中国科学院微生物研究所,中国微生物学会,中国菌物学会
文章信息
- Lulu Jiang, Mingjia Zhang, Meizhu Meng, Mingjie Xie. 2018
- 姜路路, 张铭嘉, 孟美竹, 谢明杰. 2018
- Honokiol kills Candida albicans through ROS accumulation and cell membrane destruction
- 和厚朴酚通过ROS的积累和破坏细胞膜杀死白色念珠菌
- Acta Microbiologica Sinica, 58(3): 511-519
- 微生物学报, 58(3): 511-519
-
文章历史
- 收稿日期:2017-09-06
- 修回日期:2017-10-28
- 网络出版日期:2017-11-14
Candida species are the most common fungal pathogens and they are responsible for both superficial and systemic infection[1]. Among the Candida species, Candida albicans is the predominant species that causes invasive candidiasis in most countries[2]. Candida albicans can become an opportunistic pathogen in immunocompromised patients, some immunologically weak individuals, and even in healthy persons[3].
Current drug therapies for Candida albicans infections are often toxic if used over a long period of time, and their effectiveness is also limited. Thus, the search for new antifungal drugs from natural products is a necessity[4]. Magnolia officinalis is a traditional Chinese medicine that has been used for the treatment of diarrhea, acute pain, coughs, and urinary problems[5]. Honokiol (5, 5'-diallyl-2, 4'-dihydroxybiphenyl, 2), a neolignan compound, is primarily isolated from the dried stem, root, or branch bark of M. officinalis. Honokiol exhibits antiangiogenic, anti-inflammatory, and antitumor properties without appreciable toxicity in preclinical models[6]. Honokiol also has an antifungal activity[7]. However, the detailed antifungal mechanism of honokiol is still largely unclear. In this study, we evaluated the mechanisms of honokiol-induced cell death in C. albicans. We demonstrated that honokiol caused cell death through accumulation of reactive oxygen species (ROS) and cell membrane damaging.
1 Materials and Methods 1.1 Candida albicans strainsC. albicans (ATCC10231) was obtained from the Chinese Medical Culture Collection Center. C. albicans was grown in yeast extract peptone dextrose (YEPD) medium (10 g of yeast extract powder, 20 g of peptone and 20 g of glucose in 1 L of double distilled water) at 30 ℃ in shaking flasks to logarithmic growth phase. For all assays described below, C. albicans cells in exponential phase in YEPD medium were incubated with various concentrations of drugs. Viability of C. albicans cells were determined by a colony count determination.
1.2 Drugs and materialsHonokiol powder was purchased from Chengdu Must Bio-Technology CO., LTD. and a stock solution of 20 mg/mL was prepared with dimethyl sulfoxide (DMSO). Culture medium and chemicals were purchased from Sangon Biotech unless specifically stated.
1.3 Determination of minimum inhibitory concentration (MIC) and minimum fungicidal concentration (MFC)The MIC of honokiol against C. albicans in this assay was determined according to a standardized broth microdilution method (Clinical and Laboratory Standards Institute (CLSI) document M27-A2)[8]. Briefly, 100 μL of RPMI-1640 medium was transferred into the wells of a 96-well microdilution plate. Then, 100 μL of honokiol solution (diluted by RPMI-1640) was added in the first well of horizontal row of the plate wells. Doubled serial dilutions, where a 100 μL aliquot removed from the most concentrated well to the next well, yielded concentrations of 1024-1 μg/mL. Finally, 10 μL of C. albicans inoculum suspension (1×104 CFU/mL) was added to each well of the plate. In parallel, controls were made with the standard antifungal drug amphotericin B. The plates were incubated at 30 ℃ for 24 h, and then detected by microplate reader (Thermo Scientific Multiskan FC). The MIC90 was defined as the lowest concentration that inhibited 90% growth of Candida albicans.
To determine the MFC, 1 μL aliquots of C. albicans was subcultured with 1×MIC or 2×MIC or 4×MIC of the honokiol on Petri dishes containing SDA at 30 ℃ for 48 h. The MFC was defined as the lowest concentration that inhibited 99% growth of Candida albicans as that honokiol inhibited growth of the yeast or permitted less than 3 CFUs to occur.
Biological activity assays were performed in duplicate, and the arithmetic mean of the MIC90 and MFC were used as resutls.
1.4 Transmission electron microscopy (TEM) observation of the morphology of C. albicans cellsC. albicans cells (1×105 CFU/mL) were exposed to a series of concentrations of honokiol (0, 1×MIC, and 2×MIC) for 12 h. After washed twice with PBS, cells were fixed in 2.0% glutaraldehyde (prepared in 0.1 mol/L phosphate buffer, pH 7.0). The cells were then postfixed in 1% osmium tetroxide buffer. After dehydrated in a graded ethanol series, cells were embedded in spur resin and cut into thin sections with an ultramicrotome. The section grids were stained with saturated solutions of uranylacetate and lead citrate. TEM [JEM-ARM200F(C)] was performed at a magnification of ×10000.
1.5 Annexin V/PI double staining of C. albicansCells undergoing apoptosis were determined by the Annexin V-FITC apoptosis detection kit. C. albicans cells (1×105 CFU/mL) were exposed to different concentrations of honokiol (0, 0.5×MIC, 1.0×MIC, or 2.0×MIC) for 4 h. After washed with PBS, cells (1×106 CFU/mL) were resuspended in binding buffer with Annexin V-FITC and incubated for 10 min at room temperature protected from light. After washed with PBS, cells were resuspended in binding buffer with propidium iodide (PI). The apoptosis cells were analyzed by flow cytometry (FACSCalibur)[15].
1.6 Measurement of reactive oxygen species (ROS) generationThe ROS accumulation of C. albicans cells was determined by Reactive Oxygen Species assay kit (Beyotime). C. albicans cells (1×105 CFU/mL) were exposed to different concentrations of honokiol (0, 0.5×MIC, 1.0×MIC, or 2.0×MIC) for 2 h or 4 h, then washed with PBS. Cells were resuspended in binding buffer with DCFH-DA and incubated for 20 min at 37 ℃. After washed with PBS for 3 times, cells (1×105 CFU/mL) were resuspended in PBS then transferred to a 96-well microdilution plate and detected by Fluorescence microplate reader (Thermo) at excitation wavelength of 488 nm and emission wavelength of 525 nm. The treatment of 50 μg/mL Rosup was severed as positive control. Assays were performed at least in triplicate and repeated at least 3 times[16].
1.7 Analysis of mitochondrial membrane potentialMitochondrial membrane potential was evaluated using the mitochondrial membrane potential assay kit with JC-1(Beyotime). C. alcicans cells (1×105 CFU/mL) were exposed to different concentrations of honokiol (0, 0.5×MIC, 1.0×MIC, or 2.0×MIC) for 4 h, After washed with PBS, cells were resuspended in binding buffer with JC-1 and incubated 20 min at 37 ℃. After washed with PBS for 3 times, cells (1×105 CFU/mL) were resuspended in PBS, and then transferred to a 96-well microdilution plate. JC-1 monomers and J-aggregates were detected by Fluorescence microplate reader (Thermo). The value of JC-1 monomers/J-aggregates reflected the variation of mitochondrial membrane potential. JC-1 monomers were detected at excitation wavelength of 490 nm and emission wavelength of 530 nm; J-aggregates were detected at excitation wavelength of 525 nm and emission wavelength of 590 nm. The treatment of 2 μg/mL of carbonyl cyanide m-chloropheny lhydrazone (cccp) was severed as positive control. Assays were performed at least in triplicate and repeated at least three times[21].
1.8 Detection of membrane permeabilizationThe membrane permeabilization of C. albicans cells were detected by PI staining. C. alcicans cells (1×106 CFU/mL) were incubated with different concentrations of honokiol (0, 1×MIC, or 2×MIC) at 30 ℃. At the time of exposed for 2 h, 4 h, and 6 h, 1 mL of suspension was transfered to tubes respectively, and then washed with PBS for 3 times. Cells were resuspended in PBS with PI of 20 μg/mL and incubated 60 min at room temperature. Cells were detected by fluorescence spectrophotometer (FL1102M001) at excitation wavelength of 535 nm and emission wavelength of 615 nm. Assays were performed at least in triplicate and repeated at least three times[25].
1.9 Evaluation the effect of sorbitol and ergosterol on C. albicansTo determine whether the mechanisms of honokiol were involved in only destroying the yeast cell wall or also damaging the cell membrane, the assay was performed using medium with sorbitol or ergosterol. To evaluate the effect on the cell wall, the sorbitol was added to the culture medium in a final concentration of 0.8 mol/L. The MIC of honokiol against C. albicans in the presence of sorbitol was detected as above. Since the sorbitol is a fungal cell wall osmotic protective agent, by comparing to the standard medium, the higher MIC values observed in the medium with the addition of sorbitol implicate the cell wall might be one of the targets of honokiol. Amphotericin B (AmB) was used as the control drug[25].
To assess whether the honokiol could damage the cell membrane by binding to the fungal membrane sterols, the ergosterol was added to the culture medium in a final concentration of 400 μg/mL. The MIC of honokiol against C. albicans in the presence of ergosterol was detected as above. Compared to the standard medium, the higher MIC values observed in the medium with the addition of ergosterol implicate the fungal membrane sterols as one of the targets of honokiol. AmB was used as the control drug[16].
1.10 Statistical analysisData were analyzed by using SPSS 13.0 and Microsoft Excel 2010 software. One-way analysis of variance, using the Dunnett Multiple Comparison test, was carried out on data obtained from three independent assays performed in duplicate for each sample. Levels of statistical significance at P < 0.05 and P < 0.01 were used.
2 Results and Discussion 2.1 Minimum inhibitory concentration (MIC) and minimum fungicidal concentration (MFC) of honokiol against C. albicansThe antimicrobial activities of the products were interpreted (considered active or not), according to the criteria proposed by Morales et al[8]. In the experiment that the C. albicans was treated with a series diluted honokiol, like the positive control antifungal drug amphotericin B, the honokiol produced a strong and dose-dependent inhibitory effect on C. albicans with MIC90 and MFC values of 16 μg/mL and 32 μg/mL respectively.
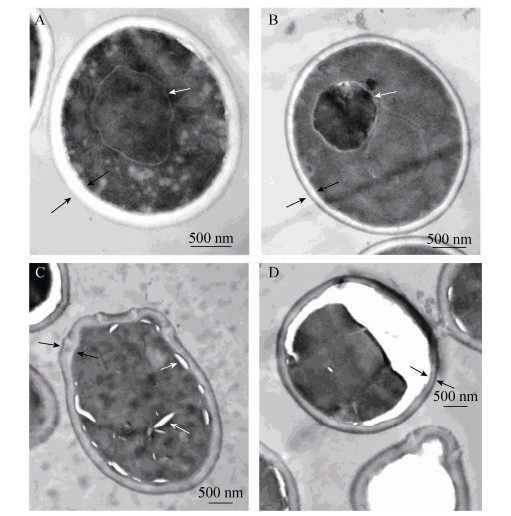
2.2 Effects of honikiol on the internal morphology of C. albicansChanges in the internal morphology of C. albicans in response to honokiol treatment were observed by TEM. Untreated C. albicans showed normal cellular morphology with a distinct cell membrane and intact membranous organelles. The cell membrane and the cell wall were smooth without any damage (Figure 1-A). In contrast, C. albicans exposed to 16 μg/mL honikiol displayed cytoplasmic coagulation phenomenon. In addition, the cell wall also became thinner compared to untreated control cells (Figure 1-B). When C. albicans was treated with 32 μg/mL honikiol, part of the cell wall of C. albicans became distorted and irregular cell walls together with the vacuoles in the cytoplasm were seen (Figure 1-C). In some of the cells that exposed to 32 μg/mL honikiol, the plasma membrane was completely destroyed, causing partial or total cytoplasmic outflow (Figure 1-D).
|
| Figure 1 Ultrastructure of honokiol-treated C. albicans cells. Samples were treated with different concentrations of honokiol. Untreated control (A), 16 μg/mL honikiol treated cells (B) and 32 μg/mL honikiol treated cells (C, D) for 12 hours, then observed with TEM. Images are shown as 1000 × magnifications. |
2.3 Effect of honokiol on C. albicans viability
Over the last decade, there have been numerous reports on apoptosis in yeasts and filamentous fungi[9-12]. Indeed, apoptosis can be induced in C. albicans by oxidative stress, exposure to acetic acid, and expression of mammalian pro-apoptotic proteins acidification[11-14]. Notably, C. albicans cells exhibit apoptotic markers that are similar to those of mammalian cells, including phosphatidylserine externalization, reactive oxygen species (ROS) accumulation, mitochondrial membrane potential dissipation, and DNA condensation and fragmentation[15].
Induction of apoptosis in C. albicans following the treatment with honikiol was examined by Annexin V-FITC/PI double straining, which specifically detects the presence of the apoptotic marker phosphatidylserine on the outler leaflet. The result showed that honikiol induced both apoptosis and necrosis in C. albicans (Figure 2). After four hours of treatment with 32 g/mL honokiol, the proportion of early apoptotic cells were decreased from 56.3% to 22.8%. In contrast, the proportion of necrotic cells increased from 4.1% to 20.3%. This suggest that honokiol could induce apoptosis in C. albicans, however, this is not the only mechanism for honokiol induced C. albicans cell death, perticularly for the high concentration of honokiol, but the necrosis could also be an alternative mechanism.

|
| Figure 2 Annexin V-FITC/PI double straining of C. albicancs treated with honokiol. Samples were treated with different concentrations of honokiol. Untreated control (A), 16, 24, 32 μg/mL honikiol treated cells (B–D) for 4 hours then stained with Annexin V-FITC. |
2.4 Effect of honokiol on reactive oxygen species (ROS) production
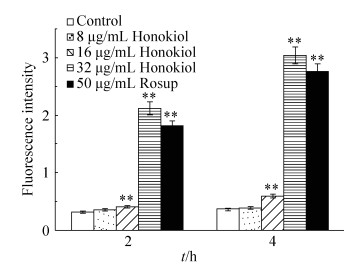
To measure the changes in ROS generation, we exploited a fluorescent molecule chloromethyl-Dichlorodihydrofluorescein diacetate (CM-H2DCFDA), which could readily enter into the cells and was sensitive to redox changes. It is deacetylated by endogenous esterase into dichlorofluorescein, which fluoresces on oxidation by ROS[16]. Candida albicans exposed to 16 μg/mL honokiol for 2 h or 4 h produced significantly more ROS than untreated C. albicans (Figure 3). Increasing the concentration of honikiol to 32 μg/mL for 4 h further increased the amount of ROS production, with the 4 h exposure time resulting in 741% increase in fluorescence intensity relative to that of untreated control cells.
|
| Figure 3 Effect of honokiol on the production of ROS in C. albicans. Samples were treated with different concentrations of honokiol for 2 or 4 hours, then stained with DCFH-DA and detected by Fluorescence microplate reader. Mean values and SD are shown for triplicate samples from three independent experiments, ** P < 0.01 vs untreated control. |
ROS, as a byproduct in cell metabolism, plays important physiological roles in the life cycle of a cell. However, if the level of ROS in the cell exceeds its antioxidant capacity, it may induce cellular damage, and eventually cell death[17]. The generation of oxygen radicals is a key event of apoptosis in yeast[18]. ROS level arises mainly because of electron leakage during aerobic respiration and environmental stress stimuli such as ultraviolet (UV) irradiation, herbicides, air pollutants, and xenobiotics[19]. Excessive ROS production can result in apoptosis or necrosis in C. albicans[20]. We have shown in this study that C. ablicans exposed to high concentration of honokiol produced rapid increase in ROS production.
2.5 Effect of honokiol on mitochondrial membrane potential (mtΔΨ)We used the vital mitochondrial dye JC-1 to investigate mitochondrial function. JC-1 selectively enters the mitochondria. In healthy cells with high mtΔΨ, it spontaneously forms complexes known as J-aggregates that exhibit intense red fluorescence. In apoptotic or unhealthy cells with low mtΔΨ, JC-1 remains in the monomeric form, which shows green fluorescence[21]. From the ratio of JC-1 monomer and J-aggregates, we can observe the mtΔΨ alteration treated with or without honokiol.
Candida albicans treated with 16 μg/mL honokiol for 4 h displayed significant loss in mitochondrial membrane potential in a dose-dependent manner (Figure 4). The dissipation of mitochondrial membrane potential is a key cellular event during early apoptosis, which leads to the opening of the transition pores of the mitochondrial membrane and the release of apoptogenic factors into the cytosol[22-23]. This suggested that loss of mitochondrial membrane potential could be an important feature associated with honokiol-induced cell death in C. albicans.

|
| Figure 4 Effect of honokiol on mitochondrial membrane potential in C. albicans. Samples were treated with different concentrations of honokiol. Different concentrations of honokiol and 2 μg/mL CCCP for 4 hours, then stained with JC-1 and detected by fluorescence microplate reader. Mean values and SD are shown for triplicate samples from three independent experiments, ** P < 0.01 vs untreated control. |
2.6 Effect of honokiol on cell membrane permeability
PI is a red fluorescent phenanthridinium intercalating dye used extensively for detecting dead cells[24]. It does not pass through the intact cell membrane. Honikiol significantly influenced the permeability of the cell membrane of C. albicans, especially at high concentration.
The cell membrane is essential for the survival of fungal cell. The changes in cellular morphology of honokiol-treated C. albicans as revealed by TEM and PI staining, indicated the damage to the cell membrane caused by honokiol. The high level of PI fluorescence observed for honokiol-treated C. albicans indicated the loss of controlled membrane permeability, especially at higher concentration of honokiol (Figure 5).

|
| Figure 5 Effect of honokiol on membrane permeability in C. albicans. C. albicans was cultured with difference concentration of honokiol (0, 16, 32 μg/mL) for 2, 4, 6 hours, then stained with PI. The fluorescence intensity was detected with fluorescent spectrophotometry after 1 hour. Mean values and SD are shown for triplicate samples from three independent experiments. **P < 0.01 vs control. |
2.7 Effect of honokiol on the cell wall and ergosterol
Sorbitol is an osmotic protector which could be used to stabilize fungi protoplasts. Specific fungal cell wall inhibitors share a distinctive characteristic in that their antifungal effects are reversed in mediums containing sorbitol. If a fungal cell wall inhibitor is added to the cells in the presence of sorbitol, the cells are protected and growth may not be inhibited[25]. This effect is manifested as increases in the MIC value for medium containing sorbitol compared to medium without sorbitol. Ergosterol is the major sterol component present in the plasma membrane of fungi and it plays the same role in fungal membranes as does cholesterol in mammalian cell membranes. Thus, these two sterols seem to exhibit similar properties.
The MIC90 of honokiol against C. albicans increased by 4 fold when sorbitol was present in the medium, and to 64 fold when sorbitol was replaced with eregosterol (Table 1). The cell wall confers cell morphology and protection of the fungal cell against environmental insults[26-28]. To investigate the action of honokiol on the fungal cell wall we performed an assay with sorbitol which has an osmoprotectant function. Smotic destabilizing agents and disrupting the cell wall lead to rearrangements of the cell wall and allow the fungal cells to survive. Our result suggested that honokiol might act by inhibiting fungal cell wall synthesis, but this may not be the key antifungal mechanism of honikiol. The effect of destroying cell membrane permeabilization involved in binding with ergosterol.
| Drugs | Sorbitol | Ergosterol | |||
| Absence | Presence | Absence | Presence | ||
| Honokiol | 16.0 | 64.0 | 16.0 | > 1024.0 | |
| Amphotericin B | 0.5 | 1.0 | 0.5 | 512.0 | |
3 Conclusions
We have demonstrated the antifungal activity of honokiol using Candida albicans as the testing fungi. Honokiol appeared to be a good antifungal agent, and its antifungal activity involved a number of mechanisms, which included induction of apoptosis and necrosis through promoting the production of ROS and dissipating mitochondrial membrane potential, as well as altering membrane permeability through the combination of ergosterol. These findings could provide a theoretical basis for the development of honokiol as an antifungal drug.
| [1] | Mayer FL, Wilson D, Hube B. Candida albicans pathogenicity mechanisms. Virulence, 2013, 4(2): 119-128. DOI:10.4161/viru.22913 |
| [2] | Turner SA, Butler G. The Candida pathogenic species complex. Cold Spring Harbor Perspectives in Medicine, 2014, 4(9): a019778. DOI:10.1101/cshperspect.a019778 |
| [3] | Kabir MA, Hussain MA, Ahmad Z. Candida albicans:A model organism for studying fungal pathogens. ISRN Microbiology, 2012, 2012: 538694. |
| [4] | Soares LA, de Cássia Orlandi Sardi J, Gullo FP, de Souza Pitangui N, Scorzoni L, Leite FS, Giannini MJSM, Almeida AMF. Anti dermatophytic therapy-Prospects for the discovery of new drugs from natural products. Brazilian Journal of Microbiology, 2013, 44(4): 1035-1041. DOI:10.1590/S1517-83822014005000011 |
| [5] | Kim SY, Kim J, Jeong SI, Jahng KY, Yu KY. Antimicrobial effects and resistant regulation of magnolol and honokiol on methicillin-resistant Staphylococcus aureus. Biomed Research International, 2015, 2015: 283630. |
| [6] | Fried LE, Arbiser JL. Honokiol, a multifunctional antiangiogenic and antitumor agent. Antioxidants & Redox Signaling, 2009, 11(5): 1139-1148. |
| [7] | Bang KH, Kim YK, Min BS, Na MK, Rhee YH, Lee JP, Bae KH. Antifungal activity of magnolol and honokiol. Archives of Pharmacal Research, 2000, 23(1): 46-49. DOI:10.1007/BF02976465 |
| [8] | Morales G, Paredes A, Sierra P, Loyola LA. Antimicrobial activity of three baccharis species used in the traditional medicine of Northern Chile. Molecules, 2008, 13(4): 790-794. |
| [9] | Madeo F, Herker E, Wissing S, Jungwirth H, Eisenberg T, Fröhlich KU. Apoptosis in yeast. Current Opinion in Microbiology, 2004, 7(6): 655-660. DOI:10.1016/j.mib.2004.10.012 |
| [10] | Herker E, Jungwirth H, Lehmann KA, Maldener C, Fröhlich KU, Wissing S, Büttner S, Fehr M, Sigrist S, Madeo F. Chronological aging leads to apoptosis in yeast. The Journal of Cell Biology, 2004, 164(4): 501-507. DOI:10.1083/jcb.200310014 |
| [11] | Côrte-Real M, Madeo F. Yeast programed cell death and aging. Frontiers in Oncology, 2013, 3(2): 283. |
| [12] | Carmona-Gutierrez D, Alavian-Ghavanini A, Habernig L, Bauer M, Hammer A, Rossmann C, Zimmermann A, Ruckenstuhl C, Büttner S, Eisenberg T, Sattler W, Malle E, Madeo F. The cell death protease Kex1p is essential for hypochlorite-induced apoptosis in yeast. Cell Cycle, 2013, 12(11): 1704-1712. DOI:10.4161/cc.24801 |
| [13] | Shrestha SK, Fosso MY, Green KD, Garneautsodikova S. Amphiphilic tobramycin analogues as antibacterial and antifungal agents. Antimicrobial Agents & Chemotherapy, 2015, 59(8): 4861-4869. |
| [14] | Cerbón J, Calderón V. Changes of the compositional asymmetry of phospholipids associated to the increment in the membrane surface potential. Biochimica et Biophysica Acta (BBA)-Biomembranes, 1991, 1067(2): 139-144. DOI:10.1016/0005-2736(91)90035-7 |
| [15] | Falcone C, Mazzoni C. External and internal triggers of cell death in yeast. Cellular and Molecular Life Sciences, 2016, 73(11/12): 2237-2250. |
| [16] | Lebel CP, Ischiropoulos H, Bondy SC. Evaluation of the probe 2', 7'-dichlorofluorescin as an indicator of reactive oxygen species formation and oxidative stress. Chemical Research in Toxicology, 1992, 5(2): 227-231. DOI:10.1021/tx00026a012 |
| [17] | Liemburg-Apers DC, Willems PHGM, Koopman WJH, Grefte S. Interactions between mitochondrial reactive oxygen species and cellular glucose metabolism. Archives of Toxicology, 2015, 89(8): 1209-1226. DOI:10.1007/s00204-015-1520-y |
| [18] | Madeo F, Fröhlich E, Ligr M, Grey M, Sigrist SJ, Wolf DH, Fröhlich KU. Oxygen stress:A regulator of apoptosis in yeast. The Journal of Cell Biology, 1999, 145(4): 757-767. DOI:10.1083/jcb.145.4.757 |
| [19] | Halliwell B, Cross CE. Oxygen-derived species:their relation to human disease and environmental stress. Environmental Health Perspectives, 1994, 102(Suppl 10): 5-12. DOI:10.1289/ehp.94102s105 |
| [20] | Perrone GG, Tan SX, Dawes IW. Reactive oxygen species and yeast apoptosis. Biochimica et Biophysica Acta (BBA)-Molecular Cell Research, 2008, 1783(7): 1354-1368. DOI:10.1016/j.bbamcr.2008.01.023 |
| [21] | Hao B, Cheng S, Clancy CJ, Nguyen MH. Caspofungin kills Candida albicans by causing both cellular apoptosis and necrosis. Antimicrobial Agents & Chemotherapy, 2013, 57(1): 326-332. |
| [22] | Green DR, Reed JC. Mitochondria and apoptosis. Science, 1998, 281(5381): 1309-1312. DOI:10.1126/science.281.5381.1309 |
| [23] | Krysko DV, Roels F, Leybaert L, D'Herde K. Mitochondrial transmembrane potential changes support the concept of mitochondrial heterogeneity during apoptosis. Journal of Histochemistry & Cytochemistry, 2001, 49(10): 1277-1284. |
| [24] | Bunthof CJ, Bloemen K, Breeuwer P, Rombouts FM, Abee T. Flow cytometric assessment of viability of lactic acid bacteria. Applied & Environmental Microbiology, 2001, 67(5): 2326-2335. |
| [25] | Leite MCA, de Brito Bezerra AP, de Sousa JP, Sarmento Guerra FQ, de Oliveira Lima E. Evaluation of antifungal activity and mechanism of action of Citral against Candida albicans. Evidence-Based Complementary and Alternative Medicine, 2014, 2014: Article ID 378280. |
| [26] | Mouyna I, Fontaine T, Vai M, Monod M, Fonzi WA, Diaquin M, Popolo L, Hartland RP, Latgé JP. Glycosylphosphatidylinositol-anchored glucanosyltransferases play an active role in the biosynthesis of the fungal cell wall. Journal of Biological Chemistry, 2000, 275(20): 14882-14889. DOI:10.1074/jbc.275.20.14882 |
| [27] | Rolli E, Ragni E, Calderon J, Porello S, Fascio U, Popolo L. Immobilization of the glycosylphosphatidylinositol-anchored Gas1 protein into the chitin ring and septum is required for proper morphogenesis in yeast. Molecular Biology of the Cell, 2009, 20(22): 4856-4870. DOI:10.1091/mbc.E08-11-1155 |
| [28] | Ene IV, Walker LA, Schiavone M, Lee KK, Martin-Yken H, Dague E, Gow NAR, Munro CA, Brown AJP. Cell wall remodeling enzymes modulate fungal cell wall elasticity and osmotic stress resistance. mBio, 2015, 6(4): e00986-15. |
 2018, Vol. 58
2018, Vol. 58