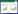中国科学院微生物研究所,中国微生物学会,中国菌物学会
文章信息
- 孙锋, 严慧聪, 汪美贞. 2019
- Feng Sun, Huicong Yan, Meizhen Wang. 2019
- 细菌群体感应调控多样性及群体感应淬灭
- Advance of the diversity of bacterial quorum sensing and quorum quenching
- 微生物学报, 59(3): 454-467
- Acta Microbiologica Sinica, 59(3): 454-467
-
文章历史
- 收稿日期:2018-05-20
- 修回日期:2018-09-27
- 网络出版日期:2018-10-09
2. 浙江省固体废物处理与资源化重点实验室, 浙江 杭州 310012
2. Zhejiang Provincial Key Laboratory of Solid Waste Treatment and Recycling, Hangzhou 310012, Zhejiang Province, China
群体感应是细菌通过信号分子分泌、识别,调控基因表达、协调菌群行为的一种细胞交流机制,这种机制赋予细菌一系列社会性能力,包括生物发光、生物膜形成、基因水平转移、蛋白酶合成、铁载体形成、芽孢产生、毒力因子分泌等[1-2]。群体感应提高了细菌群体摄取营养、抵御外敌的能力,提升了其环境适应性[3],是目前微生物学领域的研究热点和重点之一。
由于群体感应信号-受体的相对专一性、信号启动的阈值性以及调控过程的级联性等特点,群体感应干扰即群体淬灭为细菌耐药、活性污泥膨胀、生物膜堵塞等问题的解决燃起了新希望[4-7]。信号分子类似物、信号分子降解酶剂、信号受体激活剂/抑制剂、关键蛋白激活剂/抑制剂等均可以干扰或阻断群体感应[5]。群体淬灭是目前医学微生物学、食品微生物学和环境微生物学等领域的重点研发方向之一。
近来也有研究指出,简单的群体淬灭往往得不到应有效果甚至产生相反效应,这使得群体淬灭新策略研发受到了一定阻滞。然而,群体淬灭无效性往往是由于对群体感应网络复杂性了解不够所致。至此,本论文拟对已有细菌群体感应调控网络的复杂性进行阐述,并结合群体感应调控网络特点对群体淬灭策略适用性进行讨论,以期为群体感应以及群体淬灭的研究和应用提供参考和借鉴。
1 群体感应调控 1.1 等级群体感应等级群体感应系统(Hierarchical QS system),作为一种级联调控方式,指的是一个群体感应作为调节子受上级群体感应调控(图 1-A)。群体感应模式菌铜绿假单胞菌(Pseudomonas aeruginosa)具有一套典型的等级群体感应系统[3],包括位于最顶级的LasI-LasR通路以及下游的RhlI-RhlR、PqsABCDE-PqsR和AmbBCDE-IqsR通路。通过等级群体感应,协调上百个基因的转录及翻译[8-11]。
|
| 图 1 群体感应网络示意图 Figure 1 The schemes of quorum sensing network. A: The hierarchical quorum sensing; B: The parallel quorum sensing; C: The competitive quorum sensing. |
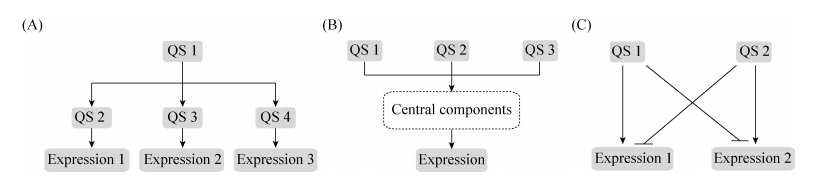
LasI-LasR位于P. aeruginosa等级群体感应系统的最顶层,由LasI蛋白合成信号分子N-3-氧十二烷酰-高丝氨酸内酯[N-(3-oxododecanoyl)-homoserine lactone, 3OC12-HSL][9],与受体蛋白LasR结合;当环境中信号分子浓度达到相应阈值后,3OC12-HSL和LasR受体结合形成LasR-3OC12-HSL复合物,调节包括弹性蛋白酶、外毒素A等大量群体性状(图 2)[8]。同时,LasR-3OC12-HSL复合物还调节许多群体感应基因的表达,包括rhlI、rhlR、pqsR以及ambBCDE (图 2-B)[11]。
|
| 图 2 P. aeruginosa群体感应信号分子结构式及群体感应网络图 Figure 2 The structure of quorum sensing signals and the quorum sensing network in P. aeruginosa. A: the structure of quorum sensing signals; B: the quorum sensing network in P. aeruginosa. |
RhlI-RhlR受LasI-LasR调控,且其与LasI-LasR通路极其类似,由RhlI蛋白合成信号分子N-丁酰-高丝氨酸内酯(N-butanoyl homoserine lactone, C4-HSL) (图 2-A)[9]。在达到相应阈值后,与RhlR受体蛋白形成RhlR-C4-HSL复合物,调控下游诸如绿脓素、鼠李糖脂等基因的表达[2]。
在PqsABCDE-PqsR通路中,PqsABCDE以邻氨基苯甲酸(Anthranilic acid)为前体物质合成2-庚基-4-喹诺酮(2-heptyl-4-quinolone, HHQ),然后通过PqsH加工合成2-庚基-3-羟基-4-喹诺酮(2-heptyl-3-hydroxy-4-quinolone, PQS)(图 2-A)[12-13],其中HHQ和PQS均能作为信号分子与PqsR结合,在达到阈值后调控约140个基因的表达[15]。PQS受体蛋白PqsR以及合成酶PqsH的表达也受LasR-3OC12-HSL调控。
此外,最近新发现的信号分子合成基因簇ambBCDE调控信号分子2-(2-羟基苯基)-噻唑-4-甲醛[2-(2-hydroxyphenyl)-thiazole-4-carbaldehyde, IQS](图 2-A)合成。值得一提的是,IQS的产生同样也受LasI-LasR通路调控,且对毒力因子、蛋白酶、鼠李糖脂等的基因表达有着显著影响[14]。
除了LasI-LasR对RhlI-RhlR、PqsABCDE-PqsR以及AmbBCDE-IqsR的调控,P. aeruginosa三个下游群体感应通路之间也存在密切关联。RhlR-C4-HSL复合物对pqsABCDE和pqsR表达起负调控,而PqsABCDE-PqsR复合物则可上调rhlI和rhlR表达[15-16]。虽然IQS的具体调控机制尚不清楚,但已经发现其能够正调节RhlI-RhlR、PqsABCDE-PqsR[10, 14]。
作为革兰氏阳性菌代表,金黄色葡萄球菌(Staphylococcus aureus)也存在等级群体感应系统,AgrACDB位于顶层,SaeR-SaeS通路位于其下游,调控α-、β-和δ-溶血素、凝固酶以及蛋白A等重要蛋白[17-18]。在鳗弧菌(Vibrio anguillarum)中,存在VanN-VanM以及VanI-VanR两套群体感应通路。VanN蛋白合成信号分子N-3-羟基己酰-高丝氨酸内酯[N-(3-hydroxyhexanoyl)-homoserine lactone, 3OHC6-HSL],与受体蛋白VanM结合;VanI合成信号分子N-3-羟基癸酰-高丝氨酸内酯[N-(3-hydroxydecanoyl)-homoserine lactone, 3OHC10-HSL],与受体蛋白VanR结合。与铜绿假单胞菌类似,VanN-VanM位于等级调控系统上游,可对VanI-VanR的信号分子合成及受体蛋白进行调控[19]。
等级群体感应最典型的特征就是多个群体感应通路自上而下呈等级分层排列,逐级调控。位于顶端的群体感应在直接调控某些基因表达的同时,也通过调控下游群体感应间接控制其他基因的表达。等级群体感应系统对基因表达的调控往往较为苛刻,即对某个基因的调控可能需要两种甚至三种信号分子的浓度同时达到阈值方可进行。这类群体感应系统优缺点十分明显,不过早启动亦不过量表达,以确保下游基因仅在非常特定的环境条件下才会表达,从而得以精确调控;但等级链中任何一个群体感应缺失都会导致下游群体感应表达的缺陷,对环境适应相对较弱。
1.2 平行群体感应平行群体感应系统(Parallel QS system),是指多信号共同输入,分别与各自的信号受体结合,同时参与同一调节子调控的复杂群体感应系统(图 1-B)。这种群体感应系统在弧菌中最为常见。
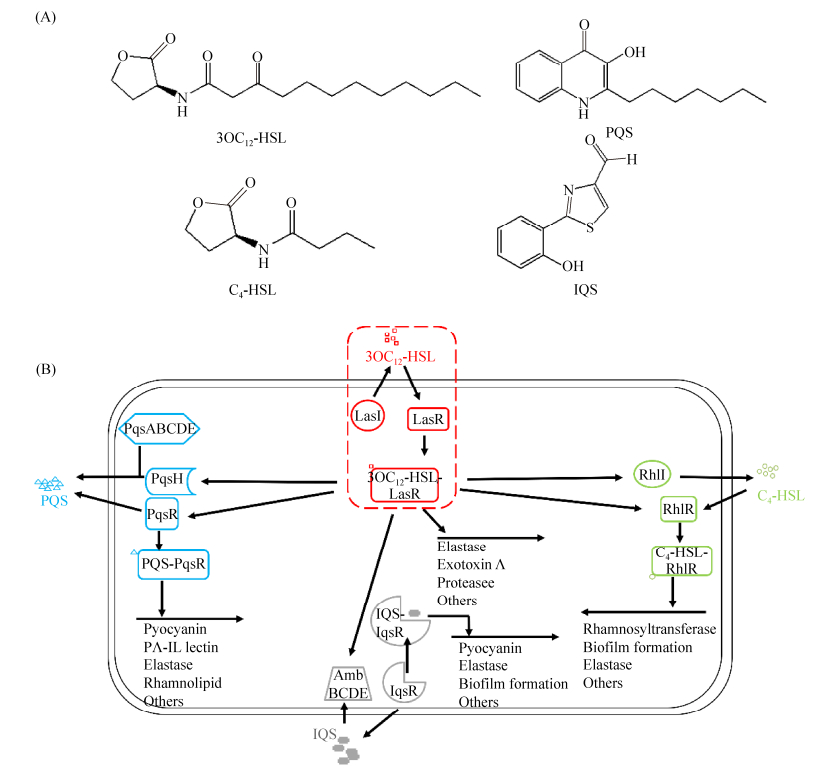
平行群体感应系统最早发现于哈维弧菌(Vibrio harveyi)中[20]。V. harveyi的平行群体感应系统由LuxM-LuxN、LuxS-LuxQ和CqsA-CqsS三个通路共同组成,通过LuxO蛋白磷酸化共同调控下游基因表达(图 3-A)。蛋白LuxM、LuxS和CqsA分别合成信号分子3-羟基丁酰-高丝氨酸内酯(3-hydroxybutanoyl-homoserine-lactone, 3OHC4-HSL)、(2S, 4S)-2-甲基-2, 3, 3, 4-羟基四氢呋喃酮-硼酸盐[(2S, 4S)-2-methyl-2, 3, 3, 4-tetrahydroxytetrahyd rofuran-borate, S-THMF-borate]和(Z)-3-氨基十一碳-2-烯-4-酮[(Z)-3-aminoundec-2-en-4-one, 3NH2C13-4O],与受体蛋白LuxN、LuxQ和CqsS结合;在低细胞密度时,LuxU自磷酸化将磷酸基团转移至LuxO,磷酸化的LuxO与σ54共同启动编码Qrr1-5的5个小RNA,促进AphA的产生,调控Ⅲ型分泌系统表达,且Qrr sRNA在RNA分子伴侣Hfq作用下破坏LuxR的正常表达[21];在高细胞密度时,多个受体蛋白分别结合各自的信号分子转变为磷酸酶,导致LuxO去磷酸化后失活,AphA受抑,且不能启动Qrr sRNA对LuxR进行干扰,LuxR得以正常翻译,呈现生物发光等群体性状[22]。
|
| 图 3 弧菌群体感应网络图 Figure 3 The schemes of quorum sensing network in Vibrio. A: V. harveyi; B: V. cholera. |
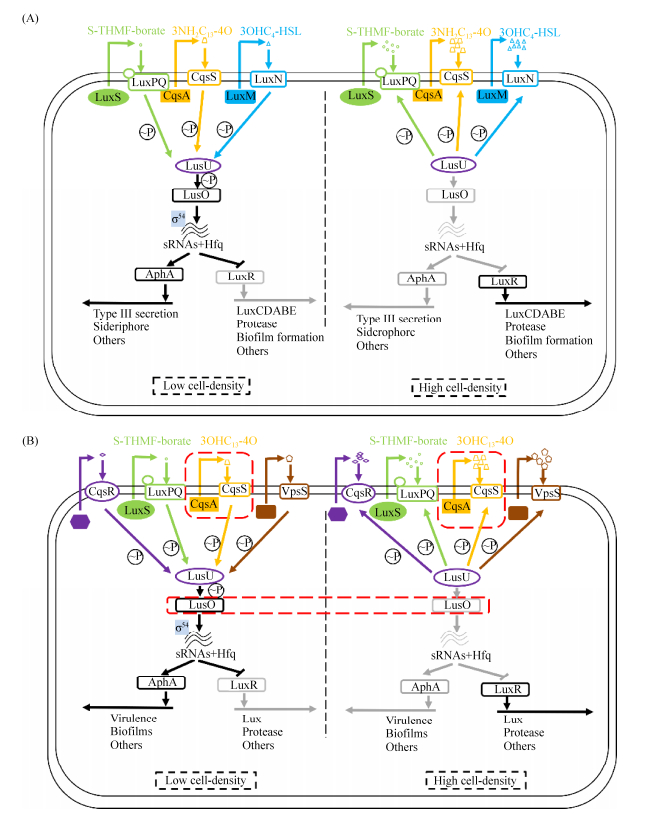
霍乱弧菌(Vibrio cholerae)也存在有类似的平行群体感应系统(图 3-B)。在V. cholerae中已知有CqsA-CqsS和LuxS-LuxPQ两个通路[6],分别由信号分子(S)-3-羟基十三烷-4-酮[(S)-3-hydroxytridecan-4-one, 3OHC13-4O]和S-THMF- borate所介导。此外,研究还发现了另外2个受体VpsS和CqsR,但目前尚不清楚与之相结合的信号分子。这4个通路共同调控LuxO蛋白磷酸化,实现对下游基因的调控。在低细胞密度时,LuxU通过自磷酸化将自身的磷酸基团转移至LuxO,启动LuxO,上调小RNA Qrr1-4转录,促进AphA、抑制HapR。高细胞密度时,受体蛋白转换为磷酸酶,LuxO活性被抑制,抑制AphA、促进HapR[23]。在V. cholerae中,AphA控制着毒力因子及生物膜等性状,HapR调控蛋白酶分泌。
虽然V. harveyi和V. cholerae的群体感应系统相似,但各自又有不同。V. harveyi群体感应更像是“重合检测器”,不同信号分子浓度会导致LuxO磷酸化水平的差异,使得V. harveyi三个群体感应转换成多个离散的LuxO~P,形成多级输出[24],V. harveyi群体感应只有在所有信号分子都存在时才能被完全诱导。但V. cholerae群体感应却是一个冗余的检测器,在V. cholerae中,四种群体感应中任意三种功能的丧失对其基因表达影响并不明显,任一单独作用都足以调节相关基因表达[25]。
平行群体感应系统通过磷酸化-去磷酸化、小RNA转录、RNA分子伴侣等将多个平行的群体感应信号整合输出。根据输入信息的差异,导致LuxO磷酸化、小RNA转录以及RNA分子伴侣的结合水平各异,从而精确调控不同基因的表达。平行群体感应很好地解决了等级群体感应适应性和精确性间的矛盾,通过将多个群体感应通路平行排列,共同控制中央调节蛋白的磷酸化,来实现相互制衡。平行群体感应一方面能防止单一群体感应通路过早表达,另一方面通过多通路共同作用提高环境适应性。
1.3 竞争型群体感应竞争型群体感应系统(Competitive QS system),指的是不同通路之间相互拮抗竞争,从而实现对相同或不同基因的调控(图 1-C)。
在枯草芽孢杆菌(Bacillus subtilis)中,存在一类由ComX信号分子介导的群体感应通路和一类由感受态和芽孢形成因子(Competence and sporulation factor, CSF)介导的群体感应通路,两者相互竞争实现对感受态和芽孢两种完全不同生命形式的调控(图 4-A)[26-27]。在感受态形成过程中,ComQ加工后形成一个由10个氨基酸组成的寡肽类信号分子ComX,膜上激酶ComP识别ComX后将转录调节子ComA磷酸化,ComA-P促进基因comS转录从而激活ComK形成感受态;同时,ComA-P启动rapA表达,其编码的RapA磷酸酶通过抑制SpoOF抑制芽孢形成[28]。在芽孢形成过程中,SpoOH调控未知蛋白合成信号分子CSF,CSF通过常见的寡肽外泌系统释放到胞外,再通过Opp多肽转运蛋白进入胞内[29]。当胞内CSF到达一定阈值后,一方面促进SpoOF磷酸化,SpoOF~P可以启动SpoOA磷酸化,从而产生芽孢;另一方面抑制ComP-ComA的信号传导,抑制感受态形成[30]。

|
| 图 4 竞争型群体感应网络图 Figure 4 The schemes of competitive quorum sensing network. A: B. subtilis; B: S. pyogene. |
化脓链球菌(Streptococcus pyogenes)中也存在竞争型群体感应系统(图 4-B)。但与B. subtilis不同,S. pyogenes两个不同群体感应受体的信号分子相同,通过受体对信号分子的竞争实现对下游基因的表达调控。在S. pyogenes中存在信号分子短链疏水性肽(Short hydrophobic peptides, SHPs),可以分别与受体蛋白Rgg2和Rgg3相结合。作为启动子的Rgg2与作为抑制子的Rgg3通过竞争DNA结合位点,实现对下游基因的调控[25, 31]。当SHPs浓度较低时,蛋白Rgg3容易占据DNA结合位点,抑制下游基因的表达。相反,当SHPs浓度较高时,能增强Rgg2、削弱Rgg3和DNA的结合,形成生物膜,并进一步诱导SHPs合成,促进下游基因表达[32]。
竞争型群感系统有且只有2个群体感应通路,且呈现非此即彼的调控效应。竞争型群体感应系统的存在,能使细菌根据环境条件以及自身生理状态呈现不同的表型。竞争型群体感应是三者中进化最为完善、环境适应度最高的系统。
2 群体淬灭群体感应调控的基因往往与细菌致病性、致腐性等性状相关,而群体感应需要信号分泌、识别、信号-受体复合体形成等过程有序高效的完成才能发生,任一过程的干扰即可影响甚至阻断群体感应即群体淬灭。根据作用机制的不同,目前群体淬灭制剂主要有:信号分子类似物、信号分子降解酶剂、信号受体激活剂/抑制剂、关键蛋白激活剂/抑制剂(表 1)。下文就不同制剂对不同群体感应干扰的有效性进行讨论。
| Name | Type | Target bacteria | References |
| Lactonase BlcC | Signal degrading enzyme | P. aeruginosa | [33] |
| AHL acylase HacA and HacB | Signal degrading enzyme | P. syringae | [34] |
| Rosmarinic acid | Signal analogue | P. aeruginosa | [35] |
| 6-gingerol | Signal analogue | P. aeruginosa | [36] |
| 4-(Dodecyloxy)-6-methyl-2H-pyran-2-one | Signal analogue | P. aeruginosa | [36] |
| Serum albumin | Signal degrading enzyme | P. aeruginosa | [37] |
| Furanone F2 | Receptor inhibitor | P. aeruginosa | [38] |
| Protoanemonin | Receptor inhibitor | P. aeruginosa | [39] |
| N-(heptyl-sulfanyl acetyl)-L-HSL | Receptor inhibitor | P. aeruginosa | [40] |
| Cranberry-derived proanthocyanidin | Receptor inhibitor | P. aeruginosa | [41] |
| Flavonoids | Receptor inhibitor | P. aeruginosa | [42] |
| Isolimonic acid and ichangin | Key protein inhibitor | V. harveyi | [43] |
| Methyl anthranilate | Receptor inhibitor | P. aeruginosa | [44] |
| Resveratrol | Key protein inhibitor | P. aeruginosa | [45] |
| N-(2-pyrimidyl)butanamide | Key protein inhibitor | P. aeruginosa | [46] |
| Carvacrol | Receptor inhibitor | Chromobacterium | [47] |
| Solonamide B | Receptor inhibitor | S. aureus | [48] |
| ω-Hydroxyemodin OHM | Receptor inhibitor | S. aureus | [49] |
| Ajoene | Key protein inhibitor | P. aeruginosa | [50] |
| Iberin | Key protein inhibitor | P. aeruginosa | [51] |
2.1 等级群体感应淬灭
等级群体感应系统中,存在一个顶级控制通路。该通路不仅直接控制相应性状表达,也通过调控下游群体感应通路间接调控其他性状的表达,对所有群体感应基因表达有着重要作用。因此,在等级群体感应中,对顶级群体感应通路进行干扰最为经济和有效。由于等级群体感应各通路之间基本不具备互补性,因此信号分子类似物、信号分子降解酶、信号受体抑制剂等均具有开发潜能。
以P. aeruginosa为例,LasI-LasR位于等级群体感应系统的顶端,干扰LasI-LasR (图 2-B红色虚线框),亦可对RhlI-RhlR、PqsABCDE-PqsR及AmbBCDE-IqsR产生干扰,效果显著。
P. aeruginosa的LasI-LasR信号分子3OC12-HSL为酰基高丝氨酸内酯类信号分子,由酰基链和高丝氨酸内酯组成。目前,通过对高丝氨酸内酯环的修饰,开发了N-3-氧十二烷酰-2-氨基环己酮[N-3-oxododecanoyl-(2-aminocyclohexanone)]、N-3-氧十二烷酰-2-氨基环戊醇[N-3-oxododecanoyl-(2-aminocyclopentanol)]等信号分子类似物;通过对酰基链的修饰,开发了N-庚基硫烷基乙酰基-高丝氨酸内酯(N-heptylsulfanylacetyl-homoserine lactone)等信号分子类似物(图 5-A)[40]。两者均通过与3OC12-HSL竞争受体结合位点,实现群体淬灭。

|
| 图 5 铜绿假单胞菌群体淬灭剂 Figure 5 Quorum quenching agents in P. aeruginosa. A: Signal analogues of 3OC12-HSL; B: Receptor inhibitors. |
除信号分子类似物外,目前还发现了一系列信号分子降解酶[5],主要有酰基侧链裂解酶和内酯环水解酶;此外,还有一部分氧化酶[5, 52-53]。较之信号类似物或小分子化合物,降解酶作用效率高、效果显著,但往往对使用条件要求苛刻,因此在复杂环境或自然环境中其应用仍存在较多问题。
除针对信号分子外,亦有学者开始进行针对信号受体淬灭剂的开发。在P. aeruginosa中,主要有呋喃酮衍生物卤化呋喃酮和棒曲霉素(图 5-B)[8]。目前,这方面开发尚待加强。
2.2 平行群体感应淬灭平行群体感应系统通常一方面具有2个以上平行群体感应通路;另一方面包含中枢整合元件(弧菌中的中枢蛋白、小RNA、RNA分子伴侣等)。对于互补性较弱的群体感应系统,群体感应通路各组分皆可作为淬灭靶点;反之,则应对信号整合元件进行干扰。
以V. cholerae为例,不同通路之间互补性较弱。CqsA-CqsS通路对毒力因子表达起主导调节作用,因此激活该通路可较好地实现对V. cholerae毒性表达的抑制(图 3-B红色虚线框)。介导CqsA-CqsS的信号分子S-3OHC13-4O由酰基尾、羟基酮以及乙基链三部分组成。针对这三部分修饰,已开发了2-羟基十二烷-3-酮(2-hydroxydodecan- 3-one)、4-羟基十四烷-5-酮(4-hydroxytetradecan- 5-one)、3-氨基十三烷-4-酮(3-aminotridecan-4-one)等具有激活CqsS活性的药物(图 6-A)[54-55]。

|
| 图 6 霍乱弧菌的群体淬灭剂 Figure 6 Quorum quenching agents in V. cholerae. A: Signal analogues; B: LuxO inhibitors with 5-thio-6-azauracil as the core structure; C: LuxO inhibitor with 2-amino-oxadiazole structure. |
LuxO是V. cholerae群体感应系统的信息整合器。以中枢LuxO为淬灭靶点,可直接控制小RNA转录,抑制基因表达,并且受到其他因素干扰较小(图 3-B红色虚线框)。目前,以LuxO为靶点的干扰剂开发较为流行,主要包括两类:一类是以5-硫代-6-氮杂尿嘧啶(5-thio-6-azauracil)为核心结构,进行不同侧链修饰的化合物(图 6-B)[56];另一类则是2-氨基-恶二唑类物质(2-amino-oxadiazole, ML366) (图 6-C)[57]。虽然两者的抑制原理相似,即通过抑制ATP酶活性抑制LuxO,从而实现V. cholerae群体淬灭[5, 56],但是后者效果明显优于前者。目前,中枢蛋白抑制是平行群体淬灭研究重点之一。
2.3 竞争型群体感应淬灭竞争型群体感应系统中,两个群体感应通路相互竞争,故激活其一即可抑制另一通路。由于竞争性群体感应优异的环境适应性,因此其淬灭研究较少。仅在S. pyogene中,研究人员开发出了两种信号分子类似物:环孢菌素A (Ciclosporin A) (图 7-A)与伐司朴达(Valspodar) (图 7-B)[58]。这两种信号分子类似物通过阻止Rgg2的激活、促进Rgg3的抑制(图 4-B红色虚线框)干扰生物膜形成,有望用于链球菌感染的治疗。
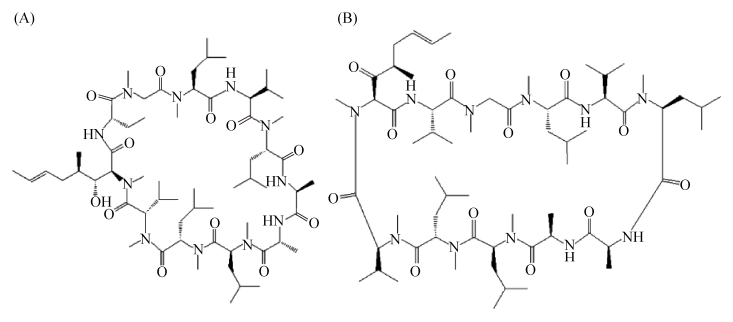
|
| 图 7 化脓链球菌的群体淬灭剂 Figure 7 Quorum quenching agents in S. pyogenes. A: Ciclosporin A; B: Valspodar. |
3 展望
经过近30年的发展,细菌群体感应在遗传学、生物化学、基因调控等方面都有了长足进步,但仍有必要加强以下几方面研究:
(1) 加强病原菌、优势菌等关键种群体感应系统的研究。病原菌、优势菌等关键种是群体淬灭的重点作用对象,如果对这些群体感应通路及关系不了解,往往会陷入群体感应应用的困境,因此有必要对复杂系统中关键种群体感应及各个群体感应通路间的关系进行研究。
(2) 开始对孤儿群体感应及调控网络进行研究。越来越多的宏基因组测序结果显示环境中存在大量的孤儿受体。这些孤儿受体的存在,是对已有群体感应通路的补充,往往会使群体淬灭不能实现,因此有必要对孤儿群体感应及调控网络进行研究。
(3) 重视群体感应环境响应及机制研究。群体感应作为一种环境变化响应机制,在不同环境条件下,会出现不同的响应。如果对群体感应环境响应及机制不理解甚至不了解,则无论正向调控群体感应亦或反向进行群体淬灭都可能导致失败,因此必须重视群体感应环境响应及机制研究。
| [1] | Papenfort K, Bassler BL. Quorum sensing signal-response systems in Gram-negative bacteria. Nature Reviews Microbiology, 2016, 14(9): 576-588. DOI:10.1038/nrmicro.2016.89 |
| [2] | Mukherjee S, Moustafa D, Smith CD, Goldberg JB, Bassler BL. The RhlR quorum-sensing receptor controls Pseudomonas aeruginosa pathogenesis and biofilm development independently of its canonical homoserine lactone autoinducer. PLoS Pathogens, 2017, 13(7): e1006504. DOI:10.1371/journal.ppat.1006504 |
| [3] | Whiteley M, Diggle SP, Greenberg EP. Progress in and promise of bacterial quorum sensing research. Nature, 2017, 551(7680): 313-320. DOI:10.1038/nature24624 |
| [4] | Reen FJ, Gutiérrez-Barranquero JA, Parages ML, O'Gara F. Coumarin: a novel player in microbial quorum sensing and biofilm formation inhibition. Applied Microbiology and Biotechnology, 2018, 102(5): 2063-2073. DOI:10.1007/s00253-018-8787-x |
| [5] | Wang JF, Ding LL, Li K, Huang H, Hu HD, Geng JJ, Xu K, Ren HQ. Estimation of spatial distribution of quorum sensing signaling in sequencing batch biofilm reactor (SBBR) biofilms. Science of the Total Environment, 2018, 612: 405-414. |
| [6] | Wang MZ, Lai BM, Dandekar AA, Yang YS, Li N, Yin J, Shen DS. Nitrogen-source stabilization of quorum sensing in the Pseudomonas aeruginosa bioaugmentation strain SD-1. Applied and Environmental Microbiology, 2017, 83(16): AEM. 00870-17. DOI:10.1128/AEM.00870-17 |
| [7] | Lai BM, Zhang K, Shen DS, Wang MZ, Shentu JL, Li N. Control of the pollution of antibiotic resistance genes in soils by quorum sensing inhibition. Environmental Science and Pollution Research, 2017, 24(6): 5259-5267. DOI:10.1007/s11356-016-8260-2 |
| [8] | Kalia VC. Quorum sensing inhibitors: an overview. Biotechnology Advances, 2013, 31(2): 224-245. |
| [9] | Singh PK, Yadav VK, Kalia M, Dohare S, Sharma D, Agarwal V. Pseudomonas aeruginosa auto inducer3-oxo-C12-HSL exerts bacteriostatic effect and inhibits Staphylococcus epidermidis biofilm. Microbial Pathogenesis, 2017, 110: 612-619. DOI:10.1016/j.micpath.2017.08.009 |
| [10] | Lai BM, Yan HC, Wang MZ, Li N, Shen DS. A common evolutionary pathway for maintaining quorum sensing in Pseudomonas aeruginosa. Journal of Microbiology, 2018, 56(2): 83-89. DOI:10.1007/s12275-018-7286-1 |
| [11] | Lee J, Zhang LH. The hierarchy quorum sensing network in Pseudomonas aeruginosa. Protein & Cell, 2015, 6(1): 26-41. |
| [12] | McGlacken GP, McSweeney CM, O'Brien T, Lawrence SE, Elcoate CJ, Reen FJ, O'Gara F. Synthesis of 3-halo-analogues of HHQ, subsequent cross-coupling and first crystal structure of Pseudomonas quinolone signal (PQS). Tetrahedron Letters, 2010, 51(45): 5919-5921. DOI:10.1016/j.tetlet.2010.09.013 |
| [13] | Reen FJ, Mooij MJ, Holcombe LJ, McSweeney CM, McGlacken GP, Morrissey JP, O'Gara F. The Pseudomonas quinolone signal (PQS), and its precursor HHQ, modulate interspecies and interkingdom behaviour. FEMS Microbiology Ecology, 2011, 77(2): 413-428. DOI:10.1111/j.1574-6941.2011.01121.x |
| [14] | Lee J, Wu JE, Deng YY, Wang J, Wang C, Wang JH, Chang CQ, Dong YH, Williams P, Zhang LH. A cell-cell communication signal integrates quorum sensing and stress response. Nature Chemical Biology, 2013, 9(5): 339-343. DOI:10.1038/nchembio.1225 |
| [15] | Diggle SP, Matthijs S, Wright VJ, Fletcher MP, Chhabra SR, Lamont IL, Kong XL, Hider RC, Cornelis P, Cámara M, Williams P. The Pseudomonas aeruginosa 4-quinolone signal molecules HHQ and PQS play multifunctional roles in quorum sensing and iron entrapment. Chemistry & Biology, 2007, 14(1): 87-96. |
| [16] | Xiao GP, He JX, Rahme LG. Mutation analysis of the Pseudomonas aeruginosa mvfR and pqsABCDE gene promoters demonstrates complex quorum-sensing circuitry. Microbiology, 2006, 152(6): 1679-1686. DOI:10.1099/mic.0.28605-0 |
| [17] | Mlynek KD, Sause WE, Moormeier DE, Sadykov MR, Hill KR, Torres VJ, Bayles KW, Brinsmade SR. Nutritional regulation of the Sae two-component system by CodY in Staphylococcus aureus. Journal of Bacteriology, 2018, 200(8): e00012-18. DOI:10.1128/JB.00012-18 |
| [18] | Giraudo AT, Cheung AL, Nagel R. The sae locus of Staphylococcus aureus controls exoprotein synthesis at the transcriptional level. Archives of Microbiology, 1997, 168(1): 53-58. DOI:10.1007/s002030050469 |
| [19] | Milton DL, Chalker VJ, Kirke D, Hardman A, Cámara M, Williams P. The LuxM homologue VanM from Vibrio anguillarum directs the synthesis of N-(3-hydroxyhexanoyl) homoserine lactone and N-hexanoylhomoserine lactone. Journal of Bacteriology, 2001, 183(12): 3537-3547. DOI:10.1128/JB.183.12.3537-3547.2001 |
| [20] | Henke JM, Bassler BL. Three parallel quorum-sensing systems regulate gene expression in Vibrio harveyi. Journal of Bacteriology, 2004, 186(20): 6902-6914. DOI:10.1128/JB.186.20.6902-6914.2004 |
| [21] | Lu RF, Osei-Adjei G, Huang XX, Zhang YQ. Role and regulation of the orphan AphA protein of quorum sensing in pathogenic Vibrios. Future Microbiology, 2018, 13(3): 383-391. DOI:10.2217/fmb-2017-0165 |
| [22] | Ruwandeepika HAD, Karunasagar I, Bossier P, Defoirdt T. Expression and quorum sensing regulation of Type Ⅲ secretion system genes of Vibrio harveyi during infection of Gnotobiotic Brine Shrimp. PLoS One, 2015, 10(12): e0143935. DOI:10.1371/journal.pone.0143935 |
| [23] | Jung SA, Chapman CA, Ng WL. Quadruple quorum-sensing inputs control Vibrio cholerae virulence and maintain system robustness. PLoS Pathogens, 2015, 11(4): e1004837. DOI:10.1371/journal.ppat.1004837 |
| [24] | Lorenz N, Shin JY, Jung K. Activity, abundance, and localization of quorum sensing receptors in Vibrio harveyi. Frontiers in Microbiology, 2017, 8: 634. |
| [25] | Hurley A, Bassler BL. Asymmetric regulation of quorum-sensing receptors drives autoinducer-specific gene expression programs in Vibrio cholerae. PLoS Genetics, 2017, 13(5): e1006826. DOI:10.1371/journal.pgen.1006826 |
| [26] | Omony J, de Jong A, Krawczyk AO, Eijlander RT, Kuipers OP. Dynamic sporulation gene co-expression networks for Bacillus subtilis 168 and the food-borne isolate Bacillus amyloliquefaciens: a transcriptomic model. Microbial Genomics, 2018, 4(2): e000157. |
| [27] | Grossman AD. Genetic networks controlling the initiation of sporulation and the development of genetic competence in Bacillus subtilis. Annual Review of Genetics, 1995, 29: 477-508. DOI:10.1146/annurev.ge.29.120195.002401 |
| [28] | Okada M, Sato I, Cho SJ, Iwata H, Nishio T, Dubnau D, Sakagami Y. Structure of the Bacillus subtilis quorum-sensing peptide pheromone ComX. Nature Chemical Biology, 2005, 1(1): 23-24. DOI:10.1038/nchembio709 |
| [29] | Gubaev A, Hilbert M, Klostermeier D. The DNA-gate of Bacillus subtilis gyrase is predominantly in the closed conformation during the DNA supercoiling reaction. Proceedings of the National Academy of Sciences of the United States of America, 2009, 106(32): 13278-13283. DOI:10.1073/pnas.0902493106 |
| [30] | Fujiya M, Musch MW, Nakagawa Y, Hu SE, Alverdy J, Kohgo Y, Schneewind O, Jabri B, Chang EB. The Bacillus subtilis quorum-sensing molecule CSF contributes to intestinal homeostasis via OCTN2, a host cell membrane transporter. Cell Host & Microbe, 2007, 1(4): 299-308. |
| [31] | Parashar V, Aggarwal C, Federle MJ, Neiditch MB. Rgg protein structure-function and inhibition by cyclic peptide compounds. Proceedings of the National Academy of Sciences of the United States of America, 2015, 112(16): 5177-5182. DOI:10.1073/pnas.1500357112 |
| [32] | Junges R, Salvadori G, Shekhar S, Åmdal HA, Periselneris JN, Chen T, Brown JS, Petersen FC. A quorum-sensing system that regulates Streptococcus pneumoniae biofilm formation and surface polysaccharide production. mSphere, 2017, 2(5): e00324-17. DOI:10.1128/mSphere.00324-17 |
| [33] | Khan MSA, Zahin M, Hasan S, Husain FM, Ahmad I. Inhibition of quorum sensing regulated bacterial functions by plant essential oils with special reference to clove oil. Letters in Applied Microbiology, 2009, 49(3): 354-360. DOI:10.1111/lam.2009.49.issue-3 |
| [34] | Shepherd RW, Lindow SE. Two dissimilar N-acyl-homoserine lactone acylases of Pseudomonas syringae influence colony and biofilm morphology. Applied and Environmental Microbiology, 2009, 75(1): 45-53. DOI:10.1128/AEM.01723-08 |
| [35] | Corral-Lugo A, Daddaoua A, Ortega A, Espinosa-Urgel M, Krell T. So different and still so similar: the plant compound rosmarinic acid mimics bacterial homoserine lactone quorum sensing signals. Communicative & Integrative Biology, 2016, 9(2): e1156832. |
| [36] | Kim HS, Lee SH, Byun Y, Park HD. 6-Gingerol reduces Pseudomonas aeruginosa biofilm formation and virulence via quorum sensing inhibition. Scientific Reports, 2015, 5: 8656. DOI:10.1038/srep08656 |
| [37] | Sheffield WP, Eltringham-Smith LJ, Bhakta V. Fusion to human serum albumin extends the circulatory half-life and duration of antithrombotic action of the kunitz protease inhibitor domain of protease nexin 2. Cellular Physiology and Biochemistry, 2018, 45(2): 772-782. DOI:10.1159/000487168 |
| [38] | Liu HB, Lee JH, Kim JS, Park S. Inhibitors of the Pseudomonas aeruginosa quorum-sensing regulator, QscR. Biotechnology and Bioengineering, 2010, 106(1): 119-126. |
| [39] | Bobadilla Fazzini RA, Skindersoe ME, Bielecki P, Puchalka J, Givskov M, Martins dos Santos VAP. Protoanemonin: a natural quorum sensing inhibitor that selectively activates iron starvation response. Environmental Microbiology, 2013, 15(1): 111-120. DOI:10.1111/emi.2013.15.issue-1 |
| [40] | Persson T, Hansen TH, Rasmussen TB, Skindersø ME, Givskov M, Nielsen J. Rational design and synthesis of new quorum-sensing inhibitors derived from acylated homoserine lactones and natural products from garlic. Organic & Biomolecular Chemistry, 2005, 3(2): 253-262. |
| [41] | Maisuria VB, Lopez-de Los Santos Y, Tufenkji N, Déziel E. Cranberry-derived proanthocyanidins impair virulence and inhibit quorum sensing of Pseudomonas aeruginosa. Scientific Reports, 2016, 6: 30169. DOI:10.1038/srep30169 |
| [42] | Paczkowski JE, Mukherjee S, McCready AR, Cong JP, Aquino CJ, Kim H, Henke BR, Smith CD, Bassler BL. Flavonoids suppress Pseudomonas aeruginosa virulence through allosteric inhibition of quorum-sensing receptors. Journal of Biological Chemistry, 2017, 292(10): 4064-4076. DOI:10.1074/jbc.M116.770552 |
| [43] | Vikram A, Jesudhasan PR, Jayaprakasha GK, Pillai SD, Patil BS. Citrus limonoids interfere with Vibrio harveyi cell-cell signalling and biofilm formation by modulating the response regulator LuxO. Microbiology, 2011, 157(1): 99-110. DOI:10.1099/mic.0.041228-0 |
| [44] | Calfee MW, Coleman JP, Pesci EC. Interference with Pseudomonas quinolone signal synthesis inhibits virulence factor expression by Pseudomonas aeruginosa. Proceedings of the National Academy of Sciences of the United States of America, 2001, 98(20): 11633-11637. DOI:10.1073/pnas.201328498 |
| [45] | Chen TT, Sheng JY, Fu YH, Li MH, Wang JS, Jia AQ. 1H NMR-based global metabolic studies of Pseudomonas aeruginosa upon exposure of the quorum sensing inhibitor resveratrol. Journal of Proteome Research, 2017, 16(2): 824-830. DOI:10.1021/acs.jproteome.6b00800 |
| [46] | Furiga A, Lajoie B, El Hage S, Baziard G, Roques C. Impairment of Pseudomonas aeruginosa biofilm resistance to antibiotics by combining the drugs with a new quorum-sensing inhibitor. Antimicrobial Agents and Chemotherapy, 2016, 60(3): 1676-1686. |
| [47] | Burt SA, Ojo-Fakunle VTA, Woertman J, Veldhuizen EJA. The natural antimicrobial carvacrol inhibits quorum sensing in Chromobacterium violaceum and reduces bacterial biofilm formation at sub-lethal concentrations. PLoS One, 2014, 9(4): e93414. DOI:10.1371/journal.pone.0093414 |
| [48] | Nielsen A, Månsson M, Bojer MS, Gram L, Larsen TO, Novick RP, Frees D, Frøkiær H, Ingmer H. Solonamide B inhibits quorum sensing and reduces Staphylococcus aureus mediated killing of human neutrophils. PLoS ONE, 2014, 9(1): e84992. DOI:10.1371/journal.pone.0084992 |
| [49] | Daly SM, Elmore BO, Kavanaugh JS, Triplett KD, Figueroa M, Raja HA, El-Elimat T, Crosby HA, Femling JK, Cech NB, Horswill AR, Oberlies NH, Hall PR. ω-hydroxyemodin limits Staphylococcus aureus quorum sensing-mediated pathogenesis and inflammation. Antimicrobial Agents and Chemotherapy, 2015, 59(4): 2223-2235. DOI:10.1128/AAC.04564-14 |
| [50] | Jakobsen TH, Warming AN, Vejborg RM, Moscoso JA, Stegger M, Lorenzen F, Rybtke M, Andersen JB, Petersen R, Andersen PS, Nielsen TE, Tolker-Nielsen T, Filloux A, Ingmer H, Givskov M. A broad range quorum sensing inhibitor working through sRNA inhibition. Scientific Reports, 2017, 7(1): 9857. DOI:10.1038/s41598-017-09886-8 |
| [51] | Tan SYY, Liu Y, Chua SL, Vejborg RM, Jakobsen TH, Chew SC, Li YY, Nielsen TE, Tolker-Nielsen T, Yang L, Givskov M. Comparative systems biology analysis to study the mode of action of the isothiocyanate compound iberin on Pseudomonas aeruginosa. Antimicrobial Agents and Chemotherapy, 2014, 58(11): 6648-6659. DOI:10.1128/AAC.02620-13 |
| [52] | Chowdhary PK, Keshavan N, Nguyen HQ, Peterson JA, González JE, Haines DC. Bacillus megaterium CYP102A1 oxidation of acyl homoserine lactones and acyl homoserines. Biochemistry, 2007, 46(50): 14429-14437. DOI:10.1021/bi701945j |
| [53] | Romero M, Martin-Cuadrado AB, Roca-Rivada A, Cabello AM, Otero A. Quorum quenching in cultivable bacteria from dense marine coastal microbial communities. FEMS Microbiology Ecology, 2011, 75(2): 205-217. DOI:10.1111/fem.2010.75.issue-2 |
| [54] | Kelly RC, Bolitho ME, Higgins DA, Lu WY, Ng WL, Jeffrey PD, Rabinowitz JD, Semmelhack MF, Hughson FM, Bassler BL. The Vibrio cholerae quorum-sensing autoinducer CAI-1: analysis of the biosynthetic enzyme CqsA. Nature Chemical Biology, 2009, 5(12): 891-895. DOI:10.1038/nchembio.237 |
| [55] | Bolitho ME, Perez LJ, Koch MJ, Ng WL, Bassler BL, Semmelhack MF. Small molecule probes of the receptor binding site in the Vibrio cholerae CAI-1 quorum sensing circuit. Bioorganic & Medicinal Chemistry, 2011, 19(22): 6906-6918. |
| [56] | Ng WL, Perez L, Cong JP, Semmelhack MF, Bassler BL. Broad spectrum pro-quorum-sensing molecules as inhibitors of virulence in Vibrios. PLoS Pathogens, 2012, 8(6): e1002767. DOI:10.1371/journal.ppat.1002767 |
| [57] | Hema M, Balasubramanian S, Princy SA. Meddling Vibrio cholerae murmurs: a neoteric advancement in Cholera research. Indian Journal of Microbiology, 2015, 55(2): 121-130. DOI:10.1007/s12088-015-0520-1 |
| [58] | Aggarwal C, Jimenez JC, Lee H, Chlipala GE, Ratia K, Federle MJ. Identification of quorum-sensing inhibitors disrupting signaling between Rgg and short hydrophobic peptides in Streptococci. mBio, 2015, 6(3): e00393-15. DOI:10.1128/mBio.00393-15 |
 2019, Vol. 59
2019, Vol. 59