中国科学院微生物研究所,中国微生物学会,中国菌物学会
文章信息
- Haowen Shi, Sanfeng Chen. 2018
- 石皓文, 陈三凤. 2018
- Genome-wide transcriptional analysis of the recombinant Escherichia coli 78-7 carrying a nif gene operon of Paenibacillus polymyxa WLY78
- 携带Paenibacillus polymyxa WLY78固氮基因簇(nif)的重组大肠杆菌Escherichia coli78-7转录组分析
- Acta Microbiologica Sinica, 58(3): 489-500
- 微生物学报, 58(3): 489-500
-
文章历史
- 收稿日期:2017-05-13
- 修回日期:2017-06-08
- 网络出版日期:2017-07-21
The diazotrophic Paenibacillus polymyxa WLY78 possesses a minimal and compact nif gene cluster composed of nine genes (nifBnifHnifDnifKnifEnifNnifXhesAnifV) which are organized as an operon. In contrast, the diazotrophic models Klebsiella oxytoca and Azotobacter vinelandii have 20 nif genes (nifJ, H, D, K, Y, T, E, N, X, U, S, V, Z, W, M, F, L, A, B, Q) organized several transcription units[1-2]. Our laboratory has demonstrated that the Paenibacillus nif gene operon under the control of its σ70-dependent promoter located in front of the nifB gene enabled Escherichia coli to synthesize functional nitrogenase[3]. Our recent study has revealed that in P. polymyxa WLY78, the nif genes and the non-nif genes encoding transporters for Mo, S and Fe are coordinatively and highly transcribed under N2-fixing condition compared to non-N2-fixing condition[4]. Whether the foreign Paenibacillus nif genes and the native non-nif genes encoding transporters for Mo, Fe and S in the recombinant E. coli 78-7 can be coordinatively and highly transcribed under N2-fixing condition is not known.
Here, we performed a genome-wide transcriptome analysis of the recombinant E. coli 78-7 cultured under non-N2-fixing and N2-fixing conditions. Our results revealed that the nif gene operon composed of nifBHDKENXhesAnifV is significantly expressed in E. coli in both conditions. The non-nif genes encoding transporters of Fe, S, Mo and electrons, the sufABCDSE operon and the isc system specific for synthesis of the Fe-S cluster are analysed. The transcriptions of genes involved in nitrogen metabolism, ATP synthesis and sigma factors are also analysed. This study will provide valuable information for engineering nitrogenase biosynthetic pathway into non-N2-fixing-organisms and will also provide guidance for improving nitrogenase activity in the heterologous host.
1 Materials and methods 1.1 Bacterial strains, media and growth conditionsThe recombinant E. coli 78-7, a derivative carrying the nif operon (nifBHDKENXhesAnifV) of P. polymyxa WLY78, is used in this study[3]. The medium and growth conditions for the strain are used according the reference[3].
1.2 Isolation of RNA and Quantitative real-time RT-PCRThe recombinant E. coli 78-7 was grown to OD600=0.30-0.45 at different concentrations of oxygen and ammonium, and cells were then harvested by centrifugation at 4 ℃. Isolation of total RNA and Quantitative real-time RT-PCR were performed according to the methods described by Shi et al[4]. The primers used for qRT-PCR reactions are listed in Supplementary Table S1.
1.3 Transcriptomic analysisThe construction of cDNA library and SOLiD sequencing for the total RNA were completed at the Beijing Genomics Institute (Chinese Academy of Sciences), and each sample were carried out three technical replicates. The raw reads were mapped to the reference genome (E. coli K12) using the programme BWA as formerly described[5-6]. We used DEGseq to identify differentially expressed genes from RNA-seq data[7]. Transcript level differences with adjusted P values of < 0.001 were considered to be significant. The RNA-seq sequencing data of the recombinant E. coli 78-7 have been deposited in NCBI database under accession number SRP053133.
2 Results 2.1 Genome-wide transcription analysis of the recombinant E. coli 78-7A genome-wide transcription analysis of the recombinant E. coli 78-7 cultured under non-N2-fixing and N2-fixing conditions is performed. Among 4366 genes of the recombinant E. coli 78-7, transcript abundances are increased for 1274 (28%) genes, decreased for 1381 (32%) and not changed for 1711 genes (39%) under the N2-fixing condition compared to those under the non-N2-fixing condition control (Figure 1 and Table 1). Based on log2 fold changes (P < 0.05), transcript levels for nearly 5% (204 among 4366 genes) of the recombinant E. coli 78-7 genes are changed more than 2-fold under N2-fixing condition relative to those under the non-N2-fixing condition control.

|
| Figure 1 Changes in transcriptional levels of the total genes within the recombinant E. coli 78-7 genomes. |
| RPKM | Gene numbers/E. coli 78-7 | |
| Non-N2-fixation (100 mmol/L NH4+ and Air) (100 mmol/L NH4+ and Air) | N2-fixation (without NH4+ and O2) (without NH4+ and O2) | |
| 0.00 | 292 | 301 |
| 0.00–0.25(> 0) | 48 | 56 |
| 0.25–0.50 | 75 | 86 |
| 0.50–1.00 | 192 | 187 |
| 1.00–5.00 | 901 | 907 |
| 5.00–10.00 | 585 | 558 |
| 10.00–50.00 | 1405 | 1399 |
| 50.00–100.00 | 366 | 390 |
| 100.00–500.00 | 377 | 361 |
| 500.00–1000.00 | 66 | 74 |
| 1000.00–** | 58 a | 46 a |
| a The gene number of this relative expression level includes the nif genes. | ||
2.2 Transcriptional analysis of the nitrogen fixation genes
In E. coli 78-7, the expression levels of the nine genes nifBHDKENXhesAnifV within the nif operon fall into the highest range in both non-N2-fixing and N2-fixing conditions (Figure 2 and Table 1). The mean expression levels of the nine foreign nif genes nifBHDKENXhesAnifV are much higher than those of the native E. coli genes, suggesting that the nif promoter is effectively recognized and transcribed by the E. coli RNA polymerase.

|
| Figure 2 Differential expression of the nif gene operon (nifBHDKENXhesdAnifV) in N2-fixing and non-N2-fixing conditions. A: Transcriptional analysis of the nif genes in the recombinant E. coli 78-7; B: Quantitative real-time RT-PCR analysis of nif gene expression in the recombinant E. coli 78-7. |
2.3 Transcriptional analysis of molybdate transporters
Molybdenum is necessary in bacteria for the activity of a limited number of microbial enzymes[8], including nitrogenase[9] and nitrate reductase[10]. As only trace amounts molybdate is present in the environment, bacteria employ an high-affinity energy-dependent molybdate transporter to accumulate it. The E. coli molybdate transporter is encoded by the modABCD operon, which is negatively regulated by the modE gene product in response to the intracellular molybdate concentration. In addition, E. coli has the modF gene, whose function is unknown.
In E. coli 78-7, the expression levels of the modABCE genes are similar in both the N2-fixing condition and the non-N2-fixing condition (Figure 3-A). However, the expression level of modF is significantly up-regulated in the N2-fixing condition.

|
| Figure 3 Differential expression of the genes relating to the transport, storage and regulation of molybdenum, sulfate and iron in N2-fixing and non-N2-fixing conditions. A: The mod genes encoding molybdenum transporters; B: The genes encoding sulfate transporters; C: The genes encoding iron transport, storage and regulation. |
2.4 Transcriptional analysis of sulfate transporters
Sulfur is an basic element for microorganisms, peculiarly for diazotrophs whose nitrogenase containing iron-sulfur clusters[2, 11-13]. Sulfate and thiosulfate being the preferred sulfur sources for most organisms, are taken in by sulfate permeases through membrane transporters. And sulfate permeases in bacteria belong to the SulT (Sbp/CysPTWA), SulP, CysP/(PiT) and CysZ families[13]. It is reported that sulfate can also be transported by the molybdate transport system, as it is structurally related to the oxyanion molybdate[10].
In E. coli 78-7, sulfate permeases include SulT (CysPTWA), SulP and CysZ. The SulT of E. coli 78-7 is encoded by the operon cysPTWA. E. coli also contains a sbp gene, which is located in another chromosomal region. Sbp and CysP have partially overlapping activities, and it has been suggested that both proteins interact with membrane proteins CysT and CysW of the SulT permease[13]. The cysPTWA cluster is up-regulated from 16-fold to 40-fold, but cysZ and sulP are down-regulated under the N2-fixing condition compared to under the non-N2-fixng condition (Figure 3-B). The cysK and cysM encoding cysteine synthase and the genes cysS, cysC, cysN, cysN, cysD, cysH, cysJ, cysG and cysE involved in sulfur metabolism are also up-regulated in the N2-fixing condition compared to in the non-N2-fixing condition.
2.5 Transcriptional analysis of Fe transporterIron (Fe) is also an basic element for almost all organisms and is required in cofactors of many enzymes, involving nitrogenase. under anaerobic conditions or at an acidic pH, Fe is the soluble Fe2+ form (ferrous iron). The main route for bacteria to-ferrous-iron uptake would appear to be, in many instances, via Feo (ferrous iron transport)[14]. Three proteins, FeoA, FeoB and FeoC, compose the enterobacterial Feo system. Though the functions of FeoA and FeoC remain unclear, FeoB is responsible for transporting ferrous iron. The feoABC genes constitute an operon, but in some bacteria, feoA and feoC are not always present aside feoB[14-15].
In E. coli 78-7, the foeABC operon is weakly depressed in the N2-fixing condition compared to in the non-N2-fixing condition (Figure 3-C). The fur gene encoding the ferric uptake regulator Fur as an transcriptional activator, which controls the transcription of genes involved in iron homeostasis as well as its own synthesis, is also weakly depressed in the N2-fixing condition compared to in the non-N2-fixing condition (Figure 3-C). The fecABCDE operon encoding the Fe3+ dicitrate transport system is weakly re-regulated in the N2-fixing condition. The fhuACDB operon, specifying the Fe3+ hydroxamate uptake apparatus, is transcribed at similar levels in both the non-N2-fixing and N2-fixing conditions. ftn, encoding iron storage protein, is up-regulated 1.21-fold in the N2-fixing condition. The fhuE, fhuF, and cirA genes involved in ferri-coprogen/ rhodotorulic acid, ferrioxamine B, and ferric-dihydroxy benzoate utilization are transcribed at similar levels in both the non-N2-fixing and N2-fixing conditions.
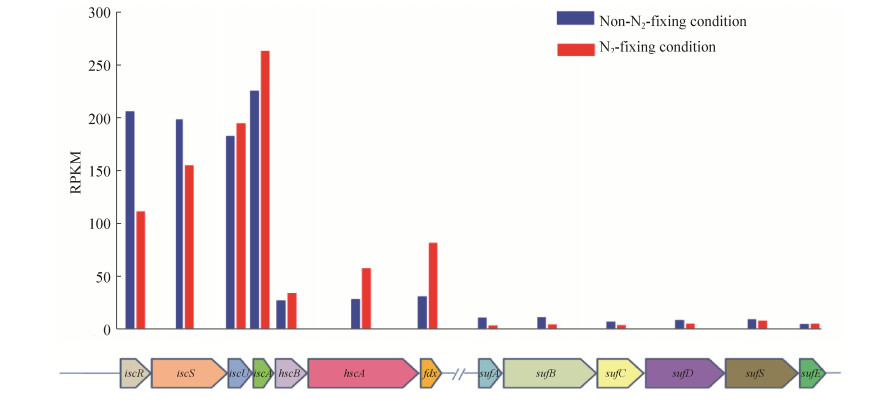
2.6 Transcriptional analysis of the iron-sulfur cluster assembly systemNitrogenase is a complex [Fe-S] enzyme, and its [Fe-S] clusters play a critical role in electron transport and in the substrates reduction driven by the free energy liberated from Mg-ATP hydrolysis[2, 11-12]. NifUS (products of nifU and nifS gene), which mobilizes S and Fe for the assembly of small Fe/S fragments, is commonly considered to be specialized for the assembly of the Fe4-S4 cluster of NifH. NifU and NifS also participate in the assembly of the FeMo-co and the P-cluster of the NifDK component of nitrogenase[16]. nifSU is widely distributed in diazotrophs, such as A. vinelandii and K. oxytoca. Except nifSU, the isc (iscR, iscU, iscS, iscA, hscB, hscA, fdx and iscX) system also plays a critical function in the assembly of the Fe-S cluster in A. vinelandii[17]. However, the Paenibacillus nif operon does not contain nifSU. The synthesis of the Fe-S cluster in E. coli 78-7 is possibly provided by E. coli iron-sulfur cluster assembly systems. There are two iron-sulfur cluster assembly systems in E. coli: the sufABCDSE operon and the isc system (iscR, iscS, iscU, iscA, hscB, hscA, fdx and orf3)[18]. This study shows that the isc system is highly transcribed in both the non-N2-fixing and N2-fixing conditions. However, the transcription level of the sufABCDSE operon is very low in both conditions (Figure 4). The data might imply that the assembly of Fe-S clusters of nitrogenase in E. coli 78-7 may be due to the main contribution of the isc system. Our results are consistent with a report stating that the suf operon and isc operon were differently expressed when E. coli cells were treated with hydrogen peroxide[19].
|
| Figure 4 Differential expression of the genes involved in the synthesis of the Fe-S cluster in N2-fixing and non-N2-fixing conditions. |
2.7 Transcriptional analysis of electron transporters
Nitrogen fixation is performed by the nitrogenase, which transfers electrons originating from low-potential electron carriers, such as ferredoxin or flavodoxin molecules, to molecular N2[20]. In K. oxytoca, the physiological electron flow to nitrogenase particularly involves the products of the nifF and nifJ genes[21]. The product of nifF gene, a flavodoxin, mediates electron transfer from the product of nifJ gene, a pyruvate-flavodoxin oxidoreductase, to the Fe protein of nitrogenase[22].
In E. coli 78-7, there are several genes encoding flavodoxins, including fldA and fldB (Figure 5-A). The fldA is weakly up-regulated 1.07-fold, while the fldB is weakly down-regulated 0.8-fold. Other genes encoding electron transport proteins such as hydN are also up-regulated in the N2-fixing condition compared to in the non-N2-fixing condition. Notably, the groEL(encoding chaperonin), which was demonstrated to play an important role in the biogenesis of the MoFe protein in the E. coli carrying K. oxytoca nif genes[23], is significantly transcribed in both the non-N2-fixing and N2-fixing conditions.

|
| Figure 5 Differential expression of the genes relating to the electron transport, ATP synthase, respiration and energy metabolism, nitrogen metabolism and specifying σ factors in the N2-fixing and non-N2-fixing conditions. A: The electron transport genes; B: The atp genes encoding ATP synthase; C: The genes involved in respiration and energy metabolism; D: The specific genes for nitrogen metabolism; E: The genes specifying σ factors. |
2.8 Transcriptional analysis of ATPase
Most biological nitrogen fixation products are catalysed by the molybdenum nitrogenase enzyme according to the following reaction: N2+8e-+16MgATP+ 8H++16MgADP→2NH3+H2+16MgADP+16Pi[24]. The nitrogen fixation process is coupled to the hydrolysis of 16 equivalents of ATP. In E. coli 78-7, except for atpB and atpI, the other atp genes are highly expressed under the N2-fixing condition compared to under the non-N2-fixing condition (Figure 5-B).
2.9 Respiration and energy metabolismNitrogen fixation was performed in anaerobic or microanaerobic conditions, as nitrogenase is very sensitive to oxygen. In E. coli 78-7, several genes, including ccmDE encoding cytochrome c biogenesis protein, appBC encoding cytochrome bd-Ⅱ oxidase subunits and cybB encoding cytochrome b561, are up-regulated from 1.15-fold to 2.87-fold. The cyoABCDE and cydAB are weakly down-regulated in the N2-fixing condition compared to in the non-N2-fixing condition. Notably, ndh encoding respiratory NADH dehydrogenase is up-regulated 10.38-fold (Figure 5-C). It has been reported that there are two transcriptional regulators controlling independent networks of oxygen-regulated gene expression in E. coli[25]. One is a two-component sensor-regulator system (ArcB-A), which represses a wide variety of aerobic enzymes under anaerobic conditions. The other is FNR, the transcriptional regulator essential for expressing anaerobic respiratory processes[26]. In this study, the E. coli fnr gene is found to be down-regulated 0.52-fold in the N2-fixing condition. arcA and arcB are down-regulated 0.36-fold and 1.7-fold, respectively, in the N2-fixing condition compared to in the non-N2-fixing condition. The data are consistent in that fnr expression in E. coli is weakly repressed by anaerobiosis, while fnr gene expression in B. subtilis is strongly activated by anaerobiosis[27]. The narIJHG encoding nitrate reductase subunits are up-regulated from 2.07-fold to 2.64-fold. The nirBCencoding nitrite reductase subunits are up-regulated. The narK encoding nitrate/nitrite transporter is weakly down-regulated in the N2-fixing condition. Our results are consistent with reports that the nasDE and narGHI were induced by the low oxygen supply in B. subtilis[28].
2.10 Transcriptional analysis of nitrogen metabolismIt has been reported that in enteric bacteria, including E. coli and K. oxytoca, the global transcriptional control of the enzymes involved in nitrogen assimilation was mediated by a two-component nitrogen regulatory (ntr) system encoded by the ntrBC genes. In E. coli 78-7, the glnAntrBC operon is up-regulated 17.96-, 6.5-and 11.98-fold, respectively, in the N2-fixing condition compared to in the non-N2-fixing condition (Figure 5-D). The first three up-regulated genes in the N2-fixing condition are nac, glnK and amtB with a 334-fold, 2154-fold and 147-fold increase, respectively. It was reported that binding of GlnK to the ammonia channel AmtB regulates the channel, thereby controlling ammonium influx in response to the intracellular nitrogen status in E. coli[29]. The nitrogen assimilation control protein (NAC) is a regulatory protein responsible for activating the transcription of operons such as hutUH, putP and ureDABCEFG, whose products supply the cell with ammonia or glutamate from histidine, proline and urea, respectively[30-31]. NAC is also responsible for repressing the transcription of the gdhA and gltBD operons, whose products are involved in assimilating ammonia under conditions of nitrogen excess or limitation[30]. The expression of the nac gene is entirely dependent on (NtrC) and σ54. In E. coli 78-7, nac is significantly differently expressed, suggesting it plays an important role in the regulation of nitrogen metabolism. gltB and gltD are up-regulated 60-and 82-fold, respectively and gdhA is up-regulated 26-fold. However, glnB is weakly down-regulated, while glnD and glnE are up-regulated 3.4-fold and 2.7-fold in the N2-fixing condition compared to in the non-N2-fixing condition.
2.11 Transcriptional analysis of the sigma factorsIn bacteria, the regulation of gene expression occurs basically at the level of transcription. Although repressors and activators can markedly affect the transcriptional efficiency, the interactions between RNA polymerase (RNAP) and the promoters decide the specificity of the transcription reaction[32]. The RNA polymerase holoenzyme (holo RNAP) in bacteria is constituted of core RNAP (α2β'βσω) and the sigma (σ) factor, and its σ factor recognizes promoter regions and then initiates transcription[33].
There are seven known σ factors in E. coli, each regulating a subset of genes with specific functions[32-33]. RpoD (σ70) is the housekeeping σ factor responsible for the expression of essential genes. Our previous results demonstrated that E. coli σ70 bound to the Paenibacillus nif gene promoter[3], indicating that the Paenibacillus nif gene operon in E. coli is recognized and transcribed by E. coli σ70-RNAP. Here, we find that rpoD is expressed at similar levels in both the non-N2-fixing and N2-fixing conditions (Figure 5-E and Supplementary Table S10). In addition, rpoN, which is mainly responsible for the transcription of genes in nitrogen utilization, is expressed at similar levels in both the non-N2-fixing and N2-fixing conditions. It was reported that the expression of rpoN was induced by nitrogen limitation. Perhaps the combination of limited oxygen and nitrogen here disturbs this regulation and makes the rpoN and other σ factors transcribe at similar levels in both the non-N2-fixing and N2-fixing conditions. RpoS (σS), a σ factor involved in gene expression in the stationary phase and many stress conditions, RpoH (σH) acting in heat shock response and RpoE (σE) involved in extra cytoplasmic/heat stress are weakly down-regulated by the N2-fixing condition. fliA(σF) directing the transcription of flagellar genes and fecI acting in iron transport are weakly transcribed in the N2-fixing condition.
3 DiscussionIn this study, the transcriptomic analysis of the whole genome of the recombinant E. coli 78-7 cultured under non-N2-fixing (air and 100 mmol/L NH4+) and nitrogen-fixing (without O2 and NH4+) conditions is implemented. Our study reveals that the nine genes nifB, nifH, nifD, nifK, nifE, nifN, nifX, hesA and nifV from Paenibacillus are significantly transcribed in E. coli under both nitrogen-fixing and non-nitrogen-fixing conditions, indicating that the σ70-dependent nif promoter of the Paenibacillus nif gene operon was efficiently recognized and transcribed by E. coli σ70-RNAP. The data are consistent with our previous demonstration using electrophoretic mobility shift assays (EMSAs) whereby E. coli σ70-RNAP bound to the Paenibacillus nif gene promoter in vitro[3]. However, the result of similar transcription levels of the nine genes nifBHDKENXhesAnifV in E. coli is very different from that in P. polymyxa WLY78 where nine genes nifBHDKENXhesAnifV are differently expressed under both condition[4], supporting that the negative regulation of nif gene transcription by ammonium and oxygen is bypassed in heterologous E. coli.
Furthermore, we investigate the transcription levels of some non-nif genes which are required for nitrogen fixation in E. coli. The mod and feoAB encoding transporters of Mo and Fe, respectively, and fldA and fldB encoding electron transporters are transcribed at low levels in both conditions. The cys genes specific for transport of S is highly up-regulated in N2-fixing condition compared to non-N2-fixing condition. The isc system specific for the synthesis of the Fe-S cluster is transcribed in medium level in both conditions, whereas suf operon specific for the synthesis of the Fe-S cluster is transcribed at very low level in both conditions. Our current results are different from those obtained in P. polymyxa WLY78 where those non-nif genes are coordinately induced with nif genes under N2-fixing condition[4].
It has been demonstrated that E. coli groEL (encoding molecular chaperones) play a role in biogenesis of MoFe protein. The level of MoFe protein is drastically reduced in an E. coli groEL mutant and overexpression of the groE operon in K. oxytoca leads to accumulation of MoFe protein[23]. In this study, we found that E. coli groEL are highly expressed in both N2-fixing and non-N2-fixing conditions, and are up-regulated by N2-fixing condition. The highly expressed groEL might play important role in nitrogen fixation of E. coli.
Most of the genes involved in nitrogen metabolism are significantly up-regulated in N2-fixing condition compared to non-N2-fixing condition. Especially, nac, glnK and amtB are significantly up-regulated in N2-fixing condition. Other genes involved in nitrogen metabolism, such as glnAntrBC operon, gltB, gltD and gdhA was up-regulated in the N2-fixing condition. Our results are consistent with the previous reports that limited nitrogen activated the expression of the specific genes for nitrogen assimilation, such as ntrBC and glnA[30-31, 34].
It is well known that the global level of nif regulation in K. oxytoca is mediated by the NtrB-NtrC two-component regulatory system[20]. The level of phosphorylated NtrC controls expression of the nifL-nifA operon which in turn controls the transcription of other 18 nif genes exception of nifL-nifA[20]. The genome of P. polymyxa WLY78 does not contain nifA and ntrC, but it has a glnR gene whose predicted product is a global regulator of nitrogen metabolism existing in Gram-positive bacteria, including Paenibacillus and Bacillus. The absence of GlnR in E. coli might explain why Paenibacillus nif gene transcription in E. coli is not regulated by ammonium and oxygen. In addition, the constitutive expression of the foreign nif genes suggests that NtrC is not involved in the regulation of nif gene transcription in E. coli, although NtrC activates the expression of NifA in enteric diazotrophic K. oxytoca.
As described above, this study reveals that the Paenibacillus nif genes are significantly transcribed in E. coli 78-7 in both N2-fixing and non-N2-fixing condition, whereas the native non-nif genes, such as Fe2+/Fe3+ transporters, electron transporters and Fe-S cluster assembly systems required for nitrogen fixation, are transcribed at low level in N2-fixing condition in E. coli. The data suggest that the highly transcribed nif genes alone are not enough to maintain high nitrogenase activity of the recombinant E. coli. The lower nitrogenase activity in E. coli than that in Paenibacillus might be caused by the low expression of those native non-nif genes of E. coli. Thus, we deduce that the nitrogenase activity of the recombinant E. coli 78-7 can be enhanced by increasing the transcription levels of the native Fe2+/Fe3+ transporters, electron transporters and Fe-S cluster assembly systems of the recombinant E. coli 78-7. The hypothesis is supported by our recent reports that Paenibacillus suf operon and pfoAB, fldA and fer (the potential electron transport genes), and K. oxytoca nifSU (Fe-S cluster assembly) and nifFJ (electron transport specific for nitrogenase) can increase nitrogenase activity of the recombinant E. coli 78-7[35].
| [1] | Dixon R, Cheng Q, Shen GF, Day A, Dowson-Day M. Nif gene transfer and expression in chloroplasts:prospects and problems. Plant and Soil, 1997, 194(1/2): 193-203. DOI:10.1023/A:1004296703638 |
| [2] | Rubio LM, Ludden PW. Biosynthesis of the iron-molybdenum cofactor of nitrogenase. Annual Review of Microbiology, 2008, 62: 93-111. DOI:10.1146/annurev.micro.62.081307.162737 |
| [3] | Wang LY, Zhang LH, Liu ZZ, Zhao DH, Liu XM, Zhang B, Xie JB, Hong YY, Li PF, Chen SF, Dixon R, Li JL. A minimal nitrogen fixation gene cluster from Paenibacillus sp. WLY78 enables expression of active nitrogenase in Escherichia coli. PLoS Genetics, 2013, 9(10): e1003865. DOI:10.1371/journal.pgen.1003865 |
| [4] | Shi HW, Wang LY, Li XX, Liu XM, Hao TY, He XJ, Chen SF. Genome-wide transcriptome profiling of nitrogen fixation in Paenibacillus sp. WLY78. BMC Microbiology, 2016, 16: 25. DOI:10.1186/s12866-016-0642-6 |
| [5] | Li H, Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics, 2009, 25(14): 1754-1760. DOI:10.1093/bioinformatics/btp324 |
| [6] | Li H, Durbin R. Fast and accurate long-read alignment with Burrows-Wheeler transform. Bioinformatics, 2010, 26(5): 589-595. DOI:10.1093/bioinformatics/btp698 |
| [7] | Wang LK, Feng ZX, Wang X, Wang XW, Zhang XG. DEGseq:an R package for identifying differentially expressed genes from RNA-seq data. Bioinformatics, 2010, 26(1): 136-138. DOI:10.1093/bioinformatics/btp612 |
| [8] | Self WT, Grunden AM, Hasona A, Shanmugam KT. Molybdate transport. Research in Microbiology, 2001, 152(3/4): 311-321. |
| [9] | Hernandez JA, George SJ, Rubio LM. Molybdenum trafficking for nitrogen fixation. Biochemistry, 2009, 48(41): 9711-9721. DOI:10.1021/bi901217p |
| [10] | Pau RN, Lawson DM. Transport, homeostasis, regulation, and binding of molybdate and tungstate to proteins. Metal Ions in Biological Systems, 2002, 39: 31-74. |
| [11] | Roberts GP, MacNeil T, MacNeil D, Brill WJ. Regulation and characterization of protein products coded by the nif (nitrogen fixation) genes of Klebsiella pneumoniae. Journal of Bacteriology, 1978, 136(1): 267-279. |
| [12] | Hu YL, Ribbe MW. Biosynthesis of nitrogenase FeMoco. Coordination Chemistry Reviews, 2011, 255(9/10): 1218-1224. |
| [13] | Aguilar-Barajas E, Díaz-Pérez C, Ramírez-Díaz MI, Riveros-Rosas H, Cervantes C. Bacterial transport of sulfate, molybdate, and related oxyanions. BioMetals, 2011, 24(4): 687-707. DOI:10.1007/s10534-011-9421-x |
| [14] | McHugh JP, Rodríguez-Qui ones F, Abdul-Tehrani H, Svistunenko DA, Poole RK, Cooper CE, Andrews SC. Global iron-dependent gene regulation in Escherichia coli. A new mechanism for iron homeostasis. Journal of Biological Chemistry, 2003, 278(32): 29478-29486. DOI:10.1074/jbc.M303381200 |
| [15] | Cartron ML, Maddocks S, Gillingham P, Craven CJ, Andrews SC. Feo-transport of ferrous iron into bacteria. Biometals, 2006, 19(2): 143-157. DOI:10.1007/s10534-006-0003-2 |
| [16] | Zhao DH, Curatti L, Rubio LM. Evidence for nifU and nifS participation in the biosynthesis of the iron-molybdenum cofactor of nitrogenase. Journal of Biological Chemistry, 2007, 282(51): 37016-37025. DOI:10.1074/jbc.M708097200 |
| [17] | Johnson DC, Dos Santos PC, Dean DR. NifU and NifS are required for the maturation of nitrogenase and cannot replace the function of isc-gene products in Azotobacter vinelandii. Biochemical Society Transactions, 2005, 33(1): 90-93. DOI:10.1042/BST0330090 |
| [18] | Johnson DC, Dean DR, Smith AD, Johnson MK. Structure, function, and formation of biological iron-sulfur clusters. Annual Review of Biochemistry, 2005, 74: 247-281. DOI:10.1146/annurev.biochem.74.082803.133518 |
| [19] | Zheng M, Wang XD, Templeton LJ, Smulski DR, LaRossa RA, Storz G. DNA microarray-mediated transcriptional profiling of the Escherichia coli response to hydrogen peroxide. Journal of Bacteriology, 2001, 183(15): 4562-4570. DOI:10.1128/JB.183.15.4562-4570.2001 |
| [20] | Dixon R, Kahn D. Genetic regulation of biological nitrogen fixation. Nature Reviews Microbiology, 2004, 2(8): 621-631. DOI:10.1038/nrmicro954 |
| [21] | Nieva-Gómez D, Roberts GP, Klevickis S, Brill WJ. Electron transport to nitrogenase in Klebsiella pneumoniae. Proceedings of the National Academy of Sciences of the United States of America, 1980, 77(5): 2555-2558. DOI:10.1073/pnas.77.5.2555 |
| [22] | Hill S, Kavanagh EP. Roles of nifF and nifJ gene products in electron transport to nitrogenase in Klebsiella pneumoniae. Journal of Bacteriology, 1980, 141(2): 470-475. |
| [23] | Govezensky D, Greener T, Segal G, Zamir A. Involvement of GroEL in nif gene regulation and nitrogenase assembly. Journal of Bacteriology, 1991, 173(20): 6339-6346. DOI:10.1128/jb.173.20.6339-6346.1991 |
| [24] | Capone DG. Marine nitrogen fixation:what's the fuss?. Current Opinion in Microbiology, 2001, 4(3): 341-348. DOI:10.1016/S1369-5274(00)00215-0 |
| [25] | Kiley PJ, Beinert H. Oxygen sensing by the global regulator, FNR:the role of the iron-sulfur cluster. FEMS Microbiology Reviews, 1998, 22(5): 341-352. DOI:10.1111/j.1574-6976.1998.tb00375.x |
| [26] | Gunsalus RP, Park SJ. Aerobic-anaerobic gene regulation in Escherichia coli:control by the ArcAB and Fnr regulons. Research in Microbiology, 1994, 145(5/6): 437-450. |
| [27] | Ramos HC, Boursier L, Moszer I, Kunst F, Danchin A, Glaser P. Anaerobic transcription activation in Bacillus subtilis:identification of distinct FNR-dependent and -independent regulatory mechanisms. The EMBO Journal, 1995, 14(23): 5984-5994. |
| [28] | Nakano MM, Hoffmann T, Zhu Y, Jahn D. Nitrogen and oxygen regulation of Bacillus subtilis nasDEF encoding NADH-dependent nitrite reductase by TnrA and ResDE. Journal of Bacteriology, 1998, 180(20): 5344-5350. |
| [29] | Radchenko MV, Thornton J, Merrick M. Control of AmtB-GlnK complex formation by intracellular levels of ATP, ADP, and 2-oxoglutarate. Journal of Biological Chemistry, 2010, 285(40): 31037-31045. DOI:10.1074/jbc.M110.153908 |
| [30] | Bender RA. The role of the NAC protein in the nitrogen regulation of Klebsiella aerogenes. Molecular Microbiology, 1991, 5(11): 2575-2580. DOI:10.1111/mmi.1991.5.issue-11 |
| [31] | Muse WB, Bender RA. The nac (nitrogen assimilation control) gene from Escherichia coli. Journal of Bacteriology, 1998, 180(5): 1166-1173. |
| [32] | Dong T, Yu R, Schellhorn H. Antagonistic regulation of motility and transcriptome expression by RpoN and RpoS in Escherichia coli. Molecular Microbiology, 2011, 79(2): 375-386. DOI:10.1111/mmi.2011.79.issue-2 |
| [33] | Helmann JD, Chamberlin MJ. Structure and function of bacterial sigma factors. Annual Review of Biochemistry, 1988, 57: 839-872. DOI:10.1146/annurev.bi.57.070188.004203 |
| [34] | Rubio LM, Rangaraj P, Homer MJ, Roberts GP, Ludden PW. Cloning and mutational analysis of the γ gene from Azotobacter vinelandii defines a new family of proteins capable of metallocluster binding and protein stabilization. Journal of Biological Chemistry, 2002, 277(16): 14299-14305. DOI:10.1074/jbc.M107289200 |
| [35] | Li XX, Liu Q, Liu XM, Shi HW, Chen SF. Using synthetic biology to increase nitrogenase activity. Microbial Cell Factories, 2016, 15: 43. DOI:10.1186/s12934-016-0442-6 |
 2018, Vol. 58
2018, Vol. 58