaseolorumo isolate 58AS/S聚类为一支,因此该菌株命名为Diaporthe sp. (图 1)。

|
| 图 1 植物内生菌Diaporthe sp.的系统发育树 Figure 1 The phylogenetic tree of the endophytic fungus Diaporthe sp.. The numbers in the brackets are gene sequence accession numbers in GenBank; The numbers of nodes indicate bootstrap values and represent the percentages of 1000 bootstrap replications in which the taxa to the right are placed together; The scale bar represents the horizontal branch lengths and the number of substitutions per nucleotide position. |
2.2 结构鉴定
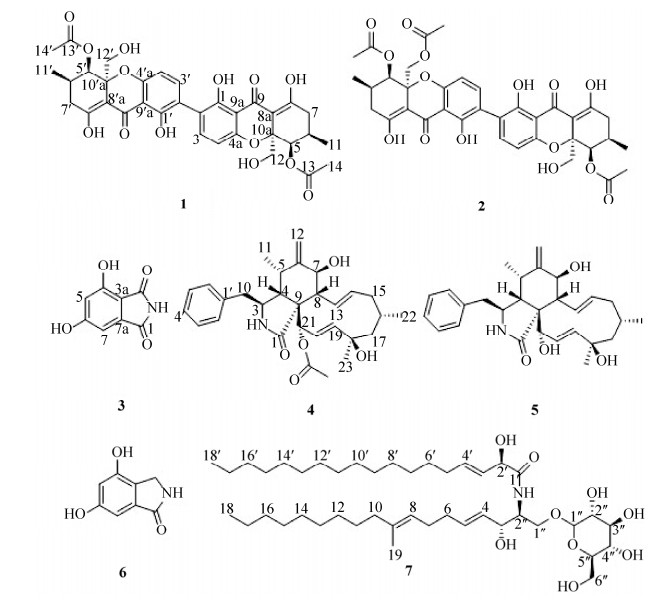
化合物1:黄色粉末,HRESIMS [M+H]+ m/z 667.2025,理论值667.2027,推测出分子式为C34H34O14,化合物谱图数据如下:1H NMR (500 MHz,CD3OD-d4) δ: 7.37 (2H,d,J=8.5 Hz,H-3,3′),6.47 (2H,d,J=8.0 Hz,H-4,4′),5.71 (2H,s,H-5,5′),3.95 (2H,d,J=13.0 Hz,H-12a,12a′),3.60 (2H,d,J=13.0 Hz,H-12b,12b′),2.51 (2H,m,H-6,6′),2.51 (2H,m,H-7a,7a′),2.35 (2H,m,H-7b,7b′),2.08 (6H,s,H-14,14′),1.05 (6H,d,J=6.0 Hz,H-11,11′)。13C NMR (125 MHz,CD3OD-d4) δ: 190.3 (C-9,9′),180.0 (C-8,8′),173.3 (C-13,13′),161.3 (C-1,1′),159.9 (C-4a,4a′),142.1 (C-3,3′),119.9 (C-2,2′),109.9 (C-4,4′),108.3 (C-9a,9a′),103.3 (C-8a,8a′),84.9 (C-10a,10a′),73.6 (C-5,5′),66.7 (C-12,12′),35.1 (C-7,7′),29.7 (C-6,6′),21.7 (C-21,21′),18.7 (C-11,11′)。谱图数据与文献[19]比对,基本一致,确定该化合物为Dicerandrol A (图 2)。
|
| 图 2 化合物1-7的结构 Figure 2 Structures of compounds 1-7. |
化合物2:黄色粉末,HRESIMS [M+H]+ m/z 709.2137,理论值709.2132,推测出分子式为C36H36O15,化合物谱图数据如下:1H NMR (500 MHz,CD3OD-d4) δ: 7.37 (2H,d,J=8.5Hz,H-3,3′),6.48 (1H,d,J=8.0 Hz,H-4),6.38 (1H,d,J=8.5 Hz,H-4′),5.71 (1H,s,H-5),5.56 (1H,s,H-5′),4.54 (1H,d,J=13.0 Hz,H-12a),4.24 (1H,d,J=13.0 Hz,H-12b),3.94 (1H,d,J=13.0 Hz,H-12a′),3.59 (1H,d,J=13.0 Hz,H-12b′),2.50 (4H,m,H-6,6′,7a,7a′),2.35 (2H,m,H-7b,7b′),2.08 (6H,s,H-14′,16′),2.03 (3H,s,H-14),1.05 (3H,d,J=6.0 Hz,H-11),1.04 (3H,d,J=6.0 Hz,H-11′)。13C NMR (125 MHz,CD3OD-d4) δ: 189.4 (C-9),189.3 (C-9′),179.6 (C-8),179.1(C-8′),172.5(C-13),172.3 (C-13′),171.8 (C-15′),160.5 (C-1,1′),159.1 (C-4a),158.7 (C-4a′),141.4 (C-3),141.2 (C-3′),119.4 (C-2′),118.9 (C-2),109.1 (C-4),108.8 (C-4′),107.5 (C-9a,9a′),102.4 (C-8a),101.5 (C-8a′),84.0 (C-10a),82.2 (C-10a′),72.7 (C-5),72.3 (C-5′),66.2 (C-12′),65.8 (C-12),34.3 (C-7),34.2 (C-7′),28.9 (C-6),28.8 (C-6′),20.8 (C-14),20.7 (C-14′),20.5 (C-16′),17.9 (C-11),17.8 (C-11′)。谱图数据与文献[19]比对,基本一致,确定该化合物为Dicerandrol B (图 2)。
化合物3:白色粉末,HRESIMS [M+H]+ m/z 180.1110,理论值180.1112,推测出分子式为C8H5NO4,化合物谱图数据如下:1H NMR (500 MHz,CD3OD-d4) δ: 6.73 (1H,s,H-7),6.51 (1H,s,H-5)。13C NMR (125 MHz,CD3OD-d4) δ: 171.0 (C-1),170.6 (C-3),166.5 (C-6),158.5 (C-4),137.9 (C-7a),109.1 (C-3a),108.6 (C-5),104.3 (C-7)。以上数据,与文献[20]报道基本一致,确定该化合物为4, 6-dihydroxy-1Hisoindole-1, 3(2H)-dione (图 2)。
化合物4:白色粉末,HRESIMS [M+Na]+ m/z 516.2731,理论值516.2725,推测出分子式为C30H39NO5,化合物谱图数据如下:1H NMR (500 MHz,CD3OD-d4) δ: 7.33 (2H,dd,J=7.5,7.5 Hz,H-3′,5′),7.26 (1H,d,J=7.5 Hz,H-4′),7.23 (2H,dd,J=7.0,7.0 Hz,H-2′,6′),5.77 (1H,dd,J=16.5,2.5 Hz,H-20),5.66 (1H,dd,J=15.5,9.5 Hz,H-13),5.55 (1H,dd,J=16.5,2.5 Hz,H-19),5.45 (1H,m,H-21),5.33 (1H,m,H-14),5.23 (1H,s,H-12a),5.01 (1H,s,H-12b),3.84 (1H,d,J=10.5 Hz,H-7),3.32 (1H,m,H-3),2.96 (1H,dd,J=10.0,10.0 Hz,H-8),2.91 (1H,dd,J=13.5,5.5 Hz,H-10a),2.74 (1H,dd,J=13.0,8.0 Hz,H-10b),2.67 (1H,qd,J=6.0,4.0 Hz,H-5),2.30 (3H,s,21-OCOCH3),2.20 (1H,dd,J=5.5,3.0 Hz,H-4),2.05 (1H,dd,J=10.0,3.5 Hz,H-15a),1.82 (1H,dd,J=14.0,2.5 Hz,H-17a),1.81 (1H,m,H-16),1.78 (1H,m,H-15b),1.57 (1H,dd,J=14.0,2.5 Hz,H-17b),1.31 (3H,s,H-23),1.05 (3H,d,J=6.5 Hz,H-22),0.60 (3H,d,J=7.0 Hz,H-11)。13C NMR (125 MHz,CD3OD-d4) δ: 177.8 (C-1),172.7 (21-OCOCH3),152.0 (C-6),139.9 (C-14),139.3 (C-19),138.5 (C-1′),131.8 (C-3′,5′),130.5 (C-2′,6′),130.1 (C-13),128.7 (C-4′),127.7 (C-20),114.3(C-12),79.3 (C-21),75.6 (C-18),73.5 (C-7),55.9 (C-17),55.9 (C-3),54.6 (C-9),50.7 (C-4),48.7 (C-8),46.0 (C-10),45.4 (C-15),34.3 (C-5),31.4 (C-23),30.1 (C-16),27.5 (C-22),21.6 (21-OCOCH3),14.5 (C-11)。以上数据与文献[21]报道基本一致,确定该化合物为cytochalasin H (图 2)。
化合物5:白色粉末,HRESIMS [M+Na]+ m/z 474.2620,理论值474.2615,推测出分子式为C28H37NO4,化合物谱图数据如下:1H NMR (500 MHz,CD3OD-d4) δ: 7.32 (2H,dd,J=7.5,7.5 Hz,H-3′,5′),7.24 (1H,d,J=7.5 Hz,H-4′),7.23 (2H,dd,J=8.0,7.0 Hz,H-2′,6′),5.83 (1H,dd,J=16.5,1.5 Hz,H-20),5.74 (1H,dd,J=16.5,2.0 Hz,H-19),5.57 (1H,dd,J=15.0,9.5 Hz,H-13),5.29 (1H,m,H-14),5.23 (1H,s,H-12a),5.02 (1H,s,H-12b),3.79 (1H,d,J=11.0 Hz,H-7),3.73 (1H,d,J=1.0 Hz,H-21),3.33 (1H,m,H-3),2.89 (1H,dd,J=10.0,10.0 Hz,H-8),2.81 (1H,dd,J=13.5,6.0 Hz,H-10a),2.76 (1H,dd,J=13.5,6.0 Hz,H-10b),2.77 (1H,m,H-5),2.62 (1H,dd,J=5.5,3.5 Hz,H-4),1.98 (1H,dd,J=11.5,4.0 Hz,H-15a),1.70 (1H,m,H-15b),1.79 (1H,m,H-17a),1.53 (1H,dd,J=13.5,3.5 Hz,H-17b),1.28 (3H,s,H-23),1.00 (3H,d,J=6.5 Hz,H-22),0.83 (3H,d,J=6.5 Hz,H-11)。13C NMR (125 MHz,CD3OD-d4) δ: 179.6 (C-1),152.2 (C-6),139.3 (C-14),138.3 (C-1′),138.2 (C-19),132.8 (C-20),131.9 (C-2′,6′),130.3 (C-3′,5′),129.9 (C-13),128.6 (C-4′),114.2 (C-12),77.5 (C-21),76.0 (C-18),73.3 (C-7),55.8(C-3),55.7(C-17),55.6 (C-9),50.7 (C-4),47.3 (C-8),45.7(C-10),45.2 (C-15),34.6 (C-5),31.9 (C-23),30.0 (C-16),27.5 (C-22),14.8 (C-11)。以上数据与文献[21]报道基本一致,确定该化合物为cytochalasin J (图 2)。
化合物6:白色粉末,HRESIMS [M+H]+ m/z 166.1280,理论值166.1285,推测出分子式为C8H7NO3,化合物谱图数据如下:1H NMR (500 MHz,CD3OD-d4) δ: 6.73 (1H,d,J=2.0 Hz,H-7),6.52 (1H,d,J=2.0 Hz,H-5),4.29 (2H,s,H-3)。13C NMR (125 MHz,CD3OD-d4) δ: 175.0 (C-1),161.3 (C-6),155.6 (C-4),136.2 (C-7a),123.9 (C-3a),108.1 (C-5),102.4 (C-7),45.1 (C-3)。以上数据与文献[20]报道基本一致,确定其为4, 6- dihydroxy-2, 3-dihydro-1H-isoindol-1-one (图 2)。
化合物7:白色粉末,FAB-MS [M-H]- m/z 752,分子式为C43H79NO9,1H NMR (500 MHz,CD3OD-d4) δ: 0.90 (6H,t,J=6.5 Hz,Me-18,18′),1.29 (34H,m,H-12-17,H-7′-17′),1.36-1.42 (4H,m,H-11,6′),1.60 (3H,s,Me-19),1.98 (2H,t,J=7.5 Hz,H-10),2.02 (2H,m,H-5′),2.02 (2H,m,H-6),2.08 (2H,m,H-7),3.20 (1H,dd,J=9.0,7.5 Hz,H-2′′),3.27 (1H,m,H-4′′或5′′),3.28 (1H,m,H-4′′或5′′),3.36 (1H,dd,J=9.5,8.0 Hz,H-3′′),3.67 (1H,dd,J=11.5,5.0 Hz,H-6′′),3.71 (1H,dd,J=10.0,3.5 Hz,H-1),3.86 (1H,d,J=12.0 Hz,H-6′′),3.97 (1H,dt,J=5.5,3.5 Hz,H-2),4.12 (1H,dd,J=10.5,3.5 Hz,H-1),4.14 (1H,dd,J=7.5,5.5 Hz,H-3),4.27 (1H,d,J=7.5 Hz,H-1′′),4.43 (1H,d,J=6.0 Hz,H-2′),5.14 (1H,t,J=7.0 Hz,H-8),5.46 (1H,dd,J=15.5,7.0 Hz,H-4),5.49 (1H,dd,J=16.0,6.0 Hz,H-3′),5.72 (1H,dt,J=15.0,6.5 Hz,H-5),5.83 (1H,dt,J=15.5,7.0 Hz,H-4′)。13C NMR (125 MHz,CD3OD-d4) δ: 175.5 (C-1′),136.7 (C-9),134.7 (C-4′),134.5 (C-5),131.0 (C-4),129.0 (C-3′),124.9 (C-8),104.7 (C-1′′),78.0 (C-3′′),77.9 (C-5′′),75.0 (C-2′′),74.1 (C-2′),72.9 (C-3),71.6 (C-4′′),69.7 (C-1),62.7 (C-6′′),54.6 (C-2),40.8 (C-10),33.8 (C-6),33.5 (C-5′),33.1 (C-16,16′),30.3-30.9 (C-12,13,14,15,6′,7′,8′,9′,10′,11′,12′,13′,14′,15′),29.2 (C-11),28.8 (C-7),23.8 (C-17,17′),16.2 (C-19),14.5 (C-18,18′)。以上数据与文献[22]报道基本一致,确定该化合物为Cerebroside C (图 2)。
3 讨论经查阅相关文献,美国的Clardy等[19]2001年最早报道了Dicerandrol类化合物,其显示了非常好的抗细菌和细胞毒活性。韩国的Lim等[23]2010年再次报道化合物1对7种水稻白叶枯病菌(Xanthomona oryza)具有不同程度抑制活性,尤其是对X. oryzae KACC 10331和X. oryzae KACC 10876两株菌活性最强,MIC值分别为2和8 μg/mL,与阳性对照药2, 4-DAPG (MIC:8 μg/mL)活性相当。化合物1对各种革兰氏阳性菌抑制活性较强,其中对枯草芽孢杆菌Bacillus subtilis KCTC 1021的活性最强,MIC值为0.125 μg/mL,远远低于阳性药卡那霉素的最低抑菌浓度值(MIC:0.5 μg/mL);另外,化合物1对白色念珠菌Candida albicans KCTC 7965也具有很强抑制活性,其MIC值2 μg/mL,显著强于阳性对照药两性霉素B (MIC: 128 μg/mL),表明化合物1对多病原菌表现出非常好的抑菌广谱性。
已有研究显示Dicerandrol类化合物主要分离自植物内生菌菌株Phomopsis longicolla中,尚未见到其他科属的真菌产生此类化合物的报道,本研究首次从Phomopsis longicolla以外的Diaporthe sp.中发现具有很强抑菌活性的Dicerandrol类化合物,为微生物源杀菌剂的开发利用提供了新的微生物来源。目前关于艾纳香内生真菌的研究相对较少,仅唐青等2017年对贵州产艾纳香内生真菌的生物多样性进行了相关报道,显示艾纳香中含有丰富的内生真菌资源,其报道的艾纳香内生真菌中也有间座壳属真菌(Diaporthe),与本实验中获得的内生真菌基本一致,进一步验证了本研究中菌株鉴定的合理性。另外,关于艾纳香内生菌次生代谢产物的研究,目前尚未见相关报道,而研究显示,植物内生真菌中蕴藏着巨大的生物合成基因簇,能够合成各种结构新颖、活性广泛的先导化合物[24],本研究为首次对黎族药用植物艾纳香内生真菌次生代谢产物进行研究,将为艾纳香中丰富的内生真菌资源的深入挖掘提供借鉴。本研究除了Dicerandrol类化合物,还发现了细胞松弛素类、苯并五元内酰胺类以及糖鞘脂类活性化合物,目前发现的细胞松弛素类化合物超过100种[25],大多具有广泛的生物活性,如细胞毒、抑菌、抗寄生虫、抗病毒以及植物毒素等[26]。糖鞘脂类化合物亦具有一定诱导植物毒素积累的活性。
综上所述,本研究从一株艾纳香内生菌中获得7个次生代谢产物(图 2),结合文献,发现大部分化合物具有不同的生物活性,尤其是抑菌及植物毒素方面,提示寄主植物艾纳香可能会通过内生真菌产生的次生代谢产物来协助抵御外界环境中的各种病原菌对植物的侵袭,以及抑制其他种类植物的生长,对研究内生真菌与寄主植物的互作关系具有重要意义。
| [1] | Cheung F. TCM: Made in China. Nature, 2011, 480(7378): S82-S83. DOI:10.1038/480S82a |
| [2] | Li LY, Sun BD, Zhang GS, Deng H, Wang MH, Tan XM, Zhang XY, Jia HM, Zhang HW, Zhang T, Zou ZM, Ding G. Polyketides with different post-modifications from desert endophytic fungus Paraphoma sp.. Natural Product Research, 2018, 32(8): 939-943. DOI:10.1080/14786419.2017.1371166 |
| [3] | Aly AH, Debbab A, Proksch P. Fungal endophytes - secret producers of bioactive plant metabolites. Die Pharmazie- An International Journal of Pharmaceutical Sciences, 2013, 68(7): 499-505. |
| [4] | Deshmukh SK, Verekar SA, Bhave SV. Endophytic fungi: a reservoir of antibacterials. Frontiers in Microbiology, 2014, 5: 715. |
| [5] | Hawksworth DC, Rossman AY. Where are all the undescribed fungi?. Phytopathology, 1997, 87(9): 888-891. DOI:10.1094/PHYTO.1997.87.9.888 |
| [6] | Li G, Kusari S, Lamshoöft M, Schüffler A, Laatsch H, Spiteller M. Antibacterial secondary metabolites from an endophytic fungus, Eupenicillium sp. LG41. Journal of Natural Products, 2014, 77(11): 2335-2341. DOI:10.1021/np500111w |
| [7] | Cook D, Gardner DR, Pfister JA. Swainsonine-containing plants and their relationship to endophytic fungi. Journal of Agricultural and Food Chemistry, 2014, 62(30): 7326-7334. DOI:10.1021/jf501674r |
| [8] | Cook D, Gardner DR, Lee ST, Pfister JA, Stonecipher CA, Welsh SL. A swainsonine survey of North American Astragalus and Oxytropis taxa implicated as locoweeds. Toxicon, 2016, 118: 104-111. DOI:10.1016/j.toxicon.2016.04.033 |
| [9] |
Li LY, Zhang XY, Sun BD, Deng H, Zou ZM, Ding G. Phenolic acid analogs from the endophytic fungus Embellisia chlamydospora isolated from desert medicinal plant Artemisia desertorum. Mycosystema, 2018, 37(1): 88-94.
(in Chinese) 李露莹, 张晓燕, 孙炳达, 邓晖, 邹忠梅, 丁刚. 沙漠药用植物沙蒿内生真菌Embellisia chlamydospora中酚酸类化合物研究. 菌物学报, 2018, 37(1): 88-94. |
| [10] | de Boer HJ, Cotingting C. Medicinal plants for women's healthcare in southeast Asia: a meta-analysis of their traditional use, chemical constituents, and pharmacology. Journal of Ethnopharmacology, 2014, 151(2): 747-767. DOI:10.1016/j.jep.2013.11.030 |
| [11] |
Wen Q, Pang YX, Hu X, Yang Q, Zhang YB, Zou J. Study on antibacterial activity of extracts from residues of Blumea balsamifera (L.) DC. in vitro. Journal of Guangdong Pharmaceutical University, 2015, 31(6): 713-716.
(in Chinese) 闻庆, 庞玉新, 胡璇, 杨全, 张影波, 邹婧. 艾纳香残渣不同提取部位体外抑菌活性研究. 广东药学院学报, 2015, 31(6): 713-716. |
| [12] | Kusari S, Lamshöft M, Zühlke S, Spiteller M. An endophytic fungus from Hypericum perforatum that produces hypericin. Journal of Natural Products, 2008, 71(2): 159-162. |
| [13] |
Song B, Zhu XF, Xu BQ, Kader AK, Yang S. Identification of Kolra fragrant pear calyx-end black spot pathogen and its sequence analysis of ITS, GPD and EF-1α. Acta Horticulturae Sinica, 2016, 43(2): 329-336.
(in Chinese) 宋博, 朱晓锋, 徐兵强, 阿布都克尤木·卡德尔, 杨森. 库尔勒香梨果萼黑斑病病原鉴定及其ITS、GPD和EF-1α序列分析. 园艺学报, 2016, 43(2): 329-336. |
| [14] | White TJ, Bruns T, Lee S, Taylor J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics//Innis MA, Gelfand DH, Sninsky JJ, White TJ. PCR Protocols: A Guide to Methods and Applications. Amsterdam: Elsevier, 1990: 315-322. |
| [15] | Kumar S, Stecher G, Li M, Knyaz C, Tamura K. MEGA X: molecular evolutionary genetics analysis across computing platforms. Molecular Biology and Evolution, 2018, 35(6): 1547-1549. DOI:10.1093/molbev/msy096 |
| [16] | Saitou N, Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Molecular Biology and Evolution, 1987, 4(4): 406-425. |
| [17] | Kimura M. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. Journal of Molecular Evolution, 1980, 16(2): 111-120. DOI:10.1007/BF01731581 |
| [18] | Felsenstein J. Confidence limits on phylogenies: an approach using the bootstrap. Evolution, 1985, 39(4): 783-791. DOI:10.1111/j.1558-5646.1985.tb00420.x |
| [19] | Wagenaar MM, Clardy J. Dicerandrols, new antibiotic and cytotoxic dimers produced by the fungus Phomopsis longicolla isolated from an endangered mint. Journal of Natural Products, 2001, 64(8): 1006-1009. DOI:10.1021/np010020u |
| [20] | Lü WW, Gao YJ, Su MZ, Luo Z, Zhang W, Shi GB, Zhao QC. Isoindolones from Lasiosphaera fenzlii Reich. and their bioactivities. Helvetica Chimica Acta, 2013, 96(1): 109-113. DOI:10.1002/hlca.201200279 |
| [21] | Shang Z, Raju R, Salim AA, Khalil ZG, Capon RJ. Cytochalasins from an Australian marine sediment-derived Phomopsis sp. (CMB-M0042F): acid-mediated intramolecular cycloadditions enhance chemical diversity. The Journal of Organic Chemistry, 2017, 82(18): 9704-9709. DOI:10.1021/acs.joc.7b01793 |
| [22] | Koga J, Yamauchi T, Shimura M, Ogawa N, Oshima K, Umemura K, Kikuchi M, Ogasawara N. Cerebrosides A and C, sphingolipid elicitors of hypersensitive cell death and phytoalexin accumulation in rice plants. Journal of Biological Chemistry, 1998, 273(48): 31985-31991. DOI:10.1074/jbc.273.48.31985 |
| [23] | Lim C, Kim J, Choi JN, Ponnusamy K, Jeon Y, Kim SU, Kim JG, Lee C. Identification, fermentation, and bioactivity against Xanthomonas oryzae of antimicrobial metabolites isolated from Phomopsis longicolla S1B4. Journal of Microbiology and Biotechnology, 2010, 20(3): 494-500. |
| [24] | Zhang Q, Wang SQ, Tang HY, Li XJ, Zhang L, Xiao J, Gao YQ, Zhang AL, Gao JM. Potential allelopathic indole diketopiperazines produced by the plant endophytic Aspergillus fumigatus using the one strain-many compounds method. Journal of Agricultural and Food Chemistry, 2013, 61(47): 11447-11452. DOI:10.1021/jf403200g |
| [25] | Chen CM, Zhu HC, Li XN, Yang J, Wang JP, Li GT, Li Y, Tong QY, Yao GM, Luo ZW, Xue YB, Zhang YH. Armochaeglobines A and B, two new indole-based alkaloids from the arthropod-derived fungus Chaetomium globosum. Organic Letters, 2015, 17(3): 644-647. DOI:10.1021/ol503666b |
| [26] | Scherlach K, Boettger D, Remme N, Hertweck C. The chemistry and biology of cytochalasans. Natural Product Reports, 2010, 27(6): 869-886. DOI:10.1039/b903913a |
 2019, Vol. 59
2019, Vol. 59