中国科学院微生物研究所,中国微生物学会,中国菌物学会
文章信息
- Li Chen, Jianke Li, Msatoshi Shirota, Kouhei Ohnishi, Akinori Kiba, Yasufumi Hikichi. 2018
- 陈立, 李建科, 成田雅敏, 大西浩平, 木厂章范, 曳地康史. 2018
- Involvement of GALA effectors in Ralstonia solanacearum disease development towards two host plants
- Ⅲ型效应子GALA对青枯菌在两种寄主植物上致病性的影响
- Acta Microbiologica Sinica, 58(1): 131-141
- 微生物学报, 58(1): 131-141
-
文章历史
- 收稿日期:2017-02-13
- 修回日期:2017-03-28
- 网络出版日期:2017-05-12
2. Research Institute of Molecular Genetics, Kochi University, Nankoku, Kochi 783-8502, Japan;
3. Laboratory of Plant Pathology and Biotechnology, Kochi University, Nankoku, Kochi 783-8502, Japan
2. 高知大学分子遗传研究中心, 日本 南国市 783-8502;
3. 高知大学植物病理研究所, 日本 南国市 783-8502
Many Gram-negative pathogenic bacteria infect plant and animals through the type Ⅲ secretion system (T3SS) to cause symptoms on their respective hosts[1-2]. The T3SS is a syringe needle-like structure consisting of inner and outer membrane rings and a protruding filament called a pilus which works as a conduit to deliver an array of bacterial proteins into host cells[3-5]. Those bacterial proteins, named type Ⅲ effectors (T3Es) are injected into the cytosol of eukaryotic cells to modulate host defense signaling pathways and to promote disease. However, some effectors are recognized by a cognate resistance protein, thereby triggering host defenses, resulting in a rapid and intense host defense response known as the hypersensitive response (HR)[6]. Previous researches focused on the role of the T3Es which could modify various plant cellular function to promote the fitness of the pathogen[7-8], however limited success was achieved.
Ralstonia solanacearum is a Gram-negative β-proteobacterium pathogenic to plants and responsible for the development of bacterial wilting disease on more than 200 plant species from 50 botanical families, including economical crops such as eggplant, tomato, tobacco and banana[9]. It has been ranked the top two most important bacterial plant pathogen, following the first one Pseudomonas syringae. This pathogen endangers the food safety in tropical and subtropical agriculture, especially in China, Bolivia, Bangladesh and Uganda. It has been reported that T3Es are involved in controlling the host invasion stages but the mechanism remains unclear[10].
It is a tough work to study the physiological and molecular functions of the T3Es[11-13]. So far, only a few number of T3E of R. solanacearum strain GMI1000 have been biochemically characterized, such as GALA family (seven effectors), AWR family (five effectors). GALAs possess an F-box domain and Leu-rich repeat (LRR) which interact with Arabidopsis ASK proteins, mimicing plant ubiquitin E3 ubiquitin ligases[14-17]. Single gala mutants derived from GMI1000 barely affected their pathogenicity, whereas the septuple mutant shows less pathogenic on Arabidopsis and delayed virulence on tomato.
In present study, we isolated a R. solanacearum strain OE1-1 from Japan. Previous genomic analysis showed that GALA effectors from strain OE1-1 and GMI1000 shared high homologous and identity (97%-99%). Strain OE1-1 (race 1, biovor 3) causes lethal wilting disease on tobacco and tomato whereas GMI1000 results in wilting disease on Arabidopsis and tomato[18]. For GMI1000, none of the seven single gala mutants are affected in their pathogenicity, whereas the septuple mutant shows less pathogenic on Arabidopsis and delayed virulence on tomato. To our best knowledge, the contribution of GALA effectors to pathogenicity of R. solanacearum strain OE1-1 remains unknown. In this study, we constructed single and multiple deletion mutants of gala generated from OE1-1, investigated the role of these effectors by observing the phenotypes of infected host plants and measuring the internal bacterial multiplication. Tobacco and tomato were chosen as the susceptible host plants for OE1-1 instead of Arabidopsis. Through comparing and analysis, we found that GALA effectors of OE1-1 were essential for disease on tobacco but not on tomato which was different from GMI1000. Single gala mutants of OE1-1 barely affected their virulence on tobacco, but the absence of all seven gala genes resulted in delayed virulence on tobacco.
1 Material and methods 1.1 Bacterial strains, plasmids and growth conditionsThe bacterial strains and plasmids used for this study are described in Table 1. R. solanacearum strain OE1-1 (race 1, biovar 3) and its derivative mutants were streak on BG medium (1.0% of bacto peptone, 0.1% of yeast extract, 0.1% of casamino acids, 0.5% of glucose, and 1.5% of agar) and incubate at 28 ℃ for 2 days. B medium (1.0% of bacto peptone, 0.1% of yeast extract, and 0.1% of casamino acids) was used to inoculate with cells of a selected single colony and incubated overnight at 28 ℃. Escherichia coli DH12S (Invitrogen Corp., Carlsbad, CA, USA) and S17-1 were grown on Luria-Bertani (LB) medium (Miller, 1992; 1.0% tryptone, 0.5% yeast extract, and 0.5% NaCl) and incubate at 37 ℃ overnight. Escherichia coli DH12S was used for plasmid construction and S17-1 was used in conjugation experiments. Antibiotics were used at the following concentrations: ampicillin (Ap), 100 μg/mL; kanamycin (Km), 50 μg/mL; polymyxin B (PB), 50 μg/mL. And 5-bromo-4-chloro-3-indolyl-beta-D-galactopy-ranoside (X-gal), 40 μg/mL, isopropyl-beta-D-thiogalactopyranosiade (IPTG), 100 μmol/L.
| Strains | Description | References |
| R. solanacearum | ||
| OE1-1 | Wild type (race 1 biovar 3) | [19] |
| RK7007 | OE1-1Δgala3 | This study |
| RK7008 | OE1-1 Δgala2 | This study |
| RK7009 | OE1-1 Δ gala6, 7 | This study |
| RK7017 | OE1-1 Δ gala4, 5, 6, 7 | This study |
| RK7018 | OE1-1 Δ gala3, 4, 5, 6, 7 | This study |
| RK7019 | OE1-1 Δ gala2, 3, 4, 5, 6, 7 | This study |
| RK7020 | OE1-1 Δ gala1 | This study |
| RK7022 | OE1-1 Δ gala1, 2, 3, 4, 5, 6, 7 | This study |
| RK7024 | OE1-1 Δ gala7 | This study |
| RK7025 | OE1-1 Δ gala4 | This study |
| RK7026 | OE1-1 Δ gala5 | This study |
| RK7036 | OE1-1 Δ gala6 | This study |
| E. coli DH12S | araD 139 Δ(ara, leu) 7697 ΔlacX74 galU galK mcrA Δ(mrr -hsdRMSmcrBC) rps L deoR Ø80dlacZΔM15 nupG recA1/F9proAB1 lacIq ZΔM15 | Invitrogen |
| S17-1 | recA pro hsdR RP4-2-Tc::Mu-Km::Tn 7 | [20] |
| Plasmids | ||
| pBluescript Ⅱ KS+ | Ampicillin resistance | Stratagene |
| pK18mobsacB | Kanamycin resistance, oriT sacB | [21] |
| pKP0914-1 | Deletion of rsp0914 (gala1) | This study |
| pKP0672-1 | Deletion of rsp0672 (gala2) | This study |
| pKP0028-1 | Deletion of rsp0028 (gala3) | This study |
| pKC1800-1 | Deletion of rsc1800 (gala4) | This study |
| pKC1801-1 | Deletion of rsc1801 (gala5) | This study |
| pKC1356-1 | Deletion of rsc1356 (gala6) | This study |
| pKC1357-1 | Deletion of rsc1357 (gala7) | This study |
1.2 Construction of single deletion mutant
Two 500-bp fragments were PCR amplified by PrimeStar HS DNA polymerase (TaKaRa Bio, Otsu, Japan) using two pairs of primers (Table 2): OEC(P)xxxxA51 and OEC (P) xxxxB51 primers for upstream fragment, and OEC(P)xxxxA31 and OEC(P)xxxxB31 for downstream fragment. The amplified PCR fragments were run on 0.8% agarose gel electrophoresis and purified by E.Z.N.A. Gel Extraction Kit (Omega Bio-Tek, Doraville, GA, USA), then cloned on pre-digested vector pBluescript Ⅱ KS(+)/EcoR Ⅴ to generate pBC(P)xxxx-5 and pBC(P)xxxx-3 plasmids. The EcoR Ⅰ-BamH Ⅰ fragment of pBC(P)xxxx-5 and the BamH Ⅰ-Hind Ⅲ fragment of pBC(P)xxxx-3 were ligated to pre-digested vector pK18mobsacB/EcoR Ⅰ- Hind Ⅲ to generate pKC(P)xxxx. Plasmid DNA was purified using GenElute Plasmid Miniprep Kit (Sigma-Aldrich, St. Louis, MO, USA). The sequences of the plasmids were determined with Bigdye terminator v3.1 cycle sequencing kit (Applied Biosystems, Foster City, CA, USA) with primer either M13-47 (5′-TGTAAAACGACGGCC AGT-3′) or RV-M (5′-CAGGAAACAGCTATGAC C-3′) and analyzed with Applied Biosystems 3130 genetic analyzer (Applied Biosystems).
| Primers | Sequences (5′→3′) | References |
| OEC1356A51 | CCGAGCCGAAGCTGATCGGCCACG | This study |
| OEC1356B51 | ggatccCGCCGTCTCCGTTACCTATCCATG | This study |
| OEC1356A31 | ggatccATGTGACGTGTGCTTGAGGTGC | This study |
| OEC1356B31 | ATATAGGGATGGGAAGCGCTG | This study |
| OEC1357A51 | CCAAGGTGCTGGAGGCCAATAC | This study |
| OEC1357B52 | ggatccCAGCTCCACTGCATGACCATG | This study |
| OEC1357A31 | ggatccGGCGGGGCGGCACTGTTTGCCTTG | This study |
| OEC1357B31 | CTCTCTCCTTGTGTGATCGATCCATC | This study |
| OEC1800A51 | TCACGGCGGCGGATGTCGAGCGCG | This study |
| OEC1800B51 | ggatccCGGCGGTGCCACCCACCGCGCCCG | This study |
| OEC1800A31 | ggatccGGCACCGCCATCGGGCCCG | This study |
| OEC1800B31 | CCGGATAGTTGCCCGCGCGC | This study |
| OEC1801A51 | GACATCGGCAACAACGGCATC | This study |
| OEC1801B51 | ggatccGCGCCACGCCCAGTCTGCTC | This study |
| OEC1801A31 | ggatccACATGCCGGTGAGTTTGCCGGCG | This study |
| OEC1801B31 | AGACCCACCTGCTGTGGGTGCCG | This study |
| OEP0028A51 | GACCTGGATGCTCGTGCTGCGCG | This study |
| OEP0028B51 | ggatccTTCCGTCCGTGGCTCCGGCAAACG | This study |
| OEP0028A31 | ggatccGGGGTGCCAGGGCATCCTCGCAAC | This study |
| OEP0028B31 | CGACAAATTCCTGATCGCCTGATC | This study |
| OEP0914A51 | CACTACGGAAACGAGGTCGCATTCAC | This study |
| OEP0914B51 | ggatccGGCAGTGCGATCGTCCTTTCTG | This study |
| OEP0914A31 | ggatccGATCGCCCCGCGCCGGGCAGGACG | This study |
| OEP0914B31 | GCCTTCGCAATCCGGGCCGTGGGCGCG | This study |
| OEP0672A51 | TAATCAAAAGTGACTCCGAAGTGC | This study |
| OEP0672B51 | ggatccGGAAGCCAGCCCGCCCAGGGGTG | This study |
| OEP0672A31 | ggatccCGCTGCGGGACCGTTCTGCAACG | This study |
| OEP0672B31 | GTGGTGGAGCACGATGCCGTGTTCG | This study |
pKC(P)xxxx was transferred from E. coli S17-1 into R. solanacearum strain. Deletion mutant strains were generated through two consecutive homologous recombination events (Figure 1). A donor E. coli S17-1 containing pKC(P)xxxx plasmid (1 mL) and a recipient R. solanacearum OE1-1 strain (1 mL) were incubated overnight in LB medium with kanamycin and B medium, respectively, and then mixed together. After centrifugation, cells were suspended in 5 mL of 10 mmol/L autoclaved MgSO4, and collected on a 0.45 μm-membrane filter. The membrane was incubated on BG agar medium at 28 ℃ overnight. Cells grown on the membrane were immersed in 10 mmol/L MgSO4 solution, and spread on BG supplemented with kanamycin and polymyxin B. Cells from several colonies were grown in B media for 5 h and spread on B agarose media supplemented with 10% sucrose. Colonies were replicated on both BG agar with polymyxin B and BG agar with kanamycin and polymyxin B. Clones, which grew only on BG agar with polymyxin B, were subjected to colony PCR (Figure 2-A). Paq5000 polymerase with 10×Paq PCR buffer (Stratagene) and 10×PCR enhancer (Invitrogen Corp.) were used for colony PCR under the condition 85 ℃ for 5 min, 35 repeats of 95 ℃ for 20 s, 60 ℃ for 20 s and 72 ℃ for 1 min.

|
| Figure 1 Construction of deletion mutant of target gala gene |
|
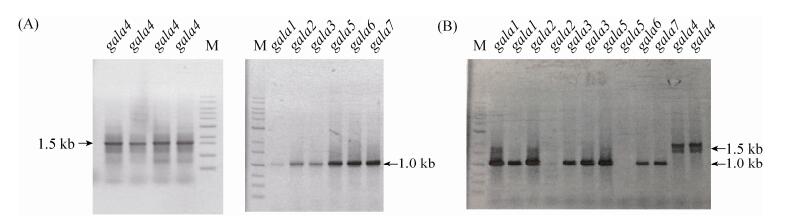
| Figure 2 Colony PCR results of mutant. A: colony PCR results of single GALA deletion mutant; B: colony PCR results of RK7022 deletion mutant |
1.3 Construction of multiple deletion mutants
A strategy was exactly same for single gene deletion. Instead of OE1-1, the already-constructed deletion mutant was used for conjugation with E. coli S17-1 containing pK18mobsacB-based plasmid. They were incubated respectively overnight in LB medium with kanamycin and B medium respectively and mixed together. After centrifugation, cells were suspended in 5 mL of 10 mmol/L autoclaved MgSO4, and collected on a 0.45 μm-membrane filter. The membrane was incubated on BG agar medium at 30 ℃ overnight. Cells grown on the membrane were immersed in 10 mmol/L MgSO4 solution, and spread on BG supplemented with kanamycin and polymyxin B. Cells from several colonies were grown in B media for 5 h and spread on B agarose media supplemented with 10% sucrose. Colonies were replicated on both BG agar with polymyxin B and BG agar with kanamycin and polymyxin B. Clones, which grew only on BG agar with polymyxin B, were subjected to colony PCR (Figure 2-B).
1.4 Plant testsR. solanacearum cells were incubated overnight in B medium and resuspended in 10 mmol/L MgSO4 at an optimal density at 600 nm (OD600) of 0.1 (1.4×108CFU/mL). For root-cutting inoculation, bacterial suspension was poured onto the root wounded plants to achieve a final concentration of 107 CFU/g. Plants were cultivated in a temperature-controlled culture room at 25 ℃ under 10000 lux (16 h light/8 h dark). Virulence assays were tested on four-week old tomato (Lycopersicon esculentum cv. Moneymaker) and tobacco (Nicotiana tabacum cv. Bright Yellow) respectively. Tomato plants were cultivated in rock-wool (Nittobo) and tobacco plants were cultivated in pots containing a mixture of vermiculite/peat moss (3:1). Each bacterial inoculation was tested on at least 4 plants and was carried out multi-replicates. Disease symptoms were scored daily for 16 days. Plants were rated according to a scale ranging: 0=no wilting; 1=1 to 25% wilting; 2=26 to 50% wilting; 3=51 to 75% wilting; and 4=76 to 100% wilting or dead.
1.5 Bacterial population dynamics in infiltrated leaf tissueInternal bacterial populations were determined at selected time intervals after inoculation. Bacterial cell suspensions (at 106 CFU/mL) in 10 mmol/L MgSO4 were infiltrated into plant leaves. Plants were grown at 25 ℃ (16 h light/8 h dark). Leaves were sampled every day. Leaf disks (an area of 0.38 cm2) were cut from infiltrated area by a sterile borer, transferred by a sterile forceps into a sterile tube containing 500 μL of sterile 10 mmol/L MgSO4, and crushed at 3000 r/min for 60 s with a 5 mm-diameter zirconia bead using Micro smash MS-100 (TOMY SEIKO). Standard 10-fold dilution plating onto B agar supplemented with PB was carried out. Colonies were counted after 2-day incubation at 28 ℃, and the bacterial populations were calculated as CFU/cm2 of leaf area.
2 Results 2.1 Comparison of GALA effectors between strain OE1-1 and GMI1000In 2006, Angot et al. identified a group of seven genes from R. solanacearum GMI1000 that contained a conserved GAxALA sequence in their Leucine-rich repeats (LRR) and named as "GALA" protein[15]. GMI1000 contains 74 effectors of which several effectors are very much conserved in phylotype Ⅰ Japanese strain OE1-1[22]. Genomic analysis of the gala genes in strain OE1-1 showed some interesting features: four of the gala gene gala4, gala5, gala6 and gala7 (RSc1800, RSc1801, RSc1356, RSc1357) located on the chromosome whereas gala1, gala2 and gala3 (RSp0914, RSp0672, RSp0028) lived on the megaplasmid. The GALA family effectors are mostly 458-1029aa in size (Table 3). Members of this family contain a Leucine-rich repeat and a F-box domain. Comparison analysis of GALA effector sequences from OE1-1 revealed that they all shared high identity and similarity with GMI1000 (97%-99%).
| Gene in OE1-1 | GALA | Size/aa | Features | Distribution in Rs species a | Identities to GMI1000/% |
| RSp0914 | GALA1 | 655 | Leucine Rich Repeats | Variable | 99 |
| RSp0672 | GALA2 | 1029 | F-box proteins | Conserved | 98 |
| RSp0028 | GALA3 | 505 | Conserved | 97 | |
| RSc1800 | GALA4 | 458 | Conserved | 99 | |
| RSc1801 | GALA5 | 533 | Conserved | 99 | |
| RSc1356 | GALA6 | 604 | Conserved | 97 | |
| RSc1357 | GALA7 | 646 | Variable | 99 | |
| a:Distribution is considered "conserved" when the gene is found in >90% of a set of 45 R. solanacearum strains through comparative genomic hybridization, otherwise it is considered "variable". | |||||
2.2 GALA effectors are important for disease on tobacco
Deciphering effector function is essential to understand the molecular interaction between pathogens and their hosts[23-25]. It has been reported that multigenic family members contribute to pathogenicity synergistically but dispensable individually. Our experiments verified this consecution in Japanese R. solanacearum strain OE1-1. Plants were inoculated with deletion mutants using the root-cutting method described in "Materials and methods". Deletion of single effector gene from gala family did not affect the bacterial pathogenicity on tobacco (Figure 3-A). The lack of phenotype of single mutants could be explained by a functional overlap of these proteins. To test this hypothesis, multiple deletion mutants were constructed and tested on tobacco. Plants inoculated with the septuple deletion mutant RK7022 showed 2-days delayed wilting symptom on tobacco, but eventually wilted in 18 days (Figure 3-B, 3-C). Consecutive deletion mutants RK7019 (∆gala2-7), RK7018 (∆gala3-7) and RK7017(∆gala4-7) affected the R. solanacearum's pathogenicity on tobacco variously.

|
| Figure 3 Pathogenicity test of OE1-1 and mutants on tobacco (Nicotiana tabacum cv. Bright Yellow). A: pathogenicity test of single deletion mutants on tobacco. B: pathogenicity test of multiple deletion mutants on tobacco. Bacterial suspension was poured onto the root wounded tobacco plants to achieve a final concentration of 107 CFU/g. Disease symptoms were scored daily for 18 days. Plants were rated according to a scale ranging of 0 to 4 (0: no wilting; 1: 1%-25% wilting; 2: 26%-50% wilting; 3: 51%-75% wilting; 4: 76%-100% wilted). Each bacterial inoculation was tested on at least 4 plants and was repeated in triplicate. The average and standard error were calculated. C: tobacco plants inoculated with OE1-1 (left) and RK7022 (right). Pictures were taken 9 days post-inoculation |
The internal bacterial population was measured by leaf-infiltration, since this methodology was reported to be more sensitive and quantitative than plant disease scoring[17]. As shown in Figure 4-A, no statistical significant difference of bacterial growth was found among single deletion mutants and wild type except RK7025 (∆gala4), RK7026 (∆gala5), RK7036 (∆gala6) and RK2024 (∆gala7) (P < 0.05). These results suggested that OE1-1 with the deletion of gala4, 5, 6, 7 affected the cell growth much more than other galas. These data correlated with the phenotypes of the single mutants and supported an important role of gala4, 5, 6, 7 in R. solanacearum OE1-1 virulence.

|
| Figure 4 Bacterial population in leaves infiltrated with OE1-1 and mutants. A: bacterial population of single deletion mutants. B: bacterial population of multiple deletion mutants. Bacterial cell suspensions (106 CFU/mL) of OE1-1 and mutants were infiltrated into leaves of tobacco (Nicotiana tabacum cv. Bright Yellow). Each assay was repeated in three successive trials, and four plants were treated within each trial. The average and standard error were calculated. Statistically significant groups were calculated using t-test (P < 0.05) |
To better understand the functional role, the bacterial growth of multiple deletion mutants were measured in tobacco leaves (Figure 4-B). The multiplication capacity of the multiple mutants was reduced with the consequent deletion of the galas. The bacterial growth of RK7022 was significant reduced compared with the wild type strain. As shown in Figure 5, the initial bacterial growth of RK7022 increased from 5.01-log to 7.11-log after 3 days inoculation while the bacterial growth of wild type varied from 5.03-log to 7.38-log. Wild type strain OE1-1 exhibited 0.64-log preservation of bacterial growth over RK7022 after 2 days inoculation (P < 0.01). These results indicated that GALA effectors jointly contributed to the growth of bacterial population.

|
| Figure 5 Bacterial population in leaves infiltrated with OE1-1 and RK7022. Bacterial cell suspensions (106CFU/mL) of OE1-1 and RK7070 were infiltrated into leaves of tobacco (Nicotiana tabacum cv. Bright Yellow). Each assay was repeated in three successive trials, and four plants were treated within each trial. The average and standard error were calculated. Statistically significant groups were calculated using t-test (P < 0.01) |
2.3 GALA effectors contribute differently towards host plants
We also evaluated the combined contribution of the gala gene family in pathogenicity towards different host plants. All the deletion mutants were tested on tomato. Angot et al. (2006) reported that single deletion mutations of gala did not affect R. solanacerum strain GMI1000 pathogenicity on tomato, but the septuple deletion mutant resulted in a significant reduction of virulence[15]. In the terms of strain OE1-1, tomato inoculated with single deletion mutants and septuple deletion mutant showed similar wilting progression as wild type in 18 days (Figure 6). These results demonstrated that gala genes of OE1-1 were essential for bacterial pathogenicity on tobacco but not on tomato.

|
| Figure 6 Pathogenicity test of OE1-1 and RK7022 on tomato (Lycopersicon esculentum cv. Moneymaker). Bacterial suspension was poured onto the root wounded tomato plants to achieve a final concentration of 107 CFU/g. Disease symptoms were scored daily for 18 days. Plants were rated according to a scale ranging of 0 to 4 (0: no wilting; 1: 1%-25% wilting; 2: 26%-50% wilting; 3: 51%-75% wilting; 4: 76%-100% wilted). Each bacterial inoculation was tested on at least 4 plants and was carried out multi-replicates. The average and standard error were calculated |
3 Discussion
In this study, we revealed the involvement of GALA effectors on pathogenicity in the Japanese R. solanacearum strain OE1-1. Although strain OE1-1 and GMI1000 both belong to phylotype I, they exhibit different pathogenicity towards host plants. The cumulative disruption of the seven gala genes strongly affects virulence of R. solanacearum strain GMI1000 on Arabidopsis and less on tomato[15]. While in our study, the septuple GALA mutant (RK7022) resulted in delayed virulence (2-day) on tobacco in comparison to wild type strain. No significant difference of wilting symptom was found between OE1-1 and RK7022 when inoculated on tomato. This suggested that GALA effectors from different R. solanacearum strains contributed in restriction of host range. Furthermore, we found the multiple mutants derived from OE1-1 showed reduced bacterial growth with the consequent deletion of galas in tobacco, but tobacco plants inoculated with mutants eventually died. These results suggested that the effect of GALAs appeared to be additive for full virulence of R. solanacearum. One GALA protein might be the virulence determinant, whereas the other members of the family act synergistically, reinforcing this function. Similar results are reported from AWR family and HLK family. The deletion of all awr genes severely impairs its capacity to multiply in host plants while AWR2 is the major contributor to virulence[17]. The absence of all three hlk genes exhibits reduced virulence on tomato and HLK2 appears to be more important than other two members[22]. These features of the multigenic family suggest that R. solanacearum virulence involves a small number of effectors with a key effect and many effectors with a weak, additive contribution.
In Arabidopsis, F-box proteins are the essential adaptor proteins linking the protein to be ubiquitinated to the SCF E3-ubiquitin ligase via their interaction. GALAs contain a plant-like F-box domain could alter protein levels through host ubiquitination pathway in Arabidopsis[26]. Genome analysis in Arabidopsis and rice reveals the presence of F-box proteins with a C-terminal lectin-related domain homologous with Nictaba, a jasmonate-inducible lectin from tobacco that is shown to interact with the core structure of high-mannose and complex N-glycans[27]. Over-expression of OsDRF1 which containing a highly conserved F-box domain in tobacco results in enhanced disease resistance against tomato mosaic virus (ToMV)[28]. Whether the GALAs of OE1-1 use the similar strategy to promote disease in tobacco need more information to explain. Future complementation studies and a yeast two-hybrid (Y2H) screen would enable us to understand the GALAs function in the interaction between R. solanacearum OE1-1 and tobacco.
| [1] | Deslandes L, Genin S. Opening the Ralstonia solanacearum type Ⅲ effector tool box:insights into host cell subversion mechanisms. Current Opinion in Plant Biology, 2014, 20: 110-117. DOI:10.1016/j.pbi.2014.05.002 |
| [2] | Huet G. Breeding for resistances to Ralstonia solanacearum. Frontiers in Plant Science, 2014, 5: 715. |
| [3] | Muthamilarasan M, Prasad M. Plant innate immunity:an updated insight into defense mechanism. Journal of Biosciences, 2013, 38(2): 433-449. DOI:10.1007/s12038-013-9302-2 |
| [4] | van der Linden L, Bredenkamp J, Naidoo S, Fouché-Weich J, Denby KJ, Genin S, Marco Y, Berger DK. Gene-for-gene tolerance to bacterial wilt in Arabidopsis. Molecular Plant-Microbe Interactions, 2013, 26(4): 398-406. DOI:10.1094/MPMI-07-12-0188-R |
| [5] | Dudler R. Manipulation of host proteasomes as a virulence mechanism of plant pathogens. Annual Review of Phytopathology, 2013, 51: 521-542. DOI:10.1146/annurev-phyto-082712-102312 |
| [6] | Khan M, Subramaniam R, Desveaux D. Of guards, decoys, baits and traps:pathogen perception in plants by type Ⅲ effector sensors. Current Opinion in Microbiology, 2016, 29: 49-55. DOI:10.1016/j.mib.2015.10.006 |
| [7] | Delga A, le Roux C, Deslandes L. Plant immune receptor decoy:pathogens in their own trap. Oncotarget, 2015, 6(18): 15748-15749. DOI:10.18632/oncotarget.v6i18 |
| [8] | Le Roux C, Huet G, Jauneau A, Camborde L, Trémousaygue D, Kraut A, Zhou BB, Levaillant M, Adachi H, Yoshioka H, Raffaele S, Berthomé R, Couté Y, Parker JE, Deslandes L. A receptor pair with an integrated decoy converts pathogen disabling of transcription factors to immunity. Cell, 2015, 161(5): 1074-1088. DOI:10.1016/j.cell.2015.04.025 |
| [9] | Peeters N, Guidot A, Vailleau F, Valls M. Ralstonia solanacearum, a widespread bacterial plant pathogen in the post-genomic era. Molecular Plant Pathology, 2013, 14(7): 651-662. DOI:10.1111/mpp.2013.14.issue-7 |
| [10] | Guidot A, Prior P, Schoenfeld J, Carrère S, Genin S, Boucher C. Genomic structure and phylogeny of the plant pathogen Ralstonia solanacearum inferred from gene distribution analysis. Journal of Bacteriology, 2007, 189(2): 377-387. DOI:10.1128/JB.00999-06 |
| [11] | Jacobs JM, Babujee L, Meng FH, Milling A, Allen C. The in planta transcriptome of Ralstonia solanacearum:conserved physiological and virulence strategies during bacterial wilt of tomato. mBio, 2012, 3(4): e00114-12. |
| [12] | Peeters N, Carrère S, Anisimova M, Plener L, Cazalé AC, Genin S. Repertoire, unified nomenclature and evolution of the Type Ⅲ effector gene set in the Ralstonia solanacearum species complex. BMC Genomics, 2013, 14: 859. DOI:10.1186/1471-2164-14-859 |
| [13] | Bonas U, Lahaye T. Plant disease resistance triggered by pathogen-derived molecules:refined models of specific recognition. Current Opinion in Microbiology, 2002, 5(1): 44-50. DOI:10.1016/S1369-5274(02)00284-9 |
| [14] | Kajava AV, Anisimova M, Peeters N. Origin and evolution of GALA-LRR, a new member of the CC-LRR subfamily:from plants to bacteria?. PLoS ONE, 2008, 3(2): e1694. DOI:10.1371/journal.pone.0001694 |
| [15] | Angot A, Peeters N, Lechner E, Vailleau F, Baud C, Gentzbittel L, Sartorel E, Genschik P, Boucher CA, Genin S. Ralstonia solanacearum requires F-box-like domain-containing type Ⅲ effectors to promote disease on several host plants. Proceedings of the National Academy of Sciences of the United States of America, 2006, 103(39): 14620-14625. DOI:10.1073/pnas.0509393103 |
| [16] | Remigi P, Anisimova M, Guidot A, Genin S, Peeters N. Functional diversification of the GALA type Ⅲ effector family contributes to Ralstonia solanacearum adaptation on different plant hosts. New Phytologist, 2011, 192(4): 976-987. DOI:10.1111/j.1469-8137.2011.03854.x |
| [17] | Solé M, Popa C, Mith O, Sohn KH, Jones JDG, Deslandes L, Valls M. 2012. The awr gene family encodes a novel class of Ralstonia solanacearum type Ⅲ effectors displaying virulence and avirulence activities. Molecular Plant-Microbe Interactions, 2012, 25(7): 941-953. DOI:10.1094/MPMI-12-11-0321 |
| [18] | Liu YQ, Kanda A, Kiba A, Hikichi Y, Ohnishi K. Distribution of avirulence genes avrA and popP1 in 22 Japanese phylotype I strains of Ralstonia solanacearum. Journal of General Plant Pathology, 2009, 75(5): 362-368. DOI:10.1007/s10327-009-0189-6 |
| [19] | Kanda A, Ohnishi S, Tomiyama H, Hasegawa H, Yasukohchi M, Kiba A, Ohnishi K, Okuno T, Hikichi Y. Type Ⅲ secretion machinery-deficient mutants of Ralstonia solanacearum lose their ability to colonize resulting in loss of pathogenicity. Journal of General Plant Pathology, 2003, 69(4): 250-257. DOI:10.1007/s10327-003-0041-3 |
| [20] | Simon R, Priefer U, Pühler A. A broad host range mobilization system for in vivo genetic engineering:transposon mutagenesis in Gram negative bacteria. Biotechnology, 1983, 1(9): 784-791. DOI:10.1038/nbt1183-784 |
| [21] | Schäfer A, Tauch A, Jäger W, Kalinowski J, Thierbach G, Pühler A. Small mobilizable multi-purpose cloning vectors derived from the Escherichia coli plasmids pK18 and pK19:selection of defined deletions in the chromosome of Corynebacterium glutamicum. Gene, 1994, 145(1): 69-73. DOI:10.1016/0378-1119(94)90324-7 |
| [22] | Chen L, Shirota M, Zhang Y, Kiba A, Hikichi Y, Ohnishi K. Involvement of HLK effectors in Ralstonia solanacearum disease development in tomato. Journal of General Plant Pathology, 2014, 80(1): 79-88. DOI:10.1007/s10327-013-0490-2 |
| [23] | Cui F, Wu S, Sun W, Coaker G, Kunkel B, He P, Shan L. The Pseudomonas syringae type Ⅲ effector AvrRpt2 promotes pathogen virulence via stimulating Arabidopsis auxin/indole acetic acid protein turnover. Plant Physiology, 2013, 162(2): 1018-1029. DOI:10.1104/pp.113.219659 |
| [24] | Wei CH, Chen JJ, Kuang HH. Dramatic number variation of R genes in Solanaceae species accounted for by a few R gene subfamilies. PLoS One, 2016, 11(2): e0148708. DOI:10.1371/journal.pone.0148708 |
| [25] | Sarris PF, Duxbury Z, Huh SU, Ma Y, Segonzac C, Sklenar J, Derbyshire P, Cevik V, Rallapalli G, Saucet SB, Wirthmueller L, Menke FLH, Sohn KH, Jones JDG. A plant immune receptor detects pathogen effectors that target WRKY transcription factors. Cell, 2015, 161(5): 1089-1100. DOI:10.1016/j.cell.2015.04.024 |
| [26] | Vierstra RD. The ubiquitin/26S proteasome pathway, the complex last chapter in the life of many plant proteins. Trends in Plant Science, 2003, 8(3): 135-142. DOI:10.1016/S1360-1385(03)00014-1 |
| [27] | Lannoo N, Peumans WJ, Van Damme EJM. Do F-box proteins with a C-terminal domain homologous with the tobacco lectin play a role in protein degradation in plants?. Biochemical Society Transactions, 2008, 36(5): 843-847. DOI:10.1042/BST0360843 |
| [28] | Cao YF, Yang YY, Zhang HJ, Li DY, Zheng Z, Song FM. Overexpression of a rice defense-related F-box protein gene OsDRF1 in tobacco improves disease resistance through potentiation of defense gene expression. Physiologia Plantarum, 2008, 134(3): 440-452. DOI:10.1111/ppl.2008.134.issue-3 |
 2018, Vol. 58
2018, Vol. 58