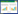中国科学院微生物研究所,中国微生物学会,中国菌物学会
文章信息
- 王若琳, 徐伟芳, 王飞, 周小磊, 郑月, 江鸿森, 谢洁. 2019
- Ruolin Wang, Weifang Xu, Fei Wang, Xiaolei Zhou, Yue Zheng, Hongsen Jiang, Jie Xie. 2019
- 桑树内生拮抗菌的分离鉴定及其对桑断枝烂叶病的生防初探
- Isolation and identification of an antagonistic endophytic bacterium from mulberry for biocontrol against Boeremia exigua
- 微生物学报, 59(11): 2130-2143
- Acta Microbiologica Sinica, 59(11): 2130-2143
-
文章历史
- 收稿日期:2018-11-28
- 修回日期:2019-04-19
- 网络出版日期:2019-07-11
2. 西南大学生物技术学院, 农业农村部蚕桑生物学与遗传育种重点实验室, 重庆 400715
2. Key Laboratory of Sericultural Biology and Genetic Breeding, Ministry of Agriculture, College of Biotechnology, Southwest University, Chongqing 400715, China
桑树作为传统经济林木,在我国种植历史悠久。桑叶可养蚕、入药,并兼具观赏及生态保护价值[1]。然而,桑树常遭受到多种病原微生物侵染,导致桑叶产量降低、桑树发育不良等现象。我国的桑树病害约有200种,包括真菌病、细菌病及病毒病等,其中真菌病害所占比例最大,高达80%左右[2]。2011年在广西西部蚕区的桑园较大范围流行桑断枝烂叶病,该病主要危害桑树嫩枝和叶片,染病的桑树新枝出现灰褐色病斑、且易折断,桑叶也因出现灰褐斑而穿孔破烂。该病流行广,危害严重,部分植株发病率高达90%,枝发病率也达50%,导致桑叶严重减产,给桑农造成了较大经济损失[3]。桑断枝烂叶病的病原菌为Boeremia exigua,属于真菌中的子囊菌门、盘菌亚门、座囊菌纲、格孢菌亚纲、格孢菌腔目、Didymellaceae、Boeremia,菌丝为无色或褐色,有隔,合轴分枝,多为锐角二叉分枝,老龄菌丝呈褐色,藕节状膨大,载孢体为分生孢子器,单腔室,分生孢子无色,单孢,椭圆形、梨形,内常含油球[3]。现有防治桑断枝烂叶病的方法主要是物理防治和化学防治。物理防治主要包括及时修剪病、弱、枯枝和桑叶,清除田间杂草,雨季及时疏水,改善桑园通风透光性,降低田间湿度等。化学防治包括施加化肥,使桑枝条健壮,增强抗病性;喷洒化学农药,杀灭病原菌等。物理防治人工投入大、成本高,且防效有限;化学防治其农药降解周期长,严重污染环境,并且会给养蚕业带来不良影响,因此寻找绿色、环保的桑断枝烂叶病防治方法已经刻不容缓。生物防治是利用有益生物或其代谢产物控制有害生物种群的侵染、繁殖,以防止或减轻病害发生[4],具有环保、持久等优点。有关植物病害生物防治的相关研究结果表明,利用植物内生菌及其代谢产物防治宿主感染病原微生物,是近年来备受青睐的生防新策略[5–7]。
植物内生菌(Endophyte)指其生活史的一定阶段或全部阶段定殖于植物器官、组织内部以及细胞间隙,但不引起宿主植物出现明显病症的微生物[8]。内生菌作为植物微生态系统的重要组成部分,几乎可定殖于所有植物的不同组织部位,它们之间存在共生、竞争、对抗等各种关系[9]。健康桑树中存在大量内生菌,其构筑了桑树的健康屏障[10]。本课题组前期研究结果表明,内生成团泛菌SWg2菌株能稳定存在于桑树体内,且能提升桑树对桑疫病的抵抗能力[11];内生芽孢杆菌7PJ-16菌株能够定殖于桑树体内,提高桑树对桑椹菌核病的抵抗力,并可促进桑苗生长[12]。山东农业大学牟志美、路国兵等人从桑树根、茎、叶以及花蕾中分离获得多株对植物病原菌具有拮抗作用的内生细菌,其中洋葱伯克霍尔德氏菌(Burkholderia cepacia) LU10-1能长期定殖于桑树体内,且定殖过程中拮抗性能未发生改变[13]。然而,有关利用桑树内生菌防治桑断枝烂叶病的相关研究还未见报道。
生防菌防治植物病害的主要机制包括竞争、拮抗作用和诱导植株产生抗性等,其中,拮抗作用是内生菌的重要生防机制。生防芽孢杆菌产生的主要拮抗物质包括脂肽类化合物和聚酮类化合物。前者主要通过非核糖体多肽合成途径(nonribosomal peptide synthetases, NRPSs)合成,后者则主要依赖于聚酮合酶途径(polyketide synthases, PKS)合成[14]。其中脂肽类物质报道最多的是伊枯草菌素(iturin)、丰原素(fengycin)和表面活性素(surfactin) 3种抑菌活性物质[15-17]。伊枯草菌素、丰原素均有抗真菌活性,而表面活性素兼具抗细菌和真菌活性。Iturin家族成员,以iturin A、C, bacillomycin D、F、L、LC和mycosubtilin为代表,因其对多种植物病原真菌具有较强的拮抗活性而被广泛研究[18]。Zhao等的研究表明B. subtilis SG6产生的丰原素和表面活性素对禾谷镰刀菌的生长具有抑制作用[19]。Vitullo等发现分离菌株B. amyloliquefaciens BO7产生的表面活性素对尖孢镰刀菌具有较强的抑菌活性[20]。
本研究拟以桑断枝烂叶病病原菌Boeremia exigua GXH1为靶标菌,从农桑8号的健康桑树中分离、筛选拮抗细菌,测试拮抗菌的抑菌范围与其活性发酵滤液的热稳定性,并通过扩增拮抗菌株抑菌活性物质合成相关基因以及检测菌株代谢产物中可能的脂肽化合物种类,初步探究抑菌机理,为利用桑树内生菌防治桑断枝烂叶病奠定坚实的前期研究基础。
1 材料和方法 1.1 供试材料与培养基供试桑树材料:健康桑树农桑8号(桑树品种)采自云南省红河自治州蒙自草坝镇(N 23°23′14″,E 103°23′51″)。
供试病原菌:Boeremia exigua GXH1、核盘菌(Sclerotinia sclerotiorum) PZ-2、桑椹核地杖菌(Scleromitrula shiraiana) SXSG-5为本实验室分离保存。灰霉菌(Botrytis cinerea) SWU5、大丽花轮枝孢(Verticillium dahlia) SWU6、榆梢枯长喙霉(Ceratocystis ulmi) SWU10、木霉属菌(Trichoderma viridae) SWU11、烟草疫霉(Phytophthora nicotianae) SWU20、大丽轮枝菌(Verticillium dahlia) SWU23、链铬孢(Alternaria sp.) SWU26、白僵菌(Beauveria bassiana) SWU40、绿僵菌(Metarhizium anisopliae) SWU42为实验室收集保存菌株。
培养基:PDA培养基(g/L):土豆200.0,葡萄糖20.0,琼脂15–20;WA培养基(g/L):琼脂15–20;高氏一号培养基(g/L):可溶性淀粉20.0,KNO3 1.0,K2HPO4 0.5,MgSO4·7H2O 0.5,NaCl 0.5,FeSO4·7H2O 0.1,琼脂15–20;PDB培养基(g/L):土豆200.0,葡萄糖20.0;LB培养基(g/L):胰蛋白胨10.0,酵母粉5.0,NaCl 10.0,琼脂15–20;发酵培养基(g/L):土豆200.0,麦芽糖20.0,蛋白胨10.0,Na2HPO4 1.5,(NH4)2SO4 5.0;Landy培养基参考文献[21]配制;以上培养基均在121 ℃灭菌20 min备用。
1.2 桑树内生菌的分离纯化利用组织培养法[10]进行桑树内生菌的分离。具体方法为:取长约3–5 cm的桑枝茎段,浸没于75%的酒精,取出后于酒精灯上燃尽酒精,将该茎段在PDA培养基滚一圈后进行培养,以培养基上未生长微生物判定表面消毒彻底。将消毒后的茎段置于无菌培养皿中,用无菌刀片将样品分层剖开,切成小块置于PDA培养基、WA培养基和高氏培养基,于25 ℃培养,逐日观察,待其长出细菌时,及时挑取,釆用平板划线法进行纯化。
1.3 桑树内生细菌的保种与发酵用接种环挑取单菌落于PDB培养基中,28 ℃摇床180 r/min振荡培养24 h,取500 μL培养液加入装有500 μL 60%甘油的冻存管中,置于–80 ℃冰箱中保存;同时,用接种环挑取单菌落于PDB培养基中,28 ℃摇床180 r/min振荡培养4 d,随后在12000 r/min条件下离心30 min,取上清备用。
1.4 内生拮抗菌的筛选 1.4.1 初筛: 以桑断枝烂叶病病原菌B. exigua GXH1为供试靶标菌,采用抑菌圈法[22]筛选对桑断枝烂叶病病原菌具拮抗作用的内生细菌。具体方法为:将B. exigua GXH1菌饼接种在新鲜PDA平板中央,并用8 mm直径的打孔器在距离菌饼中心30 mm处打孔,孔内加入200 μL内生细菌发酵上清液,25 ℃培养7 d,测量抑菌圈直径,以此判断内生菌发酵液的抑菌活性。 1.4.2 复筛: 采用平板对峙法[23]对初筛有拮抗效果的菌株进行复筛。具体方法为:在直径为90 mm的PDA平板中心接种直径为5 mm的病原菌饼,同时在距中央25 mm的对称处划线接种拮抗细菌,以不接种拮抗细菌的平板为对照,各设3个重复,22 ℃培养7 d后测量病原菌的菌落直径,计算抑菌率。复筛确定抑菌效果显著且稳定的菌株进行后续研究。

|
将拮抗菌株NPJ13接种于PDB培养基,参照方法1.3制备拮抗菌株无菌发酵上清液,取等量无菌发酵上清液分别在40、60、80和100 ℃处理30 min,冷却至室温后,参照方法1.4用抑菌圈法检测其对病原真菌B. exigua GXH1菌株的抑菌活性。具体方法为:在新鲜PDA平板中央接种B. exigua GXH1菌饼,并在距离菌饼中心20 mm处用8 mm直径的打孔器打孔,将100 μL经不同温度处理后的拮抗菌发酵上清液注入孔中,阳性对照为注入未经热处理的拮抗菌发酵上清液平板,阴性对照为未加发酵滤液的PDA空白平板,每组设置3个重复,置于25 ℃培养箱中培养5 d后,用十字交叉法测量病原菌菌落直径,根据方法1.4.2中的公式计算抑菌率(其中对照组直径为阴性对照组直径)。
1.7 拮抗菌株抑菌谱检测将具显著而稳定拮抗作用的分离株接种于发酵培养基,参照1.3方法进行发酵培养,收集发酵液,10000 r/min离心30 min,将上清液过滤膜(D=0.22 μm)制备无菌发酵滤液,采用菌丝生长速率法[30]检测拮抗菌株无菌发酵滤液的抑菌谱。具体方法为:将PDA培养基高压灭菌后冷却至50 ℃左右,拮抗菌无菌发酵上清液按5%的比例混入制备检测平板,以未加发酵滤液的PDA平板作为空白对照,接入直径5 mm的核盘菌PZ-2、桑椹核地杖菌SXSG-5等12种病原菌菌饼,每个处理3个重复,于25 ℃恒温培养,逐日观察,参照1.4.2中公式计算抑菌率。根据病原菌生长速度,核盘菌PZ-2、灰霉菌SWU5、大丽花轮枝孢SWU6、榆梢枯长喙霉SWU10、木霉属菌SWU11、烟草疫霉SWU20、大丽轮枝菌SWU23、链格孢菌SWU26、桑椹核地杖菌SXSG-5在培养5 d时测算抑菌率,桑断枝烂叶病病原菌GXH1、白僵菌SWU40、绿僵菌SWU42在培养7 d时测算抑菌率。
1.8 拮抗菌株抑菌机理的初探 1.8.1 拮抗菌株对桑断枝烂叶病菌GXH1菌丝形态的影响: 将复筛中对照组平板和拮抗菌株处理组平板用于菌丝形态观察。用灭菌牙签挑取培养7 d对照组病原菌正常生长菌丝,以及测试组处于抑菌带边缘的病原菌菌丝制片,在光学显微镜下观察拮抗菌株对桑断枝烂叶病菌GXH1菌丝形态的影响。 1.8.2 抑菌活性物质合成关键基因的检测: 以NPJ13菌株基因组为模板,参考文献[31-33],扩增抑菌物质合成关键基因,包括2种次级代谢产物合成关键酶基因I型聚酮合酶基因(PKSI)、非核糖体多肽合成酶基因(NRPS),以及4种非核糖体途径合成的脂肽抗生素:表面活性素(Sfp、Srfc)、伊枯草菌素A (ItuD),以及丰原素(FenB)等6种活性次级代谢产物合成关键编码基因的保守区域,引物序列详见表 1。| Antibitic | Target gene |
Primers | Primer sequences (5′→3′) |
| PKS I | PKSI | KSF KSR | GCGATGGATCCNCAGCAGCG GTGCCGGTNCCGTGNGYYTC |
| NRPS | NRPS | NRPSF NRPSR | GCNGGYGGYGCNTAYGTNCC CCNCGDATYTTNACYTG |
| Surfactin | Sfp | SfpF SfpR | ATGAAGATTTACGGAATTTA TTATAAAAGCTCTTCGTACG |
| Iturin A | ItuD | ItuD1F ItuD1R | GATGCGATCTCCTTGGATGT ATCGTCATGTGCTGCTTGAG |
| Surfactin | Srfc | Sur3F Sur3R | ACAGTATGGAGGCATGGTC TTCCGCCACTTTTTCAGTTT |
| Fengycin | FenB | FenBF1 FenBR1 | CCTGGAGAAAGAATATACC GTACCY GCTGGTTCAGTTKGATCACAT |
PKSI扩增程序:94 ℃ 5 min;94 ℃ 1 min,59 ℃ 1 min,72 ℃ 1 min,共35个循环;72 ℃ 10 min。NRPS扩增程序:94 ℃ 5 min;94 ℃ 1 min,50 ℃ 1 min,72 ℃ 1 min,共35个循环;72 ℃ 10 min。Sfp、ItuD、Srfc扩增程序:95 ℃ 5 min;94 ℃ 1 min,55 ℃ 1 min,72 ℃ 1 min,共30个循环;72 ℃ 10 min。FenB扩增程序:95 ℃ 5 min;95 ℃ 1 min,65 ℃ 40 s,72 ℃ 1 min,共30个循环;72 ℃延伸10 min。
1.8.3 抑菌活性物质的初步分离与检测: 将新鲜的拮抗菌接种至Landy培养基,置于恒温摇床25 ℃、200 r/min培养3 d获得拮抗菌株活性发酵液。采用酸沉淀法[34]提取活性发酵液中脂肽类化合物。具体方法为:新鲜的拮抗菌株发酵液于4 ℃、8000 r/min离心20 min,用浓HCl将上清液pH调至2.0,4 ℃静置沉淀12 h,4 ℃、8000 r/min离心20 min后,加适量甲醇充分溶解沉淀,静置3 h后于4 ℃、8000 r/min离心10 min,用直径为0.22 μm的微孔滤膜过滤,取滤液于60 ℃烘箱中使甲醇充分挥发,再进行样品冻干处理,得到活性发酵液脂肽类化合物的粗提物,最后进行高效液相色谱串联质谱仪(LC-MS)分析。LC-MS分析使用Agilent 1290 Infinity Ultra Performance Liquid Chromatography与Agilent 6545 UHD and Accurate-Mass Q-TOF质谱联用仪,其中色谱柱型号为Waters XSelect® HSS T3 (2.5 μm,100 mm×2.1 mm)。流动相为A–水溶液(含0.1%甲酸)和B–乙腈(含0.1%甲酸);柱温设为25 ℃,进样量为4 μL,流速设为0.35 mL/min。优化的色谱梯度:0–2 min,5% B;2–10 min,5%–95% B;10–15 min,95% B;15–18 min,5% B。质谱使用正离子模式结合负离子模式;干燥气体流量:10 L/min;干燥气体温度:325 ℃;雾化器压力:20 psig;碰撞电压:120 V;skimmer voltage,45 V。质谱的采集范围50–1500 m/z,在质谱采集中打入参比离子监测质量轴的准确性。进一步鉴别拮抗菌株代谢物采用MS/MS,collision energy使用10、20、40 V三个能量。数据系统分析运用Agilent Masshunter Qualitative Analysis B.07.00 software。 2 结果和分析 2.1 桑树内生菌的分离与拮抗菌的筛选从健康农桑8号的茎中共分离获得内生细菌17株,初筛结果表明6株对桑断枝烂叶病菌B. exigua GXH1具有拮抗效果。复筛结果显示其中5株仍对B. exigua GXH1具有稳定的抑菌活性,尤以NPJ13菌株的抑菌活性最为显著,22 ℃培养7 d后抑菌率可达94.29% (表 2),故选择NPJ13菌株开展后续研究。
| Strain No. | Inhibition rate/% (x±s, n=3) |
| NPJ13 | 94.29±1.86 |
| NPJ1-1 | 45.33±2.06 |
| NPJ12 | 27.34±1.76 |
| NWJ1 | 25.31±2.53 |
| NWJ2-2 | 15.67±0.78 |
| Inhibition rates were calculated as a percentage of growth inhibition as compared to control test organism at 7 days after inoculation. Tests were conducted in triplicate and results varied as indicated by standard deviation. | |
2.2 拮抗菌株的菌种鉴定 2.2.1 形态特征及生理生化特征: NPJ13菌株在LB培养基上28 ℃培养24 h,菌落呈乳白色,表面稍有褶皱,边缘不整齐,有黏性,不透明(图 1-A);染色结果显示NPJ13菌株为革兰氏阳性、杆状细菌,能形成芽孢(图 1-B,C)。NPJ13可以水解葡萄糖、淀粉、明胶、蔗糖和甘露醇,能够还原硝酸盐(表 3)。

|
| 图 1 NPJ13菌株的形态学特征 Figure 1 The morphological features of strain NPJ13. A: the colony characteristic of strain NPJ13 on the LB after 24 h; B: gram staining result; C: spores staining result. |
| Test items | Results |
| Mannose | + |
| Simon’s phosphate | – |
| Semi-solid agar | – |
| Gas of glucose | – |
| Lactose | – |
| Nitrate reduction | + |
| Starch hydrolysis | + |
| Maltose | – |
| Gelatine liquefication test | + |
| Arabinose | – |
| Xylose | – |
| Gelation | + |
| Sucrose | + |
| Glycerinum | – |
| Sorbitol | + |
| Sulfuretted hydrogen | – |
| +: positive (growth or reaction); –: negative (no growth or no reaction). | |
2.2.2 NPJ13菌株16S rDNA、gyrA和gyrB基因序列测定及系统发育分析: 以NPJ13菌株基因组为模板,扩增获得NPJ13菌株的16S rDNA、gyrA和gyrB序列分别长度为1399 bp、1127 bp和1363 bp,GenBank登录号分别为MH884062、MK564524、MK564525。基于16S rDNA的系统发育分析结果表明,NPJ13菌株与多株Bacillus velezensis的相似性达到99%,并与登录号为AY603658、KY694464的Bacillus velezensis处于系统发育树的同一最小分枝(图 2-A)。基于gyrA基因序列的系统发育分析结果表明,NPJ13菌株与登录号为EU138622的Bacillus velezensis处于系统发育树的同一最小分枝(图 2-B);基于gyrB基因序列的系统发育分析结果表明,NPJ13菌株与登录号为DQ903176的Bacillus velezensis处于系统发育树的同一最小分枝(图 2-C)。结合其形态学、生理生化特征及基于16S rDNA、gyrA和gyrB基因序列的系统发育分析结果,将NPJ13鉴定为贝莱斯芽孢杆菌(Bacillus velezensis),命名为B. velezensis NPJ13。
|
| 图 2 NPJ13菌株基于16S rDNA(A)、gyrA(B)、gyrB(C)基因的系统发育树 Figure 2 Phylogenetic trees of NPJ13 strain based on 16S rDNA(A), gyrA(B) and gyrB(C) genes sequences. Numbers at the nodes represent the bootstrap values based on neighbor-joining analyses of 1000 resampled datasets. The scale bar indicates 0.5% sequence divergence. Target strain was labeled in bold. |
2.3 NPJ13菌株发酵滤液热稳定性检测
热稳定性检测结果显示NPJ13菌株发酵液具有较强的热稳定性,未经热处理的活性发酵液抑菌率为51.85%,80 ℃热处理30 min后抑菌率仍保持有44.44%,100 ℃热处理30 min后抑菌率仅降至33.33% (表 4)。良好的热稳定性,将有利于后续研究制定活性物质的分离纯化方案,更有利于B. velezensis NPJ13菌株用于田间防治桑断枝烂叶病。
| T/℃ | Inhibition rate/% (x±s, n=3) |
| Positive control | 51.85±0.97 |
| 40 | 48.15±1.31 |
| 60 | 46.38±0.87 |
| 80 | 44.44±2.42 |
| 100 | 33.33±0.87 |
| The strain NPJ13 cell-free fermentation supernatant treated at 40, 60, 80 and 100 ℃ for 30 min respectively. Observed the results at the fifth day after B. exigua GXH1 inoculation. | |
2.4 拮抗菌株NPJ13抑菌谱检测
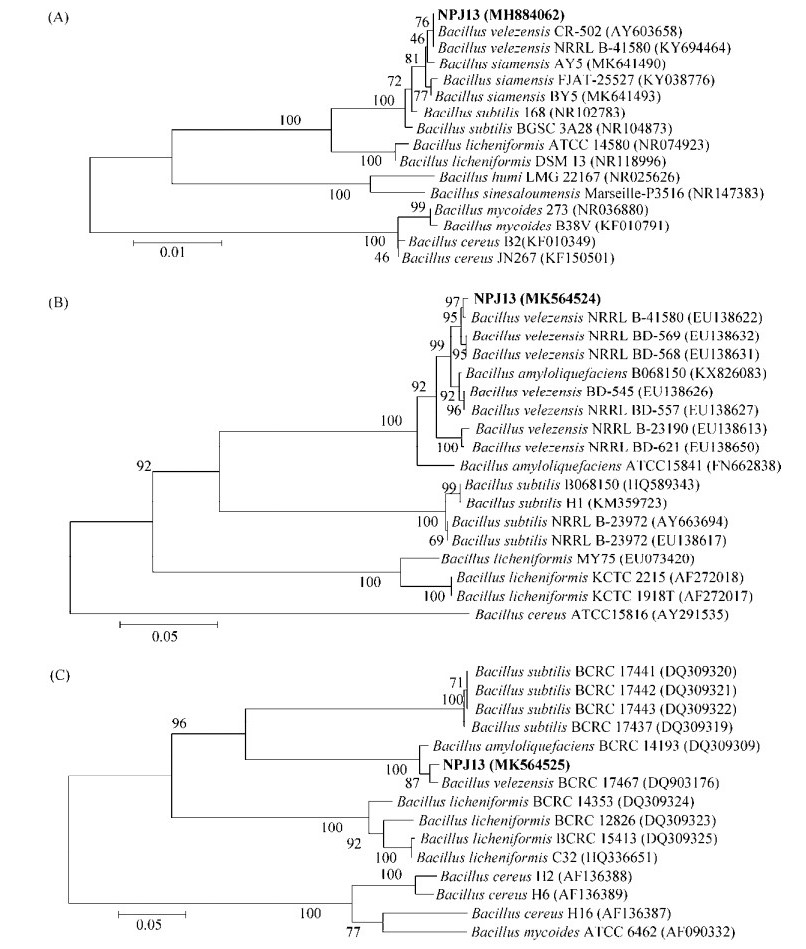
抑菌谱测试结果表明NPJ13菌株的无菌发酵滤液对12种病原真菌有不同程度的抑制作用,其中对桑断枝烂叶病菌GXH1和灰霉菌SWU5的抑制率最高,分别可达92.12%、91.55% (表 5,图 3);对核盘菌PZ-2、核地杖菌SXSG-5的抑菌率超过70%;而对榆梢枯长喙霉SWU10、白僵菌SWU40和绿僵菌SWU42的抑菌率亦超过50% (表 5)。较广的抑菌谱,使得NPJ13菌株亦可作为其他病害生物防治的候选菌株。
| Pathogen | Inhibition rate/% (x±s, n=3) |
| Botrytis cinerea SWU5 | 91.55±0.64 |
| Verticillium dahlia SWU6 | 40.23±0.69 |
| Ceratocystis ulmi SWU10 | 67.99±6.91 |
| Trichoderma viridae SWU11 | 39.46±22.63 |
| Phytophthora nicotianae SWU20 | 35.95±0.64 |
| Verticillium dahlia SWU23 | 25.47±8.80 |
| Alternaria alternata SWU26 | 19.54±0.21 |
| Sclerotinia sclerotiorum PZ-2 | 79.41±4.97 |
| Scleromitrula shiraiana SXSG-5 | 80.79±4.05 |
| Boeremia exigua GXH1* | 92.12±1.39 |
| Beauveria bassiana SWU40* | 64.00±7.37 |
| Metarhizium anisopliae SWU42* | 59.00±5.07 |
| Inhibition rates were calculated as a percentage of growth inhibition as compared to control test organism at 5 days after inoculation for most organisms (*: 7-day). Tests were conducted in triplicate and results varied as indicated by standard deviation. | |
|
| 图 3 NPJ13菌株发酵上清液对桑断枝烂叶病菌GXH1和灰霉菌SWU5的抑菌效果 Figure 3 The antagonistic activity of cell-free fermentation supernatant of strain NPJ13 against B. exigua GXH1 and Botrytis cinerea SWU5. A: B. exigua GXH1 was cultured on the PDA plate for 7 days; B: B. exigua GXH1 was cultured for 7 days on the PDA plate containing cell-free fermentation supernatant of strain NPJ13; C: B. cinerea SWU5 was cultured on PDA plate for 5 days; D: B. cinerea SWU5 was cultured for 5 days on the PDA plate containing cell-free fermentation supernatant of strain NPJ13. |
2.5 NPJ13菌株对桑断枝烂叶病菌GXH1菌丝的影响
拮抗菌对B. exigua GXH1菌体生长影响的镜检结果表明,NPJ13菌株对病菌菌丝的生长有明显的破坏作用。对照组菌丝体生长繁茂、平滑修长,光滑匀整,颜色为褐色(图 4-A);而受拮抗菌NPJ13影响的B. exigua GXH1菌丝体大部分发生畸变,菌丝缠绕、扭曲,部分菌丝膨大、透明度增加甚至断裂,局部现串珠状细胞,内含物多数溢出(图 4-B)。

|
| 图 4 NPJ13菌株对桑断枝烂叶病菌GXH1菌株菌丝的影响 Figure 4 Effect of strain NPJ13 on mycelium of B. exigua GXH1. A: The mycelial morphology of control group observed with optical microscope after cultured for 7 days; B: The mycelial morphology of treatment group observed with optical microscope after cultured for 7 days. |
2.6 拮抗菌株抗菌次级代谢产物合成相关基因的检测结果
抗菌次级代谢产物合成相关基因扩增结果显示,NPJ13菌株的基因组中能检测到PKSI、NRPS、Sfp、ItuD、Srfc这5种与抑菌活性物质合成相关的基因(表 6)。由此初步推测拮抗菌株NPJ13可通过产生表面活性素、伊枯草菌素A、聚酮类化合物等抗菌次级代谢产物抑制B. exigua GXH1生长。
| Material | Gene | NPJ13 |
| PKSⅠ | PKSI | + |
| NRPS | NRPS | + |
| Surfactin | Sfp | + |
| Iturin A | ItuD | + |
| Surfactin | Srfc | + |
| Fengycin | FenB | – |
| +: positive; –: negative. | ||
2.7 抑菌活性物质的初步分析
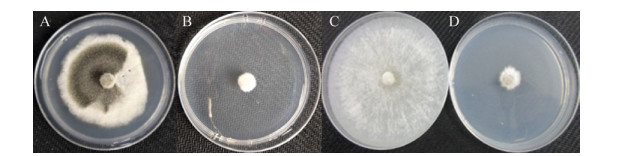
LC-MS分析结果显示NPJ13菌株脂肽粗提物在m/z值为1008.65、1022.67和1036.68处有离子峰(簇)出现,这3个离子峰的分子量依次相差14 (脂肪酸链结构“-CH2-”),分别对应于C13–C15的表面活性素正离子峰(图 5-A);在m/z值为1064.68、1070.63和1076.59处有离子峰(簇)出现,这3个离子峰均对应于1031–1082范围内的伊枯草菌素类脂肽化合物(图 5-B)。通过LC-MS质谱图分析表明,菌株NPJ13发酵液中含有脂肽类化合物表面活性素和伊枯草菌素。
|
| 图 5 菌株NPJ13脂肽类物质的LC-MS分析 Figure 5 LC-MS analysis of lipopeptide compounds by strain NPJ13. A: surfactin; B: iturin. |
3 小结和讨论
本研究从桑树品种农桑8号健康茎中分离得到17株内生细菌,并从中筛选获得一株对桑断枝烂叶病菌抑菌作用稳定且显著的内生细菌NPJ13,鉴定其为贝莱斯芽孢杆菌,命名为B. velezensis NPJ13。抑菌谱检测结果表明B. velezensis NPJ13菌株活性发酵滤液对灰霉病菌SWU5、核地杖菌SXSG-5、核盘菌PZ-2及烟草疫霉SWU20等12种常见病原真菌具有不同程度的抑制作用,且其抑菌活性发酵液具有较好的热稳定性,经80 ℃处理30 min后,抑菌活性仍较为显著,且NPJ13菌株可能通过合成表面活性素、伊枯草菌素A、聚酮类化合物等次级代谢产物,抑制病原菌生长。
芽孢杆菌是一类广泛分布于土壤、水体等多种自然环境,亦作为植物内生菌存在于多种植物体内[35]。芽孢杆菌可以产生多种抗菌化合物,包括表面活性素、丰原素和伊枯草菌素等脂肽类抗生素[15]。Arima等报道了枯草芽孢杆菌能分泌抗菌物质脂肽类抗生素表面活性素[36]。Cao等在枯草芽孢杆菌SQR9中鉴定出BmyA、ppsE、ituD、fenD、bamD、albA、srfAB、qk和yndJ等9个脂肽合成相关基因[37]。Surendra等从玉米内生拮抗菌芽孢杆菌中检测到伊枯草菌素A和丰原素等抑菌活性物质合成相关基因[30]。桑建伟等从健康香蕉植株根部中分离得到一株内生解淀粉芽孢杆菌BEB17,能有效拮抗香蕉枯萎病菌,并从其代谢产物中分离到表面活性素、丰原素和伊枯草菌素三类脂肽类化合物以及Bacillibactin、Difficidin和Bacillaene三类聚酮类化合物[38]。贝莱斯芽孢杆菌(Bacillus velezensis)作为芽孢杆菌属的一个重要成员,对植物生长、抗病虫和诱导系统抗病性等方面起着重要作用[39]。宗英等从感染赤霉病的麦穗上筛选到1株可高效抑制小麦赤霉病菌禾谷镰刀菌的B. velezensis JS25R,在温室条件下该菌能有效降低小麦赤霉病的发病率、发病程度和病情指数[40]。KIM等对B. velezensis CAUB946菌株的抗性相关次级代谢产物分析发现其可通过产生脂肽、PKS和溶杆菌素来防治烟草黑胫病、水稻纹枯病和棉花枯萎病[41]。后续研究将在本文研究基础上,评估B. velezensis NPJ13菌株对桑树的安全性,检测其田间防病效果,并分离纯化获得NPJ13菌株发酵液的主要抑菌活性成分,深入探究该菌株对桑断枝烂叶病的抑制相关机理,为在生产中利用该菌株进行桑断枝烂叶病的生物防治奠定坚实的研究基础。
| [1] |
Dai YW, Zhu H, Du HZ, Zhang JB, Wen BY. Economic value and ecological function of mulberry (Morus alba L.). Protection Forest Science and Technology, 2009(1): 78-80.
(in Chinese) 戴玉伟, 朱弘, 杜宏志, 张剑斌, 温宝阳. 论桑树资源经济价值和生态功能. 防护林科技, 2009(1): 78-80. DOI:10.3969/j.issn.1005-5215.2009.01.033 |
| [2] |
Pu GQ, Huang YJ, Mao JP, Zhang YJ. List of Chinese mulberry diseases (Ⅰ). China Sericulture, 2012, 33(2): 21-24.
(in Chinese) 浦冠勤, 黄艳君, 毛建萍, 张月季. 中国桑树病害名录(Ⅰ). 中国蚕业, 2012, 33(2): 21-24. DOI:10.3969/j.issn.1007-0982.2012.02.006 |
| [3] |
Wei HL. Identification of pathogen of mulberry snags rotten leaves disease. Master Dissertation of Guangxi University, 2015. (in Chinese) 韦海玲.桑断枝烂叶病的病原菌种类鉴定.广西大学硕士学位论文, 2015. |
| [4] |
Xiao YF, Mao RQ, Wan FH. New concept of biological control: bio-control plants used for management of arthropod pests. Chinese Journal of Biological Control, 2013, 29(1): 1-10.
(in Chinese) 肖英方, 毛润乾, 万方浩. 害虫生物防治新概念——生物防治植物及创新研究. 中国生物防治学报, 2013, 29(1): 1-10. DOI:10.3969/j.issn.2095-039X.2013.01.001 |
| [5] |
Gao XN, Chen JY, Huang LL, Qiao HP, Han QM, Kang ZS. Screening of antagonistic endophytic bacteria and their roles in control of Sclerotinia sclerotiorum in canola. Chinese Journal of Pesticide Science, 2010, 12(2): 161-167.
(in Chinese) 高小宁, 陈金艳, 黄丽丽, 乔宏萍, 韩青梅, 康振生. 油菜菌核病内生拮抗细菌的筛选及防病作用研究. 农药学学报, 2010, 12(2): 161-167. DOI:10.3969/j.issn.1008-7303.2010.02.08 |
| [6] |
Dou GM, Liu XY, Zhang YD, Ma YC. Screening and identification of antagonistic endophytes against Sclerotinia sclerotiorum. Guangdong Agricultural Sciences, 2013(7): 78-82.
(in Chinese) 窦桂铭, 刘晓瑜, 张银东, 马玉超. 大豆菌核病拮抗内生细菌的筛选与鉴定. 广东农业科学, 2013(7): 78-82. DOI:10.3969/j.issn.1004-874X.2013.07.024 |
| [7] | Kumar S, Kaushik N. Endophytic fungi isolated from oil-seed crop Jatropha curcas produces oil and exhibit antifungal activity. PLoS One, 2013, 8(2): e56202. DOI:10.1371/journal.pone.0056202 |
| [8] |
Fang ZJ, Zhang XX, Ma LA. Advances in plant endophytes. Journal of Yangtze University (Natural Science Edition), 2018, 15(10): 41-45.
(in Chinese) 方珍娟, 张晓霞, 马立安. 植物内生菌研究进展. 长江大学学报(自然科学版), 2018, 15(10): 41-45. DOI:10.3969/j.issn.1673-1409.2018.10.012 |
| [9] | Porras-Alfaro A, Bayman P. Hidden fungi, emergent properties: endophytes and microbiomes. Annual Review of Phytopathology, 2012, 49: 291-315. |
| [10] |
Ren HS, Xu WF, Wang AY, Zuo WD, Xie J. Research on biodiversity of endophytic bacteria and the antagonistic endophytes in mulberry. Journal of Southwest University (Natural Science Edition), 2017, 39(1): 36-45.
(in Chinese) 任慧爽, 徐伟芳, 王爱印, 左伟东, 谢洁. 桑树内生细菌多样性及内生拮抗活性菌群的研究. 西南大学学报(自然科学版), 2017, 39(1): 36-45. |
| [11] | Xie J, Shu P, Strobel G, Chen J, Wei JH, Xiang ZH, Zhou ZY. Pantoea agglomerans SWg2 colonizes mulberry tissues, promotes disease protection and seedling growth. Biological Control, 2017, 113: 9-17. DOI:10.1016/j.biocontrol.2017.06.010 |
| [12] | Xu WF, Ren HS, Ou T, Lei T, Wei JH, Huang CS, Li T, Strobel G, Zhou ZY, Xie J. Genomic and functional characterization of the endophytic Bacillus subtilis 7PJ-16 strain, a potential biocontrol agent of mulberry fruit sclerotiniose. Microbial Ecology, 2019, 77(3): 651-663. DOI:10.1007/s00248-018-1247-4 |
| [13] |
Mu ZM, Lu GB, Ji XL, Gai YP, Wang YW, Gao HJ, Zha CY. Identification and colonization of an antagonistic endophytic Burkholderia cepacia Lu10-1 isolated from mulberry. Acta Microbiologica Sinica, 2008, 48(5): 623-630.
(in Chinese) 牟志美, 路国兵, 冀宪领, 盖英萍, 王彦文, 高绘菊, 查传勇. 桑树内生拮抗细菌Burkholderia cepacia Lu10-1的分离鉴定及其内生定殖. 微生物学报, 2008, 48(5): 623-630. DOI:10.3321/j.issn:0001-6209.2008.05.010 |
| [14] |
Gong AD. Isolation and antagonistic mechanism analyses of biocontrol agents against Fusarium and Aspergillus species. Doctor Dissertation of Huazhong Agricultural University, 2015. (in Chinese) 宫安东.镰刀菌和黄曲霉菌生防菌的分离及拮抗机理研究.华中农业大学博士学位论文, 2015. |
| [15] |
Gao XW, Yao SY, Pham H, Vater J, Wang JS. Inhibitive substance produced by Bacillus subtilis B2 strain. Chinese Journal of Biological Control, 2003, 19(4): 175-179.
(in Chinese) 高学文, 姚仕义, Pham H, Vater J, 王金生. 枯草芽孢杆菌B2菌株产生的抑菌活性物质分析. 中国生物防治, 2003, 19(4): 175-179. |
| [16] |
Qian CD, Li BQ, Zhao T, Guo QG, Lu XY, Li SZ, Ma P. Isolation and stability analysis of lipopeptides produced by Bacillus subtilis strain BAB-1. Journal of Agricultural Science and Technology, 2009, 11(6): 69-74.
(in Chinese) 钱常娣, 李宝庆, 赵添, 郭庆港, 鹿秀云, 李社增, 马平. 枯草芽孢杆菌BAB-1脂肽类化合物的分离及稳定性分析. 中国农业科技导报, 2009, 11(6): 69-74. DOI:10.3969/j.issn.1008-0864.2009.06.012 |
| [17] |
Xie YL, Ma LZ, Xu ZW, Du Z, Gao XW. Molecular identification of Bacillus strains isolated from extreme dry-sand environment in Qinghai Chaidamu region and its lipopeptide compound analysis. Microbiology China, 2012, 39(8): 1079-1086.
(in Chinese) 谢永丽, 马莉贞, 徐志伟, 杜卓, 高学文. 青海柴达木极端干旱沙地分离芽孢杆菌的分子鉴定及拮抗活性分析. 微生物学通报, 2012, 39(8): 1079-1086. |
| [18] | Stein T. Bacillus subtilis antibiotics: structures, syntheses and specific functions. Molecular Microbiology, 2005, 56(4): 845-857. DOI:10.1111/j.1365-2958.2005.04587.x |
| [19] | Zhao PC, Quan CS, Wang YG, Wang JH, Fan SD. Bacillus amyloliquefaciens Q-426 as a potential biocontrol agent against Fusarium oxysporum f. sp. spinaciae. Journal of Basic Microbiology, 2014, 54(5): 448-456. DOI:10.1002/jobm.201200414 |
| [20] | Vitullo D, Di Pietro A, Romano A, Lanzotti V, Lima G. Role of new bacterial surfactins in the antifungal interaction between Bacillus amyloliquefaciens and Fusarium oxysporum. Plant Pathology, 2012, 61(4): 689-699. DOI:10.1111/j.1365-3059.2011.02561.x |
| [21] | Wei YH, Chu IM. Enhancement of surfactin production in iron-enriched media by Bacillus subtilis ATCC 21332. Enzyme and Microbial Technology, 1998, 22(8): 724-728. DOI:10.1016/S0141-0229(98)00016-7 |
| [22] |
Xie J, Xia T, Lin LP, Zuo WD, Zhou ZY. Isolation and identification of an antagonistic endophytic bacterial strain from mulberry. Science of Sericulture, 2009, 35(1): 121-125.
(in Chinese) 谢洁, 夏天, 林立鹏, 左伟东, 周泽扬. 一株桑树内生拮抗菌的分离鉴定. 蚕业科学, 2009, 35(1): 121-125. DOI:10.3969/j.issn.0257-4799.2009.01.020 |
| [23] |
Zhang YP, Ling LJ, Zhao Y, Hou YQ. Study on antagonistic activity on P. infestans exhibited from Pseudomonas sp. HC5. Gansu Agricultural Science and Technology, 2018(1): 33-36.
(in Chinese) 张艳萍, 令利军, 赵瑛, 厚毅清. 假单胞菌HC5对马铃薯晚疫病菌的抑制作用研究. 甘肃农业科技, 2018(1): 33-36. DOI:10.3969/j.issn.1001-1463.2018.01.011 |
| [24] | 布坎南RE, 吉本斯NE.伯杰细菌鉴定手册.第8版.中国科学院微生物研究所《伯杰细菌鉴定手册》翻译组, 译.北京: 科学出版社, 1984. |
| [25] | 东秀珠, 蔡妙英. 常见细菌系统鉴定手册. 北京: 科学出版社, 2001. |
| [26] | 沈萍, 陈向东. 微生物学实验. 第4版. 北京: 高等教育出版社, 2007. |
| [27] | Weisburg WG, Barns SM, Pelletier DA, Lane DJ. 16S ribosomal DNA amplification for phylogenetic study. Journal of Bacteriology, 1991, 173(2): 697-703. DOI:10.1128/jb.173.2.697-703.1991 |
| [28] | Chun J, Bae KS. Phylogenetic analysis of Bacillus subtilis and related taxa based on partial gyrA gene sequences. Antonie van Leeuwenhoek, 2000, 78(2): 123-127. DOI:10.1023/A:1026555830014 |
| [29] | Yamamoto S, Harayama S. PCR amplification and direct sequencing of gyrB genes with universal primers and their application to the detection and taxonomic analysis of Pseudomonas putida strains. Applied and Environmental Microbiology, 1995, 61(3): 1104-1109. |
| [30] | 方中达. 植病研究方法. 第3版. 北京: 中国农业出版社, 1998. |
| [31] |
Wang YT, Zhang CB, Qi L, Jia XQ, Lu WY. Diversity and antimicrobial activities of cultivable bacteria isolated from Jiaozhou Bay. Acta Microbiologica Sinica, 2016, 56(12): 1892-1900.
(in Chinese) 王怡婷, 张传波, 齐麟, 贾晓强, 卢文玉. 胶州湾沉积物可培养细菌的多样性及其抑菌活性. 微生物学报, 2016, 56(12): 1892-1900. |
| [32] | Chung S, Kong H, Buyer JS, Lakshman DK, Lydon J, Kim SD, Roberts DP. Isolation and partial characterization of Bacillus subtilis ME488 for suppression of soilborne pathogens of cucumber and pepper. Applied Microbiology and Biotechnology, 2008, 80(1): 115-123. DOI:10.1007/s00253-008-1520-4 |
| [33] | Gond SK, Bergen MS, Torres MS, White Jr JF. Endophytic Bacillus spp. produce antifungal lipopeptides and induce host defence gene expression in maize. Microbiological Research, 2015, 172: 79-87. DOI:10.1016/j.micres.2014.11.004 |
| [34] | Luo CP, Liu XH, Zhou HF, Wang XY, Chen ZY. Nonribosomal peptide synthase gene clusters for lipopeptide biosynthesis in Bacillus subtilis 916 and their phenotypic functions. Applied and Environmental Microbiology, 2015, 81(1): 422-431. DOI:10.1128/AEM.02921-14 |
| [35] |
Fang X, Xu WF, Niu N, Ou T, Wang F, Zuo WD, Xie J. Screening, identification and optimization of fermentation conditions of an antagonistic endophytic bacterium from mulberry. Acta Microbiologica Sinica, 2018, 58(12): 2147-2160.
(in Chinese) 方翔, 徐伟芳, 牛娜, 欧婷, 王飞, 左伟东, 谢洁. 一株桑树内生拮抗菌的分离、鉴定及发酵条件优化. 微生物学报, 2018, 58(12): 2147-2160. |
| [36] | Arima K, Kakinuma A, Tamura G. Surfactin, a crystalline peptidelipid surfactant produced by Bacillus subtilis: Isolation, characterization and its inhibition of fibrin clot formation. Biochemical and Biophysical Research Communications, 1968, 31(3): 488-494. DOI:10.1016/0006-291X(68)90503-2 |
| [37] | Cao Y, Xu ZH, Ling N, Yuan YJ, Yang XM, Chen LH, Shen B, Shen QR. Isolation and identification of lipopeptides produced by B. subtilis SQR 9 for suppressing Fusarium wilt of cucumber. Scientia Horticulturae, 2012, 135: 32-39. DOI:10.1016/j.scienta.2011.12.002 |
| [38] |
Sang JW, Yang Y, Chen YP, Cai JM, Lu CM, Huang GX. Antibacterial activity analysis of lipopeptide and polyketide compounds produced by endophytic bacteria Bacillus amyloliquefaciens BEB17. Acta Phytopathologica Sinica, 2018, 48(3): 402-412.
(in Chinese) 桑建伟, 杨扬, 陈奕鹏, 蔡吉苗, 陆翠梅, 黄贵修. 内生解淀粉芽孢杆菌BEB17脂肽类和聚酮类化合物的抑菌活性分析. 植物病理学报, 2018, 48(3): 402-412. |
| [39] |
Cai GL, Zhang F, Ouyang YX, Zhao CS, Peng XH, Jiang AM. Research progress on Bacillus velezensis. Northern Horticulture, 2018(12): 162-167.
(in Chinese) 蔡高磊, 张凡, 欧阳友香, 赵昌松, 彭宣和, 江爱明. 贝莱斯芽孢杆菌(Bacillus velezensis)研究进展. 北方园艺, 2018(12): 162-167. |
| [40] |
Zong Y, Zhao YJ, Liu Y, Yang QL. Study on the inhibitory effect of Bacillus velezensis on Fusarium graminearum. Journal of Nuclear Agricultural Sciences, 2018, 32(2): 310-317.
(in Chinese) 宗英, 赵月菊, 刘阳, 杨庆利. 一株贝莱斯芽孢杆菌抑制禾谷镰刀菌的研究. 核农学报, 2018, 32(2): 310-317. |
| [41] | Kim SY, Lee SY, Weon HY, Sang MK, Song J. Complete genome sequence of Bacillus velezensis M75, a biocontrol agent against fungal plant pathogens, isolated from cotton waste. Journal of Biotechnology, 2017, 241: 112-115. DOI:10.1016/j.jbiotec.2016.11.023 |
 2019, Vol. 59
2019, Vol. 59