中国科学院微生物研究所,中国微生物学会,中国菌物学会
文章信息
- 沈碧妙, 吴淘栩, 朱俊杰, 徐琳娜, 刘小香, 朱军莉. 2018
- Bimiao Shen, Taoxu Wu, Junjie Zhu, Linna Xu, Xiaoxiang Liu, Junli Zhu. 2018
- rpoS基因缺失突变对荧光假单胞菌胁迫耐受性、群体感应及腐败活性的影响
- Effects of rpoS deletion on stress resistance, quorum sensing and spoilage potential of Pseudomonas fluorescens
- 微生物学报, 58(9): 1563-1572
- Acta Microbiologica Sinica, 58(9): 1563-1572
-
文章历史
- 收稿日期:2017-10-30
- 修回日期:2017-12-18
- 网络出版日期:2018-01-05
2. 杭州医学院基础医学部, 浙江 杭州 310053;
3. 浙江工商大学食品与生物工程学院, 浙江省食品安全重点实验室, 浙江 杭州 310035
2. Faculty of Basic Medicine, Hangzhou Medical College, Hangzhou 310053, Zhejiang Province, China;
3. Food Safety Key Laboratory of Zhejiang Province, College of Food Science and Biotechnology, Zhejiang Gongshang University, Hangzhou 310035, Zhejiang Province, China
微生物活动是大多数食品在贮藏、加工及流通过程中腐败的主要原因。由于微生物单因素腐败作用而导致全世界约1/4的食品供应和30%捕获鱼类损失[1]。荧光假单胞菌(Pseudomonas fluorescens)是假单胞菌属中具有代表性的食品腐败菌[2],属于革兰氏阴性好氧或兼性厌氧杆菌,适应温度范围广,正常生长温度为25–30℃,但4℃也能快速增殖[3]。冷藏的水产品、肉制品、牛奶等一旦被荧光假单胞菌污染,该菌便会大量繁殖,逐渐演变为优势菌群[4-5]。研究表明,三文鱼、罗非鱼、大菱鲆及鲶鱼等在有氧冷藏过程中的优势腐败菌均为荧光假单胞菌[6-9]。荧光假单胞菌产生的腐败产物活性强,能大量产生蛋白酶、脂肪酶、生物膜、嗜铁素及其他代谢产物,促使食品中的蛋白和脂肪成分进一步被分解,生成胺(氨)、醛、酮、酯和有机酸等各种具有腐臭特征的产物,导致色泽改变,品质变劣,甚至产生毒性[2, 8]。研究腐败菌腐败活性调控机制对于保证食品及其储存的质量和安全具有重要意义。
在食品加工及贮藏过程中,人们采取一系列的措施来灭活或者抑制微生物的生长,包括氧化剂、乙醇、高温、低温、酸碱、高盐以及食品保鲜剂等。对胁迫条件的耐受能力是细菌产生腐败作用的关键环节。RNA聚合酶的选择性sigma因子RpoS,也称作sigma S或者sigma 38,协助RNA聚合酶识别启动子序列并启动下游相关基因的表达,起到基因转录调节的作用[10]。RpoS是目前发现的细菌应答多元胁迫反应重要的调控蛋白,在细菌进入生长稳定期后大量表达。RpoS最初发现于大肠杆菌(Escherichia coli),rpoS基因突变可影响该细菌对饥饿、高温、低pH及氧化等多种胁迫环境的耐受性[11-12]。在不同细菌中,RpoS对胁迫耐受性的影响往往是多样性的[13]。
群体感应(quorum sensing,QS)是一种细菌细胞间信息传递的机制,即细菌在生长繁殖过程中通过合成、分泌自诱导物(autoinducer,AI)作为信号分子,检测细菌浓度;当AI浓度随着细菌群体密度达到一定阈值时,信号分子与受体蛋白结合后,引起受体蛋白构象发生变化,从而开启细胞密度依赖的特定基因表达机制来调控特定的生物学功能[14-15]。革兰氏阴性细菌中群体感应信号分子主要为高丝氨酸内酯类(N-acyl-Lhomoserine lactones,AHLs)[15]。RpoS与QS都和细胞密度相关,这2个调控系统之间存在有相关性。在铜绿假单胞菌(Pseudomonas aeruginosa)中,40%以上的群体感应控制基因也受RpoS调控[16]。另外,研究发现,在鱼、牛肉、牛奶、豆芽等食品品质劣变过程中均检测到或者发现群体感应信号分子浓度逐步增加[17-18]。腐败菌能够利用群体感应系统调控腐败活性,如挥发性盐基氮、生物胺、生物膜、胞外酶等的产生[18-20]。
为了确定RpoS在荧光假单胞菌致腐败过程中的作用,本论文通过构建鱼源荧光假单胞菌的rpoS基因缺失突变株,研究RpoS在荧光假单胞菌致腐败过程中的作用,为靶向控制细菌腐败作用和探索高效水产品储存、保鲜技术提供理论基础。
1 材料和方法 1.1 菌株、质粒与培养条件本研究中用到的菌株和质粒见表 1。荧光假单胞菌ZJL511从腐败冷藏鱼中分离获得[21],本实验室保存。荧光假单胞菌及其突变株在28 ℃、LB或NB培养基中培养。大肠杆菌在37 ℃的LB培养基中培养。
| Strains or plasmids | Characteristics | Reference or source |
| Escherichia coli | ||
| DH5α | F–,
 80dlacZΔM15, Δ(lacZYA-argF)U169, deoR, recA1,
endA1, hsdR17(rk–, mk+), phoA, supE44,
λ–, thi-1, gyrA96, relA1 80dlacZΔM15, Δ(lacZYA-argF)U169, deoR, recA1,
endA1, hsdR17(rk–, mk+), phoA, supE44,
λ–, thi-1, gyrA96, relA1 |
Invitrogen |
| β2163 | F–, RP4-2-Tc::Mu ΔdapA::(erm-pir) | Demarre et al. (2005) [23] |
| Pseudomonas fluorescens | ||
| ZJL511 | Isolated from spoiled refrigerated turbot | Laboratory collection |
| ΔrpoS | ZJL511, in-frame deletion of rpoS | This study |
| Plasmids | ||
| pLP12 | oriTRP4 oriVR6K vmi480 PBAD, Cmr | Luo et al. (2015) [22] |
| pLP12-rpoS | pLP12 derivative containing rpoS bp 1–51 fused in-frame to bp 757–1005, Cmr | This study |
| Primers | Sequence (5ʹ→3ʹ) | |
| rpoS-MF1 | ATACCTACGTCAGTGCCTACG | This study |
| rpoS-MR1 | GTCATCGTCCTGCAACAGCTCGAGAACCTCATCGTCGATGTC | This study |
| rpoS-MF2 | GACATCGACGATGAGGTTCTCGAGCTGTTGCAGGACGATGAC | This study |
| rpoS-MR2 | CTAACTTGTACTGACTCAGTCG | This study |
| rpos3-TF | GTTCTCCATCGCCTTCCGTTAT | This study |
| rpos3-TR | ACCTTCGGAAATACCATTGGC | This study |
1.2 主要试剂
高保真DNA聚合酶(PrimeSTAR Max DNA Polymerase),Taq DNA Polymerase,T4 DNA Ligase,pMD18-T质粒载体购自TaKaRa公司;质粒提取试剂盒及凝胶回收试剂盒购自Tiangen公司;氯霉素,L-阿拉伯糖,D-葡萄糖,信号分子标准品C4-HSL、C6-HSL、C8-HSL、C10-HSL、C12-HSL和C14-HSL购自Sigma公司;酵母粉(Yeast Extract,Oxoid公司);蛋白胨(Typtone,Oxoid公司);琼脂粉(Agar,Biosharp公司);NB培养基,平板计数琼脂(PCA)均购自青岛海博生物技术有限公司。引物合成和测序均由生工生物工程(上海)股份有限公司完成。其他试剂均为国产分析纯。
1.3 构建荧光假单胞菌rpoS基因缺失突变株首先,根据GenBank中荧光假单胞菌的基因组序列设计引物rpos3-TF/TR,以ZJL511的基因组DNA为模板扩增出包括rpoS及上下游基因在内的一长段DNA序列并测序(2337 bp),根据该序列设计用于基因缺失突变的引物(表 1)。参考Luo等(2015)[22]的方法,通过同源双交换法构建荧光假单胞菌rpoS基因的框内缺失突变株。首先PCR扩增rpoS基因上下游同源臂片段。通过引物rpoS-MF1和rpoS-MR1扩增338 bp的上游同源臂片段,rpoS-MF2和rpoS-MR2扩增446 bp下游同源臂。切胶回收上下游同源臂片段,通过引物rpoS-MF1和rpoS-MR2进行融合PCR,将上下同源臂片段相连接,连接片段克隆到自杀质粒pLP12得到pLP12-rpoS,测序证明正确后电转化导入大肠杆菌β2163中。然后通过结合转移将pLP12-rpoS从供体大肠杆菌β2163转入荧光假单胞菌ZJL511中,供体菌因为营养缺陷在不含有二氨基庚二酸的LB平板上不能生长,用含有30µg/mL氯霉素和0.3% D-葡萄糖的LB平板上筛选单交换子。L-阿拉伯糖能够诱导自杀质粒上的毒性基因vmi480表达,在LB平板中添加0.4% L-阿拉伯糖筛选双交换子。缺失突变株通过PCR筛选和测序鉴定。
1.4 胁迫耐受性测定参考Liu等(2012)[24]的方法,在NB培养基中培养荧光假单胞菌野生型及突变株至稳定生长期,调整起始细胞数为106–107 CFU/mL,将细菌暴露于以下胁迫条件:(A) 10 mmol/L H2O2;(B) 47 ℃;(C) 12% (V/V)乙醇;(D) 150 μg/mL结晶紫;(E) 175 mmol/L醋酸;(F) 20% (M/V) NaCl,在不同的时间点取样,0.1 mol/L磷酸缓冲液(pH 7.0) 10倍梯度稀释后平板计数琼脂进行菌落计数。每个菌株设3个平行,每个胁迫实验重复至少2次。
1.5 AHLs信号分子检测参考綦国红等[25]的方法提取AHLs信号分子,荧光假单胞菌野生型和突变株于50 mL LB培养基中培养24 h,10000×g、4℃离心10 min收集上清液。将上清液与等体积酸化乙酸乙酯(含0.1%冰醋酸)混合,涡旋振荡,静置分层后取有机相。按同法重复3次,旋转蒸发至干,用适量甲醇溶解烧瓶内壁上的AHLs信号分子,–20℃保存备用。参考Zhao等(2016)[26]的方法通过LC-MS/MS检测AHLs的含量,采用Agilent液质联用分析仪1290 infinityII-6460 Triple Quad和RRHD Eclipse Plus C18色谱柱(50 mm×2.1 mm×1.8 μm)进行分析。提取的信号分子经C18色谱柱分离后进入质谱,采用电喷雾离子源正离子模式和多反应监测(MRM)模式,外标法进行定量分析,6种合成的AHL标品C4-HSL、C6-HSL、C8-HSL、C10-HSL、C12-HSL和C14-HSL作为混合外标定量。
1.6 三文鱼汁腐败实验三文鱼购自杭州近江水产市场,并在1 h内冰上运至实验室。参考Dalgaard等(1995)[27]的方法制备灭菌三文鱼汁。称取500 g三文鱼片绞碎后,加水500 mL,搅拌均匀后煮沸,冷却后过滤。将滤液4200 r/min离心30 min,取上清液。在上清液中加入0.1 mol/L磷酸缓冲液,调pH为6.6。每升鱼汁中加入1.6 g氧化三甲胺、40 mg L-半胱氨酸及40 mg L-甲硫氨酸,混合均匀后于121 ℃灭菌15 min。按照4–5 lg(CFU/mL)的接种量接种过夜培养的荧光假单胞菌野生型和突变株,以添加生理盐水的样品作为对照,4 ℃贮存1、2、3、4、5和6 d后取样测定菌落总数(TVC)和挥发性盐基氮(TVB-N)的生成量。菌落数采用稀释平板计数法进行。TVB-N含量测定参考Zhu等(2015)[28]的半微量定氮法测定,以mg/100 mL鱼汁来表示。
2 结果和分析 2.1 荧光假单胞菌rpoS基因缺失突变株的构建为研究RpoS在荧光假单胞菌致腐败过程中的作用,通过同源双交换的方法成功构建了荧光假单胞菌rpoS基因的框内缺失突变株ΔrpoS。rpoS基因完整的开放阅读框为1005 bp,PCR检测和测序分析证明突变株的rpoS基因开放阅读框缺失了705 bp的片段。
2.2 RpoS影响荧光假单胞菌对胁迫条件的耐受性克服各种环境不利因素的胁迫是食品腐败菌存活从而导致腐败的首要环节。本研究对野生型菌株和缺失突变株ΔrpoS在不同胁迫条件下的存活率进行了检测,这些胁迫条件分别为10 mmol/L H2O2、47 ℃、15%乙醇、150μg/mL结晶紫、175 mmol/L醋酸和20% NaCl。实验结果如图 1所示,rpoS缺失突变导致荧光假单胞菌对10 mmol/L H2O2和15%乙醇的耐受性显著降低,随着处理时间的延长,差异越来越明显,在处理45 min时,野生型株对H2O2的耐受性是突变株的42.4倍,对乙醇的耐受性是突变株的19.1倍。相反地,rpoS突变株对150μg/mL结晶紫和175 mmol/L醋酸的耐受性却有一定程度增强,但是不影响细菌对47℃和20% NaCl的耐受性。这一研究结果表明,在荧光假单胞菌中,RpoS也是调控多元胁迫耐受性的重要调节因子。

|
| 图 1 荧光假单胞菌野生型及突变株不同胁迫条件下存活率检测 Figure 1 Survival assay of P. fluorescens cells under different stress conditions. The wild-type strain (open circle) and in-frame deletion mutant ΔrpoS (closed diamond) were exposed to 10 mmol/L H2O2 (A), 15% ethanol (B), 175 mmol/L acetic acid (C), 150 μg/mL crystal violet (D), 47 ℃ (E) and 20% NaCl (F). The initial population of P. fluorescens were ca. 106–107 CFU/mL. Survival percentage was obtained by dividing the surviving population by the initial population, which corresponds to 100%. Data are mean±SD of three independent experiments. |
2.3 RpoS调节荧光假单胞菌群体感应信号分子AHL的合成
应用LC-MS/MS检测了野生型株及其突变株ΔrpoS的AHL信号分子(表 2)。根据保留时间和信号分子质谱检测过程中的特征性碎片,共鉴定出6种AHLs:C4-HSL (m/z 172),C6-HSL (m/z 200),C8-HSL (m/z 228),C10-HSL (m/z 256),C12-HSL (m/z 284)和C14-HSL (m/z 312)。其中C10-HSL、C12-HSL和C14-HSL三种长链信号分子在突变株中的生成量明显高于野生型,证明rpoS基因的缺失突变可导致长链信号分子的合成量升高,RpoS对于长链AHLs信号分子的合成具有调控作用。
| AHLs | Retention time/(min) | Precursor ion/(m/z) | Product ion/(m/z) | AHL concentration/(μg/L) | |
| Wild type | ΔrpoS | ||||
| C4-HSL | 0.78 | 172 | 102 | 18.0 | 12.7 |
| C6-HSL | 10.89 | 200 | 102 | 168.4 | 146.0 |
| C8-HSL | 12.32 | 228 | 102 | 203.0 | 217.2 |
| C10-HSL | 13.74 | 256 | 102 | 811.5 | 1495.3 |
| C12-HSL | 15.02 | 284 | 102 | 764.1 | 1211.7 |
| C14-HSL | 16.10 | 312 | 102 | 1085.2 | 2067.0 |
2.4 RpoS调节荧光假单胞菌对灭菌三文鱼汁的腐败作用
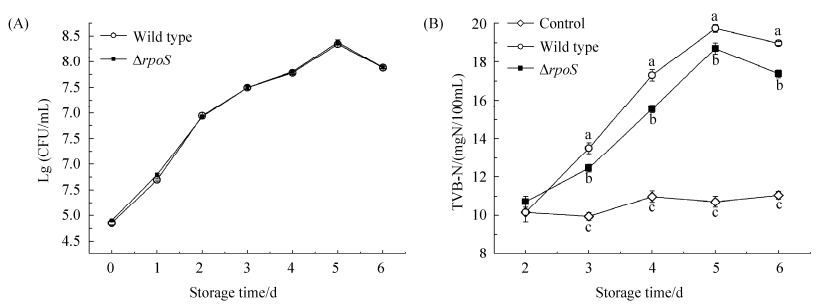
荧光假单胞菌是冷藏三文鱼的特定腐败菌[6],为了确定RpoS是否调节荧光假单胞菌对于三文鱼的腐败活性,其野生型株和rpoS突变株分别接种于4℃贮存的灭菌三文鱼汁中,比较在贮存过程中细菌的TVC和TVB-N的差异。结果表明,野生型和突变株在贮存过程中的生长曲线几乎完全重合,生长速度无显著差异(图 2-A)。TVB-N是评价水产品新鲜度的重要指标,野生型株和突变株接种于灭菌三文鱼汁后,TVB-N值在4℃贮存第2天无显著差异,但第3天开始突变株的TVB-N显著低于野生型(图 2-B,P < 0.05)。该结果表明rpoS缺失突变导致荧光假单胞菌对三文鱼汁的腐败能力降低,RpoS对于荧光假单胞菌的腐败能力存在重要的调控作用。
|
| 图 2 荧光假单胞菌野生型及突变株接种于灭菌三文鱼汁后4 ℃贮存过程中TVC (A)以及TVB-N (B)含量测定 Figure 2 Effect of RpoS on TVC (A) and TVB-N (B) of P. fluorescens in sterilized salmon muscle juice stored at 4 ℃. Data is expressed as means±standard deviations. Different letters at a given time indicate significant differences at P < 0.05 between the various conditions at that time. |
3 讨论
对胁迫环境的耐受性是食品腐败菌存活并且导致腐败的关键环节,研究调节胁迫耐受性的关键基因对于了解细菌的腐败机制具有重要意义。本研究结果显示rpoS基因缺失很大程度上降低了荧光假单胞菌对于10 mmol/L H2O2和15%乙醇的耐受性,但是对150 μg/mL结晶紫和175 mmol/L醋酸的耐受性反而有一定程度的增强,对47 ℃和20% NaCl的耐受性没有影响。这些结果表明在荧光假单胞菌中RpoS对于食品加工过程的胁迫条件具有重要的调控作用。RpoS在其他细菌中的研究也表明sigma S参与调节多种胁迫条件的耐受性,不过由于种属的差异性,RpoS参与调节的胁迫耐受性的种类往往存在多样性[13]。RpoS很可能是通过调节下游胁迫反应相关基因的表达从而实现对于胁迫耐受性的调控作用[29]。不少研究表明RpoS调节抗氧化蛋白、热激蛋白、细胞壁多糖等合成,从而影响细菌对于环境胁迫的耐受性[16, 30]。
rpoS基因缺失除了影响荧光假单胞菌的胁迫耐受性,还促进了胞外AHL信号分子的合成,表明RpoS对AHL群体感应的负调控作用。也有报道在恶臭假单胞菌(Pseudomonas putida)中的类似情况,rpoS基因缺失促使细菌的AHL信号分子的生成量增加[31]。在铜绿假单胞菌中,尽管有不少关于RpoS与群体感应系统的调控关系的文献报道,但是二者的关系仍然不很明确,多数报道显示群体感应直接或者间接调控rpoS基因的表达[16],但是也有报道称RpoS负调控群体感应基因rhlI,反过来RpoS却不受群体感应RhlR-RhlI调控[32]。群体感应参与调节微生物的多种生理过程,包括分泌胞外酶、嗜铁素及毒素,产生粘附因子、鞭毛及生物被膜等[33-34]。越来越多的证据显示细菌的群体感应参与食品腐败过程。温和气单胞菌(Aeromonas sobria)的AHL群体感应参与冷藏大菱鲆的腐败过程[19]。普城沙雷氏菌(Serratia plymuthica)的AHL群体感应信号参与胡萝卜片的腐败过程[35]。有研究发现从大黄鱼中分离的荧光假单胞菌能够通过分泌群体感应信号分子来抑制波罗的海希瓦氏菌(Shewanella baltica)生物被膜的产生和腐败能力[25]。因此,在荧光假单胞菌中RpoS通过调节AHL信号分子的合成,从而进一步调节腐败作用,但具体的调节机制还需进一步研究。
另外,检测了荧光假单胞菌野生型和rpoS基因缺失突变株在冷藏三文鱼汁中的腐败活性。研究结果表明rpoS基因缺失突变对于细菌在冷藏鱼汁中的生长没有影响,但是显著降低了TVB-N的生成量。TVB-N是评价水产品新鲜度的一个重要指标,该结果证明RpoS能够调控荧光假单胞菌对于水产品的腐败能力。TVB-N主要是氨基酸分解代谢的产物,RpoS很可能直接或者间接调节荧光假单胞菌的氮代谢相关基因的表达。在铜绿假单胞菌中,基因芯片研究结果表明RpoS调控大量氨基酸代谢相关基因的表达[16]。另外,群体感应也可调节细菌胞外蛋白酶的分泌。在类鼻疽伯克氏菌(Burkholderia pseudomallei)中,群体感应系统负向调控胞外蛋白酶和嗜铁素的分泌。在本研究中,荧光假单胞菌的RpoS也可能通过群体感应间接调控胞外蛋白酶的分泌,从而影响TVB-N的生成[34]。
综上所述,rpoS基因缺失不仅影响荧光假单胞菌对于食品加工及贮存过程中的胁迫耐受性,还调节群体感应信号分子AHL的合成以及在三文鱼汁中的腐败能力。RpoS对荧光假单胞菌这些腐败相关特性的调节证明它是一个重要的细菌腐败作用调节蛋白。本研究首次揭示了RpoS在调节细菌致腐败过程中的作用,对于靶向控制细菌腐败作用和探索高效水产品保鲜技术具有重要意义。
| [1] | Ghaly AE, Dave D, Budge S, Brooks MS. Fish spoilage mechanisms and preservation techniques: review. American Journal of Applied Sciences, 2010, 7(7): 859-877. DOI:10.3844/ajassp.2010.859.877 |
| [2] | Remenant B, Jaffrès E, Dousset X, Pilet MF, Zagorec M. Bacterial spoilers of food: behavior, fitness and functional properties. Food Microbiology, 2015, 45(Pt A): 45-53. |
| [3] | Shen Q, Yang Q, Cheung HY. Hydrophilic interaction chromatography based solid-phase extraction and MALDI TOF mass spectrometry for revealing the influence of Pseudomonas fluorescens on phospholipids in salmon fillet. Analytical and Bioanalytical Chemistry, 2015, 407(5): 1475-1484. DOI:10.1007/s00216-014-8365-8 |
| [4] | Olanya OM, Ukuku DO, Niemira BA. Effects of temperatures and storage time on resting populations of Escherichia coli O157: H7 and Pseudomonas fluorescens in vitro. Food Control, 2014, 39: 128-134. DOI:10.1016/j.foodcont.2013.11.006 |
| [5] | Andreani NA, Carraro L, Martino ME, Fondi M, Fasolato L, Miotto G, Magro M, Vianello F, Cardazzo B. A genomic and transcriptomic approach to investigate the blue pigment phenotype in Pseudomonas fluorescens. International Journal of Food Microbiology, 2015, 213: 88-98. DOI:10.1016/j.ijfoodmicro.2015.05.024 |
| [6] |
Li TT, Ding T, Zou ZY, Zhou K, Yi SM, Li JR. Differential prevalence of spoilage bacteria in salmon fillets during refrigerated storage and identification of predominant spoilage bacterial species. Modern Food Science and Technology, 2015, 31(4): 36-41.
(in Chinese) 李婷婷, 丁婷, 邹朝阳, 周凯, 仪淑敏, 励建荣. 0 ℃冷藏下三文鱼片菌相变化规律及特定腐败菌的分离鉴定. 现代食品科技, 2015, 31(4): 36-41. |
| [7] |
Guo QY, Xu Z, Yang XS. Identification and growth dynamics of specific spoilage organisms in chilled tilapia. Progress in Fishery Sciences, 2009, 30(4): 117-123.
(in Chinese) 郭全友, 许钟, 杨宪时. 冷藏罗非鱼特定腐败菌的鉴定和生长动力学. 渔业科学进展, 2009, 30(4): 117-123. DOI:10.3969/j.issn.1000-7075.2009.04.018 |
| [8] |
Cui FC, Li TT, Liu MS, Ma Y, Li JR. Quorum-sensing phenomenon in Pseudomonas fluorescens isolated from turbot and spoilage characteristics using different carbon sources. Modern Food Science and Technology, 2015, 31(12): 49-55.
(in Chinese) 崔方超, 李婷婷, 刘明爽, 马燕, 励建荣. 大菱鲆荧光假单胞菌的群体感应现象及不同碳源培养下的腐败特性研究. 现代食品科技, 2015, 31(12): 49-55. |
| [9] | Maull KD, Hickey ME, Lee JL. The study and identification of bacterial spoilage species isolated from catfish during refrigerated storage. Journal of Food Processing and Technology, 2012: S11-003. DOI:10.4172/2157-7110.S11-003 |
| [10] | Dodd CER, Aldsworth TG. The importance of RpoS in the survival of bacteria through food processing. International Journal of Food Microbiology, 2002, 74(3): 189-194. DOI:10.1016/S0168-1605(01)00679-1 |
| [11] | Landini P, Egli T, Wolf J, Lacour S. sigmaS, a major player in the response to environmental stresses in Escherichia coli: role, regulation and mechanisms of promoter recognition. Environmental Microbiology Reports, 2014, 6(1): 1-13. DOI:10.1111/1758-2229.12112 |
| [12] | Schellhorn HE. Elucidating the function of the RpoS regulon. Future Microbiology, 2014, 9(4): 497-507. DOI:10.2217/fmb.14.9 |
| [13] | Dong T, Schellhorn HE. Role of RpoS in virulence of pathogens. Infection and Immunity, 2010, 78(3): 887-897. DOI:10.1128/IAI.00882-09 |
| [14] | Asfahl KL, Schuster M. Social interactions in bacterial cell-cell signaling. FEMS Microbiology Reviews, 2017, 41(1): 92-107. DOI:10.1093/femsre/fuw038 |
| [15] | Papenfort K, Bassler BL. Quorum sensing signal-response systems in Gram-negative bacteria. Nature Reviews Microbiology, 2016, 14(9): 576-588. DOI:10.1038/nrmicro.2016.89 |
| [16] | Schuster M, Hawkins AC, Harwood CS, Greenberg EP. The Pseudomonas aeruginosa RpoS regulon and its relationship to quorum sensing. Molecular Microbiology, 2004, 51(4): 973-985. DOI:10.1046/j.1365-2958.2003.03886.x |
| [17] | Skandamis PN, Nychas GJE. Quorum sensing in the context of food microbiology. Applied and Environmental Microbiology, 2012, 78(16): 5473-5482. DOI:10.1128/AEM.00468-12 |
| [18] | Zhu JL, Zhao AF, Feng LF, Gao HC. Quorum sensing signals affect spoilage of refrigerated large yellow croaker (Pseudosciaena crocea) by Shewanella baltica. International Journal of Food Microbiology, 2016, 217: 146-155. DOI:10.1016/j.ijfoodmicro.2015.10.020 |
| [19] | Li TT, Cui FC, Bai FL, Zhao GH, Li JR. Involvement of acylated homoserine lactones (AHLs) of Aeromonas sobria in spoilage of refrigerated Turbot (Scophthalmus maximus L.). Sensors, 2016, 16(7): 1083. DOI:10.3390/s16071083 |
| [20] | Gui M, Wu RY, Liu L, Wang S, Zhang L, Li PL. Effects of quorum quenching by AHL lactonase on AHLs, protease, motility and proteome patterns in Aeromonas veronii LP-11. International Journal of Food Microbiology, 2017, 252: 61-68. DOI:10.1016/j.ijfoodmicro.2017.04.005 |
| [21] | Liu XX, Shen BM, Du P, Wang N, Wang JX, Li JR, Sun AH. Transcriptomic analysis of the response of Pseudomonas fluorescens to epigallocatechin gallate by RNA-seq. PLoS One, 2017, 12(5): e0177938. DOI:10.1371/journal.pone.0177938 |
| [22] | Luo P, He XY, Liu QT, Hu CQ. Developing universal genetic tools for rapid and efficient deletion mutation in Vibrio species based on suicide T-vectors carrying a novel counterselectable marker, vmi480. PLoS One, 2015, 10(12): e0144465. DOI:10.1371/journal.pone.0144465 |
| [23] | Demarre G, Guérout AM, Matsumoto-Mashimo C, Rowe-Magnus DA, Marlière P, Mazel D. A new family of mobilizable suicide plasmids based on broad host range R388 plasmid (IncW) and RP4 plasmid (IncPα) conjugative machineries and their cognate Escherichia coli host strains. Research in Microbiology, 2005, 156(2): 245-255. DOI:10.1016/j.resmic.2004.09.007 |
| [24] | Liu XX, Li JR, Yang Y, Chen XQ. Exposure of Pseudomonas aeruginosa to green tea polyphenols enhances the tolerance to various environmental stresses. World Journal of Microbiology and Biotechnology, 2012, 28(12): 3373-3380. DOI:10.1007/s11274-012-1149-4 |
| [25] |
Qi GH, Dong MS, Chen XH, Jiang M. Research on relationship between quorum sensing signal molecules and characteristics of spoilage in Pseudomonas spp. isolated from fish. Scientia Agricultura Sinica, 2007, 40(7): 1486-1491.
(in Chinese) 綦国红, 董明盛, 陈晓红, 姜梅. 鱼源假单胞菌群体感应信号分子与腐败特性相关关系的研究. 中国农业科学, 2007, 40(7): 1486-1491. DOI:10.3321/j.issn:0578-1752.2007.07.024 |
| [26] | Zhao AF, Zhu JL, Ye XF, Ge YY, Li JR. Inhibition of biofilm development and spoilage potential of Shewanella baltica by quorum sensing signal in cell-free supernatant from Pseudomonas fluorescens. International Journal of Food Microbiology, 2016, 230: 73-80. DOI:10.1016/j.ijfoodmicro.2016.04.015 |
| [27] | Dalgaard P. Qualitative and quantitative characterization of spoilage bacteria from packed fish. International Journal of Food Microbiology, 1995, 26(3): 319-333. DOI:10.1016/0168-1605(94)00137-U |
| [28] | Zhu SQ, Wu HH, Zeng MY, Liu ZY, Wang Y. The involvement of bacterial quorum sensing in the spoilage of refrigerated Litopenaeus vannamei. International Journal of Food Microbiology, 2015, 192: 26-33. DOI:10.1016/j.ijfoodmicro.2014.09.029 |
| [29] | Guan JY, Xiao X, Xu SJ, Gao F, Wang JB, Wang TT, Song YH, Pan JF, Shen XH, Wang Y. Roles of RpoS in Yersinia pseudotuberculosis stress survival, motility, biofilm formation and type Ⅵ secretion system expression. Journal of Microbiology, 2015, 53(9): 633-642. DOI:10.1007/s12275-015-0099-6 |
| [30] | Osiriphun Y, Wongtrakoongate P, Sanongkiet S, Suriyaphol P, Thongboonkerd V, Tungpradabkul S. Identification and characterization of RpoS regulon and RpoS-dependent promoters in Burkholderia pseudomallei. Journal of Proteome Research, 2009, 8(6): 3118-3131. DOI:10.1021/pr900066h |
| [31] | Bertani I, Venturi V. Regulation of the N-acyl homoserine lactone-dependent quorum-sensing system in rhizosphere Pseudomonas putida WCS358 and cross-talk with the stationary-phase RpoS sigma factor and the global regulator GacA. Applied and Environmental Microbiology, 2004, 70(9): 5493-5502. DOI:10.1128/AEM.70.9.5493-5502.2004 |
| [32] | Kayama S, Murakami K, Ono T, Ushimaru M, Yamamoto A, Hirota K, Miyake Y. The role of rpoS gene and quorum-sensing system in ofloxacin tolerance in Pseudomonas aeruginosa. FEMS Microbiology Letters, 2009, 298(2): 184-192. DOI:10.1111/fml.2009.298.issue-2 |
| [33] | Antunes LCM, Ferreira RBR, Buckner MMC, Finlay BB. Quorum sensing in bacterial virulence. Microbiology, 2010, 156(8): 2271-2282. DOI:10.1099/mic.0.038794-0 |
| [34] | Wongtrakoongate P, Tumapa S, Tungpradabkul S. Regulation of a quorum sensing system by stationary phase sigma factor RpoS and their co-regulation of target genes in Burkholderia pseudomallei. Microbiology and Immunology, 2012, 56(5): 281-294. DOI:10.1111/mim.2012.56.issue-5 |
| [35] | Wevers E, Moons P, van Houdt R, Lurquin I, Aertsen A, Michiels CW. Quorum sensing and butanediol fermentation affect colonization and spoilage of carrot slices by Serratia plymuthica. International Journal of Food Microbiology, 2009, 134(1/2): 63-69. |
 2018, Vol. 58
2018, Vol. 58