中国科学院微生物研究所,中国微生物学会,中国菌物学会
文章信息
- 杨阳阳, 蔡璐璐, 邹丽芳, 陈晓斌, 陈功友. 2018
- Yangyang Yang, Lulu Cai, Lifang Zou, Xiaobin Chen, Gongyou Chen. 2018
- 水稻条斑病菌pilT基因在致病性中的功能分析
- Roles of pilT gene in pathogenicity of Xanthomonas oryzae pv.oryzicola on rice
- 微生物学报, 58(5): 773-783
- Acta Microbiologica Sinica, 58(5): 773-783
-
文章历史
- 收稿日期:2017-05-16
- 修回日期:2017-06-14
- 网络出版日期:2017-07-21
Gongyou Chen, gyouchen@sjtu.edu.cn
水稻条斑病菌(Xanthomonas oryzae pv. oryzicola,Xoc)是水稻稻黄单胞菌种下致病变种之一,引起水稻细菌性条斑病(bacterial leaf streak,BLS),近年来该病发生日趋严重,成为水稻的第四大病害[1-2]。该病原菌从水稻叶片的气孔或伤口侵入,在薄壁细胞间繁殖与危害,因受叶脉限制产生条斑病症状。随着高产杂交水稻品系的大面积推广,以及生产上无有效的抗条斑病种质资源,使水稻安全生产受到严重的威胁。Xoc能够成功侵染其寄主水稻,主要依靠胞外酶类(extracellular enzymes)、胞外多糖(exoplysaccharides,EPS)、生物膜(biofilm)、鞭毛(flagellum)以及Ⅲ型效应蛋白(type Ⅲ secretion system effectors,T3SEs)等毒性因子[3]的帮助。
Type IV pili (T4P)是革兰氏阴性菌产生的直径为5–8 nm、长约为20 μm的长纤维复合体,是细菌表面的毛状附属物[4]。Ralstonia solanacearum、Xylella fastidiosa、Acidovorax citrullih和Pseudomonas species等细菌都能产生T4P。数十个基因参与T4P的合成和调节,大多数被命名为pil/fim基因。革兰氏阴性菌的T4P参与细菌活动,如蹭行运动(twitching motility)、生物膜的形成、宿主表面粘附及定殖、细菌毒性、DNA摄取及蛋白分泌等,这些细菌活动都需要具有ATP酶活性的PilT、PilU等蛋白水解ATP提供能量[5]。T4P在同源六聚体环PilT蛋白水解ATP作用下收缩以发生颤动运动、吸附以及细胞间信息的传递。PilT属于ATP酶家族的成员,具有核苷酸结合模体,与T4P的合成有关。PilT紧贴细胞内膜上,与T4P相连,是T4P收缩所必需的分子马达。PilT是六聚体ATP酶,通过分解ATP提供给纤毛运动的能量,从而保证纤毛在固体表面上运动,同时PilT还有协助纤毛组装的作用。在Pseudomonas aeruginosa中,静态条件下,pilT突变体相比野生型可以形成更加密集的生物膜,揭示了pilT基因与生物膜形成相关[6]。在Geobacter sulfurreducens中,PilT具有ATP酶活性,能够为细胞外电子转移提供能量[7]。研究表明,Ⅳ型菌毛及Twitching motility影响P. aeruginosa的致病性,丧失Twitching motility或Ⅳ型菌毛的突变体致病性丧失或减弱。Twitching motility还参与生物被膜的形成,而形成生物被膜则是难以根治铜绿假单胞菌感染的最主要的根源[8]。
T4P及其在动物致病菌中的致病性已有很多研究报道[9],但T4P在植物病原菌中的作用很少涉及,近几年人们开始关注这些表面附属物与植物细菌病原物毒力的关系。PilT作为形成Ⅳ型菌毛过程中重要的亚基之一,对于揭示Ⅳ型菌毛在Xoc中的作用具有重要意义。
1 材料和方法 1.1 材料 1.1.1 菌株和质粒::菌株和质粒的特性见表 1。大肠杆菌(Escherichia coli)于LB培养基中37 ℃培养,Xoc菌株于NA或NB培养基中28 ℃培养。NY培养基用于游动性测定,XOM3培养基用于hrpG基因等的诱导表达[10]。生长所需抗生素浓度:氨苄青霉素(Ap) 100 μg/mL,卡那霉素(Km) 20 μg/mL,利福平(Rif) 75 μg/mL。
| Strain and plasmid | Relevant characteristics | Source |
| X.oryzae pv. oryzicola | ||
| RS105 | Wild-type, Chinese race 2; Rifr | This lab |
| RΔpilT | pilT deletion mutant of RS105; Rifr | This study |
| CRΔpilT | RΔpilT containing a functional pilT gene; Rifr Kmr | This study |
| RΔhrcV | hrcV deletion mutant of RS105; Rifr | This lab |
| E. coli | ||
| DH5α | φ90, lacZΔm15, recA1, host of pHM1 | Invitrogen |
| Plasmids | ||
| pUFR034 | IncW, Mob(p), Mob+, LacZa+, PK2 replicon, Kmr | This lab |
| pKMS1 | Suicidal vector for deletion mutant; mob, oriV, sacB, Kmr | This lab |
| pKΔpilT | A fusion ligated in pKMS1 for pilT deletion mutant; Kmr | This study |
| pUFR-pilT | The full length of pilT gene in pUFR034; Kmr | This study |
1.1.2 供试植物材料::
烟草品种为本氏烟,水稻品种为感病品种IR24,本实验室保存,种植于上海交通大学农业与生物学院温室。烟草生长4–6周时用于HR反应测定;水稻生长2–3周时用于水渍症状测定,生长2个月大小时用于病斑长度测定。
1.1.3 主要试剂和仪器::Xoc基因组DNA提取试剂盒、质粒提取试剂盒购于Axygen公司,总RNA提取试剂盒、反转录试剂盒购于TransGen公司,实验中所用的限制性内切酶、修饰酶购于TaKaRa公司。冷冻离心机、PCR仪和分光光度计购于Eppendorf公司,凝胶成像系统购于Bio-Rad公司。
1.1.4 引物::表 2为本研究中所用的引物,根据GenBank登录的基因序列用Primer 5.0软件设计,引物由上海捷瑞生物公司合成。
| Primer | Sequence 5′→3′ (restriction sites underlined) | Description |
| upF | TCCCCCGGGAATACGGCCACATCCTCACC | A 700 bp upstream fragment of pilT gene |
| upR | CGGGATCCGGCGTGCTCCTCTGGAGCAG | |
| downF | CGGGATCCGCGCGTCGGCAGGCATGCAG | A 500 bp downstream fragment of pilT gene |
| downR | GCTCTAGAGTAGTGCAGCAGAAAGGCCA | |
| pilT-F | AATACGGCCACATCCTCACC | Primers to identify the pilT mutant |
| pilT-R | GCAGCGCGTAGTGCAGCAGA | |
| pilT com-F | CGGGATCCATGAGCACCATCGACTTCAC | A 1131 bp complementary fragment of pilT gene |
| pilT com-R | GGATCCTTACAGATCTTCTTCAGAAATAAGTTTTTGTTCTCGAACTTCGGAAATCTCCAC | |
| gyrb rRNA-F | CGGCACTTACGACTCCAGCAAG | A 121 bp gyrb rRNA gene fragment for qPCR |
| gyrb rRNA-R | CGACCAGGATTTTCACCACGATG | |
| rpoD-F | CGACAACACCACCAACATCAATC | A 140 bp rpoD gene fragment for qPCR |
| rpoD-R | GCTTACCGACCTCTTCCAACG | |
| hrpG-F | GTTGCTCCGCGACGAAAATAC | A 199 bp hrpG gene fragment for qPCR |
| hrpG-R | CTTGCGCAGCTTGTAGATATG | |
| hrpX-F | GGCGAHGTTGTCTTHGCTC | A 209 bp hrpX gene fragment for qPCR |
| hrpX-R | GACCTCATCGTCGGCTCCATC | |
| hrcC-F | TGGCAATCGCCGAAACGGTATCCAC | A 200 bp hrcC gene fragment for qPCR |
| hrcC-R | CATGCTCAGGATCTGGGGTGCAA | |
| rpfG-F | CGACAACACCACCAACATCAATC | A 164 bp rpfG gene fragment for qPCR |
| rpfG-R | GCTTACCGACCTCTTCCAACG | |
| clp-F | CGACAACACCACCAACATCAATC | A 167 bp clp gene fragment for qPCR |
| clp-R | GCTTACCGACCTCTTCCAACG | |
| pilA-F | TCGGTGGAAGTTGGGTATCG | A 130 bp pilA gene fragment for qPCR |
| pilA-R | GGTATCGAAGCGCTCATCCA | |
| pilC-F | AAGGGACATAGATGGGTGCG | A 110 bp pilC gene fragment for qPCR |
| pilC-R | CGAGCCTAAGGTCAATCCCA | |
| pilT-F | CGACAACACCACCAACATCAATC | A 154 bp pilT gene fragment for qPCR |
| pilT-R | GCTTACCGACCTCTTCCAACG |
1.2 pilT基因敲除突变体的构建和分子验证
本实验通过无标记双交换敲除的方法[11],以RS105 gDNA为模板,利用表 2中的引物pilT-up-F/R和pilT-down-F/R,扩增目的基因的上下游同源片段,上下游同源片段经BamHI单酶切后连接,连接后的片段经Sma I和Xba I双酶切消化处理后,再连接在经相同酶切处理的pKMS1载体上,将重组质粒转化进入DH5α菌株中,LB+Km平板上挑取单克隆测序。将测序正确的重组质粒通过电转化方式导入RS105感受态细胞中,涂布在含有Km+Rif的NAN (NA中不含有蔗糖)平板上。28 ℃培养3–5 d后得到单交换子,将单交换子接种到NAN液体培养基中培养12 h后,取100 μL涂布到NAS (NA中含有10%蔗糖)平板上,28 ℃培养3–5 d后,挑取单菌落分别于NA+Rif和NA+Km+Rif的平板上生长,能在NA+Rif平板上生长但不能在NA+Km+Rif平板上生长的单菌落,即为敲除突变体,命名为RΔpilT。
获得的突变体经PCR和Southern杂交验证。用pilT-up-F和pilT-down-R为引物,以敲除突变体基因组DNA为模板进行PCR扩增。pilT敲除突变体产生1200 bp的DNA片段,比野生型小1131 bp。经PCR筛选验证的突变体用Southern杂交验证。将突变体RΔpilT和野生型RS105菌株的基因组DNA用Not I和Sma Ⅰ酶切处理,同时将pilT基因上游700 bp片段标记成地高辛探针,按照Southern杂交的方法进行杂交,探针的标记、杂交和检测过程参照Roche的DIG试剂盒说明书。
1.3 pilT基因功能互补子的构建利用引物pilT com-F和pilT com-R,以RS105的基因组DNA为模板,PCR扩增得到1131 bp DNA片段,经BamH Ⅰ酶切消化处理后,连接在经相同酶切处理的pUFR034载体上,获得重组质粒pUFR-pilT。经酶切及测序验证后,将该质粒通过电转化的方法导入敲除突变体RΔpilT中,挑取单克隆,经Western杂交[12]验证正确后,获得功能互补子CRΔpilT。
1.4 水稻上致病性和烟草上过敏反应测定待测菌株单菌落接种于含有相应抗生素的NB培养基中,28 ℃振荡培养24 h后,取一定比例菌液转接入新鲜的NB培养基中,继续培养12 h后,收集菌体,无菌水洗涤1次,调整菌液浓度为OD600=2.0,针刺接种于生长2个月的IR24水稻叶片,15 d后观察病斑扩展情况,并统计病斑长度。同样,调整菌液浓度为OD600=0.6,用无针头的注射器注射接种于生长状况良好的IR24水稻幼苗叶片上,3 d后观察叶片上水渍状病斑(water soaking,WS)的产生情况。
将上述菌液调整OD600=0.1,同样用无针头的注射器注射接种于生长4–6周的本氏烟叶片上,24 h内观察过敏性反应产生情况。上述实验重复3次,每次处理5片叶。
1.5 细菌游动性检测按照1.4中的方法准备待测菌株。调整菌液浓度为OD600=0.5,用移液器取2 μL待测菌液,接种于含有0.5%葡萄糖的NY半固体培养基上,水平放置,28 ℃培养3–4 d后,观察细菌的游动圈,每个样品设置3个重复,统计细菌游动圈的直径,并计算平均值[13]。
1.6 细菌生物膜检测采取两种方法进行细菌生物膜测定实验。取浓度OD600=2.0的待测菌株5 mL于试管中,室温静置3 d,用移液枪将试管内的菌液吸出,用等体积的无菌水洗涤试管2次,加入6 mL结晶紫,染色30 min。将结晶紫吸出,用等体积的无菌水洗涤试管3次;于37 ℃将试管烘干,观察试管周围的紫色圈,即为生物膜。每个菌株设置3个重复[14]。
取浓度OD600=0.5的待测菌液200 μL于96孔板中,28 ℃培养12 h,将菌液弃去,无菌水洗1次,加入210 μL 1%的结晶紫,染色15–20 min后弃去结晶紫,无菌水洗2次,加入210 μL无水乙醇溶解后,用酶标仪读取数值。
1.7 Real-time PCR突变体和野生型菌株单菌落接种于含有相应抗生素的NB培养基中,28 ℃振荡培养24 h后,取一定比例菌液转接入新鲜的NB培养基中,继续培养12 h后,收集菌体,XOM3洗涤沉淀1次后,调整菌液OD600=1.0;XOM3诱导培养3 h后,按照TransGen公司总RNA提取试剂盒说明书提取细菌总RNA,经无RNase的DNase I充分消化,去除gDNA的污染,之后反转录成cDNA;用表 2中的引物检测hrpG、rpfG、clp、pilA等基因的表达水平。
2 结果和分析 2.1 水稻条斑病菌pilT基因缺失突变体及功能互补子的构建为了研究Xoc RS105菌株中pilT基因的功能,本研究利用带有反向筛选标记的自杀载体pKMS1,对pilT基因进行无标记敲除。以RS105的gDNA为模板,通过引物对pilT-up-F/R和pilT-down-F/R,分别扩增出pilT基因上下游同源臂pilT up和pilT down (图 1-A)。将同源臂构建在自杀载体pKMS1上,获得重组质粒pK∆pilT。通过电转化,将重组质粒导入RS105菌株中,经过2次同源臂交换,获得pilT基因敲除突变体RΔpilT。

|
| 图 1 Xoc pilT突变体的构建及分子验证 Figure 1 The construction of Xoc pilT mutant and molecular analysis. Schematic diagram about the construction of the Xoc pilT mutant. A: Black region stands for the left arm of the pilT gene locus (white region) and the grey presents the right arm. The left and right arms were fused in pKMS1 and the knock-out mutant was obtained after two steps recombination occurred; B: The pilT mutants were verified by PCR; C: The pilT mutants were verified by Southern blotting; D: The complementation of pilT mutant was verified by Western blotting. |
用pilT-up-F和pilT-down-R对野生型和突变体基因组DNA进行PCR扩增,野生型获得预期2331 bp大小的DNA条带,突变体因缺失1131 bp而得到1200 bp大小的条带(图 1-B)。Southern杂交结果显示:与野生型相比,突变体缺失1个1131 bp的信号(图 1-C)。PCR和Southern杂交结果证实,pilT缺失突变体RΔpilT构建正确。同时,PCR扩增pilT基因全长并构建在pUFR034载体上,导入RΔpilT突变体,进行功能互补,命名为CRΔpilT。CRΔpilT经Western杂交验证正确后进行后面的实验研究(图 1-D)。
2.2 pilT基因是水稻条斑病菌Xoc菌株在寄主水稻上的全毒性所必需的为了检测R∆pilT突变体在水稻上的毒性,我们分别在水稻幼苗期和成株期进行了毒性测定实验。针刺接种成株期感病水稻品种IR24,2周后观察病斑形成情况。野生型RS105菌株在针刺点上下扩展形成较长的条斑症状;而突变体R∆pilT仅在针刺部位形成条斑症状,并不扩展;功能互补子CR∆pilT与野生型菌株症状相同(图 2-A)。同时将这3个菌株注射接种3周龄的感病水稻品种IR24,3 d后观察水稻叶片上水渍状病斑的形成情况,发现野生型RS105菌株、突变体R∆pilT和功能互补子CR∆pilT在水稻上均有致病性,形成水渍状病斑,但4–5 d后观察发现野生型RS105菌株和功能互补子CR∆pilT能够上下扩展,形成明显的条斑症状,而突变体R∆pilT仅在注射点形成水渍状病斑,不能上下扩展(图 2-C)。这些结果表明,pilT基因缺失并未使Xoc丧失在寄主水稻上的致病性,只是显著削弱了病原菌在水稻上的毒性,pilT基因是Xoc在水稻全毒性上所必需的。非寄主烟草上HR测定结果显示,突变体R∆pilT激发烟草产生HR的能力减弱(图 2-D)。

|
| 图 2 Xoc pilT突变体在水稻和烟草上的反应 Figure 2 Responses of tobacco and rice to Xoc pilT mutant. A: Symptoms caused by RS105, R∆pilT and CR∆pilT (OD600=0.3; approximately 1×108 CFU/mL) on inoculated leaves of the host rice IR24 by leaf-needling inoculation. The photograph was taken 15 d after infiltration; B: Lesion lengths of rice bacterial streak caused by Xoc strains in rice. The lesion lengths were measured at 15 d post-inoculation. Data are the means standard deviation from three replications. (P < 0.05, student's t test); C: Water-soaked symptoms on leaves of the rice IR24. The photographs were taken 4 d post-inoculation with Xoc strains (1×108 CFU/mL) using needleless syringes; D: Induction of the hypersensitive response (HR) on the leaves of non-host tobacco by RS105, R∆pilT and CR∆pilT. The photograph was taken 24 h after infiltration. |
2.3 pilT基因影响水稻条斑病菌Xoc菌株的游动性
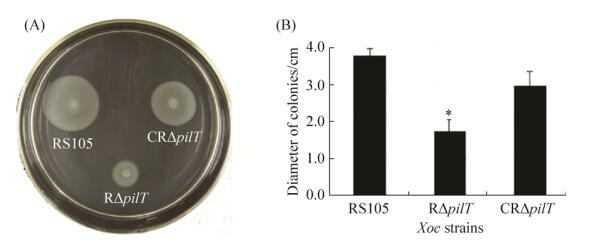
植物病原细菌的游动性能够使病原菌获得更多更好的营养物质,逃避不利的寄主环境,与毒性呈正相关。为了检测pilT突变体对Xoc菌株游动性的影响,本研究检测了野生型RS105菌株、突变体R∆pilT以及功能互补子CR∆pilT的游动性。实验发现,在半固体NY培养基上,与野生型RS105菌株相比,R∆pilT突变体的游动性显著下降,功能互补子CR∆pilT的游动能力部分回补(图 3-A)。菌落扩散后的直径统计显示,突变体的游动性较野生型下降了50%,互补菌株恢复至野生型水平的80% (图 3-B)。该结果说明,pilT基因影响Xoc菌株的游动性。
|
| 图 3 Xoc菌株游动性测定 Figure 3 Determination of motility of Xoc strains. A: Cell were inoculated onto NY supplemented 0.5% glucose plates and photographed after 3–4 d of incubation at 28 ℃; B: Data are the means standard deviation from three replications. (P < 0.05, student's t test). |
2.4 pilT基因影响水稻条斑病菌Xoc菌株的生物膜形成
R∆pilT突变体致病性降低的同时,也会对生物膜的形成产生一定的影响,因此本研究检测了pilT缺失突变体生物膜的形成情况。玻璃试管表面的附着生长实验显示,R∆pilT突变体形成的生物膜明显多于野生型RS105以及功能互补子CR∆pilT,其中功能互补子CR∆pilT形成的生物膜略多于野生型RS105菌株(图 4-A)。96孔板方法测定生物膜形成情况的实验中,定量检测了Xoc菌株生物膜的形成,R∆pilT突变体形成的生物膜明显多于野生型RS105以及功能互补子CR∆pilT,功能互补子CR∆pilT部分回补形成的生物膜略多于野生型RS105菌株(图 4-B)。实验结果说明,pilT基因影响Xoc菌株的生物膜形成。

|
| 图 4 Xoc菌株生物膜形成测定 Figure 4 Determination of biofilm formation of Xoc strains. A: Representative photographs of biofilm formation assay for RS105, R∆pilT and CR∆pilT strains grown statically in glass tubes; B: Data are the means standard deviation from three replications. (P < 0.05, student's t test). |
2.5 pilT基因影响Xoc菌株相关基因的表达
为了进一步解析pilT基因突变影响Xoc毒性的分子机理,本研究使用qRT-PCR来检测野生型RS105菌株和突变体R∆pilT中主要毒性相关基因的表达情况。水稻条斑病菌hrpG和hrpX两个基因主要调控hrp基因的表达,决定病原菌在寄主植物上的致病性,而hrcC与三型分泌系统(type Ⅲ secretion system,T3SS)的形成相关,同样影响菌株的毒性。实验结果显示,与野生型菌株RS105相比,hrpG、hrpX和hrcC的表达水平在突变体R∆pilT中显著降低(图 5)。这表明,pilT突变体在寄主水稻上致病性降低与hrpG、hrpX和hrcC的转录表达降低有关。在突变体R∆pilT中,clp、rpfG、pilA和pilC的表达量同样明显低于在野生型菌株RS105中的表达量(图 5)。已有研究指出,Xoc rpfG突变体显著影响DSF信号分子的生物合成水平,改变Xoc细胞的群体形态,生物膜聚集,而pilA和pilC与菌体的游动性有关。以上实验结果表明,pilT基因通过影响水稻条斑病菌Xoc相关基因的表达,从而影响毒性、游动性以及生物膜的合成等相关表型。

|
| 图 5 pilT影响相关基因的表达 Figure 5 Relative genes expression levels in RΔpilT and RS105 strain. Similar results were observed in two independent experiments with three replicates. Asterisk indicates statistically significant differences between the WT and mutants. (P < 0.05, student's t test). |
3 讨论
本实验中,为了研究Xoc RS105菌株中pilT基因的功能,我们通过无标记敲除方式[11]构建了pilT基因的缺失突变体RΔpilT。研究发现,与野生型RS105菌株相比,RΔpilT在感病水稻上的致病性显著降低,功能互补子CR∆pilT在水稻上的毒性恢复至野生型水平(图 2)。
研究pilT基因在病原菌致病性中的功能时,我们发现与野生型RS105菌株相比,突变体菌株R∆pilT在半固体NY培养基上的游动能力显著降低,功能互补子CR∆pilT游动能力部分回补(图 3);R∆pilT形成的生物膜明显多于野生型RS105菌株,功能互补子CR∆pilT部分恢复至野生型水平(图 4)。植物病原细菌的游动性和生物膜的产量都与病原菌的毒性相关,影响其在寄主水稻上的致病性[15]。qRT-PCR实验发现,pilA和pilC的基因的转录表达水平在突变体R∆pilT中显著降低,PilA是构成T4P的主要亚基,PilT能够为PilA蛋白的组装与拆卸提供能量[16]。在本研究中,pilT突变体能够继续形成T4P,但该突变体缺乏提供菌毛回缩和游动所需的ATP能量,导致游动性降低。同样,c-di-GMP与病原菌的游动性和生物膜合成相关,当菌体内c-di-GMP含量升高时,细菌的游动性明显减弱,而菌体内c-di-GMP的含量是受rpfG调控的,RpfG可以降解c-di-GMP[17]。在突变体R∆pilT中,rpfG的表达量明显降低,导致菌体内的c-di-GMP含量升高,细菌游动性降低,群体形态发生改变,突变体形成更加致密的生物膜[18-19]。这些结果表明,突变体R∆pilT可能通过影响游动性、生物膜形成进而影响病原菌的致病性。
在进一步解析pilT基因影响Xoc菌株毒性的分子机理时,我们利用qRT-PCR检测了野生型RS105菌株和突变体R∆pilT中主要毒性相关基因的表达情况。结果显示,与hrp基因表达调控相关的两个主要调控因子hrpG和hrpX基因的表达量明显降低,并且与T3SS形成相关的hrcC基因表达同样明显降低(图 5),这3个基因都是水稻条斑病菌全毒性所必需的基因[20-21]。由此我们推断,pilT基因主要通过影响hrp基因表达导致其在水稻上致病性降低。对于pilT基因如何影响hrp基因的表达以及在T4P形成中的作用机制,有待进一步探讨。
| [1] | Zou LF, Wang XP, Xiang Y, Zhang B, Li YR, Xiao YL, Wang JS, Walmsley AR, Chen GY. Elucidation of the hrp clusters of Xanthomonas oryzae pv. oryzicola that control the hypersensitive response in nonhost tobacco and pathogenicity in susceptible host rice. Applied and Environmental Microbiology, 2006, 72(9): 6212-6224. DOI:10.1128/AEM.00511-06 |
| [2] |
Pei JG, Zou LF, Zou HS, Chen GY. Functional analysis of xopQ1Xocgene from Xanthomonas oryzae pv. oryzicola in pathogenesis on rice. Scientia Agricultura Sinica, 2010, 43(17): 3538-3546.
(in Chinese) 裴俊国, 邹丽芳, 邹华松, 陈功友. 水稻条斑病菌xopQ1Xoc在病程中功能的初步研究. 中国农业科学, 2010, 43(17): 3538-3546. DOI:10.3864/j.issn.0578-1752.2010.17.007 |
| [3] | Büttner D, Bonas U. Regulation and secretion of Xanthomonas virulence factors. FEMS Microbiology Reviews, 2010, 34(2): 107-133. DOI:10.1111/j.1574-6976.2009.00192.x |
| [4] | Mattick JS. Type IV pili and twitching motility. Annual Review of Microbiology, 2002, 56: 289-314. DOI:10.1146/annurev.micro.56.012302.160938 |
| [5] | Craig L, Pique ME, Tainer JA. Type IV pilus structure and bacterial pathogenicity. Nature Reviews Microbiology, 2004, 2(5): 363-378. DOI:10.1038/nrmicro885 |
| [6] | Chiang P, Burrows LL. Biofilm formation by hyperpiliated mutants of Pseudomonas aeruginosa. Journal of Bacteriology, 2003, 185(7): 2374-2378. DOI:10.1128/JB.185.7.2374-2378.2003 |
| [7] | Speers AM, Schindler BD, Hwang J, Genc A, Reguera G. Genetic identification of a PilT motor in Geobacter sulfurreducens reveals a role for pilus retraction in extracellular electron transfer. Frontiers in Microbiology, 2016, 7: 1578. |
| [8] | Costerton JW, Stewart PS, Greenberg EP. Bacterial biofilms: a common cause of persistent infections. Science, 1999, 284(5418): 1318-1322. DOI:10.1126/science.284.5418.1318 |
| [9] | Bieber D, Ramer SW, Wu CY, Murray WJ, Tobe T, Fernandez R, Schoolnik GK. Type IV pili, transient bacterial aggregates, and virulence of enteropathogenic Escherichia coli. Science, 1998, 280(5372): 2114-2118. DOI:10.1126/science.280.5372.2114 |
| [10] | Guo W, Zou LF, Li YR, Cui YP, Ji ZY, Cai LL, Zou HS, Hutchins WC, Yang CH, Chen GY. Fructose-bisphophate aldolase exhibits functional roles between carbon metabolism and the hrp system in rice pathogen Xanthomonas oryzae pv. oryzicola. PLoS One, 2012, 7(2): e31855. DOI:10.1371/journal.pone.0031855 |
| [11] | Zou HS, Yuan L, Guo W, Li YR, Che YZ, Zou LF, Chen GY. Construction of a Tn5-tagged mutant library of Xanthomonas oryzae pv. Current Microbiology, 2011, 62(3): 908-916. DOI:10.1007/s00284-010-9804-1 |
| [12] |
Zhao Q, Zou LF, Zou HS, Li YR, Chen GY. Genetic modification of a biocontrol agent Pseudomonas fluorescens 2P24 by a harpin coding gene. Chinese Journal of Biological Control, 2012, 28(1): 87-94.
(in Chinese) 赵倩, 邹丽芳, 邹华松, 李玉蓉, 陈功友. 利用编码harpin蛋白的基因遗传改良生防荧光假单胞菌2P24. 中国生物防治学报, 2012, 28(1): 87-94. |
| [13] | Cai LL, Zou LF, Ge L, Xue XB, Zou HS, Chen GY. An inner membrane protein (Imp) of Xanthomonas oryzae pv. oryzicola functions in carbon acquisition, EPS production, bacterial motility and virulence in rice. Journal of Integrative Agriculture, 2014, 13(12): 2656-2668. DOI:10.1016/S2095-3119(14)60915-1 |
| [14] | Ficarra FA, Grandellis C, Galván EM, Ielpi L, Feil R, Lunn JE, Gottig N, Ottado J. Xanthomonas citri ssp. citri requires the outer membrane porin OprB for maximal virulence and biofilm formation. Molecular Plant Pathology, 2017, 18(5): 720-733. DOI:10.1111/mpp.2017.18.issue-5 |
| [15] | Bahar O, Goffer T, Burdman S. Type IV pili are required for virulence, twitching motility, and biofilm formation of Acidovorax avenae subsp. citrulli. Molecular Plant-Microbe Interactions, 2009, 22(8): 909-920. DOI:10.1094/MPMI-22-8-0909 |
| [16] | Craig L, Li J. Type IV pili: paradoxes in form and function. Current Opinion in Structural Biology, 2008, 18(2): 267-277. DOI:10.1016/j.sbi.2007.12.009 |
| [17] | Ryan RP, Fouhy Y, Lucey JF, Crossman LC, Spiro S, He YW, Zhang LH, Heeb S, Cámara M, Williams P, Dow JM. Cell-cell signaling in Xanthomonas campestris involves an HD-GYP domain protein that functions in cyclic di-GMP turnover. Proceedings of the National Academy of Sciences of the United States of America, 2006, 103(17): 6712-6717. DOI:10.1073/pnas.0600345103 |
| [18] |
Yin FQ, Zhao YC, Liu CH, Qian GL, Fan JQ, Hu BS, Liu FQ. Analysis of the flgD and figE genes regulated by diffusible signal factor in Xanthomonas oryzae pv. oryzicola. Acta Microbiologica Sinica, 2011, 51(7): 891-897.
(in Chinese) 殷芳群, 赵延存, 刘春晖, 钱国良, 范加勤, 胡白石, 刘凤权. 水稻细菌性条斑病菌中受DSF调控的鞭毛基因flgD、flgE的功能分析. 微生物学报, 2011, 51(7): 891-897. |
| [19] | Zhao YC, Qian GL, Fan JQ, Yin FQ, Zhou YJ, Liu CH, Shen Q, Hu BS, Liu FQ. Identification and characterization of a novel gene, hshB, in Xanthomonas oryzae pv. oryzicola co-regulated by quorum sensing and clp. Phytopathology, 2012, 102(3): 252-259. DOI:10.1094/PHYTO-06-11-0169 |
| [20] | Tsuge S, Nakayama T, Terashima S, Ochiai H, Furutani A, Oku T, Tsuno K, Kubo Y, Kaku H. Gene involved in transcriptional activation of the hrp regulatory gene hrpG in Xanthomonas oryzae pv. oryzae. Journal of Bacteriology, 2006, 188(11): 4158-4162. DOI:10.1128/JB.00006-06 |
| [21] | Tsuge S, Terashima S, Furutani A, Ochiai H, Oku T, Tsuno K, Kaku H, Kubo Y. Effects on promoter activity of base substitutions in the cis-acting regulatory element of HrpXo regulons in Xanthomonas oryzae pv. oryzae. Journal of Bacteriology, 2005, 187(7): 2308-2314. DOI:10.1128/JB.187.7.2308-2314.2005 |
 2018, Vol. 58
2018, Vol. 58